Open Access Article
Open Access ArticleIdentification and in situ removal of an inhibitory intermediate to develop an efficient phytosterol bioconversion process using a cyclodextrin-resting cell system†
Da Wang‡
 ,
Jian Zhang‡,
Dan-Dan Cao,
Xuedong Wang
,
Jian Zhang‡,
Dan-Dan Cao,
Xuedong Wang * and
Dongzhi Wei
* and
Dongzhi Wei
State Key Laboratory of Bioreactor Engineering, New World Institute of Biotechnology, East China University of Science and Technology, Shanghai 200237, China. E-mail: xdwang@ecust.edu.cn; Fax: +86-21-64250068; Tel: +86-21-64253715
First published on 15th July 2021
Abstract
A classically versatile steroid intermediate, 9α-hydroxyandrost-4-ene-3,17-dione (9α-OH-AD), can be obtained by phytosterol (PS) bioconversion using Mycobacterium. In this study, a cyclodextrin-resting cell reaction system with a high concentration of PS (50 g L−1) was used to produce 9α-OH-AD. However, the inhibitory effect of metabolic intermediates is a key factor limiting production efficiency. After the separation and identification of a series of metabolic intermediates, it was found that 4-ene-3-keto steroids, which are the first metabolites of sterol side-chain degradation, accumulated at the beginning of the bioprocess and had a remarkable inhibitory effect on bioconversion. The bioconversion rate was greatly improved when 5 g L−1 of macroporous adsorbent resin D101 was added to the reaction system in the initial phase. A certain amount of resin acted as a reservoir to remove the inhibitory intermediate in situ and facilitated the bioconversion process, and the 9α-OH-AD space–time yield increased to 8.51 g L−1 d−1, which was 23.15% higher than that without resin addition (6.91 g L−1 d−1) after 72 h bioconversion. In summary, we identified an inhibitory intermediate that limits the bioconversion rate and provided a solution based on resin adsorption for improving 9α-OH-AD production efficiency in a commercial-scale process.
1. Introduction
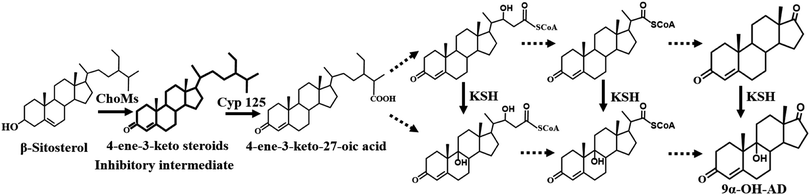
In the past decades, researchers have focused on the microbial side-chain degradation of phytosterols (PS) or cholesterol to produce steroid intermediates.1,2 9α-Hydroxyandrost-4-ene-3,17-dione (9α-OH-AD) is one of the intermediates that can further be chemically modified to steroid drugs, such as hydrocortisone and eplerenone.3 However, the bioavailability of PS and the cytotoxicity of intermediate metabolites are the key factors that limit production efficiency, particularly at high substrate concentrations.4Currently, the synthesis efficiency of steroid intermediates is enhanced by a variety of metabolic regulation methods. Genetic engineering modification of the production strains is a widely used method to improve bioconversion. Yao et al. reported an engineered Mycobacterium-transformed PS to 9α-OH-AD.5 In addition, diverse sterol-solubilizing reaction systems, including a cyclodextrin complexing system,4,6 biocompatible ionic liquid system,7 microdispersion/emulsification system, and surfactant addition,8,9 were established to improve the poor water solubility of PS. Among them, the cyclodextrin-resting cell system is an ideal method that has been successfully used for the production of a variety of steroid intermediates. It was observed that not only was the expression of genes related to PS metabolism influenced but the degradation of steroid intermediates was also inhibited in this reaction system.10,11 Nonetheless, an extended bioconversion period and a lower reaction rate still exist, especially at high PS concentrations. The inhibitory effect of metabolic intermediates is a key factor limiting production efficiency.4 Thus, many resin-based in situ removal methods have been used to alleviate the inhibition of intermediates and improve the bioconversion efficiency of many steroids, such as androsta-1,4-diene-3,17-dione and testosterone.12–14
In situ product removal (ISPR) is a common method used for the simultaneous separation of inhibitory products during fermentation and biotransformation. Macroporous resins are promising candidates for ISPR materials and have many advantages, such as alleviated feedback suppression, low cost, and high phase stability.15,16 In recent years, macroporous resins have been successfully used to produce various metabolites, such as diepoxin ζ, biogenic amines, botrallin and TMC-264.17–19 However, the study on the influence of adsorption resin on the performance of Mycobacterium to produce 9α-OH-AD from PS at high substrate concentrations has not been published and requires further investigation.
In this study, 9α-OH-AD was obtained from PS through biotransformation using the cyclodextrin-resting cell system at a high substrate concentration. We evaluated the effect of the adsorption resin on the reaction efficiency of 9α-OH-AD production. The ISPR system with different resin types, times, and amounts was measured to optimize the bioconversion process. Furthermore, we investigated the mechanism by which the adsorption resin improved phytosterol bioconversion efficiency, and we separated and identified the inhibitory intermediate that limits the bioconversion rate. Using the in situ removal strategy, we were able to provide a solution for improving 9α-OH-AD production efficiency at high substrate concentrations.
2. Materials and methods
2.1 Microorganisms, medium and reagents
Mycobacterium neoaurum NwIB-HK86 (devoid of 3-ketosteroid-1,2-dehydrogenase activity; 3-ketosteroid-9-hydroxylase and cholesterol oxidases overexpressed) can bioconvert PS to produce 9α-OH-AD as the main product. MYC/01 seed medium was used as an amplification medium for microorganisms, as previously described by Gao et al.4 The seeds were then inoculated at 5% (v/v) into 500 mL shake flasks containing 100 mL of MYC/04 broth and cultured at 30 °C for 72 h on a 200 rpm shaker. MYC/04 were composed of (g L−1) K2HPO4·3H2O, 0.5; MgSO4·7H2O, 0.5; glucose, 30; sodium citrate, 2.8; ammonium ferric citrate, 0.05; starch, 2; corn steep powder, 3; and PS, 0.1. The initial pH was controlled at 8.0.The reagents described in the above medium composition were purchased from Shanghai Titan Scientific Co., Ltd. (Shanghai, China) or Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). 9α-OH-AD, AD standards were obtained from Sigma (Shanghai, China). PS and HP-β-CD were purchased from Shanxi Sciphar Natural Products Co., Ltd. (Shangluo, China) and Shandong Binzhou Zhiyuan Biotechnology Co., Ltd. (Binzhou, China), respectively. The organic solvents used in the mobile phase, e.g., methanol, acetonitrile, isopropanol, were all HPLC grade.
2.2 Preparation of resting cells and macroporous resins
Resting cells were prepared according to the method described by Gao et al.4 Ten types of macroporous adsorbent resins were employed as carriers for the in situ adsorption technique during the production of 9α-OH-AD, and were purchased from Shanghai Huazhen Technology Co., Ltd. (Shanghai, China). The physical and chemical properties of the resins are listed in Table S1.† The resins were pre-treated by soaking in ethanol at 30 °C for 48 h, then washed three times with deionised water for 24 h each, followed by drying at 55 °C to a constant weight.2.3 Bioconversion with ISPR by addition of macroporous resins
Precisely, weighed resin was added to the reaction solution at the beginning of the bioconversion (except for experiments exploring the optimum time to add resin). The transformation of PS was performed under non-sterile conditions with a 10 mL PB (20 mM, pH = 8.0) system consisting of 100 g L−1 wet cells, 50 g L−1 PS, and 200 g L−1 HP-β-CD, placed on a shaker at a rotation speed of 200 rpm and 30 °C for the reaction. All experiments were repeated three times.2.4 Metabolites analytical methods
The resins were separated from the reaction system using a single layer gauze. The samples from the reaction solution and resins were extracted with ten volumes of ethyl acetate (before desorption, the resin was washed twice with deionized water to remove non-adsorbed products). After centrifugation, an appropriate amount of the upper organic phase was withdrawn and allowed to evaporate completely before detection by high-performance liquid chromatography (HPLC) with Hypersil ODS2-column. Methanol–water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v) was used as the mobile phase at a flow rate of 1 mL min−1 and UV detector at 254 nm was used to investigate the main steroidal metabolites, 9α-OH-AD et al. For the detection of phytosterols, the mobile phase and detection wavelength were changed to acetonitrile–isopropanol (70
20, v/v) was used as the mobile phase at a flow rate of 1 mL min−1 and UV detector at 254 nm was used to investigate the main steroidal metabolites, 9α-OH-AD et al. For the detection of phytosterols, the mobile phase and detection wavelength were changed to acetonitrile–isopropanol (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, v/v) and 210 nm, respectively.
30, v/v) and 210 nm, respectively.
3. Results and discussion
3.1 Transformation of PS by Mycobacterium neoaurum NwIB-HK86 under resting cell system
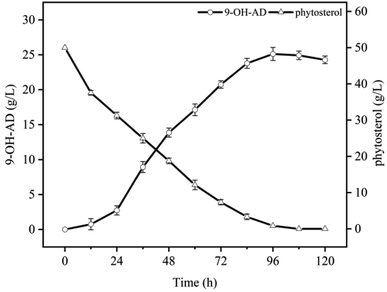
In our previous study, Mycobacterium neoaurum NwIB-HK86 efficiently produced the main product 9α-OH-AD from phytosterols in HP-β-CD-resting cell system, accompanied by more than 20% of by-products such as androstenedione (AD), 9,22-dihydroxy-23,24-bisnorchol-4-en-3-one (DHBC), and 9,24-dihydroxychol-4-en-3-one (DHC).20 Furthermore, the results demonstrated that the reaction was remarkably inhibited by high levels of substrates and metabolites. As shown in Fig. 1, 9α-OH-AD production increased slowly during the first 24 h. Subsequently, the accumulated products entered a rapid growth stage and reached the maximum level (25.1 g L−1) at 96 h. The PS was also depleted after 96 h of bioconversion, following the accumulation of 9α-OH-AD. After further incubation, 9α-OH-AD entered the degradation stage, and the yield began to decrease.3.2 Application of ISPR in the process
| Resin | 9α-OH-AD in supernatant (g L−1) | 9α-OH-AD in resins (g L−1) | Total 9α-OH-AD (g L−1) | Conversion rate of PS (%) | Space–time yield (g L−1 d−1) |
|---|---|---|---|---|---|
| a Except for the control group with no resin addition (CK), all the resins were added at a concentration of 20 g L−1 at 0 h. — means not detected. All experiments contained 50 g L−1 of phytosterols, 100 g L−1 of wet biomass, 200 g L−1 of HP-β-CD, and 10 mL of reaction solution bioconverted in 250 mL shake flasks for 3 d (error bars for standard deviations, n = 3). | |||||
| CK | 20.74 ± 0.54 | — | 20.74 ± 0.74 | 85.30 ± 0.39 | 6.91 ± 0.24 |
| D101 | 21.05 ± 0.38 | 2.23 ± 0.04 | 23.28 ± 0.86 | 92.78 ± 0.30 | 7.76 ± 0.19 |
| SP 207 | 17.90 ± 1.12 | 1.16 ± 0.01 | 19.06 ± 0.49 | 82.72 ± 0.73 | 6.35 ± 0.18 |
| HZ 820 | 12.24 ± 0.24 | 0.72 ± 0.09 | 12.96 ± 0.38 | 66.67 ± 0.65 | 4.32 ± 0.27 |
| HZ 818 | 15.94 ± 0.78 | 1.33 ± 0.03 | 17.27 ± 0.51 | 78.22 ± 0.71 | 5.76 ± 0.31 |
| HZ 806 | 6.36 ± 0.01 | 0.54 ± 0.02 | 6.90 ± 0.25 | 57.89 ± 0.57 | 2.30 ± 0.22 |
| HZ 801 | 16.24 ± 0.13 | 0.76 ± 0.05 | 17.00 ± 0.53 | 75.41 ± 0.81 | 5.67 ± 0.27 |
| HPD 826 | 3.63 ± 0.15 | 0.14 ± 0.01 | 3.77 ± 0.19 | 42.80 ± 0.55 | 1.22 ± 0.11 |
| HPD 500 | 11.83 ± 0.70 | 0.69 ± 0.04 | 12.52 ± 0.45 | 70.89 ± 1.25 | 4.17 ± 0.24 |
| HPD 300 | 7.04 ± 0.51 | 0.48 ± 0.04 | 7.52 ± 0.47 | 55.09 ± 0.68 | 2.51 ± 0.13 |
| HPD 100 | 5.71 ± 0.59 | 0.29 ± 0.01 | 6.00 ± 0.32 | 52.26 ± 0.53 | 2.00 ± 0.21 |
| D101 concentrations (g L−1) | 9α-OH-AD in supernatant (g L−1) | 9α-OH-AD in resins (g L−1) | Total 9α-OH-AD (g L−1) | Conversion rate of PS (%) | Space–time yield (g L−1 d−1) |
|---|---|---|---|---|---|
| a D101 resin was added at the beginning of the reaction. All experiments contained 50 g L−1 phytosterols, 100 g L−1 wet biomass, 200 g L−1 HP-β-CD, 10 mL reaction solution bioconverted in 250 mL shake flasks for 3 d (error bars for standard deviations, n = 3). | |||||
| 0 | 20.74 ± 0.54 | — | 20.74 ± 0.74 | 85.30 ± 0.39 | 6.91 ± 0.24 |
| 3 | 22.02 ± 0.34 | 0.23 ± 0.02 | 22.25 ± 0.51 | 89.16 ± 0.34 | 7.42 ± 0.10 |
| 5 | 24.75 ± 0.43 | 0.78 ± 0.18 | 25.53 ± 0.23 | 100 ± 0.00 | 8.51 ± 0.15 |
| 10 | 22.45 ± 0.18 | 1.26 ± 0.09 | 23.71 ± 0.50 | 94.68 ± 0.12 | 7.90 ± 0.44 |
| 20 | 21.05 ± 0.38 | 2.23 ± 0.04 | 23.28 ± 0.86 | 92.78 ± 0.30 | 7.76 ± 0.19 |
| 30 | 17.96 ± 0.58 | 2.74 ± 0.31 | 20.70 ± 0.38 | 82.98 ± 0.83 | 6.90 ± 0.15 |
| 40 | 15.88 ± 0.17 | 3.32 ± 0.29 | 19.20 ± 0.73 | 79.44 ± 0.15 | 6.40 ± 0.25 |
| 50 | 14.63 ± 0.58 | 3.71 ± 0.47 | 18.34 ± 0.49 | 76.28 ± 0.96 | 6.11 ± 0.17 |
| D101 addition times (h) | 9α-OH-AD in supernatant (g L−1) | 9α-OH-AD in resins (g L−1) | Total 9α-OH-AD (g L−1) | Conversion rate of PS (%) | Space–time yield (g L−1 d−1) |
|---|---|---|---|---|---|
| a The D101 resin was added at a concentration of 5 g L−1. All experiments contained 50 g L−1 phytosterols, 100 g L−1 wet biomass, 200 g L−1 HP-β-CD, 10 mL reaction solution bioconverted in 250 mL shake flasks for 3 d (error bars for standard deviations, n = 3). | |||||
| CK | 20.74 ± 0.54 | — | 20.74 ± 0.74 | 85.30 ± 0.39 | 6.91 ± 0.24 |
| 0 | 24.75 ± 0.43 | 0.78 ± 0.18 | 25.53 ± 0.23 | 100 ± 0.00 | 8.51 ± 0.15 |
| 12 | 23.72 ± 0.54 | 0.55 ± 0.08 | 24.27 ± 0.23 | 95.2 ± 0.37 | 8.09 ± 0.11 |
| 24 | 22.24 ± 0.64 | 0.57 ± 0.02 | 22.81 ± 0.50 | 92.02 ± 0.49 | 7.60 ± 0.07 |
| 36 | 21.25 ± 0.17 | 0.55 ± 0.07 | 21.80 ± 0.67 | 86.98 ± 0.43 | 7.27 ± 0.12 |
| 48 | 20.44 ± 0.65 | 0.31 ± 0.04 | 20.75 ± 0.38 | 84.16 ± 0.55 | 6.92 ± 0.10 |
| 60 | 20.39 ± 0.52 | 0.22 ± 0.01 | 20.61 ± 0.73 | 82.30 ± 0.83 | 6.87 ± 0.39 |
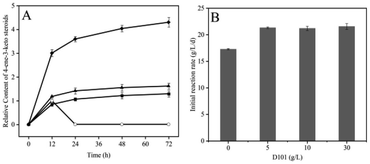
In this section, macroporous adsorbent resin D101, which favourably produced 9α-OH-AD, was selected and the conditions for its use were optimized. The results showed that the optimum time for the addition of D101 was at the beginning of the reaction, and the most suitable concentration was 5 g L−1.
3.3 Analysis of the mechanism of D101 in improving the bioconversion process
| Metabolites | In supernatant (g L−1) | In resins (g L−1) | Absorption ratio (%) |
|---|---|---|---|
| 9α-OH-AD | 24.75 ± 0.33 | 0.78 ± 0.02 | 3.05 ± 0.04 |
| AD | 0.46 ± 0.04 | 0.06 ± 0.01 | 11.54 ± 0.11 |
| DHBC | 2.99 ± 0.03 | 0.14 ± 0.02 | 4.47 ± 0.09 |
| DHC | 2.17 ± 0.17 | 0.29 ± 0.02 | 11.79 ± 0.23 |
| 4-Ene-3-keto steroids | — | 0.05 ± 0.01 | 100 ± 0.00 |
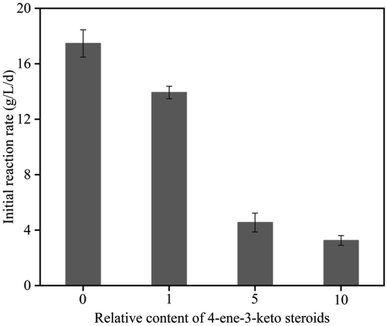
To verify if 4-ene-3-keto steroids accumulation could have an inhibitory effect on bioconversion, we exogenously supplemented 4-ene-3-keto steroids and detected its inhibitory effect on 9α-OH-AD production. The initial reaction rate decreased significantly in the presence of exogenous 4-ene-3-keto steroids (Fig. 4), and the higher the concentration of 4-ene-3-keto steroids, the lower the initial reaction rate. The initial reaction rate decreased by 19.39% to 13.93 g L−1 d−1 after adding the 4-ene-3-keto steroids at a relative concentration of 1 (4-ene-3-keto steroids content of the control group at 12 h was set to a relative concentration of 1). These results suggested that the accumulation of 4-ene-3-keto steroids had an inhibitory effect on the biotransformation process, thus reducing the space–time yield of 9α-OH-AD.
The generation of 4-ene-3-keto steroids is the initial step in the catabolism of PS by Mycobacterium neoaurum and has been demonstrated to be the rate-limiting step in PS bioconversion.22 The augmentation of cholesterol oxidase expression levels is a commonly used method to enhance the formation of 4-ene-3-keto steroids, but the increased response rate was in turn inhibited by feedback from the excessive accumulation of 4-ene-3-keto steroids. Therefore, enhancing cholesterol oxidase activity alone did not result in a sustained improvement in PS bioconversion. In this study, we demonstrated that the adsorption of 4-ene-3-keto steroids by resin accompanied with the reaction progress was a key method to help mitigate 4-ene-3-keto steroids toxicity, and achieving a balance between 4-ene-3-keto steroids production and consumption was a key factor affecting PS bioconversion.
4. Conclusions
PS bioconversion to 9α-OH-AD proceeded in a series of bioreactions and produced many intermediates that putatively inhibited cell activity. Mechanistic probing revealed that the increased space–time yield of 9α-OH-AD was mainly achieved by in situ removal of 4-ene-3-keto steroids, which is the first intermediate metabolite of sterol degradation, to alleviate its feedback inhibition rather than the adsorption of the final product 9α-OH-AD. A method of in situ adsorption of the toxic intermediate metabolite using D101 resin as a carrier in a cyclodextrin-resting cell system was first used to promote the production efficiency of 9α-OH-AD. We evaluated the types and amounts of resins added to the reaction system and optimized the addition time of the resins. The results revealed that D101 resin was the most suitable adsorption carrier for this ISPR system, and the space–time yield of 9α-OH-AD was markedly improved when it was added at a concentration of 5 g L−1 at the beginning of PS bioconversion. This study indicated that the in situ removal of the inhibitory intermediate is a potential candidate for producing 9α-OH-AD under the cyclodextrin-resting cell system at high substrate concentrations.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (31570079, 21706072).References
- W. Y. Tong and X. Dong, Recent Pat. Biotechnol., 2009, 3, 141–153 CrossRef CAS.
- R. Batth, C. Nicolle, I. S. Cuciurean and H. T. Simonsen, Plants, 2020, 9, 1144 CrossRef CAS PubMed.
- M. V. Donova and O. V. Egorova, Appl. Microbiol. Biotechnol., 2012, 94, 1423–1447 CrossRef CAS.
- X. Q. Gao, J. X. Feng, Q. Hua, D. Z. Wei and X. D. Wang, Biocatal. Biotransform., 2014, 32, 343–347 CrossRef CAS.
- K. Yao, L. Q. Xu, F. Q. Wang and D. Z. Wei, Metab. Eng., 2014, 24, 181–191 CrossRef CAS.
- L. Su, S. Xu, Y. Shen, M. Xia, X. Ren, L. Wang, Z. Shang and M. Wang, Appl. Environ. Microbiol., 2020, 86, e00441-20 CrossRef PubMed.
- J. J. Yuan, Y. X. Guan and S. J. Yao, ACS Sustainable Chem. Eng., 2017, 5, 10702–10709 CrossRef CAS.
- R. A. Mancilla, C. Little and A. Amoroso, Appl. Biochem. Biotechnol., 2018, 185, 494–506 CrossRef CAS.
- L. Zhou, H. Li, Y. Xu, W. Liu, X. Zhang, J. Gong, Z. Xu and J. Shi, Process Biochem., 2019, 87, 89–94 CrossRef CAS.
- V. Y. Shtratnikova, M. I. Schelkunov, D. V. Dovbnya, E. Y. Bragin and M. V. Donova, Appl. Microbiol. Biotechnol., 2017, 101, 4659–4667 CrossRef CAS.
- S. M. Khomutov, G. V. Sukhodolskaya and M. V. Donova, Biocatal. Biotransform., 2009, 25, 386–392 CrossRef.
- M. A. Molchanova, V. A. Andryushina, T. S. Savinova, T. S. Stytsenko, N. V. Rodina and N. E. Voishvillo, Russ. J. Bioorg. Chem., 2007, 33, 354–358 CrossRef CAS PubMed.
- W. H. Liu, C. W. Kuo, K. L. Wu, C. Y. Lee and W. Y. Hsu, J. Ind. Microbiol. Biotechnol., 1994, 13, 167–171 CrossRef CAS.
- C. Y. Lee, C. D. Chen and W. H. Liu, Appl. Microbiol. Biotechnol., 1993, 38, 447–452 CrossRef CAS.
- J. T. Dafoe and A. J. Daugulis, Biotechnol. Lett., 2014, 36, 443–460 CrossRef CAS PubMed.
- A. G. Santos, T. L. Albuquerque, B. D. Ribeiro and M. A. Z. Coelho, Biotechnol. Appl. Biochem., 2020, 1–14 Search PubMed.
- J. Zhao, Y. Li, T. Shan, Y. Mou and L. Zhou, World J. Microbiol. Biotechnol., 2011, 27, 2753–2758 CrossRef CAS.
- S. W. Han, Y. R. Choi and J. S. Shin, Catal. Lett., 2021 DOI:10.1007/s10562-021-03535-6.
- H. Luo, H. Liu, Y. Cao, D. Xu, Z. Mao, Y. Mou, J. Meng, D. Lai, Y. Liu and L. Zhou, Molecules, 2014, 19, 14221–14234 CrossRef.
- X. Q. Gao, J. X. Feng, X. D. Wang, Q. Hua and D. Z. Wei, Chem. Biochem. Eng. Q., 2015, 29, 567–573 CrossRef CAS.
- J. K. Capyk, R. Kalscheuer, G. R. Stewart, J. Liu, H. Kwon, R. Zhao, S. Okamoto, W. R. Jacobs, L. D. Eltis and W. W. Mohn, J. Biol. Chem., 2009, 284, 35534–35542 CrossRef CAS.
- K. Yao, F. Q. Wang, H. C. Zhang and D. Z. Wei, Metab. Eng., 2013, 15, 75–87 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, Table S1, Fig. S1 and S2. See DOI: 10.1039/d1ra02774c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |