Open Access Article
Open Access ArticleObtaining lignocellulosic biomass-based catalysts and their catalytic activity in cellobiose hydrolysis and acetic acid esterification reactions
Daniela Godina *ab,
Kristine Meilea and
Aivars Zhurinsha
*ab,
Kristine Meilea and
Aivars Zhurinsha
aLatvian State Institute of Wood Chemistry, Dzerbenes 27, Riga, LV-1006, Latvia. E-mail: danielagodina393@gmail.com; kristine.meile@inbox.lv; aivarsz@edi.lv
bUniversity of Latvia, Faculty of Chemistry, Jelgavas 1, Riga, LV-1004, Latvia
First published on 20th May 2021
Abstract
Global challenges prompt the world to modify its strategies and shift from a fossil-fuel-based economy to a bio-resource-based one with the production of renewable biomass chemicals. Different processes exist that allow the transformation of raw biomass into desirable bio-based products and/or energy. In this work different biochars that were obtained as a by-product from birch chip fast pyrolysis and carbonization were used as is or chemically/physically treated. These sulfonated carbon catalysts were compared to a commercially available sulfonated styrene-divinylbenzene macroreticular resin (Dowex 50W X8). Characterisation (water content and pH value, FTIR, base titration, element analysis and N2 desorption) was done to evaluate the obtained sulfonated biocarbon catalysts. Catalytic activity was tested using cellobiose (CB) hydrolysis and acetic acid esterification. For the catalytic CB hydrolysis, we tested the reaction temperature, time and CB and catalyst mass ratios. The determined optimal conditions were 120 °C and 24 h, with CB and catalyst mass ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The highest glucose yield was observed for biochar obtained from the birch chip fast pyrolysis process (BC_Py-H2SO4) – 92% within 24 h for 120 °C. Comparably high glucose yield was observed for biochar that was obtained in birch chip carbonization (BC_Carbon-H2SO4) – 86% within 24 h for 120 °C.
5. The highest glucose yield was observed for biochar obtained from the birch chip fast pyrolysis process (BC_Py-H2SO4) – 92% within 24 h for 120 °C. Comparably high glucose yield was observed for biochar that was obtained in birch chip carbonization (BC_Carbon-H2SO4) – 86% within 24 h for 120 °C.
Introduction
Global consumption of fossil resources – oil, coal and gas – is rising and the deposits themselves are dwindling. The European Green Deal1 envisages starting a transformation of the economy with the aim of climate neutrality. It is meant for the transition to chemicals that are safe and sustainable by design, too.2 The terms green energy, green products and sustainable development are increasingly being used to mean making full use of renewable natural resources instead of fossil resources, which is at the heart of a biorefinery. Today, biorefinery is defined as the sustainable and synergistic processing of biomass into marketable food and feed ingredients, products (chemicals, materials) and energy (fuel, electricity, heat).2,3 The principle of biorefining is an alternative to the oil refining industry, using renewable biomass instead of oil as a raw material.3 A biorefinery also integrates a variety of processing technologies when compared with petroleum refinery to produce multiple bio-products from various biomass sources.3,4 Such approach will help maximize the value of the biomass and minimize no value by-products.Biomass, such as wood, is a type of renewable resources that can be used not only for energy, but also for carbon-containing chemicals and materials.5 Biomass has great potential to be used as a sustainable resource to provide biofuels, biochemicals, and biomaterials.6 As the most abundant biopolymers in biomass, cellulose and hemicelluloses are the largest components of the earth's biomass.7 Thus, effectively converting these components into useful C5 sugars platform compounds, such as furfural (FF), 5-hydroxymethylfurfural (HMF), etc., is a highly desirable endeavour.8 The C6 sugars from the hydrolysis of cellulose and hemicelluloses can be further utilized to produce alcohols, lactic acid and other chemicals.9,10 The FF and HMF from the dehydration of sugars can be converted into various furan derivatives for numerous applications. Therefore, the hydrolysis of biomass into monomeric sugars and their further conversion is one of the most important research topics in biomass upgrading.11,12 Many hydrolysis agents, such as enzymes, mineral acids, and solid acids, have been employed in biomass hydrolysis.13,14 However, the enzymatic hydrolysis of biomass is slow and costly, and the hydrolysis of biomass by mineral acids are corrosive and usually produces various environmental hazards.15–17 Recently, several studies reported that biochar-based solid acid is a promising alternative to mineral acid in biomass hydrolysis and dehydration due to its high reaction activity, recyclability, and low cost.18–20
The concept of sulfonated carbon catalysts is based on carbon materials obtained by using various carbonization processes, such as pyrolysis, gasification, hydrothermal carbonization, and torrefaction.19,21 The carbonization of the raw material takes place in an oxygen-free environment, which ensures the removal of volatile substances and the formation of non-volatile carbon with undeveloped pore structures.22 It is then possible to subject the carbonized carbon materials to further physical or chemical activation producing activated carbon (AC).23 The activation process develops the porous structure by increasing pore volume and internal surface area,24 which must lead to better catalytic properties. Physical activation involves the prior carbonization of a raw material with its further activation.25 The most used physical activating agents are superheated water vapour, CO2, and oxygen or air, or a combination of all these agents. As a result, the activated biochars can be further used in various applications, such as activated carbon, soil amendments, carbon sequestration agents, and environmental adsorbents.26–28
Chemical activation is known as a one-step AC production method in which the feedstock is mixed with a concentrated chemical activation agent. The resulting mixture is then pyrolyzed.29,30 Chemical activation usually takes place at lower temperatures and for a shorter time compared to physical activation.23,31 The formation of the pore structure is better in the process of chemical activation, because chemical reagents are substances with dehydrating properties that inhibit the formation of tar during carbonization of cellulose and aromatization of the structure, as a result of which the amount of released volatiles is reduced.32 The most used biochar produced in the carbonization process modification method is sulfonation with concentrated H2SO4 or its derivatives.33
The purpose of this work is to produce and compare several biomass-based sulfonated biocarbon catalysts and evaluate their potential as solid catalysts for sugar hydrolysis. The investigated biochars are by-products of anhydrosugar targeted fast pyrolysis, thus promoting the biorefinery approach to developing new materials and catalysts.
Methods
Materials and chemicals
LC-MS LiChrosolv acetonitrile, sulfuric acid (95–97%), D-(+)-cellobiose (≥99%), D-(+)-glucose (≥99.5%), 5-hydroxymethylfurfural (≥99%) and Dowex 50W X8 resins were purchased from Merck and used without further purification. For UHPLC analysis Millipore ultrapure deionized water was used.Catalyst generation: carbonization, fast pyrolysis, chemical and physical activation of biochar
Fig. 1 demonstrates the scheme for catalyst generation from lignocellulosic biomass. In further text the scheme is described in more detail.There were two pathways for obtaining the biochar – carbonization (Carb) and fast pyrolysis (Py) process.
In case of the carbonization pathway, ground birch wood – BC (0.63–1.0 mm) was carbonized at 400 °C for 2 h. In order to obtain catalyst BC_Carb-H2SO4, chemical activation of the carbonized material was done as follows. Biochar was weighted (10 g) in a beaker and contacted with concentrated sulphuric acid (50 mL). Via periodic stirring (15 min) the concentrated sulphuric acid was mixed with carbon and placed in an oven at 80 °C overnight (20 h). After impregnation with sulphuric acid, the catalyst was suspended with 1 L deionized water and mixed for 30 minutes. Then the suspension was filtered, and the remaining catalyst was again suspended in 1 L deionized water and stirred for 30 minutes. This process was repeated in total 3 times. After that the catalyst on the filter was additionally washed with 1 L hot deionized water (90 °C) until neutral pH was reached. In all rinsing steps the pH value of the water solution was measured with a TitraLab TIM 840 automatic titration station. The catalyst was finally dried at 100 °C overnight (20 h).
The second part of the carbonized material was subjected to physical activation with steam at 900 °C with 200 mL water. After that the biochar was impregnated with concentrated sulfuric acid as described previously. The obtained biocarbon catalyst BC_Carb_Steam-H2SO4 was washed with deionized water to neutral pH and dried as described previously.
On the other hand, fast pyrolysis char and tar samples were prepared, using lignocellulose obtained from hydrolysed birch (Betula pendula) chips (0.40–0.63 mm). Material was treated with 1–5% H2SO4 from the wood oven dry mass in the form of a 30% water solution, sprayed in a paddle mixer with the mixing time 10 min in mildly acidic conditions at 120 °C for 2 h with washing up to pH 3.5 and pyrolysed by superheated steam treatment in an entrained flow thermoreactor for 2–4 s at temperature 380–400 °C.34 Pyrolysis was done to obtain levoglucosan as a main product, while leftover side products were the main object of study in this work. The insoluble pyrolysis condensate sediments were used as tar sample and consisted of recondensated lignin and furan oligomers. In the fast pyrolysis process approximately 45 wt% on dry basis of the raw material is left over as solid residue (char).
To prepare the catalyst BC_Py-H2SO4, one part of the biochar obtained as a by-product from fast pyrolysis process was washed with deionized water until neutral pH and dried. After that, the biochar was impregnated with a concentrated sulphuric acid solution at 80 °C overnight (20 h), then washed with hot deionized water to neutral pH and dried at 100 °C overnight as described previously. Second part of the pyrolysis biochar was treated with concentrated nitric acid solution at 80 °C overnight, then washed with hot deionized water to neutral pH and dried at 100 °C overnight as described previously, producing catalyst BC_Py-HNO3.
Furthermore, the condensate (bio-oil) obtained in the fast pyrolysis process was hydrolysed at 121 °C with 0.2 M H2SO4 solution and the obtained solid non-hydrolysable residue, mainly consisting of phenolic type compounds, was washed with hot deionized water to neutral pH as described previously and used as sulfonated biocarbon catalyst BC_Py-CHR.
The tar obtained in the fast pyrolysis process was carbonized at 220 °C with 20% sulfuric acid solution. The obtained residue was washed with hot deionized water to neutral pH as described previously and used as sulphured biocarbon catalyst BC_Py_Tar_Carb-H2SO4.
Catalytic activity of the obtained sulfonated biocarbon catalysts was also compared to a commercially available sulfonated styrene–divinylbenzene macroreticular resin – Dowex 50W XB (Sigma–Aldrich). The Dowex resins were purchased with an approximate moisture content of 50–55%. The Dowex resins were washed at room temperature with deionized water to neutral pH and dried at 55 °C for 5 h before storage and later use.
Catalyst characterization
Different physical and chemical characteristics of the biocarbon catalysts were determined. Moisture content in biocarbon catalysts was determined in triplicate with moisture analyser Mettler Toledo. The pH value in biocarbon catalysts was analysed according to EBC (2012) “European Biochar Certificate – Guidelines for a Sustainable Production of Biochar” and taken into account in further calculations.35 The pH value was determined in triplicate. Approximately 5 g of dried biochar was placed in a glass vessel and five times the volume (25 mL) of a 0.01 M CaCl2 solution was added. The suspension was rotated for 1 h. The obtained suspension was filtered through a Whatman filter paper (90 mm) and measured with a TitraLab TIM 840 automatic titration station pH meter.Surface area of the catalyst was measured by N2 adsorbtion over a relative pressure range (P/Po) of 0.100/0.100 (ads/des). Isotherms were obtained using a Quantachrome Nova 4200e instrument. The outgas temperature was 105 °C, the adsorption gas was nitrogen. Brunauer–Emmet–Teller (BET) and Dubinin–Radushkevich (DR) approaches were used to characterize AC porous structure for high (>0.2) (mesopores) and low (<0.2) (micropores) relative pressure regions, respectively.
Elemental analysis was performed with element analyser Elementar Vario Macro. Samples were analysed in triplicates.
Functional group analysis was done by acid–base titration with TitraLab TIM 840 automatic titration station. To approximately 0.5 g of catalyst 50 mL of a 0.075 M NaOH or NaHCO3 solution was added and left overnight at room temperature with constant mixing. The solution with biochar was then filtered and 5 mL of solution was back titrated with 0.1 M HCl until first stoichiometric point for NaOH and second stoichiometric point for NaHCO3. For each sample 3 replicates were done.
To qualitatively assess the formation of functional groups on the surface of the treated carbon, FTIR analysis (in ATR mode) was performed on the obtained biocarbon catalysts. The carbon catalysts were crushed to a fine powder and analysed directly. The FTIR data was collected using an attenuated total reflectance technique with a ZnSe and Diamond crystals on a Thermo Fisher Nicolet iS50 spectrometer. A total of 64 scans were averaged at 4 cm−1 resolution for each spectrum.
The obtained biochar surfaces were examined using an SEM Hitachi Tabletop Microscope TM3000 (Japan). The biochar catalysts were used as they were, to obtain images in different magnifications with a voltage of 15 kV.
Catalytic esterification reaction
Catalytic activity for each biochar catalyst was determined by performing esterification reaction – ethyl acetate production.36 Acetic acid esterification with ethanol is a model reaction for catalyst activity evaluation to further compare to the cellobiose hydrolysis efficiency, which is described in the next section. For experiments about 2 g of biocarbon catalyst were weighed into the conical flask, 1 mol (19 mL) of CH3COOH and 10 mol (195 mL) of ethanol were added. After stirring the reaction mixture, an aliquot (about 0.1 g) was taken, diluted with distilled water (about 20 mL), and titrated with 0.1 M KOH to determine the initial relative percentage of acetic acid. The reaction mixture was refluxed, aliquoted at regular intervals, and titrated with 0.1 M KOH to monitor the reduction in the relative acetic acid content. The esterification reaction experiments were done in duplicates.Cellobiose hydrolysis to glucose
For the determination of the catalytic activity, cellobiose hydrolysis was performed as a model reaction. Firstly, the experiments to determine CB hydrolysis reaction kinetics and the optimal conditions were done with biochar BC_Py-H2SO4 under pressurised conditions at 3 different temperatures – 103, 110 and 120 °C. The reaction time was 1, 2, 3, 6, 7 and 24 h. For each time individual samples were made. In test experiments, the mass ratio of CB and catalyst were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. In the optimal conditions (120 °C and 24 h) we tested also different CB and catalyst mass ratios – 1
5. In the optimal conditions (120 °C and 24 h) we tested also different CB and catalyst mass ratios – 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, 1
2.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; 1
5; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; 1
10; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15; 1
15; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20; 1
20; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25. The initial mass concentration of CB solution in deionized water was 2 mg mL−1. CB hydrolysate with biochar was filtered (0.45 μm nylon syringe filters Frisenette) and diluted 2 times with ACN before UHPLC analysis. All experiments were performed in triplicate.
25. The initial mass concentration of CB solution in deionized water was 2 mg mL−1. CB hydrolysate with biochar was filtered (0.45 μm nylon syringe filters Frisenette) and diluted 2 times with ACN before UHPLC analysis. All experiments were performed in triplicate.
The cellobiose hydrolysis with the rest of biochar samples was performed using the determined optimal conditions: for 24 h at 120 °C with CB and catalyst ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. All experiments were performed in triplicate.
5. All experiments were performed in triplicate.
Biochar catalyst reuse capacity was determined at 120 °C for 6 h, with CB and catalyst mass ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, using initial cellobiose mass concentration 2 mg mL−1. After each hydrolysis experiment, the used catalyst was separated by filtration (Glass filter funnel, Por. 2) and dried at 100 °C for 3 h and reused, also FTIR spectra was obtained for these reused catalysts. All experiments were performed in duplicate.
5, using initial cellobiose mass concentration 2 mg mL−1. After each hydrolysis experiment, the used catalyst was separated by filtration (Glass filter funnel, Por. 2) and dried at 100 °C for 3 h and reused, also FTIR spectra was obtained for these reused catalysts. All experiments were performed in duplicate.
Qualitative and quantitative measurements of cellobiose, glucose and their degradation products were done using a Waters Acquity H-Class Ultra High Performance Liquid Chromatography Equipment with Column and Sample Thermostat, Continuous Mobile Phase Degasser, Automatic Sample Injection Unit, Photodiode Matrix Detector (PDA), evaporative light scattering detector (ELSD) and Liquid Chromatography High Pressure Pump “Waters Acquity H-Class Quaternary Solvent Manager” (Waters, USA). CB and glucose analysis was done with evaporative light scattering detector (ELSD) using BEH amide (1.7 μm, 2.1 × 100 mm) column at 60 °C with eluent consisting of 50% ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (40
H2O (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) mixture with 0.1% NH4OH additive and 50% ACN at flow rate 0.15 mL min−1. Carbohydrate degradation product (5-hydroxymethylfurfural) concentration was determined with diode array detector (DAD) using CSH Phenyl-Hexyl (1.7 μm, 2.1 × 100 mm) column at 30 °C. The mobile phase for CSH Phenyl-Hexyl column was 0.1% formic acid in water/ACN (90
60) mixture with 0.1% NH4OH additive and 50% ACN at flow rate 0.15 mL min−1. Carbohydrate degradation product (5-hydroxymethylfurfural) concentration was determined with diode array detector (DAD) using CSH Phenyl-Hexyl (1.7 μm, 2.1 × 100 mm) column at 30 °C. The mobile phase for CSH Phenyl-Hexyl column was 0.1% formic acid in water/ACN (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) for 2.5 min followed by gradient to water/ACN (10
10) for 2.5 min followed by gradient to water/ACN (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) and an equilibration step back to the initial composition by 10 min with flow rate 0.4 mL min−1. UV/vis spectral range: 210–400 nm, wavelength for quantitative analysis was 275 nm. Identification of compounds and concentrations were determined using matching retention times and standard curves for cellobiose, glucose, 5-hydroxymethylfurfural.
90) and an equilibration step back to the initial composition by 10 min with flow rate 0.4 mL min−1. UV/vis spectral range: 210–400 nm, wavelength for quantitative analysis was 275 nm. Identification of compounds and concentrations were determined using matching retention times and standard curves for cellobiose, glucose, 5-hydroxymethylfurfural.
Conversion of cellobiose and glucose yield from CB hydrolysis after chromatography data were calculated by equitation 1 and 2 respectively. The theoretical mass concentration of glucose (γtheoretical) was calculated by equitation 3.
 | (1) |
 | (2) |
 | (3) |
Additionally, cellobiose and glucose adsorption capacity for each biocarbon and Dowex catalyst was measured at room temperature (20 °C). Catalysts were weighted and solution of known initial cellobiose or glucose concentration were added and left at 20 °C for 24 hours. The solutions were filtered using 0.45 μm nylon syringe filter Frisenette, diluted with acetonitrile and analysed with UHPLC to determine the equilibrium cellobiose and glucose concentration. The adsorption capacity (q) was calculated by equitation 4.
 | (4) |
Results and discussion
Catalyst properties
Different catalyst generation techniques produced biocarbon catalysts with different properties. Table 1 is a summary of the catalyst characterization results. Elemental analysis showed moderate H content and a comparatively high O content in all samples, but the S content was directly linked to the catalyst activation method. The chemical composition in biochar is highly dependent on the used biomass and the pyrolysis conditions.37 S, % was ≤0.5 for the catalyst, which has not undergone chemical treatment with sulfuric acid. The S, % content was similar for samples BC_Py-H2SO4 and BC_Py_Tar_Carb-H2SO4 (approximately 2% both), but twice as high for sample BC_Carb-H2SO4, which had an almost tenfold bigger total pore volume. The C content was relatively constant in all obtained biochars (from 64 to 68 ± 0.4%) since the utilised treatment methods did not influenced the carbon content.| BC_Py-H2SO4 | BC_Py-HNO3 | BC_Py_CHR | BC_Carb-H2SO4 | BC_Py_Tar_Carb-H2SO4 | BC_Carb_Steam-H2SO4 | |
|---|---|---|---|---|---|---|
| pH | 1.49 ± 0.02 | 1.95 ± 0.03 | 3.09 ± 0.02 | 1.56 ± 0.05 | 2.97 ± 0.04 | 5.03 ± 0.03 |
| H, % | 2.81 ± 0.13 | 2.97 ± 0.18 | 5.00 ± 0.05 | 3.99 ± 0.08 | 5.16 ± 0.06 | 2.23 ± 0.18 |
| S, % | 2.21 ± 0.15 | 0.18 ± 0.03 | 0.54 ± 0.07 | 4.06 ± 0.11 | 2.127 ± 0.010 | 0.10 ± 0.03 |
| O, % | 31.3 ± 0.3 | 33.6 ± 0.2 | 30.6 ± 0.4 | 30.9 ± 0.4 | 24.26 ± 0.06 | 30.6 ± 0.2 |
| mmol NaOH per g per sample | 0.079 ± 0.002 | 0.087 ± 0.005 | 0.090 ± 0.007 | 0.066 ± 0.006 | 0.10 ± 0.02 | 0.005 ± 0.002 |
| mmol NaHCO3 per g per sample | 0.0297 ± 0.0012 | 0.037 ± 0.002 | 0.008 ± 0.011 | 0.086 ± 0.002 | 0.013 ± 0.012 | 0.0005 ± 0.0002 |
| Total surface area, m2 g−1 | <2 | 2.6 | <2 | 2.8 | <2 | 1264 |
| Micropore area, m2 g−1 | 4.8 × 10−9 | 4.3 × 10−9 | 2.2 × 10−5 | 2.6 | 2.7 × 10−6 | 1477 |
| Total pore volume, cm3 g−1 | 2.9 × 10−3 | 4.6 × 10−3 | 1.4 × 10−3 | 1.2 × 10−2 | 3.3 × 10−3 | 1.3 |
| Average pore diameter, nm | — | 7.1 | 11.5 | 16.4 | 11.4 | 4.0 |
The total surface area of the obtained biocarbon catalysts was below or a little bit above 2 m2 g−1, with the exception of biochar BC_Carb_Steam-H2SO4 (1264 m2 g−1) which had been physically treated with water steam and also chemically treated with concentrated sulfuric acid. For this biocarbon catalyst the micropore area and total pore volume also was larger than for the other biochars. On the contrary, the average pore diameter was smaller. After the functional group titration results and also the S, % content for this catalyst, it could be seen that on the surface area the acid functional groups were non-existent.
For the biochar BC_Py_Tar_Carb-H2SO4 the total acidic group content was larger. The elemental analysis showed a higher amount of hydrogen (5.16%), also in FTIR spectra the signal corresponding to C–H stretching could be observed (in the region of 3000 to 2500 cm−1). This could be explained by the fact that this biochar had been obtained from tar that was obtained in the pyrolysis process, and tar from pyrolysis contains a large amount of aromatic compounds.
For the biochar BC_Py-HNO3 the total acidic group content (mmol NaOH per g per sample) was larger than for the biochars BC_Py-H2SO4 and BC_Carb-H2SO4. This can be explained by the fact that by chemical treatment with nitric acid to the surface not only the nitric groups were impregnated but also nitric acid is used for oxidising the already existent functional groups on the surface of char.
For the biochar BC_Py-H2SO4, the hydrogen, sulphur and strongly acidic group content was larger, as well as the micropore area, total pore volume and average pore diameter than for the biochar BC_Carb-H2SO4. This can be explained by the fact that for this biochar possibly the impregnated functional groups were sterically less available.
Biochar BC_Py_CHR contained a larger amount of hydrogen which with FTIR spectra corresponds to aliphatic compounds (in the region of 3000 to 2500 cm−1). The pyrolysis condensate contained different organic acids, and these acids gave a high content of strongly acidic groups.
As shown in SEM images (Fig. 2), to the biochar after fast pyrolysis (BC_Py) deep channels and pores are prominent caused by organic material valorisation during fast pyrolysis process.38 For the sulfonated biochar (BC_Py-H2SO4) the decrease in the porosity can be observed. This can be contributed with the partial oxidation, condensation and partial deconstruction of the surface by the result of impregnation with sulfuric acid. The pore blockage occurs due to the adsorption of the –SO3H groups on the surface.39 By comparing carbonised biochar (BC_Carb) with biochar obtained from fast pyrolysis (BC_Py) essential differences in char structural images were not found. This can be explained by the small difference in temperature for fast pyrolysis and carbonisation.
 | ||
| Fig. 2 SEM images of biochar obtained from fast pyrolysis – (A) (BC_Py) and sulfonated biochar – (B) (BC_Py-H2SO4). | ||
When comparing results in Table 1, mainly pore properties with obtained SEM pictures, it can be said that obtained results are mostly consistent. In SEM pictures it can be visualized that obtained biochar are heterogenous materials with high variance in surface structure.
Cellobiose hydrolysis optimal conditions
For method development of cellobiose hydrolysis, biochar BC_Py-H2SO4 was used. Reaction was performed at 103, 110 and 120 °C for 1, 2, 3, 6, 7 and 24 hours with cellobiose and catalyst mass ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The standard deviations for these obtained results were in a range of 1 to 3%.
5. The standard deviations for these obtained results were in a range of 1 to 3%.
After the obtained data (Fig. 3) it can be seen that at a higher temperature cellobiose conversion and glucose yield is much higher – at 120 °C after 3 h the CB conversion is already 44% but after 24 h the CB conversion is 100%.
 | ||
| Fig. 3 Cellobiose conversion (A) and yield of glucose (B) over time at 3 temperatures (103, 110, 120 °C) with biochar BC_Py-H2SO4. | ||
We also tested different cellobiose and catalyst mass ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, 1
2.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 1
15, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 1
20, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) with biocarbon catalyst BC_Py-H2SO4 at 120 °C for 6 h. The results are shown in Fig. 3.
25) with biocarbon catalyst BC_Py-H2SO4 at 120 °C for 6 h. The results are shown in Fig. 3.
By using a larger catalyst amount the cellobiose conversion and yield of glucose increases (Fig. 4). If the cellobiose hydrolysis is performed in 24 h at 120 °C with CB and catalyst ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, (Fig. 4) then glucose yield is the same as when the reaction is performed in 6 h with CB and catalyst ratio 1
5, (Fig. 4) then glucose yield is the same as when the reaction is performed in 6 h with CB and catalyst ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 1
20 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25. So it can be concluded that by performing CB hydrolysis with this biocarbon larger amount of catalyst is not necessary to increase the glucose yield, while the reaction time needs to be increased. Also, it can be seen that despite the total CB conversion with CB and catalyst mass ratios 1
25. So it can be concluded that by performing CB hydrolysis with this biocarbon larger amount of catalyst is not necessary to increase the glucose yield, while the reaction time needs to be increased. Also, it can be seen that despite the total CB conversion with CB and catalyst mass ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 1
15, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 1
20 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, the yield of glucose is below 100%. After the UHPLC-UV chromatographic data 5-HMF could be identified as a glucose dehydration product. However, the determined amount of 5-HMF in these hydrolysed CB solutions was below 0.1%, so it is possible that CB and glucose formed during hydrolysis are adsorbed and condensate on the surface of the biochar. After functional group titration results (Table 1) it can be seen that on the biocarbon catalysts surface exists both strong and weak acid functional sites. Although weak acid functional groups such as phenols, lactones and carboxylic acids are more selective in terms of glucose formation, the strong acid groups such as sulfonic are more accessible in polar solvents.40 It should be noted that strong acidic groups can catalyse unnecessary side reactions such as glucose degradation to 5-HMF.41
25, the yield of glucose is below 100%. After the UHPLC-UV chromatographic data 5-HMF could be identified as a glucose dehydration product. However, the determined amount of 5-HMF in these hydrolysed CB solutions was below 0.1%, so it is possible that CB and glucose formed during hydrolysis are adsorbed and condensate on the surface of the biochar. After functional group titration results (Table 1) it can be seen that on the biocarbon catalysts surface exists both strong and weak acid functional sites. Although weak acid functional groups such as phenols, lactones and carboxylic acids are more selective in terms of glucose formation, the strong acid groups such as sulfonic are more accessible in polar solvents.40 It should be noted that strong acidic groups can catalyse unnecessary side reactions such as glucose degradation to 5-HMF.41
 | ||
| Fig. 4 Cellobiose conversion and glucose yield after hydrolysis reaction with catalyst BC_Py-H2SO4 at 120 °C for 6 hours with different CB and catalyst ratios. | ||
The carbon material can incorporate large amounts of water, due to the high density of the hydrophilic functional groups. This provides good access of reactants in solution to the –SO3H groups into the carbon material, which gives high catalytic activity, despite smaller surface area. This means that the effective surface area of the carbon material in the hydrolysis process is much larger than the BET surface area. Water molecules can also adsorb to the surface of the catalyst and thus interfere with the sorption of cellobiose.42
Large molecules such as cellobiose are not densely adsorbed on surface of carbon catalyst. In literature it is claimed that carbon catalyst has to have surface area above at least 4 m2 g−1 then the carbon material can incorporate even large molecules such cellobiose into the bulk in the presence of water.42
By comparing the biochars that were obtained via fast pyrolysis process, the best results (highest glucose yield) can be obtained by performing CB hydrolysis with sulfonated biochar BC_Py-H2SO4 (Table 2). The standard deviations for these obtained results are in a range of 1 to 3%. If the biochar is impregnated with nitric acid, then the CB hydrolysis occurs, but the results are better with sulfuric acid impregnation. Nitric acid was used for carbon surface oxidation to introduce oxygen containing groups (mainly as carboxyl groups) and small amount of nitrogen functional groups on the surface. Sulfuric acid was used to perform sulfonation and introduce the –SO3H groups onto the surface of the biochar.43 It is important to note that nitric acid has not been widely studied. In general, in optimised conditions nitric acid is less effective than sulfuric acid in hemicellulose conversion into monomeric sugars.13 For the biochar that was obtained as a solid residue from fast pyrolysis condensate hydrolysis (BC_Py_CHR) the glucose yield is low. This can be explained by the fact that the pores of the obtained biochar are blocked with volatiles and other amorphous decomposition products, therefore CB and glucose conversion is so low. Also, it must be noted that for this biochar the additional chemical activation was not done, so the functional groups on the surface are obtained from the condensate hydrolysis with sulfuric acid. For the biochar BC_Py_Tar_Carb-H2SO4 it is also possible that the pores on the surface area are filled with tar and therefore blocked, but since additionally the carbonisation was done the tar components are volatilized and the pores are accessible for the CB hydrolysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the CB and glucose adsorption and CB and glucose adsorption capacity at room temperature for 24 hours
5, the CB and glucose adsorption and CB and glucose adsorption capacity at room temperature for 24 hours
| Catalyst | CB conversion, % | Glucose yield, % | CB adsorption, % | CB adsorption capacity, mg g−1 | Glucose adsorption, % | Glucose adsorption capacity, mg g−1 |
|---|---|---|---|---|---|---|
| Dowex | 24 | 26 | 5 | 106 | 8 | 100 |
| BC_Py_CHR | 29 | 24 | 23 | 124 | 25 | 120 |
| BC_Py-HNO3 | 49 | 40 | 18 | 118 | 26 | 120 |
| BC_Py_Tar_Carb-H2SO4 | 63 | 45 | 39 | 138 | 25 | 117 |
| BC_Carb_Steam-H2SO4 | 69 | 21 | 66 | 164 | 40 | 139 |
| BC_Carb-H2SO4 | 86 | 78 | 21 | 122 | 23 | 116 |
| BC_Py-H2SO4 | 100 | 92 | 31 | 133 | 23 | 120 |
Comparing the biochars obtained from birch chip carbonization it can be seen that the best results are shown by the sulfonated biochar BC_Carb_H2SO4. Even if the carbonised biochar was treated with water steam (physical treatment) the glucose yield was low. The CB and glucose adsorption for BC_Carb_Steam-H2SO4 biochar is the highest among other catalysts, so CB and glucose adsorbs on the surface of the biochar and the hydrolysis occurs very minimal.
With a larger catalyst amount the CB conversion increases, but the increase for the yield of glucose is small and if the reaction is performed at 120 °C for 24 hours with CB and catalyst ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, then the glucose yield is much higher than with CB and catalyst ratio 1
5, then the glucose yield is much higher than with CB and catalyst ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (Fig. 5 and Table 2).
25 (Fig. 5 and Table 2).
 | ||
| Fig. 5 Cellobiose conversion and glucose yield after hydrolysis reaction with catalyst BC_Carb-H2SO4 at 120 °C for 6 hours with different CB and catalyst ratios. | ||
Dowex resins for CB hydrolysis were used as a reference material to compare our obtained biomass based catalysts with a commercially available one. Low CB conversion and glucose yield (Fig. 6) can be explained by the small CB and glucose adsorption on the surface (Table 2). In literature it has been stated that –SO3H groups on the surface of commercial resin do not or minimally adsorb CB and glucose molecules. The adsorption is mainly facilitated by phenolic –OH and –COOH groups on the surface of solid catalyst via hydrogen bonding.42 The CB hydrolysis and adsorption was performed in different conditions – the CB and glucose adsorption was done at room temperature, so it is possible that temperature effects the sorption.
 | ||
| Fig. 6 Cellobiose conversion and glucose yield after hydrolysis reaction with catalyst Dowex at 120 °C for 6 hours with different CB and catalyst ratios. | ||
By comparing two biocarbon catalysts with the best results and Dowex resins, it can be seen that with biochar BC_Py-H2SO4 at 120 °C after 24 h with CB and catalyst mass ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 it is possible to completely hydrolyse cellobiose to glucose (Fig. 7).
5 it is possible to completely hydrolyse cellobiose to glucose (Fig. 7).
 | ||
| Fig. 7 Cellobiose conversion (A) and glucose yield (B) over time during CB hydrolysis reaction using biochars BC_Py-H2SO4, BC_Carb-H2SO4 and Dowex resins as catalysts. | ||
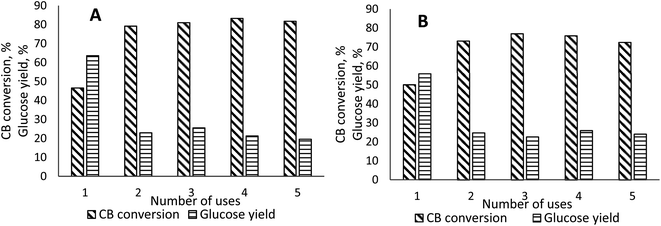
For both biochars the catalytic activity decreased after the first recycling at 120 °C for 6 hours but after the second recycling and on to the fifth the catalytic activity stayed constant (with CB conversion and glucose yield approximately 20–30%) (Fig. 8). This can be explained that after first use, irreversible adsorption of glucose occurs on surface of the catalyst and a large number of active sites remains occupied even after separation. FTIR spectra shows that after first use of catalyst there is a noticeable decrease of –SO3 group content (Fig. 9). Meaning that after the first use catalysts are being degraded. Most likely in the increased temperature and pressure, desulfonation occurred, decreasing the overall functional group content able to catalyse further reactions. In literature different biocarbon material reuse capacity has been described. For sulfonated pine chip biochar the catalytic activity decreased slightly after recycling one time and eventually lost all activity after the fourth time of reuse. Authors mentioned that there was a visible reduction of particle size of the catalyst – from granular to a fine powder. So, they claim that the reduction of catalytic activity can be explained by the acid site leaching and the significant attrition of the biochar – mass loss. Also analysis of the reused biocarbon catalysts indicated a reduction in surface area after using the catalyst four times and acid site density.20 Other scientist group explains the reduction of the reused biocarbon catalyst by the removal of some unreacted sulfonic compounds that were just adsorbed on the carbon surface.44
Biochar catalytic activity comparison in acetic acid esterification and cellobiose hydrolysis
For more detailed description about obtained biocarbon catalytic activity we also performed acetic acid esterification reaction as described previously. The obtained results are shown in Fig. 10.In esterification reaction the best results showed biocarbon catalyst BC_Py_Tar_Carb-H2SO4 – acetic acid conversion reached 72%. In contrary to CB hydrolysis this catalyst showed low glucose yield – 45%. It is possible that in esterification reaction tar dissolves in ethanol and produced ethyl acetate and so the phenolic compounds in tar raises the catalytic activity in esterification. Non-existent catalytic activity in esterification reaction showed biocarbon catalyst BC_Carb_Steam-H2SO4. After the functional group titration results for this catalyst, it could be seen that on the surface area the acid functional groups were non-existent. For this reason, the acetic acid esterification reaction did not occur. Also, in CB hydrolysis glucose yield is only 21%. The glucose and CB adsorption – 40 and 66% respectively cause the low catalytic activity for this biocarbon catalyst. Low catalytic activity in CB hydrolysis and esterification reaction was determined for biocarbon catalyst BC_Py-HNO3 – acetic acid conversion 23%. The incorporated nitric acid groups have low acidity compared to SO3H groups.45 This can be explained by –SO3H group importance in these reactions. The biochar BC_Py_CHR contained a larger amount of total functional groups but the acetic acid conversion was small – 36%. The pyrolysis condensate contained different organic acids, and these acids gave a high content of strongly acidic groups. But after the acetic acid conversion data it can be seen that these –COOH groups were less effective for the esterification reaction than the –SO3H groups. For the biochars that showed the best results for the cellobiose hydrolysis reaction – BC_Py-H2SO4 and BC_Carb-H2SO4 – we expected also high catalytic activity in acetic acid esterification reaction, but the obtained acetic acid content was 67 and 55% respectively. These results may lead to a conclusion that for the cellobiose hydrolysis and acetic acid esterification reaction different functional groups on the surface of the biochar catalyst are needed.
To confirm or deny our conclusion we firstly reused both biochars (BC_Py-H2SO4 and BC_Carb-H2SO4) in esterification reaction. The obtained results are shown in Fig. 11.
 | ||
| Fig. 11 The biocarbon catalyst reuse activity data for acetic acid esterification reaction (acetic acid content). | ||
Between each esterification the biochar was washed with deionized water until neutral pH and dried. In total we performed 9 esterification reactions. The sulfonated biochars could be used as a catalyst for up to four times with no noticeable decrease in catalytical activity. In literature several authors for their biochar catalysts also observed the decrease in catalytic activity after the fourth reuse.46
For both biochars after the fourth reuse in esterification reaction FTIR spectra were taken, and also acid–base titration was done to monitor changes in functional group content. The obtained results were compared to the biochars before esterification. The functional group content after titration is shown in Table 3. It can be seen that for both biochars the functional group content decreased after the fourth reuse in esterification reaction, and it explains the loss in catalytic activity. The FTIR spectra for both biochars also shows the decrease for –SO3 group content after the acetic acid esterification reaction.
| BC_Py-H2SO4 | BC_Py-H2SO4_E | BC_Py-H2SO4_H + E | BC_Carb-H2SO4 | BC_Carb-H2SO4_E | BC_Carb-H2SO4_H + E | |
|---|---|---|---|---|---|---|
| Mmol NaOH per g per sample | 0.079 ± 0.002 | 0.055 ± 0.012 | 0.033 ± 0.011 | 0.066 ± 0.006 | 0.030 ± 0.005 | 0.016 ± 0.007 |
| Mmol NaHCO3 per g per sample | 0.0297 ± 0.0012 | 0.017 ± 0.007 | 0.017 ± 0.010 | 0.086 ± 0.002 | 0.028 ± 0.003 | 0.030 ± 0.005 |
We performed another experiment to explain the differences between the cellobiose hydrolysis and acetic acid esterification. For both biochars that were used in cellobiose hydrolysis reaction the esterification reaction was also performed. After the functional group content results (Table 3) it can be seen that for both biochars after the esterification reaction the total acidic group (mmol NaOH per g per sample) and strongly acidic group (mmol NaHCO3 per g per sample) content decreases. When the hydrolysis and additional esterification is done than for both biochars the total acidic group content decreases again, but the strongly acidic group content stays constant. The same pattern can be seen after the FTIR spectra – the –SO3 group content (as strongly acidic groups) stays unchanged. For the biochar BC_Carbon-H2SO4 the acetic acid content after hydrolysis and additional acetic acid esterification reaction was 91%, but for the biochar BC_Py-H2SO4 it was 62%. For the biocarbon catalyst BC_Py-H2SO4 after the cellobiose hydrolysis and additional esterification reaction the total acidic group content is larger (0.033 mmol NaOH per g per sample), so it can be assumed that for the esterification reaction the strongly acidic groups do not predominantly influence the catalytic activity for this application. In the literature it is claimed that in the esterification reaction mechanism, the strong acid nature of –SO3H group makes the protonation of acetic acid molecule difficult, but when the weak acid groups such as –COOH are on the surface of the catalyst, the deprotonated form of –COOH can generate hydrogen bonding with the –OH group in the acetic acid molecule, leading a small portion of a “negative charge” to the oxygen in the acetic acid molecule. And so, this negative charge promotes the nucleophilicity of the acetic acid molecule and positively influencing the esterification reaction rate and so as the catalytic activity.46
Conclusions
In this work different biomass based chars were obtained from fast pyrolysis and slow pyrolysis (carbonization). The obtained biochars were used as is or treated with chemical treatment by impregnation with concentrated sulfuric acid or nitric acid and physical treatment with water steam. The highest cellobiose conversion level was achieved by the catalysts obtained by chemical activation with H2SO4: 78% glucose yield with catalyst BC_Carb_H2SO4 and 92% glucose yield with catalyst BC_Py-H2SO4. For a comparison, 26% glucose yield was obtained by a commercially available sulfonated resin. In the acetic acid esterification reaction, the best catalytic activity was also shown by the sulfonated biochars: the acetic acid conversion for biochar BC_Carb-H2SO4 was 55% but for BC_Py-H2SO4 – 67%.The tested biochars showed different trends in their reusability in cellobiose hydrolysis and acetic acid esterification reactions, meaning that each reaction was influenced by different functional groups. Both sulfonated biochar catalysts could be used up to four times with no noticeable decrease in catalytic activity in acetic acid esterification. In case of cellobiose hydrolysis, the tested biochars partly lost their catalytic activity after the first reuse, but they could still be reused for up to five times with cellobiose conversion remaining at 20%. Also, it is important to note that the biocarbon catalyst BC_Py-H2SO4 retained its catalytic activity and could effectively be used in esterification reaction after having been used for cellobiose hydrolysis.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by a Bio-economic grant “BioCat” from the Latvian State Institute of Wood Chemistry. Lzp-2020/2-0019 New biomass origin materials hybrid carbon composites for energy storage (BiComp).References
- The European Commission, Guidance document on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed, http://www.crl-pesticides.eu/docs/public/tmplt_article.asp?CntID=727%26LabID=100%26Lang=EN Search PubMed.
- EU Commission, J. Chem. Inf. Model., 2013, 53, 1689–1699 CrossRef PubMed.
- F. Cherubini, Energy Convers. Manage., 2010, 51, 1412–1421 CrossRef CAS.
- M. Suhag and H. R. Sharma, Iarjset, 2015, 2, 103–109 CrossRef.
- M. Balat and G. Ayar, Energy Sources, 2005, 27, 931–940 CrossRef.
- A. Bridgewater, J. Therm. Sci., 2004, 8, 21–50 CrossRef.
- F. H. Isikgor and C. R. Becer, Polym. Chem., 2015, 6, 4497–4559 RSC.
- F. Yang, Q. Liu, X. Bai and Y. Du, Bioresour. Technol., 2011, 102, 3424–3429 CrossRef CAS PubMed.
- P. Mäki-Arvela, T. Salmi, B. Holmbom, S. Willför and D. Y. Murzin, Chem. Rev., 2011, 111, 5638–5666 CrossRef PubMed.
- R. Ahorsu, F. Medina and M. Constantí, Energies, 2018, 11, 3366 CrossRef CAS.
- G. Dedes, A. Karnaouri and E. Topakas, Catalysts, 2020, 10, 743 CrossRef CAS.
- X. Tong, Y. Ma and Y. Li, Appl. Catal., A, 2010, 385, 1–13 CrossRef CAS.
- V. Oriez, J. Peydecastaing and P.-Y. Pontalier, Molecules, 2019, 24, 4273 CrossRef CAS PubMed.
- Y.-L. Loow, T. Y. Wu, J. M. Jahim, A. W. Mohammad and W. H. Teoh, Cellulose, 2016, 23, 1491–1520 CrossRef CAS.
- S. Rahmati, W. Doherty, D. Dubal, L. Atanda, L. Moghaddam, P. Sonar, V. Hessel and K. ) Ostrikov, React. Chem. Eng., 2020, 5, 2017–2047 RSC.
- D. P. Maurya, A. Singla and S. Negi, 3 Biotech, 2015, 5, 597–609 CrossRef PubMed.
- L. Capolupo and V. Faraco, Appl. Microbiol. Biotechnol., 2016, 100, 9451–9467 CrossRef CAS PubMed.
- A. d. C. Fraga, C. P. B. Quitete, V. L. Ximenes, E. F. Sousa-Aguiar, I. M. Fonseca and A. M. B. Rego, J. Mol. Catal. A: Chem., 2016, 422, 248–257 CrossRef CAS.
- B. Bermejo, A. Couto Fraga and E. F. Sousa-Aguiar, Braz. J. Chem. Eng., 2019, 36, 309–315 CrossRef CAS.
- R. Ormsby, J. R. Kastner and J. Miller, Catal. Today, 2012, 190, 89–97 CrossRef CAS.
- M. P. Maniscalco, M. Volpe and A. Messineo, Energies, 2020, 13, 4098 CrossRef CAS.
- L. H. Noszko, A. Bota, A. Simay and L. G. Nagy, Period. Polytech., Chem. Eng., 1984, 28, 293–297 CAS.
- A. Ahmadpour and D. D. Do, Carbon, 1996, 34, 471–479 CrossRef CAS.
- E. Yasuda, M. Inagaki, K. Kaneko, M. Endo, A. Oya and Y. Tanabe, Carbon Alloys, Elsevier, 2003 Search PubMed.
- H. T. Ma, H. C. Ly, V. T. T. Ho, N. B. Pham, D. C. Nguyen, K. T. D. Vo and P. D. Tuan, Vietnam J. Sci. Technol., 2017, 55, 494 CrossRef.
- J. Skubiszewska-Zięba, B. Charmas, M. Kołtowski and P. Oleszczuk, J. Therm. Anal. Calorim., 2017, 130, 15–24 CrossRef.
- N. Hagemann, K. Spokas, H. P. Schmidt, R. Kägi, M. A. Böhler and T. D. Bucheli, Water, 2018, 10, 1–19 CrossRef.
- B. Sajjadi, W.-Y. Chen and N. O. Egiebor, Rev. Chem. Eng., 2019, 35, 735–776 CAS.
- M. S. Reza, C. S. Yun, S. Afroze, N. Radenahmad, M. S. A. Bakar, R. Saidur, J. Taweekun and A. K. Azad, Arab. J. Basic Appl. Sci., 2020, 27, 208–238 CrossRef.
- K. Ukanwa, K. Patchigolla, R. Sakrabani, E. Anthony and S. Mandavgane, Sustainability, 2019, 11, 6204 CrossRef CAS.
- T. D. Burchell, Carbon Materials for Advanced Technologies, Elsevier, 1999 Search PubMed.
- Y. Gao, Q. Yue, B. Gao and A. Li, Sci. Total Environ., 2020, 746, 141094 CrossRef CAS PubMed.
- X. Cao, S. Sun and R. Sun, RSC Adv., 2017, 7, 48793–48805 RSC.
- A. Zhurinsh, G. Dobele, J. Rizhikovs, J. Zandersons and K. Grigus, J. Anal. Appl. Pyrolysis, 2013, 103, 227–231 CrossRef CAS.
- EBC, European Biochar Certificate - Guidelines for a Sustainable Production of Biochar, European Biochar Foundation (EBC), Arbaz, Switzerland, 2012, http://www.european-biochar.org/en/download Search PubMed.
- X. N. Tian and X. S. Zhao, Sulfonic acid catalysts based on porous carbons and polymers, 2008, pp. 1347–1350 Search PubMed.
- S. Wijitkosum and P. Jiwnok, Appl. Sci., 2019, 9, 3980 CrossRef CAS.
- A. Y. Elnour, A. A. Alghyamah, H. M. Shaikh, A. M. Poulose, S. M. Al-Zahrani, A. Anis and M. I. Al-Wabel, Appl. Sci., 2019, 9, 1149 CrossRef CAS.
- X. Xiong, I. K. M. Yu, S. S. Chen, D. C. W. Tsang, L. Cao, H. Song, E. E. Kwon, Y. S. Ok, S. Zhang and C. S. Poon, Catal. Today, 2018, 314, 52–61 CrossRef CAS.
- P. Fernández, J. M. Fraile, E. García-Bordejé and E. Pires, Catalysts, 2019, 9, 804 CrossRef.
- G. S. Foo, A. H. Van Pelt, D. Krötschel, B. F. Sauk, A. K. Rogers, C. R. Jolly, M. M. Yung and C. Sievers, ACS Sustainable Chem. Eng., 2015, 3, 1934–1942 CrossRef CAS.
- S. Suganuma, K. Nakajima, M. Kitano, D. Yamaguchi, H. Kato, S. Hayashi and M. Hara, J. Am. Chem. Soc., 2008, 130, 12787–12793 CrossRef CAS PubMed.
- J. J. Ternero-Hidalgo, J. M. Rosas, J. Palomo, M. J. Valero-Romero, J. Rodríguez-Mirasol and T. Cordero, Carbon, 2016, 101, 409–419 CrossRef CAS.
- S. Carlier and S. Hermans, Front. Chem., 2020, 8, 347 CrossRef CAS PubMed.
- R. P. Rocha, M. F. R. Pereira and J. L. Figueiredo, Catal. Today, 2013, 218–219, 51–56 CrossRef CAS.
- A. P. da Luz Corrêa, R. R. C. Bastos, G. N. da Rocha Filho, J. R. Zamian and L. R. V. da Conceição, RSC Adv., 2020, 10, 20245–20256 RSC.
| This journal is © The Royal Society of Chemistry 2021 |