Open Access Article
Open Access ArticleRoom temperature facile synthesis of olivine-Co2SiO4 nanoparticles utilizing a mechanochemical method
Phuong Q. H. Nguyen a,
Dongzhou Zhangab,
Robert Rappa,
John P. Bradleya and
Przemyslaw Dera*a
a,
Dongzhou Zhangab,
Robert Rappa,
John P. Bradleya and
Przemyslaw Dera*a
aHawaii Institute of Geophysics and Planetology, University of Hawai'i at Manoa, 1680 East-West Road, Honolulu, HI 96822, USA. E-mail: pdera@hawaii.edu; Fax: +1-808-956-3188; Tel: +1-808-956-6347
bGeoSoiEnviro CARS, Argonne National Laboratory, University of Chicago, Argonne, IL 60439, USA
First published on 9th June 2021
Abstract
Co2SiO4 is a ceramic pigment and promising battery material of significant technological interest, as well as a model end-member of one of the most important mineral families in the Earth's crust and upper mantle. All previously developed methods for synthesis of Co2SiO4 require high-temperature processing, which promotes grain growth, while the nanocrystalline form is required for some important technological applications. Here, we report a successful method for synthesizing nanocrystalline Co2SiO4 via a simple and inexpensive high-energy ball milling mechanochemical process. Products of the synthesis were characterized by a combination of XRD and TEM, and their crystal structures and elemental compositions are reported.
Silicates with M2SiO4 formula and olivine-structure, where M is mainly Mg and Fe, and containing small amounts of Mn, Ni, Co and Ca, are of fundamental importance in geology and mineralogy, as they are predominant minerals in the crust and upper mantle of the Earth. The olivine structure is one of the most robust crystallographic arrangements, able to accommodate not only a variety of metal cations, but also other types of anionic groups. Naturally occurring silicates recognized as minerals include simple binary compounds: forsterite (Mg2SiO4), fayalite (Fe2SiO4), tephroite (Mn2SiO4), liebenbergite (Ni2SiO4), and larnite (Ca2SiO4), as well as Ca-containing ternary silicates: monticellite (CaMgSiO4), kirschsteinite (CaFeSiO4) and glaucochroite (CaMnSiO4). In addition to silicates, germanate GeO42−, SiS42−, and GeS42− as well as phosphate PO43− compounds can crystallize in the olivine structure.
The Fe- and Mg- end-members of the naturally occurring (Fe1−xMgx)2SiO4 solid solution have been the subject of intensive studies in mineralogy, as refractories, lithium battery materials, and for carbon sequestration.1–3 Mg2SiO4 occurs in three well-known equilibrium polymorphs, referred to as α (forsterite, orthorhombic Pnma olivine structure), β (wadsleyite, orthorhombic Imma spinelloid structure), and γ (ringwoodite, cubic spinel structure) phases. The latter two of these polymorphs (β and γ) are stable at elevated pressure and temperature conditions, yet both can be metastably quenched. One of the less-studied olivine-type silicates, Co2SiO4, also exists in three structural forms, analogous to Mg2SiO4 phases. Ringwood et al. determined that the transformation to the ringwoodite form of the Co2SiO4 takes place at 7 GPa and 700 °C and Morimoto et al. synthesized, analysed, and compared the crystal structures of all three Co2SiO4 polymorphs.4,5
Olivine-type Co2SiO4 has been synthesized by a variety of traditional methods, including precipitation, hydrothermal, and sol–gel, all of which involve multi-step synthesis and/or heating to above 500 °C,6–8 resulting in crystal growth and particle aggregation. For example, Co2SiO4 can be produced by heating a mixture of Co3O4 and SiO2 at 1350 °C.4 Yatabe et al. synthesized Co2SiO4 by sintering an amorphous precipitate, formed by mixing sodium metasilicate and cobalt nitrate solutions, at 1200 °C.8 Taguchi et al. synthesized Co2SiO4 by hydrothermal reaction of CoCl2·6H2O and Na2SiO3·9H2O, and subsequent calcination of the resulting precursor at 950 °C.7 Guo et al. also synthesized Co-olivine nanoparticles by solid-state reaction of cobalt acetate and tetraethyl orthosilicate followed by calcination at 500–700 °C.9 Stoia et al. used a sol–gel method and tetraethylorthosilicate, cobalt nitrate and organic diols to synthesize Co2SiO4/SiO2 nanocomposite after calcination at 700 °C.6 Finally, Bayat et al. recently reported synthesis of Co2SiO4 nanostructures and nanocomposites via the sol–gel method using cobalt(II) acetate tetrahydrate, tetraethyl orthosilicate, NH3 and carbohydrate at calcination temperatures of 500–700 °C.10
The nanoparticulate Co2SiO4 was shown to exhibit improved optical and electrochemical performance.9,10 The conventional ceramic synthetic processes, which result in grain sizes exceeding 1 micrometer, cannot produce the grain shapes and morphologies required for some of technological applications. Mechanochemical activation by high-energy milling has become a widely used method for solid state synthesis, representing an alternative to high temperature processes.11 The recent report of successful mechanochemical synthesis of Fe2SiO4 raises the strong probability that other olivine compounds might be obtained using a similar approach.12 To this end, we have investigated the one-step synthesis of a stoichiometric mixture of CoO and SiO2 at ambient temperature. To the best of our knowledge, this approach for synthesizing olivine-structured Co2SiO4 has not been tried before.
Using a high-energy Retsch MM400 oscillating mill, mixtures of CoO and SiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) were loaded in 25 mL tungsten carbide (WC) jars, and mechanically milled dry with two 15 mm diameter WC balls (40
1 molar ratio) were loaded in 25 mL tungsten carbide (WC) jars, and mechanically milled dry with two 15 mm diameter WC balls (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ball to powder ratio) at oscillating frequency of 30 Hz, for various times, ranging from 5 to 180 minutes. Because the linear motion component in the grinding jars of oscillating mills is more pronounced, compressive stress is enhanced at the expense of the shear component, when compared to the more common planetary mills. An optional annealing stage involving heating of the milled product at 500, 750, or 1000 °C for 12 hours, was also carried out for the 180 minute-milled sample. Milling experiments performed in air or under controlled atmosphere (Ar) showed no difference in processing outcomes. Macroscopic temperature monitoring of the milling jar using an attached thermocouple indicated that a maximum temperature of 75 °C was reached during milling trials. The issue of thermal evolution during ball milling has been a subject of many previous studies, and the term “ambient temperature process” is quite widely accepted in the mechanochemical literature. For example, Schmidt et al. discusses the temperature progression in a mixer ball mill, while Takacs and McHenry et al. talks about shaker and planetary mills. Our temperature monitoring results are consistent with these earlier studies.15,16 The phase composition of the starting materials, milled products, and annealed samples were tested using Bruker D8 Advance high-resolution powder diffraction with 3 kW CuKα source and LynxEye XE detector in Bragg-Brentano parafocusing geometry.
1 ball to powder ratio) at oscillating frequency of 30 Hz, for various times, ranging from 5 to 180 minutes. Because the linear motion component in the grinding jars of oscillating mills is more pronounced, compressive stress is enhanced at the expense of the shear component, when compared to the more common planetary mills. An optional annealing stage involving heating of the milled product at 500, 750, or 1000 °C for 12 hours, was also carried out for the 180 minute-milled sample. Milling experiments performed in air or under controlled atmosphere (Ar) showed no difference in processing outcomes. Macroscopic temperature monitoring of the milling jar using an attached thermocouple indicated that a maximum temperature of 75 °C was reached during milling trials. The issue of thermal evolution during ball milling has been a subject of many previous studies, and the term “ambient temperature process” is quite widely accepted in the mechanochemical literature. For example, Schmidt et al. discusses the temperature progression in a mixer ball mill, while Takacs and McHenry et al. talks about shaker and planetary mills. Our temperature monitoring results are consistent with these earlier studies.15,16 The phase composition of the starting materials, milled products, and annealed samples were tested using Bruker D8 Advance high-resolution powder diffraction with 3 kW CuKα source and LynxEye XE detector in Bragg-Brentano parafocusing geometry.
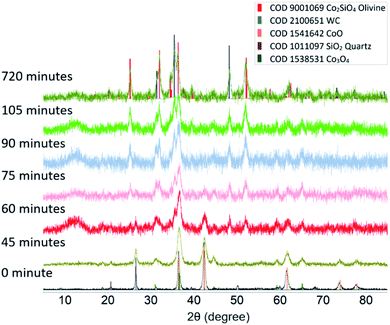
Fig. 1 shows a comparison of the powder XRD patterns of the starting mixture and samples milled for different duration. The cobalt oxide starting material was found to contain 13% of spinel-type Co3O4. Short milling times (less than 45 minutes) only produce changes in the average grain size of the sample and lattice strain, which as indicated by increasing diffraction peak width, but no new peaks are observed. Milling for 60 minutes results in the appearance of the characteristic pattern for orthorhombic olivine. Complete conversion of starting materials to Co2SiO4 was observed after 75 minutes. The width of the diffraction peaks of the product phase was similar to the width of the peaks for the precursors after milling for 45 minutes, indicative of sub-micrometer grain size. Milling for 720 minutes does not produce any further changes in the Co-olivine product, but results in increased contamination of the sample with WC debris from the grinding elements.
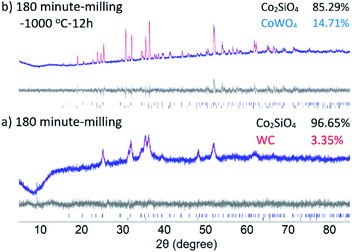
The milling process in an oscillating mill is believed to proceed at only slightly elevated average temperatures (below 100 °C), though individual ball-and-sample impacts may involve significant localized heating. Therefore, our solid-state milling-induced synthesis of Co2SiO4 is essentially a room temperature reaction. In order to test the effect of annealing on the mechanochemically-synthesized Co-olivine, a series of heating experiments, at 500, 750 and 1000 °C for 12 h was conducted. Results of powder XRD analysis of the annealed samples are shown in Fig. 2. Heating at 500 °C produces only minor changes in the grain size (grain growth) and anneals some of the residual strain, as evidenced by the changes in the peak widths. A marked change in the peak width of the olivine phase (significant grain growth) was observed after heating above 750 °C. Further heating to 1000 °C induced a reaction of the WC debris with the olivine sample, resulted in the formation of approximately 15% CoWO4.
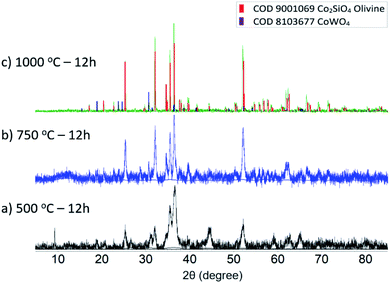 | ||
Fig. 2 Powder XRD spectra of ball milled 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio CoO and SiO2 mixture after annealing for 12 hours at (a) 500 °C (b) 750 °C (c) 1000 °C. 1 molar ratio CoO and SiO2 mixture after annealing for 12 hours at (a) 500 °C (b) 750 °C (c) 1000 °C. | ||
Fig. 3 illustrates the results of Rietveld refinements of the 180- minute-milled sample both with and without annealing, which converge to a final-figure-of-merit Rwp = 3.128 and 1.886, respectively. Rietveld analysis was conducted using Bruker TOPAS 5.13 The starting models for the Co2SiO4, WC, CoWO4 structures were taken from PDF 00-015-0865, PDF 00-051-0939, and PDF 00-015-0867, respectively.14 Refinement included optimization of background, phase fractions, unit cell parameters, peak profiles (controlled by grain size and strain models), site occupancies for non-oxygen atoms, and atomic displacement parameters. Fractional atomic coordinates were kept fixed at the literature values. Refined unit cell parameters are in excellent agreement with literature data,5 and refined site occupancy factors for Si and Co sites were very close to 1.0 (Table 1). Quantitative analysis of grain size using the Hall-Williamson method implemented in TOPAS 5 and refined peak profiles indicated an increase in average grain size from 96 nm in the 180 minute-milled sample to 250 nm after heat treatment.
| 105 min | 180 min | Heat 750 °C | Heat 1000 °C | |
|---|---|---|---|---|
| a Standard deviations in parentheses are in unit of the last digit stated. | ||||
| a (Å) | 4.79(9) | 4.79(6) | 4.785(14) | 4.7855(9) |
| b (Å) | 10.34(19) | 10.31(13) | 10.30(3) | 10.311(2) |
| c (Å) | 5.98(11) | 6.01(8) | 6.006(17) | 6.0105(12) |
| V (Å3) | 296.2(10) | 296.8(8) | 295.8(6) | 296.38(12) |
| SOF [Co_1] | 1.01(4) | 1.02(3) | 1.003(19) | 0.964(13) |
| SOF [Co_2] | 1.02(4) | 0.99(3) | 0.995(16) | 0.955(11) |
| SOF [Si] | 1.00(6) | 1.00(7) | 0.991(5) | 0.992(4) |
| Si–O bond (Å) | 1.614(22) | 1.613(22) | 1.612(21) | 1.613(12) |
| Co–O bond (Å) | 2.145(71) | 2.143(69) | 2.142(68) | 2.141(69) |
| Rietveld (Rwp) | 2.203 | 1.886 | 2.458 | 3.128 |
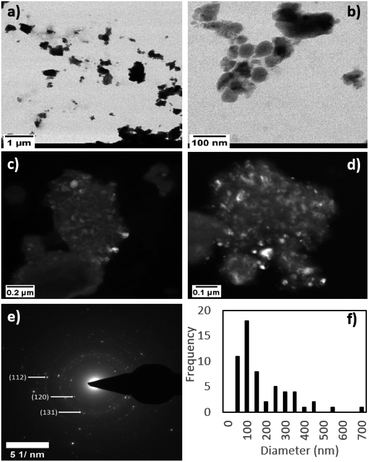
In order to determine the elemental composition and assess chemical homogeneity of the milled product, we used an 80–300 keV high-base Titan (FEI Thermo-Fisher) scanning transmission electron microscope equipped with a solid state Si(Li) energy-dispersive X-ray detector (Genesis 4000, EDAX). Brightfield and darkfield images were acquired in scanning transmission (“STEM”) mode and the crystal structures of grains were assessed using selected area electron diffraction (SAED). Compositions were measured by energy-dispersive X-ray spectroscopy (“EDS”) and quantified using a Cliff-Lorimer thin-film approximation and correction factors (“K factors”) derived from thin film standards. Representative STEM micrographs of the mechanochemically-synthesized Co2SiO4 after 180 minutes of milling time at different magnifications are displayed in Fig. 4a–d. The product consists of particles that show fairly broad size distribution, with many smaller grains aggregated together in large clumps. Attempt to break out these clumps ultrasonically produced little significant change in the end product. A representative SAED pattern of the milled sample (Fig. 4e) shows high degrees of spottiness in the diffraction rings, consistent with the nanocrystalline nature of the as-synthesized Co2SiO4. Indexing of the SAED pattern revealed the presence of (112), (120), and (131) single crystal peaks of Co2SiO4. Particle size analysis performed with the TEM images (Fig. 4f) indicates an average particle size of 157 nm with a broad range in size distribution (28 to 651 nm diameter).
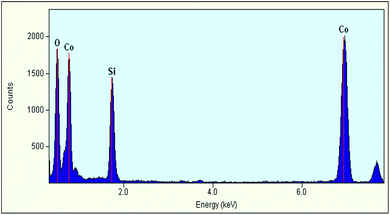
An EDS spectrum obtained with the TEM instrument on the Co2SiO4 sample milled for 180 minutes (Fig. 5) depicts the sole presence of Co, Si, and O elements. Quantitative analysis of elemental mass percentages based on this spectrum suggests an empirical formula of Co2SiO4 (Table 2). WC debris, despite being detected by powder XRD (Fig. 1), was not present in the EDS spectrum. This observation further confirms our suspicion that the milling product is a mixture of olivine Co2SiO4 and contaminant WC.
| Element | Weight% | Atomic% | Uncert.% |
|---|---|---|---|
| O (K) | 27.74 | 53.44 | 0.38 |
| Si (K) | 15.26 | 16.75 | 0.19 |
| Co (K) | 56.98 | 29.79 | 0.42 |
We conducted a series of comparative experiments with grinding jars and balls made of stainless steel. In all of these experiments, we noted (based on XRF results and Rietveld refinements) that the product olivine phase incorporates some amount of iron into its crystal structure. The details of these experiments will be discussed in a separate publication.
In summary, this communication presents a straightforward means of synthesizing nanocrystalline Co2SiO4 directly from oxide precursors. No traces of the high-pressure forms of Co2SiO4 were detected in any of the synthesis experiments, suggesting that the peak impact conditions were not high enough to promote polymorphic transformation. This synthesis has been shown to proceed at ambient temperature, and without contingent inert atmospheric control. Although WC debris is present in the final milled sample, the nature of the product raises the plausibility that WC can be removed by the development of a proper solid–solid separation technique. Annealing of the milled product at high temperatures results in a solid-state substitution reaction between the as-synthesized olivine Co2SiO4 and WC, leading to the formation CoWO4 species. Given the nanocrystalline nature of the product and simple reaction activation by milling, it is recommended that the as-synthesized Co2SiO4 merits further study in material development applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support of this work provided by the Office of Naval Research, Department of Navy's Historically Black Colleges and Universities/Minority Institutions, the Materials for Thermal and Chemical Extreme program, grant number FOA N00014-19-S-F004. RR has been supported by NSF EAR grant 1829273.Notes and references
- V. Goldschmidt, Ind. Eng. Chem., 1938, 30(1), 32–34 CrossRef CAS.
- M. Leonardi, M. Villacampa and J. C. Menéndez, Chem. Sci., 2018, 9(8), 2042–2064 RSC.
- Q. R. Miller, H. T. Schaef, J. P. Kaszuba, G. Gadikota, B. P. McGrail and K. M. Rosso, Environ. Sci. Technol. Lett., 2019, 6(8), 431–442 CrossRef CAS.
- A. Ringwood, Nature, 1963, 198(4875), 79–80 CrossRef CAS.
- N. Morimoto, M. Tokonami, M. Watanabe and K. Koto, Am. Mineral., 1974, 59(5–6), 475–485 CAS.
- M. Stoia, M. Stefanescu, T. Dippong, O. Stefanescu and P. Barvinschi, J. Sol-Gel Sci. Technol., 2010, 54(1), 49–56 CrossRef CAS.
- H. Taguchi, Y. Takeda and H. Shibahara, Mater. Lett., 2002, 52(6), 412–416 CrossRef CAS.
- J. Yatabe, T. Sugizaki, T. Ikawa and T. Kageyama, J. Ceram. Soc. Jpn., 1997, 105(1218), 188–191 CrossRef CAS.
- P. Guo and C. Wang, RSC Adv., 2015, 5(86), 70661–70667 RSC.
- S. Bayat, A. Sobhani and M. Salavati-Niasari, J. Mater. Sci.: Mater. Electron., 2018, 29(9), 7077–7089 CrossRef CAS.
- Q. Zhang, S. Ge, H. Xue, X. Wang, H. Sun and A. Li, RSC Adv., 2014, 4(102), 58260–58264 RSC.
- V. Šepelák, M. Myndyk, M. Fabián, K. L. Da Silva, A. Feldhoff, D. Menzel, M. Ghafari, H. Hahn, P. Heitjans and K. D. Becker, Chem. Commun., 2012, 48(90), 11121–11123 RSC.
- A. A. Coelho, J. Appl. Crystallogr., 2018, 51(1), 210–218 CrossRef CAS.
- S. Gates-Rector and T. Blanton, Powder Diffr., 2019, 34(4), 352–360 CrossRef CAS.
- R. Schmidt, H. M. Scholze and A. Stolle, Int. J. Ind. Chem., 2016, 7, 181 CrossRef.
- L. Takacs and J. S. McHenry, J. Mater. Sci., 2006, 41, 5246 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |