Open Access Article
Open Access ArticleEffect of H2SiF6 modification of IM-5 on catalytic performance in benzene alkylation with ethylene†
Yunping Zhai ,
Junwen Chen,
Yongrui Wang*,
Yibin Luo
,
Junwen Chen,
Yongrui Wang*,
Yibin Luo and
Xingtian Shu
and
Xingtian Shu
State Key Laboratory of Catalytic Material and Reaction Engineering, Research Institute of Petroleum Processing, Sinopec, Beijing 100083, China. E-mail: wangyr.ripp@sinopec.com
First published on 20th May 2021
Abstract
Ethylbenzene (EB) is an important bulk chemical intermediate. The vapor-phase process is considered to be more efficient than the liquid-phase process when using dilute ethylene (e.g. FCC or DCC off-gas) as the feed due to its high ethylene space velocity. However, realizing a balance between reducing the xylene formation and enhancing the EB selectivity is still a challenge due to the poor performance of ZSM-5 at low reaction temperature. This study concerns an IM-5 zeolite (IMF topology) modified by H2SiF6, with 89% ethylbenzene selectivity, 98.6% total EB + DEB selectivity and only 540 ppm of xylene at 330 °C. IM-5 zeolites with different Si/Al2 ratios (40–170) were prepared by H2SiF6 modification and their catalytic performance in vapor phase alkylation of benzene with ethylene was investigated. There was an obvious decrease in the acid sites and acid strength of IM-5 in the H2SiF6 treatment process, which led to a slight decrease in ethylbenzene selectivity and a significant decline in xylene yield. Under the conditions of complete ethylene conversion, the selectivity to EB + DEB increased from 96.1% to 98.6% in the parent I-40 and modified IM-5. Compared with ZSM-5 that has a similar acidity, the slightly bigger channel opening makes IM-5 more conductive to the formation and diffusion of DEB while xylene may present adverse effects. The 120 hour-lifetime test showed that IM-5 (I-110) has superior activity, equivalent stability, higher DEB selectivity and a much lower xylene selectivity in comparison with ZSM-5. The catalytic performance of the IM-5 zeolite in the vapor phase process provides a new choice for the production of ethylbenzene.
1. Introduction
As one of the most important intermediates in the petrochemical industry, ethylbenzene (EB) is mainly used to produce styrene.1–3 Ethylbenzene is mainly produced by the alkylation of benzene with ethylene. The process was mainly catalyzed by the corrosive Friedel–Crafts catalyst (mainly referring to AlCl3–HCl) in the past. However, zeolite-based catalysts are now commonly applied, including ZSM-5 (for vapor-phase alkylation), beta (for-liquid-phase alkylation) and MCM-22 (for liquid phase alkylation).3Compared to the liquid-phase process, the ZSM-5 catalyzed vapor-phase process has better flexibility in the selection of ethylene resources, including the dilute ethylene from FCC, DCC and ethane cracking units in refineries.4,5 Moreover, the medium pore ZSM-5-based catalysts have been proven to suppress the formation of bulkier products due to steric hindrance, resulting in better EB selectivity and catalyst stability.5,6 However, the two types of 10-member ring channel systems (0.51 nm × 0.55 nm and 0.53 nm × 0.56 nm) in the ZSM-5 structure exhibits stronger resistance for the pore diffusion process, limiting the mass transport to and from the acid sites.7 Thus, a relatively higher reaction temperature (e.g. 360–420 °C) is usually adopted in the vapor-phase process.8 Under the given temperature, more xylenes, residues and other undesired by-products are generated through side reactions, such as cracking, isomerization or disproportionation.9 It is believed that most part of xylene is generated by the side reactions, e.g. EB isomerization, on the strong acid sites of catalysts. Furthermore, the formation of xylene can also be accelerated when raising the reaction temperature.10 Xylene is one of the most important by-products and their boiling points are very close to that of ethylbenzene. There are no economic ways to separate xylene from ethylbenzene.11 As a result, the purity of EB is affected. Therefore, reducing the reaction temperature and decreasing the strength of zeolite acid sites are both effective ways to enhance the selectivity in benzene alkylation with ethylene. However, it is difficult for ZSM-5 to realize the goal due to its narrow 3D 10-ring pore system. The strong diffusion resistance to aromatics and quick ethylene oligomerization at a relative low reaction temperature will lead to a rapid deactivation of ZSM-5.12,13 Therefore, developing an alternative catalyst is crucial for the synthesis of ethylbenzene. Taking into consideration of the steric hindrance and mass transport, the IM-5 (framework type: IMF) zeolite may be an ideal candidate for vapor phase alkylation of benzene with ethylene as its slightly bigger channel opening and adjustable acidity.
IM-5 was firstly synthesized by Benazzi in 1998 with 1,5-bis(N-methyl-pyrrolidinium) pentane as a structure-directing agent14 and its crystal structure was resolved by an enhanced charge flipping structure-solution algorithm method in 2007.15 In contrast to ZSM-5, which channel structure consists of intersectional straight and sinusoidal ten-membered ring channels (5.1 × 5.5 Å, 5.3 × 5.6 Å) and an intersection cavity of about 8.6 Å, IM-5 consists of two 2D channel systems. One 2D system has channel diameters of 5.5 × 5.6 Å and 5.3 × 5.4 Å, while the other has channel diameters of 4.8 × 5.4 Å and 5.1 × 5.3 Å. These two 2D systems are connected by a channel with a diameter of 5.3 × 5.9 Å from each other and generate a bigger cavity (10.4 Å) in the channel intersections.16 With similar acidic properties (e.g. similar acid strength and Brønsted to Lewis acid site ratio),17 a better thermal and hydrothermal stability18,19 as well as higher adsorption capacity of alkyl aromatic hydrocarbon20 compared to ZSM-5, IM-5 is expected to replace ZSM-5 in many petrochemical processes. The catalytic performance of IM-5 zeolite has been investigated in alkane hydro-isomerization,21 methylation of toluene,22,23 disproportionation of toluene24 and alkylation of benzene with ethanol25 as well as alkylation with methanol.26 However, the application of IM-5 zeolite in the vapor phase alkylation of benzene with ethylene has not been reported.
The catalytic performance of the zeolites depends upon the acidity controlled by the framework Si/Al2 ratio, which can be regulated either during synthesis or by post-treatment dealumination methods. Steam treatments, SiCl4 treatment, reaction with chelating agents such as ammonium hexafluorosilicate (NH4SiF6), oxalic acid, etc. and leaching with mineral acid are some of the common post-synthesis methods used to control the acidity of the zeolites. Due to the crystallization of pure IM-5 is possible only from synthesis mixtures with a narrow range of SiO2/Al2O3,27 thus, it is an effective way to regulate the Si/Al2 ratio of IM-5 by post-treatment method. Compared with other methods, the dealumination with hexafluorosilicic acid (H2SiF6) is an effective way to increase Si/Al2 ratio while preserving the structural and textural properties of the zeolite.28 Moreover, the effect of H2SiF6 treatment on the physical and chemical properties of IM-5 has not been systematically studied, even though it is of great significance for establishing the correlation between the properties of zeolites and its catalytic performance on vapor-phase alkylation of benzene with ethylene. Herein, we report an IM-5 zeolite (IMF topology) modified by H2SiF6, with an 89% ethylbenzene selectivity, 98.6% total EB + DEB selectivity and with only 540 ppm of xylene at 330 °C. A series of IM-5 zeolites with different Si/Al2 ratios (40–170) were prepared by H2SiF6 modification and their catalytic performance in vapor phase alkylation of benzene with ethylene was explored. The structural and acidity properties of IM-5 before and after modification by H2SiF6 were investigated. Characterization techniques such as X-ray diffraction, X-ray fluorescence, N2 adsorption–desorption isotherms measurement, scanning electron microscopy, magic angle spinning nuclear magnetic resonance, and pyridine-adsorption IR measurement were adopted to characterize the changes in structural and acidities for the modified materials. The results suggest that H2SiF6 treatment is an effective way to increase the Si/Al2 ratio of IM-5. The ethylated benzene (EB + DEB) selectivity can be further increased and the formation of coke can be inhabited at an optimum Si/Al2 ratio. Moreover, reducing the reaction temperature can increase the total EB + DEB selectivity and reduce xylene selectivity. The unique pore structure of IM-5 played a key role in contributing to a higher EB + DEB selectivity and a much lower xylene yield compared with ZSM-5 with a similar Si/Al2 ratio under equivalent stability.
2. Experimental
2.1 Synthesis and preparation
IM-5 zeolite was synthesized by traditional hydrothermal synthesis as reported in the literature method.14 The as-synthesized IM-5 zeolite was firstly calcined at 823 K for 5 h to remove the organic templates and then ion exchanged 3 times with an NH4Cl solutions at 353 K for 3 h to obtain the parent H-IM-5 via subsequent calcination at 823 K for 4 h. IM-5 zeolites with different SiO2/Al2O3 ratio was prepared by treating the parent H-IM-5 (SiO2/Al2O3 = 40, denoted as I-40) with H2SiF6 aqueous solution at 353 K for 60 min. The concentration of H2SiF6 in the aqueous solution was varied from 0.05 to 0.20 mol L−1. All samples were filtrated and the solids were washed with deionized water, dried at 393 K for 12 hours and calcined at 823 K for 3 h to obtain H-IM-5 samples with different SiO2/Al2O3 ratio. The samples were designated as I-80, I-110, I-130 and I-170 according to their SiO2/Al2O3 ratio, respectively. The H-ZSM-5 zeolite (SiO2/Al2O3 = 110, denoted as Z-110) was obtained from the Research Institute of Petroleum Processing in order to investigate the effect of zeolite topology on product distribution in benzene alkylation with ethylene over IM-5 and ZSM-5.2.2 Catalyst characterization
The elemental analysis of all samples were conducted with an X-ray fluorescence (XRF) spectrometer. X-ray diffraction (XRD) patterns of all samples were recorded on a Siemens D5000 diffractometer using nickel-filtered Cu Kα radiation. The relative crystallinity (R.C.) of IM-5 zeolite was calculated based on the sum of relative peak area of the reflection at 2θ range of 22.1–25.4 of the prepared sample with that of the parent IM-5 (whose R.C. was assumed as 100%). Physical adsorption/desorption of nitrogen on the samples were performed with an adsorption analyzer. The total surface area of each sample was determined with the Brunauer–Emmett–Teller (BET) method, and the micropore volume and external surface area were determined with the t-plot method. The mesopore size distributions profile and the mesopores volume of the samples were calculated with the Barrett–Joyner–Halenda (BJH) desorption branch of the isotherms. Chemical adsorption of NH3 on samples were carried out with an adsorption analyzer (Autochem 2920, Micromeritics, USA). Pyridine infrared (Py-IR) spectra of the samples in the range of 1700–1300 cm−1 were recorded on a Fourier transform infrared FT-IR spectrometer with a resolution of 0.35 cm−1. The morphologies and particle sizes of the samples were measured by using a scanning electron microscope (SEM: Quanta200F, FEI, USA) at an accelerating voltage of 20 kV. In a typical GC-MS analysis, 200 mg of the used catalyst was completely dissolved in 2 mL of 40% HF solutions and neutralized with NaOH. CH2Cl2 (Aldrich, 99.9%) was employed to extract the organic species from the resulting solutions. The GC-MS total ion chromatograms of extracted organic phases were recorded on a Varian CP 3800 gas chromatograph equipped with a Varian 320-MSD mass-selective detector, using electron impact ionization at 70 eV.2.3 Catalytic evaluation
The vapor-phase alkylation of benzene with ethylene was carried out in a fixed-bed reactor with an internal diameter of 12 mm. Analytical grade (99 wt%) benzene and ethylene were used. Samples were loaded and pretreated with N2 flow at 400 °C for 2 h prior to the reaction. Benzene was firstly introduced into the reactor and then the ethylene was introduced when benzene was detected in the outlet of the condenser. The reaction temperature varied from 300 °C to 360 °C and the reaction pressure was 0.8 MPa. The ethylene weight hourly space velocity (WHSV) was 0.5 h−1 and the molar ratio of benzene to ethylene was 8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The products were analyzed by an on-line Agilent 7890A gas chromatograph, equipped with a flame ionization detector and 60 m cross-linked methyl silicone capillary column innowax (0.32 mm). The ethylene conversion and product selectivity were calculated as follows:
1. The products were analyzed by an on-line Agilent 7890A gas chromatograph, equipped with a flame ionization detector and 60 m cross-linked methyl silicone capillary column innowax (0.32 mm). The ethylene conversion and product selectivity were calculated as follows:| Ethylene conversion (%) = (Xethylene before reaction − Xethylene after reaction)/Xethylene before reaction × 100% |
| Selectivity (%) = (amount of i% in the products)/(∑amounts of i% in the products) × 100% |
3 Results and discussion
3.1 Textural and physicochemical characterization
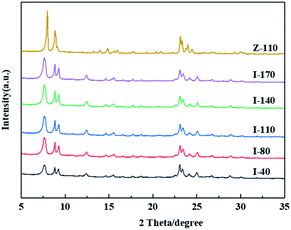
The crystal structure of IM-5 zeolites and the reference ZSM-5 are shown in Fig. 1. The diffraction peaks observed in the 2θ range of 22.1–25.4 of all IM-5 samples indicate the typical IMF structure29 without detection of any other phases. Moreover, there is no obvious decline in the crystallinity of post modified IM-5 zeolites compared with I-40, which illustrates that the structure of IM-5 was not destroyed during H2SiF6 treatment. In addition, ZSM-5 (Z-110) exhibits typical characteristic patterns of the MFI topology without other impurity crystal phase observed.SEM was employed to analyse the changes of microscopic morphology of IM-5 zeolites before and after H2SiF6 treatment. As shown in Fig. 2, all IM-5 samples exhibit rectangular shape with crystal size of 200–250 nm in length and about 80 nm in width, which is in good agreement with that of ref. 27. In addition, all IM-5 samples have a smooth surface and dense structure, with no amorphous material found after H2SiF6 treatment. The results further confirm that there was no serious damage to the IM-5 structure during H2SiF6 treatment. The crystal size of ZSM-5 is in the range of 400–800 nm, which is incomparable to that of IM-5 zeolite. In addition, an aggregation of small crystals was observed in both IM-5 and ZSM-5 samples. Which would be further improved by the N2 adsorption–desorption isotherms. Fig. 3(a) displays the N2 adsorption–desorption isotherms of IM-5 and ZSM-5 samples. The corresponding data are summarized in Table 1. The curves clearly indicate that the combination characteristic of type I and type IV isotherms with a closed hysteresis loop at high relative pressure of 0.45–0.99 of both IM-5 and ZSM-5 samples. This suggest that the mesopores are mainly intergranular pores generated by the accumulation of nanosized zeolite crystals.30–32 This is further verified in the mesopore size distributions from BJH method (Fig. 3(b)).
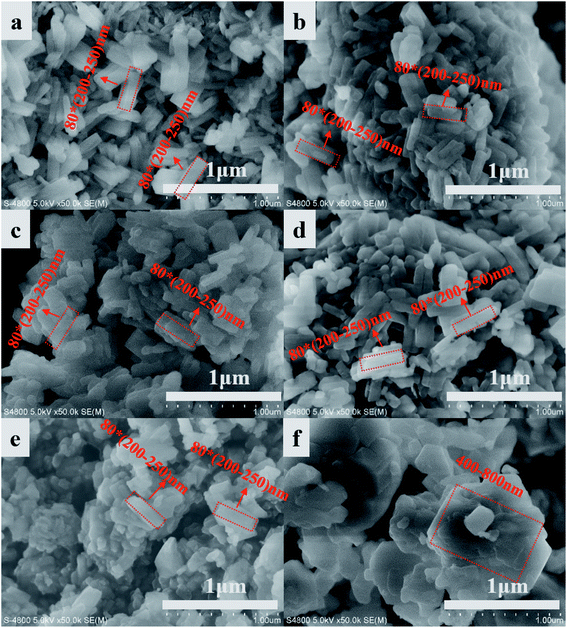 | ||
| Fig. 2 SEM images and particle size distribution of IM-5 and ZSM-5: I-40 (a), I-80 (b), I-110 (c), I-140 (d), I-170 (e) and Z-110 (f). | ||
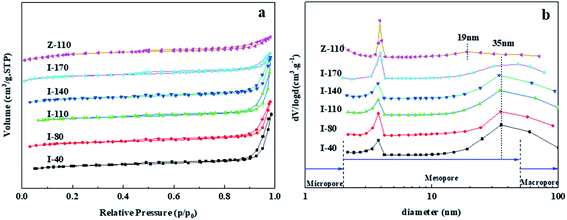 | ||
| Fig. 3 N2 adsorption–desorption isotherms (a) and BJH pore size distribution curves (b) for IM-5 and ZSM-5 samples. | ||
| Samples | C(H2SiF6) mol L−1 | R.C.a (%) | Si/Al2b | HFc (×100) | Textural properties | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stotal (m2 g−1) | Smicro (m2 g−1) | Sext (m2 g−1) | Vtotal (cm3 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | |||||
| a Relative crystallinity.b Si/Al2: molar ratio detected by XRF.c HF: hierarchical factor, defined as (Vmicro/Vtotal) × (Smeso/SBET). | ||||||||||
| I-40 | 0 | 100 | 40 | 5.06 | 343 | 295 | 48 | 0.373 | 0.135 | 0.238 |
| I-80 | 0.05 | 99.8 | 84 | 4.33 | 317 | 277 | 40 | 0.379 | 0.130 | 0.249 |
| I-110 | 0.10 | 99.6 | 110 | 4.83 | 313 | 264 | 49 | 0.392 | 0.121 | 0.271 |
| I-140 | 0.15 | 99.4 | 132 | 6.89 | 316 | 259 | 57 | 0.309 | 0.118 | 0.191 |
| I-170 | 0.20 | 98.2 | 170 | 5.50 | 264 | 223 | 41 | 0.291 | 0.103 | 0.188 |
| Z-110 | 110 | 5.20 | 370 | 341 | 29 | 0.238 | 0.158 | 0.080 | ||
The mesopores are centered around 35 nm and 19 nm for IM-5 and ZSM-5, respectively. The parent I-40 sample has a large specific surface area of 343 cm2 g−1 and micropore surface area of 295 cm2 g−1. With the increase of SiO2/Al2O3 ratio, the specific surface area, micropore surface area and micropore volume of IM-5 are all decrease, which might be associated with dealumination during the H2SiF6 treatment process. However, the mesopore volume of IM-5 firstly increase and then decrease with increasing SiO2/Al2O3 ratio. I-110 has the largest mesopore volume of 0.271 cm3 g−1. Z-110 has a specific surface area of 370 cm2 g−1 and micropore volume of 0.158 cm3 g−1, larger than 264 cm2 g−1 and 0.121 cm3 g−1 of I-110. However, the mesopore volume of Z-110 is only about 0.08 cm3 g−1. The hierarchical factor (HF), defined as (VMicro/Vtotal) × (Smeso/SBET), which is one of the very important factor in describing structural properties of zeolites, was calculated for the tested samples (Table 1). The HF (×100) values increased with Si/Al2 ratio increasing from 80 to 140. Further increasing Si/Al2 ratio to 170, the HF began to decrease.
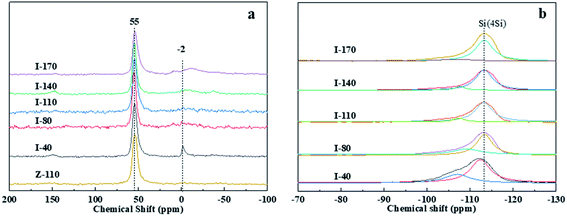
Fig. 4(a) shows the 27Al MAS NMR spectra of IM-5 samples before and after H2SiF6 treatment and ZSM-5 sample. Two types of Al coordinate centered at around 55 ppm and −2 ppm are observed in the I-40, for which tetrahedral-coordinated framework Al and octahedral-coordinated extra-framework Al are assigned respectively.27 The extra-framework Al of IM-5 samples after the H2SiF6 treatment disappeared, indicating that the extra-framework Al of I-40 was removed out and no additional extra-framework Al was formed during acid treatment. The dealumination of parent IM-5 can be further confirmed by the information obtained from the 29Si MAS NMR spectra. As shown in Fig. 4(b), the two signals at about −107 and −113 ppm are assigned Si (3Si, 1Al) and Si (4Si, 0Al) respectively. After the H2SiF6 treatment, the positions of peaks in the spectra of all IM-5 samples are not change, but the intensity of the Si (3Si, 1Al) signal is remarkably reduced with the increases of H2SiF6 concentration. The results indicate that the dealumination first occurred in the Si (3Si, 1Al) opposition.
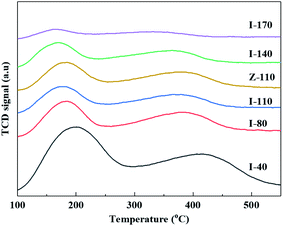
The SiO2/Al2O3 ratios of the parent and post modified IM-5 samples are listed in Table 1. Clearly, the SiO2/Al2O3 of IM-5 samples increased greatly with the increase of H2SiF6 concentration. There are no distinct differences in the NH3-TPD curves between I-110 and Z-110, indicating that the strength of acid sites between them is similar. It is in good accordance with the results obtained by Martin Kubů et al.17
3.2 Acidity characterization
The NH3-TPD curves of IM-5 and ZSM-5 (Z-110) are shown in Fig. 4. The spectrum of all samples can be divided into two different NH3 desorption peaks at low and high temperatures, corresponding to weak and strong acid sites, respectively. Both the low and high temperature peaks shift to lower temperature with the increase of SiO2/Al2O3. The results demonstrate that the H2SiF6 treatment reduced both weak and strong acid strength of IM-5.The amount of acid obtained from NH3-TPD profiles are summarized in Table 2. It is clear that H2SiF6 treatment has a significant effect on the acid amount of IM-5 sample. Both the strong and the weak acid decrease with the increase of H2SiF6 concentration. The concentrations of the weak acid sites decreased to a larger extent. It is generally accepted that the strong acid sites enhance the side reactions such as EB isomerization and cracking, leading to the formation of xylene and coke. Hence, the decreased concentration of strong acid sites in H2SiF6 treated IM-5 samples is expected to improve the catalytic performance in the alkylation of benzene with ethylene reaction. The acid concentration between I-110 and Z-110 is comparable. The acidity of IM-5 and ZSM-5 was also investigated using FTIR spectroscopy with pyridine as a probe molecule. The results obtained according to the corresponding extinction coefficient of Brønsted and Lewis acid sites are listed in Table 2. It was reported that the aqueous fluorosilicate treatment led to the substitution of an Al atom by an Si atom in the zeolite framework, leading to a sharp decrease in the concentration of Brønsted acid sites ascribed to the extraction of framework Al atom.33 As shown in Table 2, both the Brønsted and Lewis acid concentration dropped with the increase of H2SiF6 concentration. The B/L ratio increased in the lower degree of dealumination. However, the values declined considerably at a higher degree of extraction of Al atom. It is common knowledge that the production of a Brønsted acid site originated from the substitution of a Si atom by an Al atom in the framework of zeolite,34 while the Lewis acid site is a coordinative unsaturated Al3+. Thus, the coordinative of Al atom in zeolite framework varied with the increase of H2SiF6 concentration, which was consistent with the 27Al NMR results. In short, increasing H2SiF6 concentration caused the total acid sites to decrease. Furthermore, there were no obvious difference in the total acid sites and B/L ratio of I-110 and Z-110 samples (Fig. 5 and Table 2).
| Sample | mmol NH3 per g | 473 K | 623 K | ||||
|---|---|---|---|---|---|---|---|
| Brønsted acid (μmol g−1) | Lewis acid (μmol g−1) | B/L | Brønsted acid (μmol g−1) | Lewis acid (μmol g−1) | B/L | ||
| I-40 | 1.30 | 180.0 | 51.4 | 3.5 | 94.4 | 36.5 | 2.6 |
| I-80 | 0.82 | 114.2 | 31.7 | 3.6 | 88.8 | 31.7 | 2.8 |
| I-110 | 0.66 | 85.2 | 23.0 | 3.7 | 79.7 | 24.2 | 3.3 |
| I-140 | 0.58 | 63.1 | 22.3 | 2.8 | 48.6 | 23.3 | 2.1 |
| I-170 | 0.28 | 33.2 | 13.4 | 2.5 | 22.4 | 12.6 | 1.8 |
| Z-110 | 0.69 | 86.1 | 26.2 | 3.3 | 80.2 | 23.6 | 3.4 |
3.3 Catalytic performance in benzene alkylation with ethylene
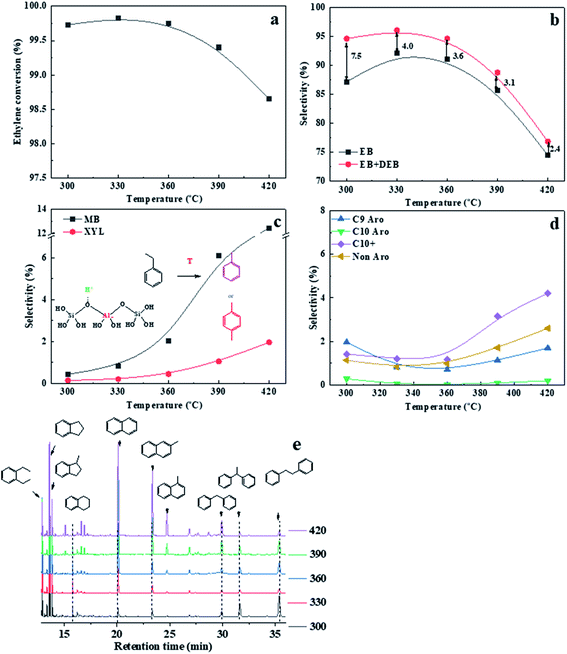
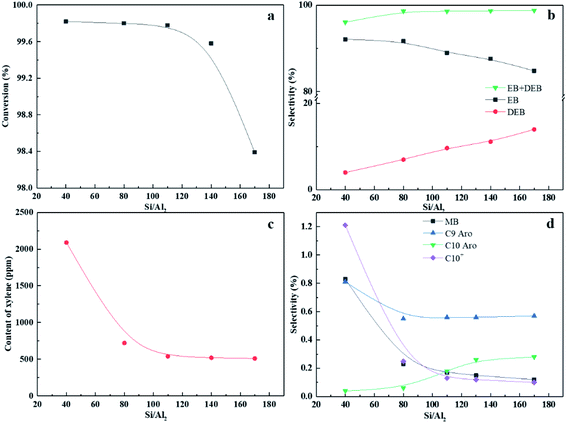
The zeolite activity declined from 99.78% to 98.39%, while the EB + DEB selectivity and xylene content kept almost remained almost unchanged after the Si/Al2 ratio was increased to 170. Thus, 110 was considered as the optimum Si/Al2 ratio for IM-5 in catalyzing benzene alkylation with ethylene. It should be noted that DEB is an exceptive by-product, which is generated by consecutive alkylation of EB with ethylene and can be used to yield EB via trans-alkylation with benzene.49 The DEB selectivity was enhanced with the increase of Si/Al2 ratio and the EB selectivity decreased as a result. This is due to the higher intrinsic reactivity of ethylbenzene compared with benzene. It is reported that the reactivity of alkyl benzene molecules increases with increasing number of alkyl groups on the benzene ring.50 Thus, the subsequent alkylation of EB with ethylene to DEB was accelerated at IM-5 with a lower amount of Brønsted acid sites.
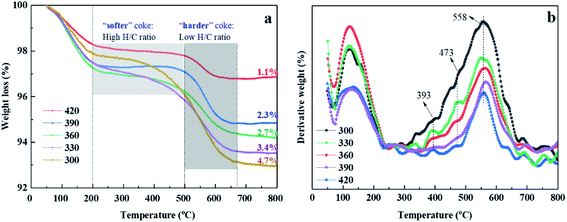
Fig. 9 shows the TGA and DTG curves of the used catalysts after 12 h on stream. With an increase in the Si/Al2 ratio, the second weight loss of the IM-5 showed a more uniform loss rate. The result suggests that the weight loss is not associated with the formation of harder coke, but likely due to the desorption or oxidation of polymerization of ethylene.51 The amount of coke deposits was calculated in the temperature range of 200 °C to 700 °C. Obviously, the coke amount formed on the IM-5 zeolite is decreased owing to the reducing of amount and strength of acid sites at first. However, I-170 sample exhibits a similar coke amount with that of I-110. This may be because the low amount of acid sites is insufficient to alkylate the benzene with ethylene. The ethylene react with each other to form ethylene oligomerization. This is also evident with the incomplete ethylene conversion (Fig. 8(a)) and higher butyl selectivity (Fig. 8(d)). In addition, it was found that there was a good correspondence between the HF factor and the carbon deposition rate over IM-5 samples. As shown in Fig. S1,† on the whole, the carbon deposition rate decreases with the increase of HF (×100). Apart from the first peak corresponding to the physical adsorption of water/light aromatics, it can be seen from the DTG curves shown in Fig. 9(b) that another two weight loss peaks are observed at 420 °C and 560 °C, which belong to the different coke species formed on various acid sites with different strengths. Moreover, the decomposition peaks shift to lower temperature at both high and low amount of acid sites, indicating that the coke species were easier to eliminate.
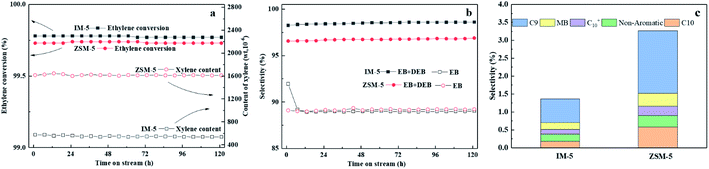
The main products of IM-5 and ZSM-5 were ethylbenzene and di-ethylbenzene. IM-5 and ZSM-5 showed a similar ethylbenzene selectivity. However, the EB + DEB selectivity (98.4%) for IM-5 is higher that of ZSM-5 (96.6%). The higher DEB selectivity on IM-5 contributed to this increase. Considering the two samples have a similar amount of acid sites as well as B/L ratio, we speculate that the pore structure of IM-5 (pore diameter: 5.4–5.9 Å) is more conductive to the formation and diffusion of DEB (d: m-DEB ≈ 5.5 Å nm, p-DEB ≈ 4.3 Å and o-DEB ≈ 7.8 Å) than ZSM-5 (pore diameter: 5.3–5.6 Å). Compared to ZSM-5 the diffusion properties of aromatics, such as toluene and m-xylene, in IM-5 proved to be better in the adsorption experiment.20,21
In addition, there was a large difference in xylene content between IM-5 and ZSM-5. The xylene content is at about 1600 ppm on ZSM-5 compared to 540 ppm on IM-5. The difference can be attributed to the different pore structures of IM-5 and ZSM-5. In terms of reducing of xylene yield, IM-5 zeolite exhibited a better catalytic performance than ZSM-5 in practical use.
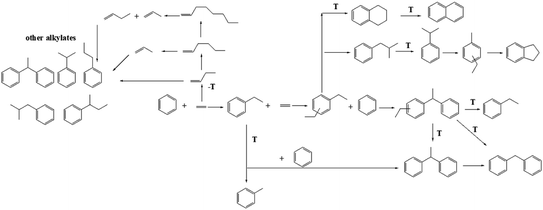
C9 and C10 aromatics were the major by-products for both IM-5 and ZSM-5 samples (Fig. 9(c)). Notably, the selectivity of C9 and C10 aromatics on ZSM-5 was higher than IM-5. In the alkylation of benzene with ethylene reaction, the active ethylene species formed on a Brønsted acid site of 10-ring member zeolite can follow two major routes: (1) it can alkylate with benzene to produce EB which can later undergo subsequent reactions to produce mainly di-ethylbenzene. (2) It can react with another one or more ethylene molecules to produce a C4, C6 species which can further transform via alkylation, oligomerization, isomerization or cracking yielding other alkylbenzenes and olefins (Scheme 1).49 Compared to the free diameter (8.6 Å) at the channel intersection in ZSM-5, a large channel intersection (10.4 Å) was formed due to much more complex channel intersections.52 This distinctive pore structure enables IM-5 to accommodate bulky intermediates in catalytic reactions. Thus, the di-ethylbenzene was much easier to form and to diffuse out of the channel system, leading to a higher DEB selectivity. Babatunde Ogunbadejo et al.53 has also found that IM-5 had a higher DEB selectivity compared to ZSM-5 during toluene alkylation with ethanol reaction due to the larger internal reaction volume of IM-5. In contrast, the diffusion rate of DEB in ZSM-5 is much slower, while the ethylene oligomerization-cracking and subsequent alkylation with benzene is accelerated, leading to a much higher C9 and C10 aromatics selectivity.
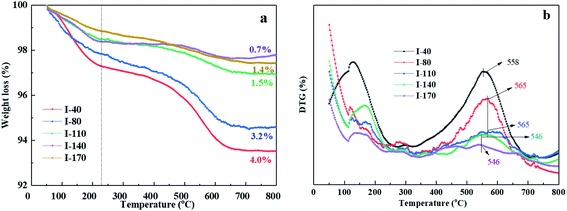
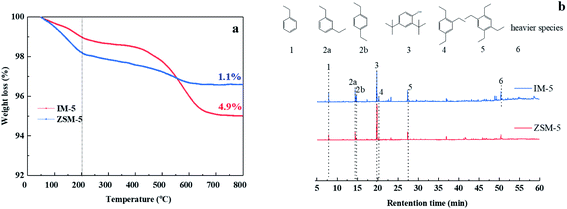
Fig. 11(a) shows the TGA curves of used IM-5 and ZSM-5 after 120 hours reaction. IM-5 has a higher coking amount (4.9%) than that of ZSM-5 (1.1%). Fig. 11(b) shows that both of the reacted IM-5 and ZSM-5 contained substantial amounts of ethyl-substituted benzenes with 1 to 4 ethyl groups. There is a lower proportion of ethyl-substituted benzenes but a higher proportion of heavier species (6) on IM-5 compared to ZSM-5. This is further evidence that IM-5 possesses a better diffusion property for di-ethylbenzene due to its larger pores size.
4. Conclusion
The reaction temperature has an important effect on the catalytic performance of IM-5 in vapor phase benzene alkylation with ethylene. It is found that both the highest ethylene conversion and EB + DEB selectivity were obtained at a reaction temperature of 330 °C over IM-5. The xylene yield is inhibited greatly with reducing of the reaction temperature. The H2SiF6 treatment could effectively decrease the total amount and strength of acid sites of IM-5 without destroying its framework. The selectivity to EB + DEB improved while the formation of xylene was inhibited and the coke amounts decreased over modified IM-5 due to the decline in acid amount and acid strength. Compared with ZSM-5 (Si/Al2 = 110), the unique pore system with larger channel crossing volume and bigger pore mouth gives IM-5 better diffusion properties, leading to superior activity, equivalent stability, similar EB selectivity, higher DEB selectivity as well as lower C9–C10 aromatics and xylene selectivity. In conclusion, IM-5 is a promising catalytic material for vapor-phase alkylation of benzene with ethylene.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The author(s) would like to thank the Department of Analysis at Sinopec in Research Institute of Petroleum Processing for its support.References
- T. F. Degnan, C. M. Smith and C. R. Venkat, Appl. Catal., A, 2001, 221, 283–294 CrossRef CAS.
- C. Perego and P. Ingallina, Catal. Today, 2002, 73, 3–22 CrossRef CAS.
- W. Yang, Z. Wang, H. Sun and B. Zhang, Chin. J. Catal., 2016, 37, 16–26 CrossRef CAS.
- Dilute ethylene alkylation with ethylene, WO2013006603A2, 2013.
- L. Zhang, Z. Wang, H. Sun and W. Yang, Ind. Catal., 2016, 24, 1–7 Search PubMed.
- X. X. Zhu, F. C. Chen, J. An, P. Zeng and L. Y. Xu, Adv. Mater. Res., 2011, 233–235, 1708–1713 CAS.
- C. H. Christensen, K. Johannsen, I. Schmidt and C. H. Christensen, J. Am. Chem. Soc., 2003, 125, 13370–13371 CrossRef CAS PubMed.
- Z. Shen, C. Ma, J. He, D. Wang, H. Sun, Z. Zhu and W. Yang, Appl. Catal., A, 2019, 577, 20–27 CrossRef CAS.
- P. G. Smirniotis and E. Ruckenstein, Ind. Eng. Chem. Res., 1995, 34, 1517–1528 CrossRef CAS.
- M. Lu, Y. Guo, Z. Zhu, W. Yang and W. Lu, Petrochem. Technol., 2001, 30, 270–274 CAS.
- S. Liu, F. Chen, S. Xie, P. Zeng, X. Du and L. Xu, J. Nat. Gas Chem., 2009, 18, 21–24 CrossRef CAS.
- S. Al-Khattaf, C. D'Agostino, M. N. Akhtar, N. Al-Yassir, N. Y. Tan and L. F. Gladden, Catal. Sci. Technol., 2014, 4, 1017–1027 RSC.
- K. Gołąbek, K. A. Tarach, U. Filek and K. Góra-Marek, Spectrochim. Acta, Part A, 2018, 192, 464–472 CrossRef PubMed.
- IM-5 zeolite, A process for its preparation and catalytic applications thereof, US6136290, 2000.
- C. Baerlocher, F. Gramm, L. Massüger, L. B. McCusker, Z. He, S. Hovmöller and X. Zou, Science, 2007, 315, 1113–1116 CrossRef CAS PubMed.
- H.-K. Min, S. H. Cha and S. B. Hong, ACS Catal., 2012, 2, 971–981 CrossRef CAS.
- M. Kubů, S. I. Zones and J. Čejka, Top. Catal., 2010, 53, 1330–1339 CrossRef.
- A. Corma, J. Mengual and P. J. Miguel, Appl. Catal., A, 2013, 460–461, 106–115 CrossRef CAS.
- A. Corma, J. Martínez-Triguero, S. Valencia, E. Benazzi and S. Lacombe, J. Catal., 2002, 206, 125–133 CrossRef CAS.
- A. Corma, A. Chica, J. M. Guil, F. J. Llopis, G. Mabilon, J. A. Perdigón-Melón and S. Valencia, J. Catal., 2000, 189, 382–394 CrossRef CAS.
- Q. Yu, Z. Huang, H. Sun, L. Li, X. Zhu, S. Ren and B. Shen, Ind. Eng. Chem. Res., 2018, 57, 14448–14459 CrossRef CAS.
- Q. Chen, Y. Wang, S. Min, M. Xuhong and S. Xingtian, Acta Pet. Sin., 2010, 26, 165–170 CAS.
- J. Chen, C. Zhang, Y. Wang, M. Sun, X. Mu and X. Shu, Acta Pet. Sin., 2013, 29, 757–765 CAS.
- M. Kubů, N. Žilková, S. I. Zones, C.-Y. Chen, S. Al-Khattaf and J. Čejka, Catal. Today, 2015, 259, 97–106 CrossRef.
- C. Yang, S. Xie, H. Liu, W. Xin, C. Feng, X. Li, S. Liu, L. Xu and P. Zeng, Catal. Lett., 2018, 148, 2030–2041 CrossRef CAS.
- X. Meng, D. Yi, L. Shi and N. Liu, Pet. Sci. Technol., 2020, 38, 501–508 CrossRef CAS.
- S. Lee, J. Catal., 2003, 215, 151–170 CrossRef CAS.
- H. Najar, M. S. Zina and A. Ghorbel, React. Kinet. Mech. Catal., 2010, 100, 385–398 CAS.
- S. H. Lee, D. K. Lee, C. H. Shin, Y. K. Park, P. A. Wright, W. M. Lee and S. B. Hong, J. Catal., 2003, 215, 151–170 CrossRef CAS.
- K. Egeblad, C. H. Christensen, M. Kustova and C. H. Christensen, Chem. Mater., 2008, 20, 946–960 CrossRef CAS.
- E. Xing, Y. Shi, W. Xie, F. Zhang, X. Mu and X. Shu, Microporous Mesoporous Mater., 2016, 236, 54–62 CrossRef CAS.
- J. Pérez-Ramírez, C. H. Christensen, K. Egeblad, C. H. Christensen and J. C. Groen, Chem. Soc. Rev., 2008, 37, 2530–2542 RSC.
- Y. He, C. Li and E. Min, Stud. Surf. Sci. Catal., 1989, 49, 189–197 CrossRef.
- J. Van der Mynsbrugge, A. Janda, L.-C. Lin, V. Van Speybroeck, M. Head-Gordon and A. T. Bell, ChemPhysChem, 2018, 19, 341–358 CrossRef CAS PubMed.
- J. Das, Y. S. Bhat and A. B. Halgeri, Ind. Eng. Chem. Res., 1993, 32, 2525–2529 CrossRef CAS.
- J. M. Serra, E. Guillon and A. Corma, A rational design of alkyl-aromatics dealkylation–transalkylation catalysts using C8 and C9 alkyl-aromatics as reactants, J. Catal., 2004, 227, 459–469 CrossRef CAS.
- S. Siffert, L. Gaillard and B. L. Su, J. Mol. Catal. A: Chem., 2000, 153, 267–279 CrossRef CAS.
- K. Becker, J. Catal., 1973, 28, 403–413 CrossRef CAS.
- G. Mirth, J. Cejka, J. Krtil and J. A. Lercher, Stud. Surf. Sci. Catal., 1994, 88, 241–248 CrossRef CAS.
- P. B. Venuto, L. A. Hamilton and P. S. Landis, J. Catal., 1966, 5, 484–493 CrossRef CAS.
- J. Čejka, B. Wichterlová and S. Bednářová, Appl. Catal., A, 1991, 79, 215–226 CrossRef.
- T. Odedairo and S. Al-Khattaf, Appl. Catal., A, 2010, 385, 31–45 CrossRef CAS.
- K. Bian, A. Zhang, H. Yang, B. Fan, S. Xu, X. Guo and C. Song, Ind. Eng. Chem. Res., 2020, 59, 22413–22421 CrossRef CAS.
- E. G. Derouane, J.-P. Gilson and J. B. Nagy, J. Mol. Catal., 1981, 10, 331–340 CrossRef CAS.
- M. Osman, L. Atanda, M. M. Hossain and S. Al-Khattaf, Chem. Eng. J., 2013, 222, 498–511 CrossRef CAS.
- W. Xue Qin and W. Xiangsheng, Acta Pet. Sin., 1994, 10, 44–48 Search PubMed.
- L. Hexuan, Z. Yaru, T. Keyi and Z. Yingxian, Chem. J. Chin. Univ., 1985, 6, 191–196 Search PubMed.
- Y. Weimin, S. Hongmin and C. Qingling, Adv. Fine Petrochem., 2002, 3, 12–14 Search PubMed.
- C. Perego and P. Ingallina, Green Chem., 2004, 6, 274–279 RSC.
- S. Al-Khattaf, M. N. Akhtar, T. Odedairo, A. Aitani, N. M. Tukur, M. Kubů, Z. Musilová-Pavlačková and J. Čejka, Appl. Catal., A, 2011, 394, 176–190 CrossRef CAS.
- Y. Du and J. Ru, Pet. Process. Petrochem., 2006, 37, 31–36 CAS.
- H.-K. Min and S. B. Hong, J. Phys. Chem. C, 2011, 115, 16124–16133 CrossRef CAS.
- B. Ogunbadejo, A. Aitani, J. Čejka, M. Kubů and S. Al-Khattaf, Chem. Eng. J., 2016, 306, 1071–1080 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02427b |
| This journal is © The Royal Society of Chemistry 2021 |