Open Access Article
Open Access ArticleReutilization of Fe-containing tailings ore enriched by iron(III) chloride as a heterogeneous Fenton catalyst for decolorization of organic dyes†
Lan Huong Nguyena,
Quoc Nguyen Ngob,
Huu Tap Van *c,
Van Nam Thaid,
Tan Phong Nguyena and
Kieu Oanh Phan Thie
*c,
Van Nam Thaid,
Tan Phong Nguyena and
Kieu Oanh Phan Thie
aFaculty of Environment-Natural Resources and Climate Change, Ho Chi Minh City University of Food Industry (HUFI), Ho Chi Minh City, Vietnam
bViet Nhat Industry Joint Stock Company, 140 Phan Anh Street, Tan Thoi Hoa Ward, Tan Phu District, Ho Chi Minh City, Vietnam
cFaculty of Natural Resources and Environment, TNU – University of Sciences (TNUS), Tan Thinh Ward, Thai Nguyen City, Vietnam. E-mail: tapvh@tnus.edu.vn
dHUTECH Institute of Applied Sciences, Ho Chi Minh City University of Technology (HUTECH), 475A Dien Bien Phu, Ward 25, Binh Thanh District, Ho Chi Minh City, Vietnam
eInstitute for Tropicalization and Environment (ITE), 57A Truong Quoc Dung Street, Phu Nhuan District, Ho Chi Minh City, Vietnam
First published on 28th April 2021
Abstract
In this study, the Fe-containing tailings (Fe-TO) ore was reutilized and enriched with FeCl3 as a heterogeneous catalyst for the Fenton process to degrade the organic dyes from aqueous solution. The determinants of the heterogeneous catalytic Fenton system which included iron modification ratio, solution pH, catalyst dosage, H2O2 dosage and initial concentration of organic dyes were systematically investigated. The modification ratio of 15% (w/w of iron), pH of 3, MFe-TO15 dosage of 0.5 g L−1 and H2O2 dosage of 840 mg L−1 were chosen as the best operational conditions for Fenton oxidation of organic dyes. The decolorization efficiency of both MB and RhB by MFe-TO15/H2O2 was higher than that of Fe-TO/H2O2 by about two times. The kinetic study showed the degradation of organic dyes well fitted the pseudo-first-order kinetic model with apparent constant rate values (Kd) following the same sequence as the degradation efficiency of organic dyes. The degradation mechanism of dyes could be attributed to adsorption due to the good-development in textural properties of the iron modified catalyst (MFe-TO) with an increase in BET surface area, pore volume and pore diameter of, respectively, 2, 5 and 5 times and leaching iron through homogeneous Fenton reaction. However, the oxidation process occurring on the MFe-TO15's surface by heterogeneous Fenton reaction which enhanced decomposition of H2O2 for continuous generation of hydroxyl radicals was the main mechanism. The key role of *OH radical in oxidation of organic dyes was further ascertained by the remarkable drop in the decolorization of both organic dyes when the various radical-scavengers, including tert-butanol and chloride were supplemented into Fenton systems. A good stability of the catalyst was obtained through leaching test with low leaching iron ratio. The applied modified catalyst remained stable through three consecutive runs. From these findings, it can be concluded that the modified material can be applied as a feasible, inexpensive and highly effective catalyst for removal of persistent organic compounds from wastewater.
1. Introduction
Rapid development of industries in recent years, especially dyestuff, leather, ceramics, and textiles industries, has generated a huge amount of wastewater containing excellent color and persistent organic matters which are poisonous to humans and eco-systems.1–3Dyes are divided into two main kinds of non-azo and azo dyes. The azo dyes include acidic, basic, reactive, disperse, sulfur and vat dye.1,4,5 Methylene blue (MB) is a popular azo basic dye which is applied for wood, paper, leather, silk and pharmaceutical industries6,7 and rhodamine B (RhB) is a typically amphoteric dye which has been commonly used in the printing, textile, scientific research, and pharmaceutical industries.8,9 Both organic dyes are toxic and exhibit serious effects on human health and aquatic organisms.9 Thus, it is necessary to remove them before they are discharged into receiving water bodies.
Recently, a number of technologies have been applied for discoloration and mineralization of dyes, such as flocculation–coagulation,10 adsorption,11 membrane filtration,12 biological method and advanced oxidation processes (AOPs).13 Among them, the AOPs have been widely used thanks to continuous generation of hydroxyl free radicals (*OH) with an extremely high oxidant potential of 2.8 eV (ref. 14) that have strong oxidation ability with its non-selectively degradation, nontoxic byproducts, popular applicability and high efficiency.4,5 The AOPs are classified into two main processes depending photochemical agents, including advanced non-photochemical oxidation processes and advanced photochemical oxidation processes.7,8,15 Of these AOPs, the process based on Fenton reactions have been proved as one of the best methods for degradation of persistent organic compounds due to possessing many advantages which consist of high efficiency, simple operation, inexpensive and popular applicability.9,16,17 However, the traditional Fenton process using homogeneous catalyst owns many disadvantages, including the narrow pH range, the generation of iron-containing sludge and low efficiency.15,18 Hence, it is essential to apply heterogeneous catalysts instead in which the active metals can be incorporated into a solid support. The applying such heterogeneous catalytic Fenton processes overcame the drawbacks of homogeneous Fenton due to its outstanding advantages such as usability, good stability, easy separation of catalysts after reaction and the formation of low amount of waste sludge.16,17,19,20
At present, there have been various heterogeneous catalysts applied for Fenton processes to decolor RhB, comprising Fe2O3–Kaolin;21 iron sludge;22 magnetic nickel ferrite;23 Fe-based metallic glass;24 natural schorl;20 Fe-loaded mesoporous MCM-41;25 natural graphite tailings;9 and Fenton processes to remove MB, such as mesoporous Fe/SiO2 prepared from rice husk pyrolytic residues;19 zero valent iron;16 Ag–Fe3O4/graphene composites;26 octahedron-like hybrids of highly graphitized carbon dopants; Fe2O3 (C–Fe2O3-2)27 and Se/Fe3O4.28 Although these catalysts possessed many advantages in degradation of persistent organic compounds but they must go through complex synthesis procedures such as doping, calcination at high temperature.15,18 Besides, these catalysts only exhibit removal efficiency of pollutants in extremely acid medium triggering much consumption of chemicals to acidify wastewater before application of Fenton.14 Therefore, it is essential to seek an inexpensive and available heterogeneous catalyst for Fenton processes to degrade the organic dyes is good alternative.
Fe-Containing tailings ore (Fe-TO) is solid waste which occurs abundantly after mining and ferrous and non-ferrous metallurgy processes. In Vietnam, there are about 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of metal mine tailings discharged into environment annually29 leading to crucial issue in tailings management because of its irreversible impact onto human health and eco-system. Thus, the reutilization of Fe-containing tailings ore as heterogeneous Fenton catalyst to decolor the organic dyes would remarkably reduce overall cost of textile wastewater treatment system. However, Fe-TO is usually impurities and contains low content of iron resulting in its unstable property and low catalytic efficiency. Therefore, to improve catalytic efficiency of Fe-TO for heterogeneous Fenton of organic dyes, the Fe-TO was enriched by iron salt in order to increase amount of iron ions in constituent of raw catalyst. Besides, the textural properties of iron-modified Fe-TO catalyst, including BET specific surface area, total pore volume and pore size were also improved leading to oxidation rate enhancement of organic dyes adsorbed on the catalyst's surface by Fenton system. Thus, aim of this study, was to prepare Fe-containing tailings ore-derived catalyst which was then modified by iron(III) chloride (FeCl3) as heterogeneous catalyst in Fenton processes for decolorization and mineralization of organic dyes from stimulated wastewater. Two organic dyes, including basic dye (Methyl blue) and amphoteric dye (Rhodamin B) were used as target compounds to evaluate decolorization efficiency of the heterogeneous catalytic Fenton. The physical–chemical properties of both original and modified catalysts were fully analyzed. The effects of various operational parameters, consisting of modification ratio of iron, solution pH, catalyst dosage, H2O2 dosage, initial organic dyes and inorganic ions concentrations onto organic dyes decolorization were systematically investigated. The stability and reusability of modified catalyst were evaluated. The kinetic and mechanism of organic dyes degradation were deeply discussed using quenching experiments.
000 tons of metal mine tailings discharged into environment annually29 leading to crucial issue in tailings management because of its irreversible impact onto human health and eco-system. Thus, the reutilization of Fe-containing tailings ore as heterogeneous Fenton catalyst to decolor the organic dyes would remarkably reduce overall cost of textile wastewater treatment system. However, Fe-TO is usually impurities and contains low content of iron resulting in its unstable property and low catalytic efficiency. Therefore, to improve catalytic efficiency of Fe-TO for heterogeneous Fenton of organic dyes, the Fe-TO was enriched by iron salt in order to increase amount of iron ions in constituent of raw catalyst. Besides, the textural properties of iron-modified Fe-TO catalyst, including BET specific surface area, total pore volume and pore size were also improved leading to oxidation rate enhancement of organic dyes adsorbed on the catalyst's surface by Fenton system. Thus, aim of this study, was to prepare Fe-containing tailings ore-derived catalyst which was then modified by iron(III) chloride (FeCl3) as heterogeneous catalyst in Fenton processes for decolorization and mineralization of organic dyes from stimulated wastewater. Two organic dyes, including basic dye (Methyl blue) and amphoteric dye (Rhodamin B) were used as target compounds to evaluate decolorization efficiency of the heterogeneous catalytic Fenton. The physical–chemical properties of both original and modified catalysts were fully analyzed. The effects of various operational parameters, consisting of modification ratio of iron, solution pH, catalyst dosage, H2O2 dosage, initial organic dyes and inorganic ions concentrations onto organic dyes decolorization were systematically investigated. The stability and reusability of modified catalyst were evaluated. The kinetic and mechanism of organic dyes degradation were deeply discussed using quenching experiments.
2. Materials and methods
2.1. Chemicals
Methylene blue (MB), Rhodamin B (RhB) were purchased from BHD Chemical LTD POOLE Company (England). Other chemicals, consisting of iron(III) chloride (FeCl3), tert-butanol (CH3)3COH, hydrochloric acid (HCl), hydrogen peroxide 30% (H2O2), sodium hydroxide (NaOH), sulfuric acid (H2SO4) obtained from Merck with analytical grade without further purification. The chemicals for determination of color consisting of K2PtCl6 and CoCl2·6H2O were obtained from Sigma-Aldrich.The stock solutions of both MB and RhB of 1000 mg L−1 were prepared by separately dissolving 1.0 g of MB and RhB in 1000 mL of deionized water. The stimulated wastewater containing organic dyes was separately prepared from stock solutions by diluting a certain amount of stock solutions in deionized water to desired concentrations depending on experimental conditions of organic dyes degradation.
2.2. Materials
The Fe-containing tailings ore (Fe-TO) was collected from residual solid waste after mining mineral ores in Thai Nguyen province, Vietnam. In the next step, the dried Fe-TO was crushed using grinder (Model RM 200, Germany) with a grinding rate of 300 rpm during 10 min. The sample was continuously sieved to achieve particles with size less than 0.25 mm. Finally, the dried Fe-TO power was held in plastic bags for further experiments.For modification of dried Fe-TO power, the procedure was performed by loading of Fe3+ ions onto original Fe-TO at ratios of 5%; 10%; 15% and 20% of Fe3+/Fe-TO (w/w). At the beginning of the process, a pre-determined amount of Fe3+ was put into Erlenmeyer containing 50 mL deionized water and placed on the magnetic agitator until the FeCl3 was completely dissolved. Next, a certain amount of original Fe-TO catalyst was supplemented into the above mixture with an adjusting pH of 11 using NaOH 1.0 N and H2SO4 1.0 N. The mixture then was transferred into 1000 mL beaker and put on magnetic stirrer with an agitation rate of 120 rpm at room temperature (25 ± 2 °C) for 6 h. Subsequently, the result mixture was filtrated using 0.45 μm filtration membrane to separate solid phase from liquid phase. The obtained solid was continuously dried at 105 °C for 2 h in an oven. Finally, the dried solid was crushed and sieved to obtain particles with diameter less than 0.5 μm. The obtained modified Fe-TOs (MFe-TOs) were stored in plastic bags and labelled as MFe-TO5; MFe-TO10; MFe-TO15 and MFe-TO20 for further usage.
2.3. Organic dyes degradation experiment
All experiments were performed according to batch-mode which used a 1000 mL glass reactor (h = 140 mm, Φ = 110 mm) containing 500 mL diluted solution of each dye at various concentrations. The initial solution pH was adjusted using NaOH 1.0 N and H2SO4 1.0 N which was followed by supplementation of a pre-determined catalyst amount (Fe-TO and MFe-TO) and certain content of commercial H2O2 solution (30% w/w) into the reactor. Then, the reactors were put on a shaker with a stirring rate of 150 rpm at room temperature (25 ± 2 °C). The 5 mL solution was withdrawn at interval time of each 5 min in whole process of 30 min and 60 min, respectively, for degradation experiments of MB and RhB. The sample was then filtered using filtration membrane (0.45 μm) to separate catalyst from liquid phase. The obtained liquid was used to determine color according to standard methods 2120C.302.4. Measurement
The decolorization of MB and RhB during the degradation experiments was analyzed according to Standard Method 2120C30 using UV-Vis spectrophotometer (Shimazu, model Z2000, Japan) at λ = 660 nm and 550 nm, respectively, for MB and RhB. Meanwhile, the mineralization degree of both MB and RhB was evaluated through chemical oxygen demand (COD) analysis according to Standard Method 5220.31 The solution pH was determined by a pH meter (SI Analytics). The concentration of iron ions leached in the solution was measured using Inductively Coupled Plasma–Optical Emission Spectrometry (ICP–OES, Model: ULTIMA EXPERT, Horiba, France). The morphology properties of original and modified heterogeneous catalysts were evaluated through Scanning Electron Microscope (SEM) and the energy dispersive X-ray spectroscopy analysis (EDS) using JSM-IT200 (InTouchScop). The textural properties of catalysts which consisted of BET specific surface area, total pore volume and pore size, were analyzed using N2 adsorption/desorption isotherm (Quantachrome Instruments version 11.0-NOVA). Meanwhile, the power X-ray diffraction (XRD) patterns were analyzed on a Bruker D8 advance X-ray diffractometer using Cu Kα as the X-ray source.All samples were measured in triplicate. The experimental data were analyzed using MS Excel and Origin 9.0 software and expressed by mean ± deviation standard.
3. Results and discussion
3.1. Effect of modification ratio on organic dyes removal
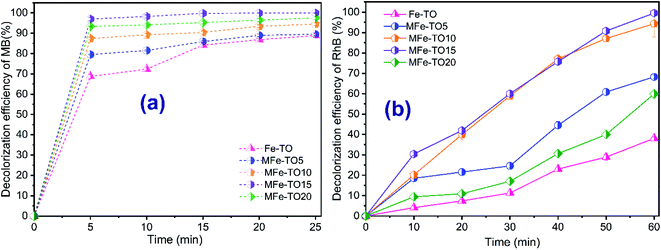
To investigate the effect of modification ratios between FeCl3 and original Fe-TO onto decolorization of MB and RhB, the batch experiments were conducted at various Fe/Fe-TO mass ratios of 5%, 10%, 15% and 20% with fixing other operational parameters. The achieved modification catalysts were denoted as MFe-TO5, MFe-TO10, MFe-TO15 and MFe-TO20. The experimental data are illustrated in Fig. 1. It can be seen from Fig. 1a that the decolorization of both MB and RhB increased when modification ratio between FeCl3 and Fe-TO rose from 0% to 15% during reaction time. The decolorization of both MB and RhB reached a peak at 99.96% and 99.55%, respectively, for MB and RhB at the modification ratio of 15%. However, when modification ratio was increased to 20%, the decolorization of RhB saw a slight different trend from MB. Specifically, at the modification ratio of 20%, the decolorization of RhB decreased clearly while the decolorization of MB had a slight growth. The decolorization efficiency reached the lowest value towards the heterogeneous catalytic Fenton using Fe-TO. Nonetheless, it is clear that the decolorization rate and the complete degradation time of MB were much faster than those of RhB when all kinds of catalysts were applied in heterogeneous Fenton systems. This result can be because the RhB had structure more complex than that of MB.The decolorization efficiency and degradation rate of both organic dyes by modified heterogeneous catalytic Fenton were higher than those of heterogeneous catalytic Fenton using original catalyst. It can be explained that the Fe-TO was successfully enriched by iron ions, so the modified heterogeneous catalytic Fenton of organic dyes was remarkably enhanced. Nevertheless, when modification ratio was further risen (20%), the decolorization efficiency witnessed a slight downward trend. The reason was because the modification ratio went up while the H2O2 dosage was unchanged, thus the excess Fe2+ ions consumed *OH according to following reactions:
| Fe2+ + H2O2 → Fe3+ + *OH + OH− | (1) |
| *OH + Fe2+ → OH− + Fe3+ | (2) |
The consumption of Fe2+ ions led to a drop in decolorization of organic dyes when the amount of catalyst rose. Besides, oxidation rate of adsorbed dyes on the catalyst's surface decreased due to the saturation of active sites on the catalysts' surface and filling iron ions into catalyst's pores. These trends are analogue to the results reported recently.32,33 Therein, Zubir et al. (2014)33 used heterogeneous Fenton with modified Go–Fe3O4 catalyst for decolorization of Acid Orange 7. The results showed that the maximum decolorization efficiency of AO7 reached at 5% of modification ratio. The decolorization efficiency of AO7 decreased when the modification ratio went up 20%. Similarly, the decolorization of Reactive Blue 181 by heterogeneous Fenton with modified fly ash catalyst decreased with the growth in modified fly ash content.32 Based on the above reported results, the MFe-TO with the modification ratio of Fe at 15% (w/w) was chosen as catalyst for heterogeneous Fenton system in the next experiments.
3.2. Effect of solution pH
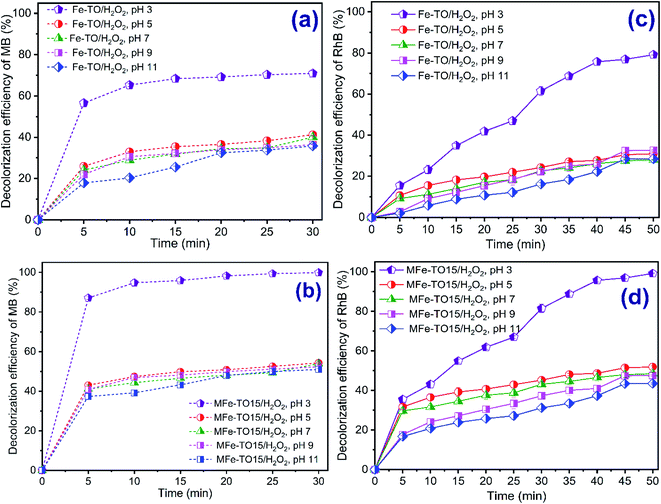
In Fenton reaction, the pH has an important effect on decomposition and content of iron ions which led to a significant influence on reaction rate and degradation efficiency of organic dyes.18,34 The solution pH controls the production rate of hydroxyl radicals and the amount of iron catalyst available in reactor.35 To study the effect of solution pH on decolorization of both MB and RhB, the experiments were carried out by varying pH values between 3 and 11 with initial concentration of each organic dye of 200 mg L−1, catalyst dosage of 1.0 g L−1, H2O2 dosage of 420 mg L−1 and contact time of 0–30 min and 0–60 min, respectively, for MB and RhB. The experimental results showed that the solution pH considerably affected the decolorization of MB and RhB by heterogeneous Fenton processes using both Fe-TO and MFe-TO15 catalysts (Fig. 2).What stands out from data in Fig. 2 is that the decolorization of both MB and RhB witnessed a considerably different trend at various pH levels in all heterogeneous Fenton systems (H2O2/Fe-TO and H2O2/MFe-TO15). Overall, the decolorization rate of both MB and RhB dropped corresponding with a growth in solution pH values. For pH of 3, the maximum decolorization of 69.91% and 62.09%, respectively, for MB and RhB by H2O2/Fe-TO system and of 99.91% and 81.52%, respectively, for MB and RhB by H2O2/MFe-TO15 system at reaction time of 30 min achieved. When the pH levels were risen to 5, 7, 9 and 11, the removal efficiencies of MB by H2O2/Fe-TO system, respectively, were 41.23%, 40.07%, 36.40%, 35.77% and 54.79%, 53.90%, 51.82% and 50.97%, respectively, for H2O2/MFe-TO15 after 30 min of reaction time. The removal efficiencies of RhB achieved, respectively, 30.94%, 28.05%, 32.58% and 28.53% for H2O2/Fe-TO system and reached 51.94%, 48.55%, 47.58% and 43.53%, respectively, for H2O2/MFe-TO15 after 50 min of contact time as pH is increased to 5, 7, 9 and 11. It is clear from the data, the decolorization of H2O2/Fe-TO was lower than that of H2O2/MFe-TO15 and removal rate of MB was higher than that of RhB in all systems. This can be due to RhB's hermaphrodite and complicated structure.
The results are in good agreement with other studies.9,14,17,36 The growth in the removal efficiency with the drop in pH level to 3 was because the Fenton reactions were favored to produce hydroxyl radicals in acid condition (eqn (3)):
| Fe2+ + H2O2 → Fe3+ + *OH + OH− | (3) |
Besides, at acid pH of 3, the stability of H2O2 was high thanks to generation of H3O2+:21
| H+ + H2O2 → H3O2+ | (4) |
Whereas, the precipitation of Fe3+ occurred faster than reduction of Fe3+ into Fe2+ which triggered a decrease in generation of Fe2+ at high pH condition.37 Also, the deactivation of the heterogeneous catalyst also produced other complexes which led to the drop in generation of hydroxyl radicals due to hydrolysis of Fe2+ and the formation of FeOOH precipitation (eqn (6)) and thus further weakened the Fenton reaction.36 Besides, the hydrogen peroxide was also automatically decomposed to form water and oxygen molecules (eqn (7) and (8)) in this condition.9,21 Lastly, at high pH, the hydroxyl radical was quickly transferred into its conjugate base *O− (eqn (9))38 which had lower active ability than that of *OH (ref. 39) leading to delay of the degradation rate of organic dyes.
| Fe3+ + H2O2 → Fe2+ + H+ + *HO2 | (5) |
| Fe2+ + H2O2 → FeO2+ + H2O | (6) |
| Fe3+ + *HO2 → Fe2+ + H+ + O2 | (7) |
| *HO + H2O2 → H2O + *HO2 | (8) |
| *HO + OH− → *O− + *H2O | (9) |
From the above results, pH of 3 was optimum and was chosen for conducting the next degradation experiments in this study.
3.3. Effect of H2O2 dosage
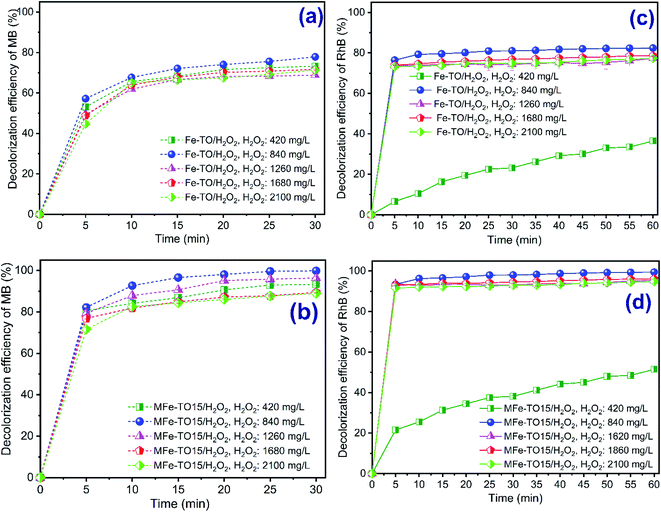
The reaction rate grows when the hydrogen peroxide concentration increases, and the H2O2 concentration depends on pollutants concentration (in term of COD). Thus, concentration of H2O2 plays a vital role in Fenton reaction.2,3 The influence of H2O2 concentration on decolorization of MB and RhB within the range from 420 to 2100 mg L−1 was studied. The heterogeneous Fenton reaction conditions were conducted at an initial concentration of each organic dye of 200 mg L−1, pH of 3, catalyst dosage of 1.0 g L−1 and contact time of 0–30 min and 0–60 min, respectively, for MB and RhB. The results are illustrated in Fig. 3. For degradation of MB, the decolorization efficiency increased from 73.30% to 77.84% and from 93.29% to 99.84% with the growth in H2O2 concentration from 420 to 840 mg L−1, respectively, for H2O2/Fe-TO and H2O2/MFe-TO15 systems. The decolorization of RhB rose from 36.49% to 82.41% and from 51.49% to 99.41% corresponding with the increase in H2O2 concentration from 420 to 840 mg L−1, respectively, for H2O2/Fe-TO and H2O2/MFe-TO15 systems. This is due to the fact that the generation of *OH radicals increased with the rising supplemented H2O2 concentration into reactors which triggered the enhancement of dyes molecules degradation (eqn (10) and (11)).9,21,36| Fe2+ + H2O2 + H+ → Fe3+ + H2O + *OH | (10) |
| Fe3+ + H2O2 → Fe2+ + H+ + *HOO | (11) |
The lowest decolorization of RhB at H2O2 concentration of 420 mg L−1 for both Fenton systems was because the low concentration of H2O2 cannot produce enough hydroxyl radicals for effective degradation of organic dyes.36 Nevertheless, when the H2O2 concentration was exceeded 840 mg L−1, the decolorization of MB saw a marginal fall. Specifically, the decolorization of MB was 68.82%, 71.62% and 71.43% for H2O2/Fe-TO system and 96.32%, 89.42% and 88.93% for H2O2/MFe-TO15 system, respectively, at the H2O2 concentration from 1260 mg L−1 to 2100 mg L−1 during 30 reaction min. Whereas, the decolorization of RhB possessed a slight different trend. At the H2O2 concentration from 1260 mg L−1 to 2100 mg L−1, there was no considerable variation towards color removal and the decolorization reached only 77.29%, 78.65% and 77.25% for H2O2/Fe-TO and 95.29%, 96.15% and 94.75% for H2O2/MFe-TO15, respectively, during 60 reaction min. However, the color removal efficiency of both MB and RhB by all Fenton systems was not distinctly different at the higher concentration of hydrogen peroxide. This was because the iron catalyst dosage was unchanged while the excess hydrogen peroxide would react with *OH to produce perhydroxyl radicals causing depletion of hydroxyl radicals (eqn (12) and (13)). Thus, the oxidization rate of organic dyes was slower.40
| H2O2 + *OH → H2O + *OOH | (12) |
| *OOH + *OH → H2O + O2 | (13) |
Analogue trends were achieved by some scholars.36,40–43 Laiju et al.,41 2014 reported the removal of COD from leachate by iron loaded mangosteen decreased with the rise in hydrogen peroxide content. The rhodamine B removal efficiency of the heterogeneous Fenton with iron loaded activated carbon catalyst fall when the H2O2 concentration went up43 and magenta MB degradation using heterogeneous Fenton's catalyst also dropped at the high H2O2 concentration of 0.44 mM.42 As a result, the decolorization of both MB and RhB maximized at 840 mg L−1 of H2O2 concentration.
Also, as can be seen from data in Fig. 3, the decolorization efficiency of H2O2/MFe-TO15 system was higher than that of H2O2/Fe-TO at all applied H2O2 concentrations. The results were because there was the existence of abundant iron ions in H2O2/MFe-TO15 system compared with H2O2/Fe-TO system which enhanced the formation of more *OH by reaction between iron ions and H2O2. Besides, the well-development in pore structure and surface area of modified catalyst also improved the organic dyes removal by adsorption mechanism (data shown in Table 1).
| Catalyst | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) |
|---|---|---|---|
| Fe-TO | 7.9870 | 0.0351 | 25.4336 |
| MFe-TO15 | 94.440 | 0.1710 | 120.006 |
In this study, the decolorization of both MB and RhB maximized in all Fenton systems at 840 mg L−1 of H2O2 concentration. Therefore, the H2O2 concentration of 840 mg L−1 was chosen for next experiments.
3.4. Catalyst properties and effect of catalyst dosage
The textural properties of Fe-TO and MFe-TO15 were determined based on the nitrogen adsorption and desorption isotherm. The calculated data are presented in Table 1. The BET surface area, pore volume and pore diameter were, respectively, 7.9870 m2 g−1, 0.0351 cm3 g−1 and 25.4336 nm for Fe-TO and 94.440 m2 g−1, 0.1710 cm3 g−1 and 120.006 nm for MFe-TO15. What stands out from data in Table 1 is that the modified heterogeneous catalyst had excellent development in textural properties with a considerable growth in SBET, pore volume and pore size by 12, 5 and 5 times, respectively. These properties of MFe-TO15 were favorable for the adsorption of pollutants by pore filling mechanism which enhanced the oxidation rate of organic dyes through adsorption by Fenton process.The SEM images illustrated the surface morphology of catalysts before and after modification at all loading ratios as indicated in Fig. S1.† It can be seen from Fig. S1† that all Fe-TO and modified MFe-TO5, MFe-TO10, MFe-TO15 and MFe-TO20 catalysts possessed the amorphous structure, rough and heterogeneous surface. The results also showed that the materials had porous structure. The MFe-TO5, MFe-TO10 and MFe-TO15 were more porous structures than Fe-TO and MFe-TO20. It might cause by the effect of Fe element (5–15% w/w) during modification process which led to change in the structure of Fe-TO. However, when the modification ratio increased to 20%, Fe ions in the IS might be released into solution during modification process. The particle size of Fe-TO was in range from several nm to more than 20 nm (Fig. S1a and b†). Compared with Fe-TO, the modified materials (MFe-TO5, MFe-TO10 and MFe-TO15) possessed many particles with the smaller and rougher structure compared with original material (Fe-TO) and MFe-TO20. The results can be due to the effect of iron ions during modification process which reacted and broke down the particles structure of original material causing a part or all the large particles to split into many small particles. This result was suitable with the textural properties analysis result of MFe-TO15 which had the well-development in BET area, pore volume and pore size compared with original material (Table 1).
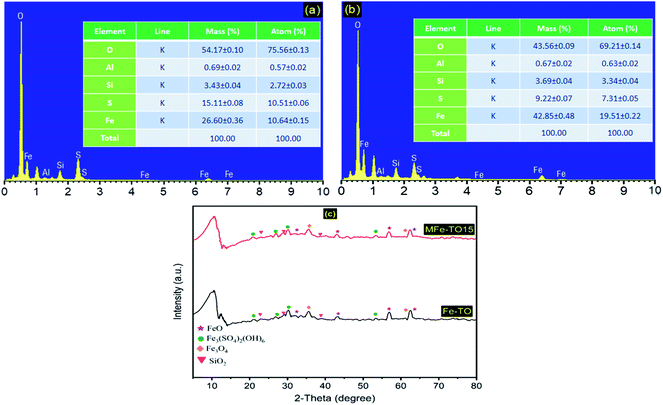
The chemical composition of the original and modified heterogeneous catalysts (Fe-TO and MFe-TO15) were observed from data in Fig. 4. The EDS result from Fig. 4a show that the major elements presence in Fe-TO and MFe-TO15 were O, Al, Si, S and Fe. When the Fe-TO was modified with iron ions, the modified material saw a remarkable change in the percentage ratio of molecular weight of Fe compared to the original material. The mass ratio of Fe in Fe-TO was 26.60 ± 0.36% (Fig. 4a) but this ratio was increased to 42.85 ± 0.48% (Fig. 4b) in MFe-TO15 constituent. Thus, it can be seen that the modification of Fe-TO catalyst by iron ions resulted in a considerable increase the content of iron ions onto Fe-TO's surface, suggesting the good attachment of iron ions onto the original Fe-TO. The results proved that iron ions were successfully loaded onto original Fe-containing tailings ore catalyst which enhanced degradation rate of organic dyes by heterogeneous Fenton systems using MFe-TO15 as catalyst.
Besides, the valence state of the iron components contained in catalysts was also determined by XRD data (Fig. 4c). As is illustrated in Fig. 4c, the iron ions existed in form of both hematite (FeO) and magnetite (Fe3O4) in catalysts. The presence of Fe3O4 in the catalysts made them have magnetic property which benefited for recovery catalysts after Fenton reactions by external magnetic field.
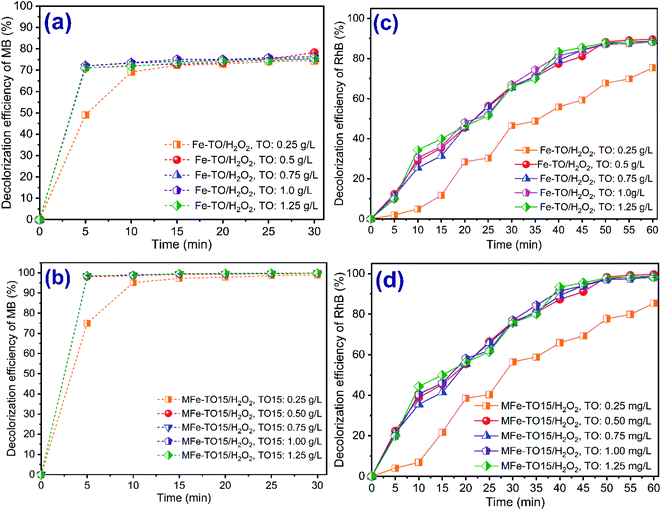
The dosage of catalyst plays an important role in practical application of heterogeneous Fenton processes for wastewater treatment with regard to economic aspect. Fig. 5 presents the effect of catalyst dosage on the decolorization of both MB and RhB using heterogeneous catalytic Fenton systems. The experiments were carried out by varying the catalysts dosage from 0.25 g L−1 to 1.25 g L−1 at initial concentration of each organic dye of 200 mg L−1, pH of 3, H2O2 dosage of 840 mg L−1 and contact time of 0–30 min and 0–60 min, respectively, for MB and RhB.
What stands out from data in Fig. 5 is that the decolorization of both MB and RhB increased with a growth in catalyst dosages from 0.25 g L−1 to 1.25 g L−1 for all Fenton systems. The color removal of MB reached the maximum of 78.27% and 99.77%, respectively, for H2O2/Fe-TO and H2O2/MFe-TO15 at 0.5 g L−1 of catalyst dosage after 30 min of degradation time (Fig. 5a and b). The similar results were also obtained by both H2O2/Fe-TO and H2O2/MFe-TO15 systems for decolorization of RhB with 89.66% and 99.66%, respectively, at 0.5 g L−1 of applied catalyst dosage after 60 min of treatment time (Fig. 5c and d). The color removal rate of MB jumped in first 5 of contact time while the RhB decolorization rate experienced the gradual increase during reaction time. The gradual growth in color removal efficiency with the increase in catalyst dosage was mainly owing to the increase in the active sites for the production of hydroxyl radicals and the formed hydroxyl radicals effectively attacked the chromophore (eqn (14)), thus enhancement of the decolorization rate of MB and RhB.9,34,36 However, there was no distinct variation in increase the decolorization of both dyes when the applied catalyst dosages further increased. The results mainly due to quenching reaction of hydroxyl radicals which happened between Fe2+ ions and *OH present in solution, as indicated in eqn (15).
| Fe2+ + H2O2 → Fe3+ + *OH + OH− | (14) |
| Fe2+ + *OH → Fe3+ + OH− | (15) |
Another reason was that the catalyst dosage increased while the hydrogen peroxide concentration was constant during reaction time leading to the exhaustion of H2O2 to react with ferrous ion species for generation of hydroxyl radicals to oxidize of organic dyes. Thus, a higher catalyst dosage also can cause the quenching reactions of hydroxyl radical due to recombination of hydroxyl radicals in aqueous solution (eqn (16)):
| *OH + *OH → H2O2 | (16) |
For comparison with other studies, the obtained results of this study were fit well. Shi et al. (2018)18 found out the catalytic efficiency of Fenton/Fe2GeS4 nanoparticle of organic dyes dropped a little along with the increase of catalyst dosage >0.3 g L−1. The mineralization of paracetamol also decreased at the H2O2/Fe2+ of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.44 From these results, the catalyst dosage of 0.5 g L−1 was chosen for conducting further degradation experiments in this work.
3.44 From these results, the catalyst dosage of 0.5 g L−1 was chosen for conducting further degradation experiments in this work.
3.5. Effect of initial dyes concentration
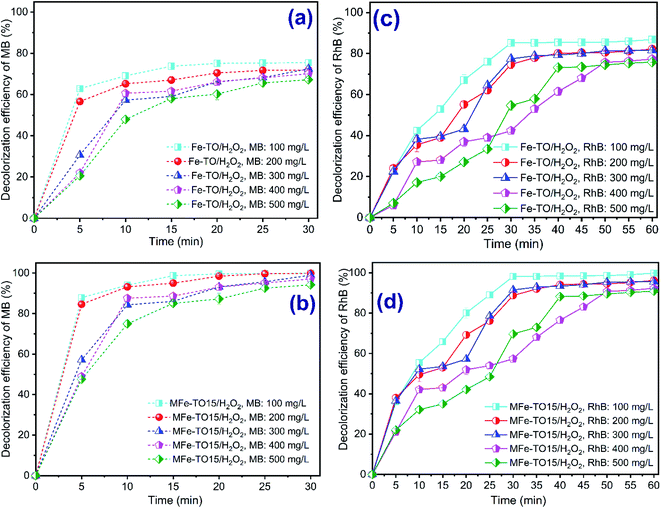
The feasibility of the heterogeneous Fenton oxidation plays an important role in practical application. Thus, the experiments to investigate the effect of initial organic dyes concentration on dyes color removal by heterogeneous Fenton systems were conducted by varying initial concentrations of each MB and RhB, ranged from 100 mg L−1 to 500 mg L−1 at pH of 3, H2O2 dosage of 840 mg L−1, catalyst dosage of 0.5 g L−1 and contact time of 0–30 min and 0–60 min, respectively, for MB and RhB. The results are demonstrated in Fig. 6. As expected, the decolorization of both MB and RhB witnessed a distinct drop with a rise in initial concentration of organic dyes. For H2O2/Fe-TO system, the decolorization rate of MB and RhB, respectively, ranged from 75.43% to 67.19% during 30 min of reaction time and from 86.83% to 75.89% during 60 min treatment time. In the case of H2O2/MFe-TO15 system, the color removal of MB and RhB, respectively, reached from 99.94% to 94.19% after 30 min of contact time and from 99.83% to 90.89% after 60 min reaction. The results are the incident property of all advanced oxidation processes.9 With the same amount of catalyst, a growth in initial concentration of dyes triggered a saturation of active sites on the catalysts' surface because of unchangeability in adsorption ability by pore filling mechanism and the reaction sites of Fe-TO and MFe-TO15.45 Another reason can be explained for this result was that the oxidation agent concentration was constant, thus amount of generated *OH would be unchanged during reaction process. Whereas, the higher concentration of organic dyes was, the more hydroxyl radicals consumed, thus overall efficiency of degradation of both MB and RhB was dropped [11, 3]. Furthermore, the degradation of intermediates which can be formed during MB and RhB decolorization process, especially at a high initial concentration of MB and RhB, led to the competitive consumption of hydroxyl radicals.93.6. Kinetic studies
The decolorization kinetics of MB and RhB in aqueous solution were investigated, using heterogeneous catalytic Fenton processes with both original and modified catalysts under optimum conditions found from the abovementioned experiments. The general equation describing the oxidation reaction of organic dyes can be written as follows:| Organic dyes + OH* → nCO2 + mH2O + intermediates | (17) |
The reaction rate in eqn (17) can be described by following kinetic equation (eqn (18)):
| r = k·[C]a·[OH*]b | (18) |
| v = k′·[C]a | (19) |
The assumption that Fenton reaction follows both pseudo-first-order and pseudo-second-order kinetics, the integral of both sides of eqn (19) (with a = 1 for pseudo-first-order and a = 2 for pseudo-second-order kinetic):
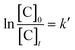 | (20) |
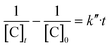 | (21) |
From the experimental data, the plot of 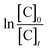 and
and 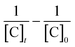 versus reaction time, the correlation coefficient (R2) were obtained, respectively, 0.9864 and 0.8057 for pseudo-first-order and pseudo-second-order kinetics. The obtained results showed that the data was fitted as a straight line and the k′ (apparent first-order rate constant) value corresponded to the slope of the straight line. Thus, in this study, the pseudo-first-order kinetic equation was employed to describe the effect of reaction conditions in the heterogeneous Fenton processes (eqn (22)):
versus reaction time, the correlation coefficient (R2) were obtained, respectively, 0.9864 and 0.8057 for pseudo-first-order and pseudo-second-order kinetics. The obtained results showed that the data was fitted as a straight line and the k′ (apparent first-order rate constant) value corresponded to the slope of the straight line. Thus, in this study, the pseudo-first-order kinetic equation was employed to describe the effect of reaction conditions in the heterogeneous Fenton processes (eqn (22)):
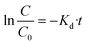 | (22) |
By plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C/C0 versus reaction time under optimum conditions, the calculated kinetic parameters for decolorization of MB and RhB by heterogeneous Fenton system using MFe-TO15 catalyst are illustrated in Fig. S2.†
C/C0 versus reaction time under optimum conditions, the calculated kinetic parameters for decolorization of MB and RhB by heterogeneous Fenton system using MFe-TO15 catalyst are illustrated in Fig. S2.†
From data in Fig. S2,† it can be seen that the Kd values of decolorization of MB were higher than those of RhB by H2O2/MFe-TO15 system. The Kd values of H2O2/MFe-TO15 systems for color removal of both organic dyes diminished in the following order: pH 3 > pH 7 > pH 9 > pH 11 (Fig. S2a†). For pH of 3, the maximum Kd values of heterogeneous catalytic Fenton of MB were 0.223 min−1 which was higher than that of the heterogeneous catalytic Fenton of RhB by about 3 times (0.086 min−1). The highest Kd values were 0.3204 min−1 and 0.0754 min−1 of H2O2/MFe-TO15 system, respectively, for decolorization of MB and RhB at same modification ratio of Fe3+/Fe-TO of 15% (Fig. S2b†). Besides, the Kd values followed the descending sequence of H2O2 dosage: 840 mg L−1 > 1260 mg L−1 > 2100 mg L−1 and the Kd maximized at a H2O2 dosage of 840 mg L−1 for MB and RhB decolorization with the Kd values of 0.2035 min−1 and 0.0593 min−1, respectively (Fig. S2c†). The Kd values reached a peak of 0.1616 min−1 and 0.1638 min−1, respectively, for the heterogeneous catalytic Fenton of MB and RhB at the catalyst dosage of 0.5 g L−1 (Fig. S2d†). The Kd values dropped from 0.2344 min−1 to 0.0942 min−1 for the heterogeneous catalytic Fenton oxidation of MB and from 0.1820 min−1 to 0.0784 min−1 for the heterogeneous catalytic Fenton oxidation of RhB when the initial organic dyes concentration increased from 100 mg L−1 to 500 mg L−1 (Fig. S2d†), respectively. In the quenching experiment using various scavengers, the heterogeneous Fenton oxidation without scavenger gave the highest Kd values of 0.1711 min−1 and 0.1284 min−1, respectively, for MB and RhB removal (Fig. S2e†).
Also, as is illustrated by Fig. S2,† the pseudo-first-order model fit well with the experimental data at whole experimental conditions with the high value of regression coefficient (R2 > 0.90) for all heterogeneous catalytic Fenton systems of both MB and RhB. Therefore, it can conclude that the heterogeneous catalytic Fenton processes of both MB and RhB in this study followed the pseudo-first-order kinetic. The results of this study were high agreement with other studies.21,24,44
3.7. Degradation mechanism studies
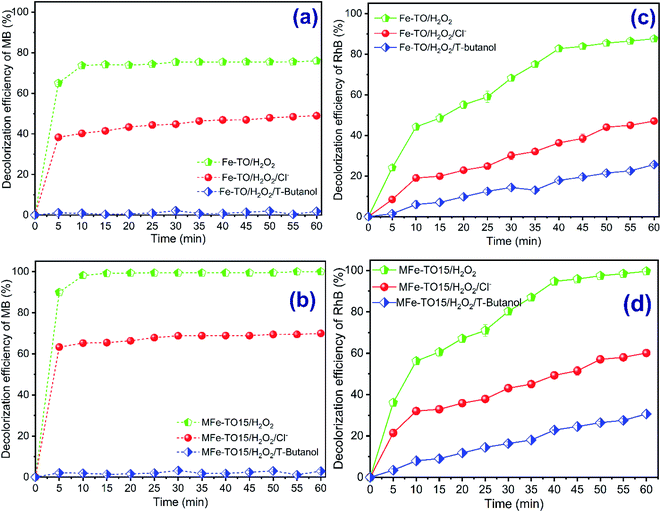
So as to verify the decolorization mechanism of both MB and RhB by contribution of hydroxyl radicals which were continuously produced during the heterogeneous catalytic Fenton process, the various scavengers, including tert-butanol and Cl− (ref. 14, 18, 29 and 44) were separately supplemented into both H2O2/Fe-TO and H2O2/MFe-TO15 systems. The *OH scavenger experiments were performed at catalyst dosage of 0.5 g L−1, H2O2 dosage of 840 mg L−1, pH of 3 and Cl− and t-butanol concentrations of 100 mg L−1 with reaction time of 60 min. The obtained results are presented in Fig. 7.As is indicated by data in Fig. 7a and b, the decolorization of both MB and RhB by H2O2/Fe-TO system reached approximately 80% while the MB and RhB were completely degraded in the H2O2/MFe-TO15 system without scavengers after 60 reaction min (Fig. 7c and d). Nonetheless, the color removal of MB and RhB saw a noticeable drop by about 40% when 100 mg L−1 of Cl− was supplemented into H2O2/Fe-TO system. Specially, the decolorization of both MB and RhB was negligible in the H2O2/Fe-TO system with addition of 100 mg L−1 of t-butanol. Similarly, there was a dramatic downward trend in decolorization of MB and RhB by H2O2/MFe-TO15 system when 100 mg L−1 of Cl− and t-butanol was separately added into Fenton reactions. The color removal of MB and RhB, respectively, was 69.97% and 60.10% for H2O2/MFe-TO15/Cl− system and only reached, respectively, 2.87% and 30.62% for H2O2/MFe-TO15/t-butanol system. The dropped decolorization efficiency with supplement of TBA elucidated that hydroxyl radicals played a vital role in the degradation of MB and RhB in the Fe-TO and MFe-TO15 heterogeneous Fenton systems.
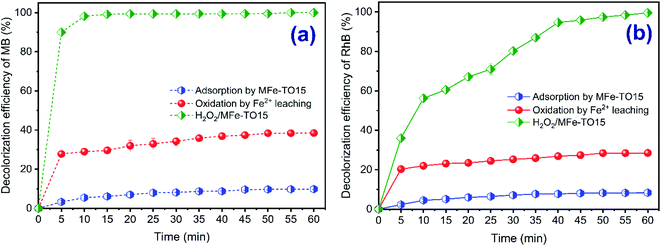
In addition, in the heterogeneous Fenton, the organic dyes removal mechanism highly depends on catalyst's adsorption property and leaching iron ions into solution through homogeneous Fenton reaction. Therefore, to assess the contribution of these mechanisms in color removal of organic dyes, the experiment was conducted at pH of 3, MFe-TO15 dosage of 0.5 g L−1, initial concentration of each dye of 200 mg L−1 and 840 mg L−1 of H2O2 dosage. The color removal of MB and RhB by various mechanisms are presented in Fig. 8. From data in Fig. 8, it is clear that there was contribution of both adsorption and homogeneous Fenton reactions in removal of dyes. However, removal efficiency of MB and RhB reached only 9.94% and 7.94%, respectively, by adsorption mechanism thanks to well-development in textural property of modification catalyst (data in Table 1). Meanwhile, removal of both MB and RhB by leaching iron through homogeneous Fenton reaction (catalyzed by the leaching Fe) was 38.5% and 28.49%, respectively. In summary, contribution of physical adsorption (i.e. pore filling) was negligible and removal efficiency of MB was higher than that of RhB due to higher adsorption capacity of MB onto catalyst's surface which can be thanks to more simple structure of MB compared with RhB. Besides, there was moderate contribution of homogeneous Fenton reaction into dyes removal. In combination with data in Fig. S4c,† it can be seen that there were both Fe2+ and Fe3+ ions liberated into aqueous solution from the modified solid catalyst. However, the released amount of Fe3+ ions were lower than those of Fe2+ ions. Together with reaction progress, the content of Fe2+ ions which was liberated in the system gradually rose and reached at maximum value of 2.5 mg L−1 after 60 min reaction. Besides, the appearance of other metals such Al, Si in MFe-TO15 constituent can also contribute in degradation of H2O2 to form more *OH radicals and increase the number of active sites on the catalyst's surface.44 From these results, it is clear that the organic dyes were partly degraded due to the liberation of Fe2+ ions into water medium. However, nearly all degradation of organic dyes was thanks to the heterogeneous Fenton oxidation reaction happened between hydrogen peroxide and enriched iron ions on the heterogeneous catalyst's surface for production of *OH radicals and the catalysis performance was mainly due to the heterogeneous effect of the catalyst (Fe-TO and MFe-TO15). The plausible mechanisms of the heterogeneous catalyst Fenton of MB and RhB decolorization can be demonstrated by eqn (23)–(27):18,44
| Fe(II)solid + H2O2 → Fe(III)solid + *OH + OH− | (23) |
| Fe(III)solid + H2O2 → Fe(III)solid + *H2O | (24) |
| Fe(III)solid + *H2O → Fe(II)solid + *OOH + H+ | (25) |
| Fe(III)solid + *OOH → Fe(II)solid + O2 + H+ | (26) |
| Fe(II)solid → Fe(III)solid | (27) |
Clearly, in above reactions chain (eqn (23)–(27)), the first reaction occurred on the catalyst's surface. The reaction was initiated by Fe2+ in solid catalyst constituent with H2O2 via Haber–Weiss mechanism (eqn (23)).8,18,46 Besides, Fe2+ (solid) was continuously regenerated through eqn (25) and (26). As a result, the *OH produced from the above reactions enhanced to oxidize the adsorbed dyes on the surface of MFe-TO15. Moreover, the H+ ions formed from Fenton reaction (eqn (25) and (26)) also formed the stable acid condition which was beneficial for the heterogeneous Fenton reactions by promotion of dissolution of iron ions from MFe-TO15 (eqn (27)).
From abovementioned results and discussion, the proposed main mechanisms for decolorization of organic dyes by the modified heterogeneous catalytic Fenton process may be included: (1) adsorption; (2) oxidation. Both mechanisms occurred simultaneously in the degradation of MB and RhB by Fenton process, which was characterized by two degradation kinetic processes, consisting of initial stage of adsorption by pore filling when the active sites on catalyst's surface was abundant and following by a rapid oxidation period of adsorbed organic dyes on the catalyst's surface thanks to *OH radicals.47 The *OH radicals were generated by leaching Fe2+ through homogeneous Fenton reaction and mainly by heterogeneous Fenton reaction on the catalyst's surface. However, the oxidation process was thanks to continuous generation of hydroxyl radicals which can attack effectively and non-selectively into aromatic rings of organic dyes to mineralize them into end products of CO2, H2O and other intermediates was determined as the main mechanism of heterogeneous Fenton process in this study. From the above results, it can conclude that the *OH radicals played a key role in degradation of organic dyes by both H2O2/Fe-TO and H2O2/MFe-TO15.
3.8. Mineralization, stability and reusability of catalyst
The COD of the dyes solution were measured both before and after heterogeneous Fenton of MB and RhB at optimal experimental conditions which obtained from above experiments. The mineralization experiment of both MB and RhB was performed at initial concentration of organic dye of 200 mg L−1, H2O2 dosage of 840 mg L−1, pH of 3 and MFe-TO15 dosage of 0.5 g L−1. The mineralization efficiency of dyes is illustrated in Fig. S3.† The data in Fig. S3† show that after 60 min of reaction time, the mineralization efficiency of both MB and RhB by H2O2/MFe-TO15 evaluated via COD removal reached, respectively, 75.43% and 60.43% which was lower compared with their decolorization efficiency. The mineralization of MB was slight higher than that of RhB in same condition. The result indicated that a high portion of the dyes was mineralized into CO2 and H2O which triggered the decrease in both color and toxicity of the solution.To investigate stability and reusability of modified catalyst, the experiments were conducted to determine amount of leaching iron into solution and removal ability of dyes after three runs. For stability evaluation of catalyst (through leaching iron), the experiment was carried out at optimized condition of pH 3, MFe-TO15 dosage of 0.5 g L−1, H2O2 dosage of 840 mg L−1 and initial organic dyes of 200 mg L−1. For reusability investigation of catalyst, the used catalyst in the first run was recovered by filtering through membrane filter (0.45 μm) and cleaned by deionized water several times with drying in oven at 70 °C for 1 h. The obtained solid was used as catalyst for next runs. Other experimental conditions consisted of pH of 3, MFe-TO15 dosage of 0.5 g L−1, H2O2 dosage of 840 mg L−1 and initial concentration of organic dyes of 200 mg L−1. The iron ions concentration released from solid catalyst into aqueous solution was measured during 60 min of reaction time. The results are illustrated in Fig. S4c.† As can be seen from data in Fig. S4c,† there was both Fe2+ and Fe3+ ions liberated into aqueous solution from the modified solid catalyst. However, the released amount of Fe3+ ions was lower than that of Fe2+ ions. Together with reaction progress, the content of Fe2+ ions which was liberated in the system gradually rose and reached at maximum value of 2.5 mg L−1 after 60 min reaction. The result exhibited low leaching ratio of iron compared with other studies, such as leaching iron of 5 mg L−1 in Fenton degradation of organic dyes by Fe2GeS4 (ref. 18) or 9.8 mg L−1 of leached iron found in heterogeneous Fenton with Fe3O4 magnetite nanoparticles after 180 reaction min.48 With the low leaching iron ratio proved that applied catalyst in this study was feasible in practical application.
The reusability of catalyst over three runs is presented in Fig S4a and b.† The data indicated that the decolorization of MB and RhB had an inconsiderable change after three consecutive runs. To be specific, the decolorization of MB reached 99.97%, 89.94% and 87.94%, respectively, for at the first run, second run and third run. Similarly, the decolorization of RhB was 99.58%, 88.94% and 87.19% over three consecutive runs after 60 reaction min. The results showed that MFe-TO15 possessed a good stability, which had an important significant for practical application at large-scale.
4. Conclusions
In the present study, the waste Fe-containing tailings ore-derived MFe-TO15 catalyst enriched by ion ions was successfully developed for the heterogeneous Fenton process of organic dyes. It was found that the MFe-TO15 heterogeneous Fenton system exhibited the high decolorization and mineralization efficiency of different organic dyes under optimal operational conditions. The decolorization rate of MB was much higher than that of RhB at same operational condition due to complicated structure of RhB compared with MB. At the most suitable operational condition of the heterogeneous catalytic Fenton of organic dyes, including the modification ration of 15%, solution pH of 3, H2O2 dosage of 840 mg L−1 and catalyst dosage of 0.5 g L−1, the color removal efficiency of MB and RhB, respectively, by H2O2/MFe-TO15 system achieved from 94.19% to 99.94% after 30 min of contact time and from 90.89% to 99.83% after 60 min reaction. The mineralization efficiency of MB and RhB achieved 75.43% and 60.43%, respectively. The Kd values of the pseudo-first-order model followed the same order as the degradation efficiency of both organic dyes by H2O2/MFe-TO15 system. Especially, the main Fe constituent enriched in modified catalyst enhanced the degradation of organic dyes of the heterogeneous Fenton by both adsorption and oxidation mechanisms. Among mechanisms, oxidation by *OH radicals which were generated by reaction between iron on catalyst surface as well as Fe2+ ions released in solution and H2O2 was main mechanism. From this study, it can conclude that the MFe-TO15 was an effective, feasible and inexpensive catalyst for Fenton oxidation of organic dyes. Moreover, this study was successfull in reutilization of a solid waste to fabricate an effective, low-cost and high applicability catalyst for AOPs to remove the persistent organic pollutants from wastewater.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was funded by Ho Chi Minh City University of Food Industry (HUFI) under grant number 56/HĐ-DCT.References
- A. Buthiyappan, A. R. Abdul Aziz and W. M. A. Wan Daud, Rev. Chem. Eng., 2016, 32, 1–47 CAS.
- N. K. Daud and B. H. Hameed, J. Hazard. Mater., 2010, 176, 938–944 CrossRef CAS PubMed.
- R. Idel-aouad, M. Valiente, A. Yaacoubi, B. Tanouti and M. López-Mesas, J. Hazard. Mater., 2011, 186, 745–750 CrossRef CAS PubMed.
- C. R. Holkar, A. J. Jadhav, D. V. Pinjari, N. M. Mahamuni and A. B. Pandit, J. Environ. Manage., 2016, 182, 351–366 CrossRef CAS PubMed.
- A. Ahmad, S. H. Mohd-Setapar, C. S. Chuong, A. Khatoon, W. A. Wani, R. Kumar and M. Rafatullah, RSC Adv., 2015, 5, 30801–30818 RSC.
- S. Yang, H. He, D. Wu, D. Chen, X. Liang, Z. Qin, M. Fan, J. Zhu and P. Yuan, Appl. Catal., B, 2009, 89, 527–535 CrossRef CAS.
- Y. Ahmed, Z. Yaakob and P. Akhtar, Catal. Sci. Technol., 2016, 6, 1222–1232 RSC.
- Y. Gao, Y. Wang and H. Zhang, Appl. Catal., B, 2015, 178, 29–36 CrossRef CAS.
- B. Cuiping, G. Wenqi, F. Dexin, X. Mo, Z. Qi, C. Shaohua, G. Zhongxue and Z. Yanshui, Chem. Eng. J., 2012, 197, 306–313 CrossRef.
- L. Liu, B. Zhang, Y. Zhang, Y. He, L. Huang, S. Tan and X. Cai, J. Chem. Eng. Data, 2015, 60, 1270–1278 CrossRef CAS.
- H. T. Van, T. M. P. Nguyen, V. T. Thao, X. H. Vu, T. V. Nguyen and L. H. Nguyen, Water, Air, Soil Pollut., 2018, 229, 393–407 CrossRef.
- D. De Jager, M. S. Sheldon and W. Edwards, Sep. Purif. Technol., 2014, 135, 135–144 CrossRef CAS.
- T. A. Nguyen and R. S. Juang, Chem. Eng. J., 2013, 219, 109–117 CrossRef CAS.
- H. T. Van, L. H. Nguyen, T. K. Hoang, T. P. Tran, A. T. Vo, T. T. Pham and X. C. Nguyen, Sep. Purif. Technol., 2019, 224, 431–442 CrossRef CAS.
- J. Herney-ramirez, M. A. Vicente and L. M. Madeira, Appl. Catal., B, 2010, 98, 10–26 CrossRef CAS.
- B. Yang, P. Zhou, X. Cheng, H. Li, X. Huo and Y. Zhang, J. Colloid Interface Sci., 2019, 555, 383–393 CrossRef CAS PubMed.
- Y. Wang, Y. Gao, L. Chen and H. Zhang, Catal. Today, 2015, 252, 107–112 CrossRef CAS.
- X. Shi, A. Tian, J. You, H. Yang, Y. Wang and X. Xue, J. Hazard. Mater., 2018, 353, 182–189 CrossRef CAS PubMed.
- J. Xiong, G. Li and C. Hu, Catal. Today, 2020, 355, 529–538 CrossRef CAS.
- H.-Y. Xu, S.-Y. Qi, Y. Li, Y. Zhao and J.-W. Li, Environ. Sci. Pollut. Res., 2013, 20, 5764–5772 CrossRef CAS PubMed.
- S. Guo, G. Zhang and J. Wang, J. Colloid Interface Sci., 2014, 433, 1–8 CrossRef CAS PubMed.
- S. Guo, Z. Yang, Z. Wen, H. Fida, G. Zhang and J. Chen, J. Colloid Interface Sci., 2018, 532, 441–448 CrossRef CAS PubMed.
- S. Q. Liu, L. R. Feng, N. Xu, Z. G. Chen and X. M. Wang, Chem. Eng. J., 2012, 203, 432–439 CrossRef CAS.
- X. Wang, Y. Pan, Z. Zhu and J. Wu, Chemosphere, 2014, 117, 638–643 CrossRef CAS PubMed.
- D. Xu, X. Sun, X. Zhao, L. Huang, Y. Qian, X. Tao and Q. Guo, Water, Air, Soil Pollut., 2018, 229, 317–326 CrossRef.
- R. Saleh and A. Taufik, J. Environ. Chem. Eng., 2019, 7, 102895 CrossRef CAS.
- B. Ren, Y. Xu, C. Zhang, L. Zhang, J. Zhao and Z. Liu, J. Taiwan Inst. Chem. Eng., 2019, 97, 170–177 CrossRef CAS.
- X. Chen, J. Mao, C. Liu, C. Chen, H. Cao and L. Yu, Chin. Chem. Lett., 2020, 31, 3205–3208 CrossRef CAS.
- N. T. Hien, L. H. Nguyen, H. T. Van, T. D. Nguyen, T. H. V. Nguyen, T. H. H. Chu, T. V. Nguyen, V. T. Trinh, X. H. Vu and K. H. H. Aziz, Sep. Purif. Technol., 2020, 233, 115961 CrossRef CAS.
- S. H. Jenkins, Water Res., 1982, 16, 1495–1496 CrossRef.
- Standard Methods For the Examination of Water and Wastewater, ed. E. W. Rice, R. B. Baird and A. D. Eaton, American Public Health Association, 2018 Search PubMed.
- D. S. Duc, Asian J. Chem., 2013, 25, 4083–4086 CrossRef CAS.
- N. A. Zubir, C. Yacou, X. Zhang and J. C. Diniz Da Costa, J. Environ. Chem. Eng., 2014, 2, 1881–1888 CrossRef CAS.
- F. Ji, C. Li, J. Zhang and L. Deng, Desalination, 2011, 269, 284–290 CrossRef CAS.
- Y. Yao, L. Wang, L. Sun, S. Zhu, Z. Huang, Y. M. WangyangLu and W. Chen, Chem. Eng. Sci., 2013, 101, 424–431 CrossRef CAS.
- K. K. Rubeena, P. Hari Prasad Reddy, A. R. Laiju and P. V. Nidheesh, J. Environ. Manage., 2018, 226, 320–328 CrossRef CAS PubMed.
- J. J. Pignatello, E. Oliveros and A. MacKay, Crit. Rev. Environ. Sci. Technol., 2006, 36, 1–84 CrossRef CAS.
- A. Babuponnusami and K. Muthukumar, Chem. Eng. J., 2012, 183, 1–9 CrossRef CAS.
- G. V. Buxton, C. L. Greenstock, W. P. Helman and A. B. Ross, J. Phys. Chem. Ref. Data, 1988, 17, 513–886 CrossRef CAS.
- N. Panda, H. Sahoo and S. Mohapatra, J. Hazard. Mater., 2011, 185, 359–365 CrossRef CAS PubMed.
- A. R. Laiju, T. Sivasankar and P. V. Nidheesh, Environ. Sci. Pollut. Res., 2014, 21, 10900–10907 CrossRef CAS PubMed.
- S. Xavier, R. Gandhimathi, P. V. Nidheesh and S. T. Ramesh, Desalin. Water Treat., 2015, 53, 109–118 CrossRef CAS.
- P. V. Nidheesh and R. Gandhimathi, Clean: Soil, Air, Water, 2014, 42, 779–784 CAS.
- H. T. Van, L. H. Nguyen, T. K. Hoang, T. T. Nguyen, T. N. H. Tran, T. B. H. Nguyen, X. H. Vu, M. T. Pham, T. P. Tran, T. T. Pham, H. D. Nguyen, H. P. Chao, C. C. Lin and X. C. Nguyen, Environ. Technol. Innovation, 2020, 18, 100670 CrossRef.
- A. Nezamzadeh-Ejhieh and M. Khorsandi, Desalination, 2010, 262, 79–85 CrossRef CAS.
- R. Gonzalez-Olmos, F. Holzer, F. D. Kopinke and A. Georgi, Appl. Catal., A, 2011, 398, 44–53 CrossRef CAS.
- J. He, X. Yang, B. Men and D. Wang, J. Environ. Sci., 2016, 39, 97–109 CrossRef CAS PubMed.
- J. A. Zazo, J. A. Casas, A. F. Mohedano and J. J. Rodríguez, Appl. Catal., B, 2006, 65, 261–268 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02939h |
| This journal is © The Royal Society of Chemistry 2021 |