Open Access Article
Open Access ArticleMagnetically recoverable catalysts for the preparation of pyridine derivatives: an overview
Ghodsi Mohammadi Ziarani
 *a,
Zohreh Kheilkordi
*a,
Zohreh Kheilkordi
 a,
Fatemeh Mohajer
a,
Fatemeh Mohajer
 a,
Alireza Badiei
a,
Alireza Badiei
 b and
Rafael Luque
b and
Rafael Luque
 *cd
*cd
aDepartment of Chemistry, Faculty of Physics and Chemistry, Alzahra University, Tehran, 1993893979, Iran. E-mail: gmohammadi@alzahra.ac.ir; Fax: +98 2188613937; Tel: +98 2188613937
bSchool of Chemistry, College of Science, University of Tehran, Tehran, Iran
cDepartamento de Quimica Organica, Universidad de Cordoba, Campus de Rabanales, Edificio Marie Curie, Córdoba, 14014, Spain. E-mail: rafael.luque@uco.es
dPeoples Friendship University of Russia (RUDN University), 6 Miklukho Maklaya str, Moscow, 117198, Russian Federation
First published on 13th May 2021
Abstract
Magnetically recoverable nano-catalysts can be readily separated from the reaction medium using an external magnet. In recent years, chemistry researchers have employed them as catalysts in chemical reactions. The high surface area, simple preparation, and modification are among their major advantages. Pyridine derivatives are an important category of heterocyclic compounds, which show a wide range of excellent biological activities, including IKK-β inhibitors, anti-microbial agents, A2A adenosine receptor antagonists, inhibitors of HIV-1 integrase, anti-tumor, anti-inflammatory, and anti-Parkinsonism. Recently, the catalytic activity of magnetic nanoparticles was investigated in multicomponent reactions in the synthesis of pyridine derivatives, which is discussed in this review.
1. Introduction
In recent decades, nanotechnology has attracted much attention in various fields.1,2 One of the most influential families of nanomaterials is magnetic nanoparticles, which have been extensively employed in different sciences, including drug delivery,3 illness recognition,4 water desalination,5 ambiance scrubbing,6 and chemical catalysis.7 Recently, magnetic nano-catalysts have attracted the consideration of many researchers due to their high activity, selectivity, availability, large surface area, low toxicity, excellent reusability, and easy separation.8,9 Magnetic nanoparticles (MNPs) have high surface-to-volume ratios, and can be functionalized with inorganic and organic compounds.10–15 The magnetic nano-catalysts can be separated by external magnetic fields.16 Fe3O4 nanoparticles can be coated with organic and inorganic materials, including silica,17 surfactants,18 polymers,17,19 cellulose,20 carbon,21 chitosan,22 as well as prepared with a core–shell structure. The coating layer on magnetic nanoparticles can be prevented from aggregation or oxidation and their stability can be increased.Heterocyclic compounds have high biological and pharmaceutical activities. Among them, pyridine derivatives are important heterocyclic compounds, which attracted the attention of scientists. Pharmaceutical molecules and natural products can be based on heterocyclic compounds such as pyridine derivatives,23 which have biological activities, such as inhibitors of HIV-1 integrase, A2A adenosine receptor antagonists, IKK-β inhibitors, anti-microbial, anti-tumor, analgesic, anti-inflammatory, and antipyretic agents.24 In continuation our research work,25–29 this contribution will be aimed to discuss the synthesis of magnetic nano-catalysts as well as their applications in the synthesis of pyridine derivatives.
2. The synthesis of pyridine derivatives by diverse magnetic catalysts
2.1. Basic magnetic catalyst
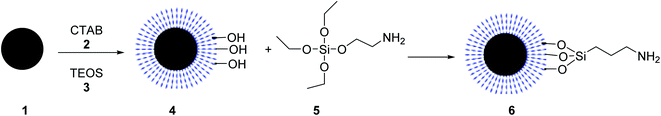
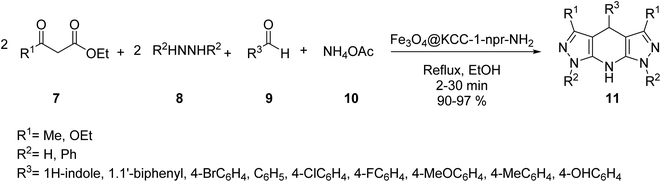
The core–shell structure of Fe3O4@KCC-1-npr-NH2 6 as an effective basic magnetic catalyst was prepared and employed in the synthesis of tetrahydro di-pyrazolopyridines by Azizi, and his co-workers. Core–shell Fe3O4@KCC-1 4 was prepared by adding cetyl trimethyl ammonium bromide (CTAB) 2 and tetraethylorthosilicate (TEOS) 3. Then, Fe3O4@KCC-1 4 was functionalized with 3-aminopropyl)triethoxysilane 5 to produce Fe3O4@KCC-1-npr-NH2 6 with excellent basic properties. Details for the preparation of Fe3O4@KCC-1-npr-NH2 6 are shown in Scheme 1. Various characterization techniques, including FT-IR, SEM, TEM, BET, and XRD, confirmed the structure of Fe3O4@KCC-1-npr-NH2 6 as magnetic nano-catalyst.30Fe3O4@KCC-1-nPr-NH2 6 was employed in the tetra-component reaction of ethyl acetoacetate 7, hydrazine hydrate 8, ammonium acetate 10, and various aromatic aldehydes 9 in ethanol under reflux condition for the synthesis of tetrahydrodipyrazolo pyridine 11 in excellent yields, short reaction times. According to obtained results, different substituents including electron-donating or electron-withdrawing groups on the aromatic ring, did not affect the product yields. All products were obtained in high purity and excellent yields. Also, the anticancer activity of tetrahydrodipyrazolo pyridine derivatives 11 was studied that some of these compounds showed good cytotoxic activity toward types of cancer cell (Scheme 2).30
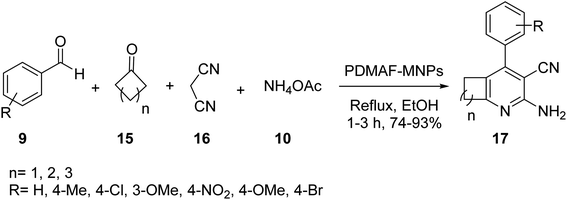
Fe3O4 MNPs 1 were also synthesized according to the literature,31 and then coated by TEOS to yield Fe3O4@SiO2 MNPs 4,32 which were modified by 3-aminoropropyl-trimethoxysilane (APTS) 5 to provide Fe3O4@SiO2-pr-NH2 MNPs 6, followed by mixing with a solution of N,N-dimethylaniline 12, and formaldehyde 13 in DMF, and then refluxed for 24 h to provide poly N,N-dimethylaniline-formaldehyde supported on silica-coated Fe3O4 MNPs (PDMAF-MNPs) 14 (Scheme 3).33
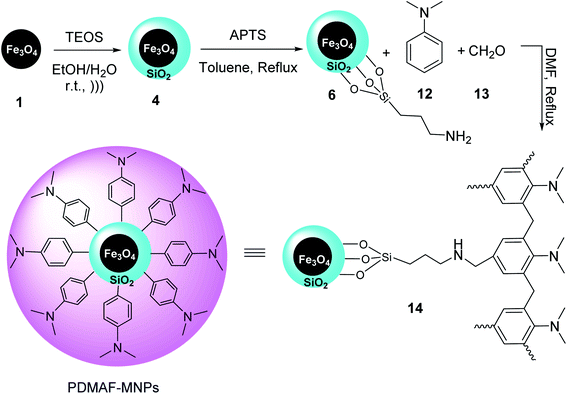 | ||
| Scheme 3 Synthesis of poly N,N-dimethylaniline-formaldehyde supported on silica-coated Fe3O4 MNPs (PDMAF-MNPs) 14. | ||
PDMAF-MNPs was investigated in the multicomponent reaction of aldehydes 9, malononitrile 16, ammonium acetate 10, and various ketones 15 under reflux condition in EtOH to obtain 2-amino-3-cyanopyridines 17 in high yields. It was demonstrated that the electron-donating groups results in low reaction yields and long reaction time (Scheme 4).33
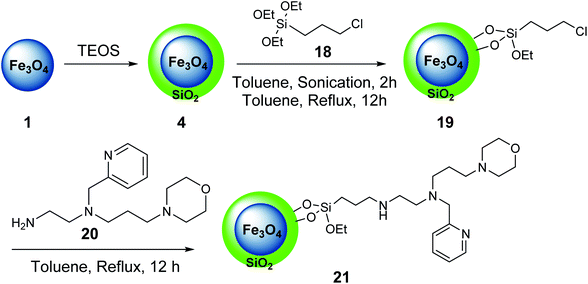
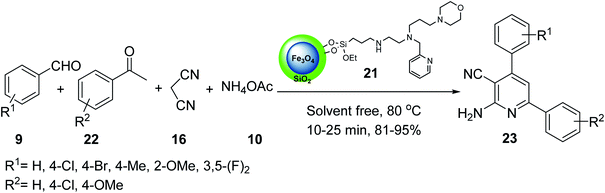
In another example, iron oxide 1 was prepared and reacted with tetraethylorthosilicate (TEOS) 3 to provide Fe3O4@SiO2 4,34 which was treated with 3-chloropropyltriethoxysilane 18 to give Fe3O4@SiO2@Pr-Cl 19, followed by the reaction with the ligand bearing morpholine tags 20 to obtain the nano-magnetic catalyst 21 (Scheme 5).35
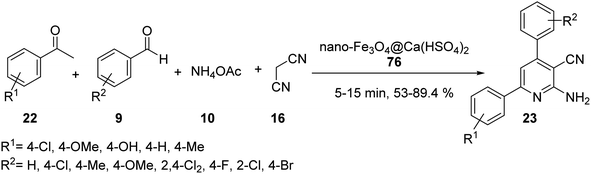
The nano-magnetic catalyst 21 was examined in the multicomponent reaction of benzaldehydes 9, acetophenone derivatives 22, malononitrile 16, and ammonium acetate 10 under the solvent-free condition in 80 °C for the preparation of 2-amino-4,6-diphenylnicotinonitriles 23 (Scheme 6).35
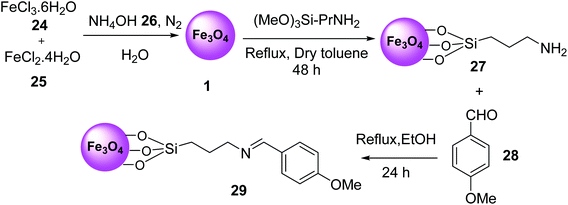
Nano-magnetic Fe3O4–Si–(CH2)3–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–Ph–OMe MNPs 29 was prepared by the reaction of Fe·Cl3·6H2O 24, FeCl2·4H2O 25, and NH4OH 26 in H2O under N2 atmosphere to prepare Fe3O4 MNPs 1, which was functionalized with aminopropyl silane 5 to provide Fe3O4–Si–[CH2]3–NH2 27, followed by modification with 4-methoxy benzaldehyde 28 under reflux conditions in ethanol for 24 h (Scheme 7).36
CH–Ph–OMe MNPs 29 was prepared by the reaction of Fe·Cl3·6H2O 24, FeCl2·4H2O 25, and NH4OH 26 in H2O under N2 atmosphere to prepare Fe3O4 MNPs 1, which was functionalized with aminopropyl silane 5 to provide Fe3O4–Si–[CH2]3–NH2 27, followed by modification with 4-methoxy benzaldehyde 28 under reflux conditions in ethanol for 24 h (Scheme 7).36
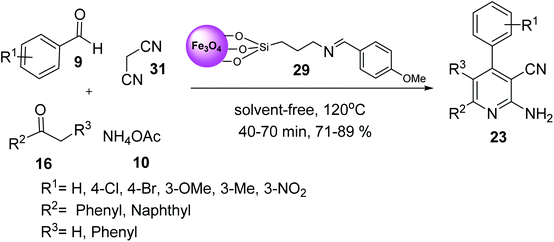
Fe3O4–Si–(CH2)3–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–Ph–OMe MNPs 29 was used in the synthesis of 2-amino-3-cyanopyridines 23 via the multicomponent reaction of various aromatic aldehydes 9, 2-acetylnaphthalene 31, or deoxybenzoin 31, malononitrile 16, and ammonium acetate 10 under solvent-free conditions at 120 °C for 40–70 min in good to high yield in short times (Scheme 8).36
CH–Ph–OMe MNPs 29 was used in the synthesis of 2-amino-3-cyanopyridines 23 via the multicomponent reaction of various aromatic aldehydes 9, 2-acetylnaphthalene 31, or deoxybenzoin 31, malononitrile 16, and ammonium acetate 10 under solvent-free conditions at 120 °C for 40–70 min in good to high yield in short times (Scheme 8).36
2.2. Acidic magnetic catalysts
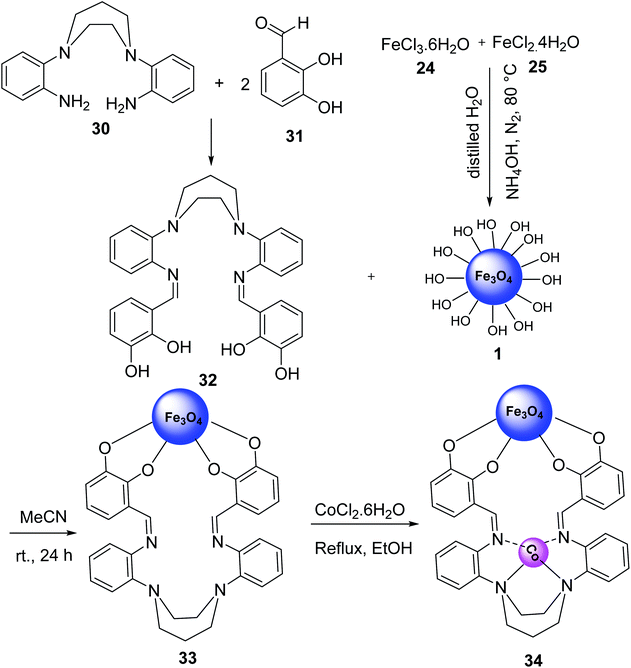
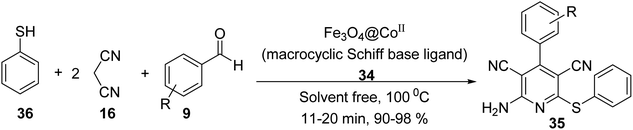
Fe3O4@CoII (macrocyclic Schiff base ligand) 34 was synthesized as an efficient and recoverable catalyst for the synthesis of thiopyridine. Macrocyclic Schiff base ligand 32 was obtained via reaction of 2,2′-(1,4-diazepane-1,4-diyl)-di-aniline 30 and 2,3-dihydroxybenzaldehyde 31 in ethanol under reflux for 24 hours. Then, a mixture of FeCl3·6H2O 24, FeCl2·4H2O 25, and NH4OH 26 was stirred in H2O under N2 gas at 100 °C to give Fe3O4 1, which was treated with macrocyclic Schiff base ligand (III) 32 to give Fe3O4-supported macrocyclic Schiff base ligand (III) 33, followed by the reaction with Co(Cl)2·6H2O EtOH under reflux for 24 hours to obtain Fe3O4@macrocyclic Schiff base ligand 34 (Scheme 9).37Fe3O4@macrocyclic Schiff base ligand 34 was employed in the synthesis of 2-amino-4-aryl-6-(phenylsulfanyl)pyridine-3,5-dicarbonitrile derivatives 35 via three-component reaction of aldehyde derivatives 9, malononitrile 16, thiophenol 36 under solvent-free conditions (Scheme 10). The catalytic activity of Fe3O4@CoII (macrocyclic Schiff base ligand) 34 was separately compared to that of Fe3O4, macrocyclic Schiff base ligand, Fe3O4@macrocyclic Schiff base ligand 33. It was demonstrated that Fe3O4@CoII 34 showed the best results.37
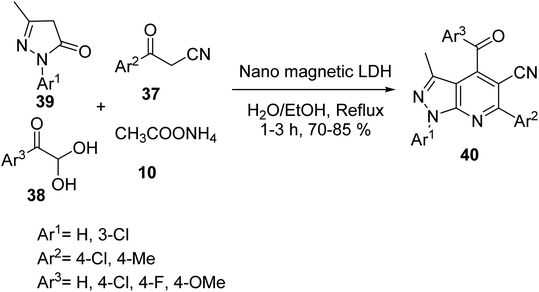
4-Aroyl-3-methyl-1,6-diaryl-1H-pyrazolo[3,4-b] pyridine-5-carbonitrile derivatives 40 were synthesized via one-pot, the four-component reaction of 1-aryl-3-methyl-1H-pyrazol-5-(4H) one 39, 3-aryl-3-oxopropanenitriles 37, arylglyoxals 38, and ammonium acetate 10 in the presence of metal oxide silica based-metal bifunctional LDH (layered double hydroxide) as a magnetic nano-catalyst in EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under the reflux conditions (Scheme 11). In addition, pyrazolo[3,4-b] pyridines 40 have biological and pharmacological activity.38
1) under the reflux conditions (Scheme 11). In addition, pyrazolo[3,4-b] pyridines 40 have biological and pharmacological activity.38
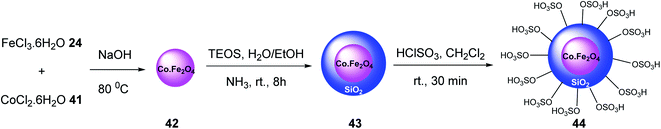
CoFe2O4@SiO2–SO3H 44 was synthesized as a reusable nano-catalyst by Hosseinzadeh et al. Initially, CoFe2O4 magnetic nanoparticles 42 were prepared according to previous works.39 Then, it was modified with tetraethylorthosilicate to provide CoFe2O4@SiO2 43,40. which was dispersed in dry CH2Cl2, and ClSO3H to give CoFe2O4@SiO2–SO3H 44 (Scheme 12).41
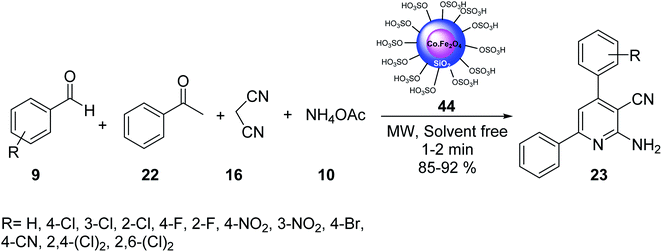
CoFe2O4@Silica MNPs 44 was used in the multicomponent reaction of aldehydes 9, acetophenone 22, malononitrile 16, and ammonium acetate 10 in solvent-free conditions under MW irradiation to provide 2-amino-4,6-diarylnicotinonitrile derivatives 23 in good yields (Scheme 13).41
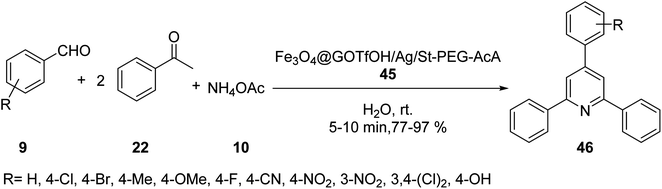
Forouzandehdel and co-workers synthesized a novel, recyclable nano-catalyst Fe3O4@GOTfOH/Ag/St-PEG-AcA 45, which was employed in the synthesis of 2,4,6-tri-arylpyridine derivatives 46 by the reaction of aldehyde derivatives 9, acetophenone 22, and ammonium acetate 10 in H2O at room temperature (Scheme 14).42
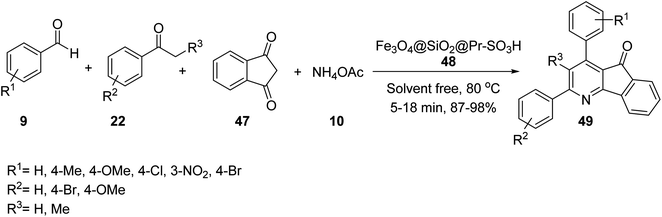
Fe3O4@SiO2@Pr-SO3H 48 was employed as heterogeneous acidic catalyst in the multicomponent reaction of 1,3-indandione 47, aromatic aldehydes 9, acetophenone or propiophenone 22, and ammonium acetate 10 under solvent-free conditions at 80 °C to obtain indeno[1,2-b]pyridines 49 (Scheme 15).43
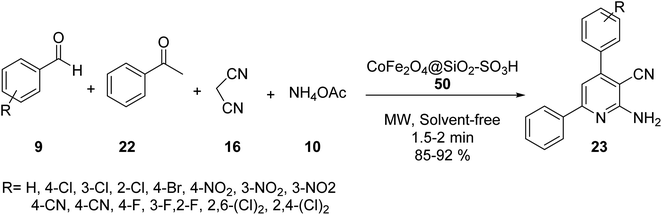
Hosseinzadeh and et al. synthesized 2,6-diaryl-substituted pyridine derivatives 23 via tetra component reaction of aldehyde derivatives 9, acetophenone 22, malononitrile 16, and ammonium acetate 10 in the presence of CoFe2O4@SiO2–SO3H 50 under microwave irradiation and solvent-free conditions (Scheme 16).44
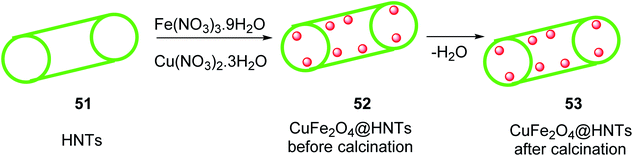
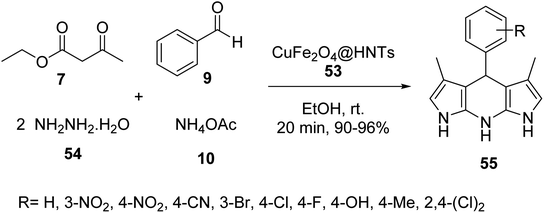
Halloysite nanotubes CuFe2O4@HNTs 53 was synthesized by the reaction of Halloysite nanotubes HNTs 51 was added to Fe(NO3)3·9H2O and 0.14 g (0.58 mmol) of Cu(NO3)2·3H2O in distilled water and stirred at room temperature for 1 h, and then the solution of NaOH was added dropwise to it for 10 min at 25 °C, followed by stirring for 2 h at 90 °C to give CuFe2O4@HNTs 52, which was separated by an external magnet, and washed four times with distilled water, dried for 4 h, and calcinated at 500 °C for 5 h to yield extra pure CuFe2O4@HNTs 53 (Scheme 17).45
The catalytic activity of CuFe2O4@HNTs 53 was tested in the synthesis of pyrazolopyridine derivatives 55 via the multicomponent reaction of ethyl acetoacetate 7, hydrazine hydrate 54, benzaldehyde 9, and ammonium acetate 10 in EtOH at room temperature for 20 min (Scheme 18).45
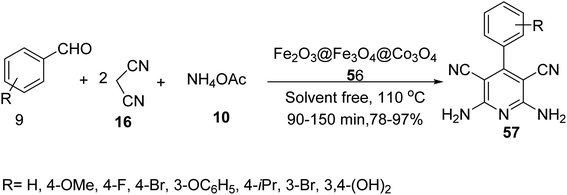
Maleki and co-workers also synthesized Fe2O3@Fe3O4@Co3O4 56 as catalyst to provide polysubstituted pyridines 57 through the pseudo-four-component reaction of aldehyde derivatives 9, malononitrile 16, and ammonium acetate 10 under solvent-free conditions at 110 °C (Scheme 19).46
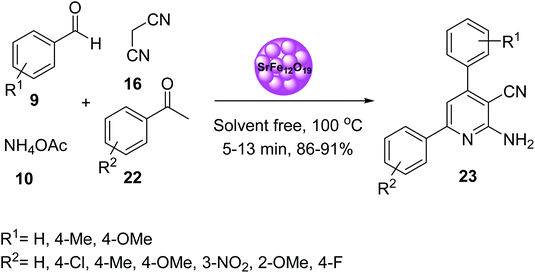
In 2019, Mohammadi and co-workers also prepared 2-amino-3-cyanopyridine 23 via multicomponent reaction of aromatic aldehydes 9, acetophenone derivatives 22, malononitrile 16, and ammonium acetate 10, in the presence of SrFe12O19 as magnetic catalyst under solvent-free conditions at 100 °C. The spectrophotometric properties of 2-amino-4,6-diphenylnicotinonitrile 23 as organo-ligand and several metal ions such as Ag+, Cd2+, Co2+, Cr3+, Cu2+, Fe3+, Hg2+, Mn2+, Ni2+, Pb2+, and Zn2+ in CH3CN solution at 25 °C was also investigated. According to the results, 2-amino-4,6-diphenylnicotinonitrile 23 exhibited a good complexation as organo-ligand with Hg2+ (Scheme 20).47
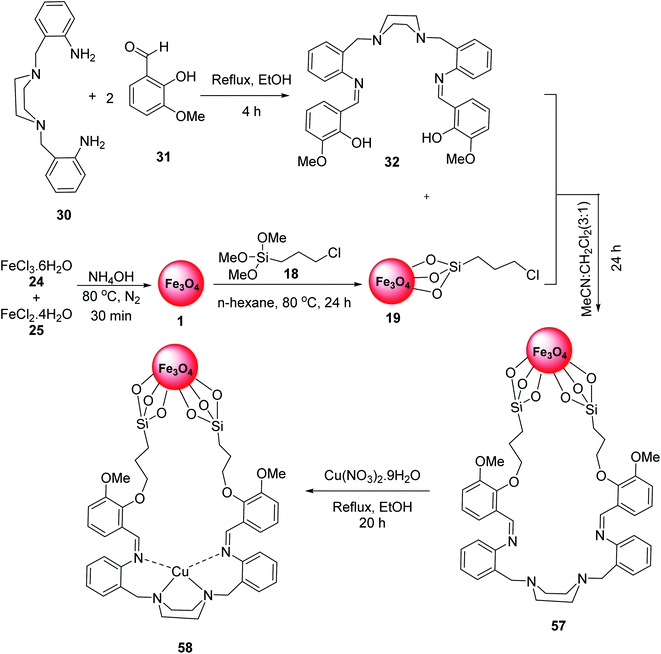
Fe3O4-supported Schiff-base copper(II) complexes 58 were reported by Mahmoudi-GomYek et al. Ligand 32 was synthesized via the reaction of 2,2′-[piperazine-1,4-diylbis-(methylene)]dianiline 30 and 2-hydroxy-3-methoxy benzaldehyde 31. The reaction of FeCl3·6H2O 24, FeCl2·4H2O 25 and NH4OH in H2O under N2 atmosphere provided Fe3O4 MNPs 1, which were functionalized by 3-chloropropyl(trimethoxy)silane (CPTMS) 18 to give Fe3O4@Si-PrCl 19. The reaction of compound 32 with Fe3O4@Si-PrCl 19 gave the compound 57, which reacted with Cu(NO3)2·9H2O to yield Fe3O4-supported Schiff-base copper(II) complex 58 (Scheme 21).48
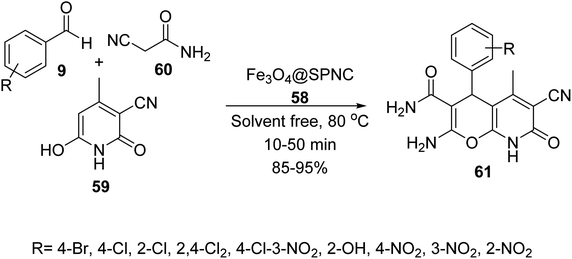
Fe3O4@SPNC 58 was used as catalyst in the synthesis of pyrano[2,3-b]pyridine-3-carboxamide derivatives 61 via the three-component reaction of aldehydes 9, 2-isocyanoacetamide 59, and 3-cyano-6-hydroxy-4-methyl-pyridin-2(1H)-one 60 under solvent-free conditions at 80 °C (Scheme 22).48
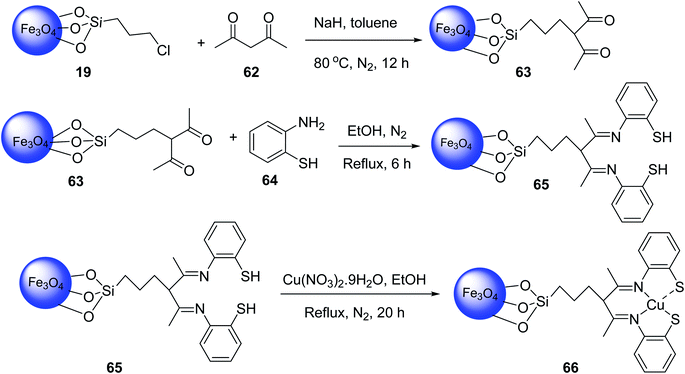
Similar Cu complexes on magnetic nanomaterials were also synthesized from Fe3O4@CPTMS MNPs 19 (ref. 49 and 50) according to the literature. The reaction of Fe3O4@CPTMS MNPs 19, acetylacetone 62 and sodium hydride in toluene at 80 °C under nitrogen atmosphere gave Fe3O4@SiO2-n-Pr-acac MNPs 63, which was reacted with 2-aminobenzenethiol 64 in EtOH under reflux condition and nitrogen atmosphere to provide Fe3O4@SiO2-acac-2ATP 65, followed by reacting with Cu(NO3)2·9H2O in ethanol under reflux and nitrogen gas for 12 h to obtain Fe3O4@SiO2-acac-2ATP-Cu(II) 66 (Scheme 23).51
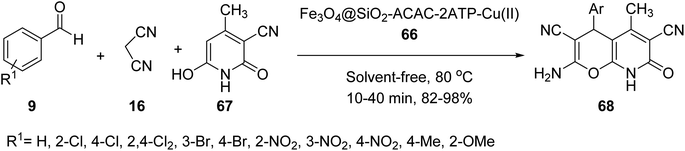
Fe3O4@SiO2-acac-2ATP-Cu(II) MNPs 66 was then employed as catalyst in the three-component reaction of aldehydes 9, malononitrile 16, and 3-cyano-6-hydroxy-4-methyl pyridine-2(1H)-one 67 under solvent-free conditions at 80 °C for the synthesis of 4H-pyrano[2,3-b]pyridine-3,6-dicarbonitrile derivatives 68 by Azarifar and co-works (Scheme 24).51
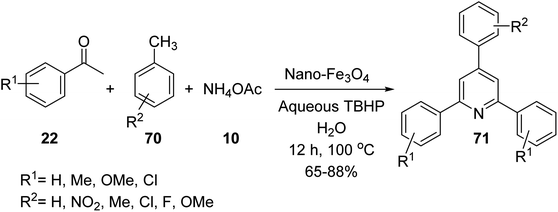
Gajaganti and his co-workers utilised nano-Fe3O4 as a catalyst in the synthesis of 2,4,6-tri-arylpyridines 71 via a three-component reaction of acetophenone derivatives 22, methyl arenes 70, and ammonium acetate 10 (Scheme 25).52
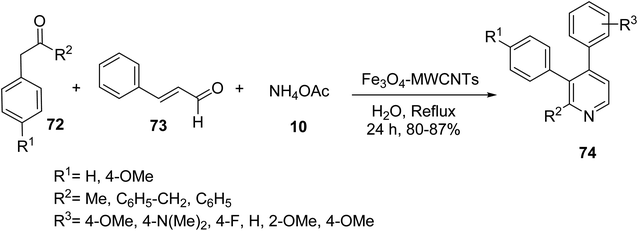
Similar Fe3O4 multi-walled carbon nanotubes (MWCNTs) were prepared and employed as catalyst in the three-component reaction of ketones 72, different cinnamaldehyde 73, and ammonium acetate 10 to synthesize the functionalized pyridines 74 (Scheme 26).53
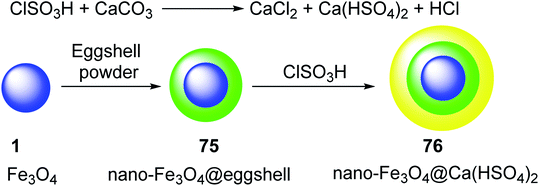
The eggshell powder was coated on the surface of magnetic nano-Fe3O4 1, to give nano-Fe3O4@eggshell 75, which was treated with ClSO3H to yield nano-magnetic acid catalyst Fe3O4@Ca(HSO4)2 76. In this process, CaCO3 from the eggshell was converted to Ca(HSO4)2 through reaction with ClSO3H (Scheme 27).54
Nano-Fe3O4@Ca(HSO4)2 76 was subsequently utilised in the synthesis of 2-amino-3-cyanopyridines 23 via four-component reaction of different benzaldehydes 9, acetophenone 22, ammonium acetate 10, and malononitrile 16 under solvent-free conditions at 90 °C for 5–15 min (Scheme 28).54
2.3. Ionic liquid-based magnetic nanomaterials
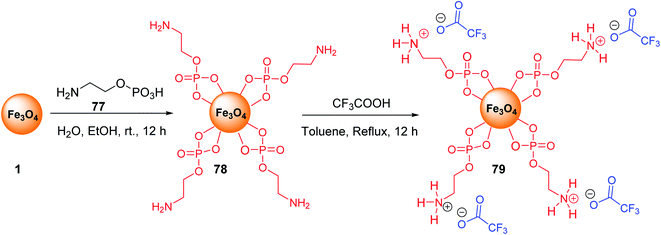
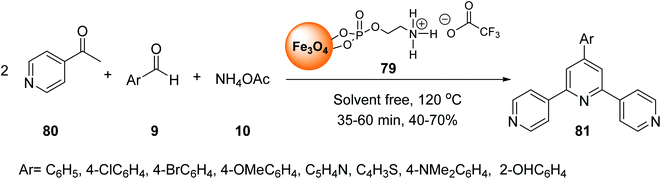
Fe3O4@O2PO2(CH2)2NH2 MNPs 78 was prepared according to the reported method.34,55 After dispersion in the ultrasonic bath, it was reacted with CF3CO2H to prepare Fe3O4@O2PO2(CH2)2NH3 CF3CO2 79 (Scheme 29).56Fe3O4@O2PO2(CH2)2NH3+ CF3CO2− 79 was employed in the multicomponent reaction between various acetyl pyridines 80, aryl aldehydes 9, and ammonium acetate 10 under solvent-free reaction conditions at 120 °C to synthesize terpyridines 81 (Scheme 30).57
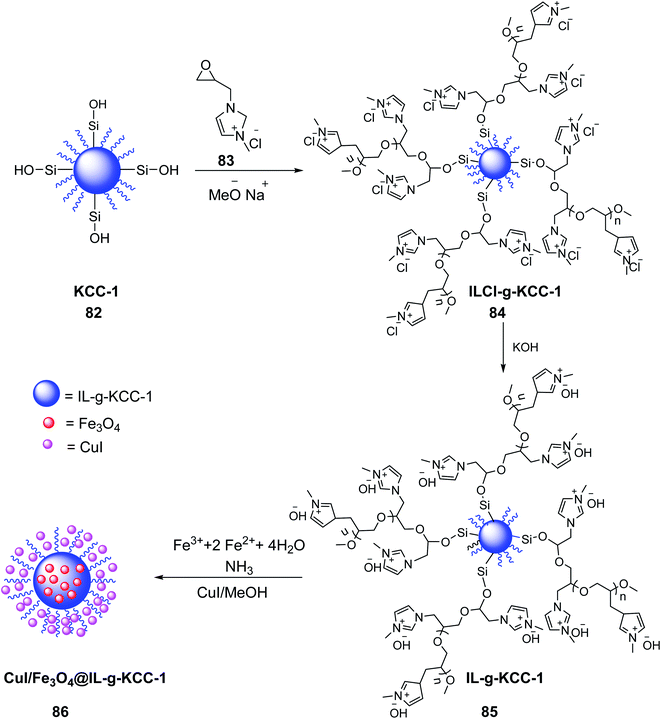
CuI/Fe3O4 NPs@Biimidazole IL-KCC-1 86 was prepared by Azizi et al. in 2020. Firstly, 1-methyl-3-(oxiran2-ylmethyl)-1H-imidazol-3-ium chloride 83 and sodium methoxide were added to the prepared KCC-1 82 in dimethylformamide (DMF), and stirred for 60 min under a nitrogen atmosphere at 60 °C. Methanol and DMF were subsequently evaporated under vacuum to obtain 1-methyl-3-(oxiran-2-yl-methyl)-1H-imidazolium chloride (ILCl-g-KCC-1) 84.58 Then, solid potassium hydroxide was added to ILCl-g-KCC-1 84 to yield IL-KCC-1 85 by replacing chloride ions with hydroxide ions. Fe3O4 NPs were subsequently doped on the substrate of IL-KCC-1 84 and treated with CuI/MeOH to obtain CuI/Fe3O4 NPs@Biimidazole IL-KCC-1 86 (Scheme 31).
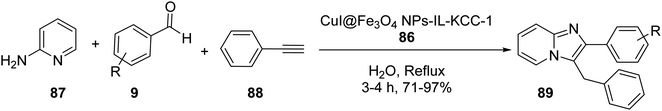
CuI/Fe3O4 NPs@IL-KCC-1 86 was investigated in the three-component reaction of 2-aminopyridine 87, aldehydes 9, phenylacetylene 88, and CTAB in H2O under reflux condition to obtaib imidazo[1,2-a]pyridines 89 in high yields (Scheme 32).59
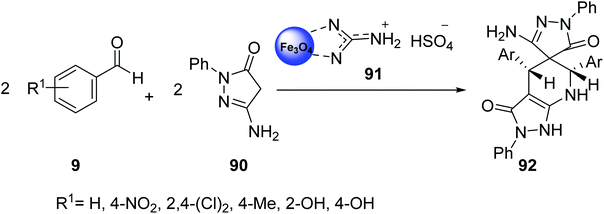
Shojaei et al. was studied the catalytic activity of guanidinium hydrogen sulfate on Fe3O4 nanoparticles 91 in the pseudo-four-component reactions of aryl aldehydes 9 with 3-amino-1-phenyl-2-pyrazolin-5-one 90 to give spiro[pyrazole-pyrazolo[3,4-b]pyridine]-dione derivatives 92 under mild conditions (Scheme 33).60
2.4. Bifunctional magnetic catalysts
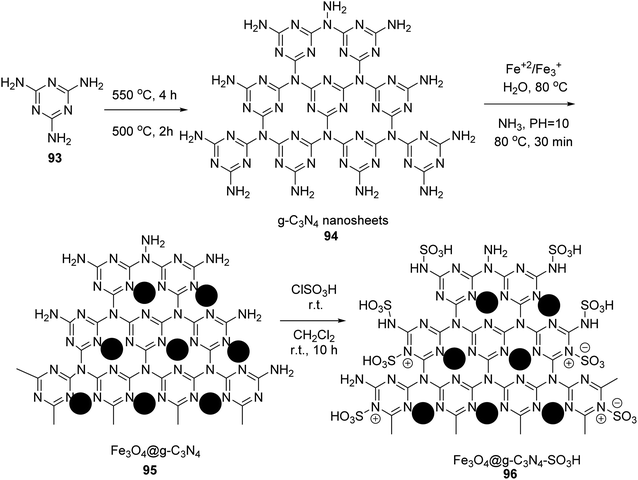
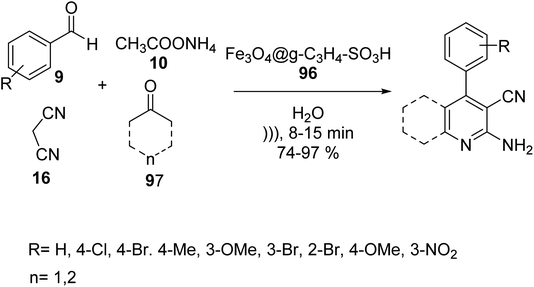
In 2019, Edrisi et al. synthesized g-C3N4 94 according to the reported method.61 g-C3N4 94 was functionalized with Fe3O4 nanoparticles62 to give Fe3O4@g-C3N4 95. Finally, Fe3O4@g-C3N4–SO3H 96 was washed with methanol and ethyl acetate and afterward dried under vacuum at 60 °C (Scheme 34).63Fe3O4@g-C3N4–SO3H 96 was then utilized in the synthesis of pyridine derivatives 98 via the one-pot multicomponent reaction of different aldehydes 9, various ketones 97, ammonium acetate 10, and malononitrile 16 in H2O under ultrasonic irradiation (Scheme 35).63
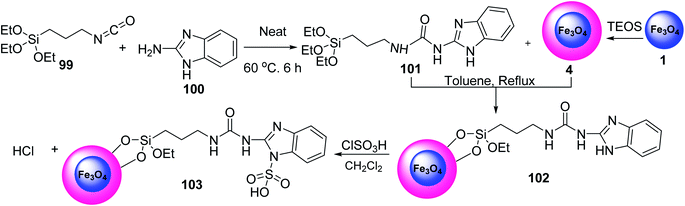
Torabi and et al. prepared Ligand 101 via the reaction of 1H-benzo[d]imidazol-2-amine 100 and compound 99 under solvent-free conditions. Fe3O4 was then functionalized with tetraethyl orthosilicate (TEOS) in toluene under reflux conditions to give Fe3O4@SiO2 4, which was reacted with ligand 101 to yield Fe3O4@SiO2@(CH2)3-urea-benzimidazole 102, followed by the reaction with chlorosulfuric acid in dichloromethane to obtain Fe3O4@SiO2@(CH2)3-urea-benzimidazole sulfonic acid 103 (Scheme 36).64
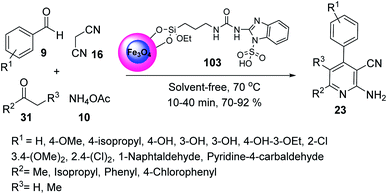
Fe3O4@SiO2@(CH2)3-urea-benzimidazole sulfonic acid 103 was employed in the synthesis of 2-amino-3-cyano pyridines 23 through the multicomponent reaction of benzaldehyde 9, malononitrile 16, methyl isopropyl ketone 31, and ammonium acetate 10 under solvent-free conditions at 70 °C (Scheme 37).64
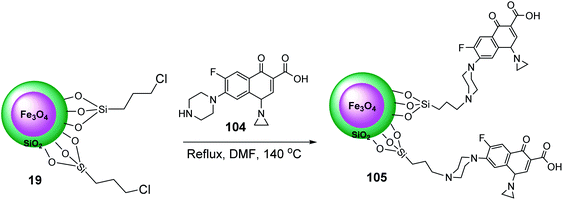
Initially, according to previous works,65 Fe3O4@SiO2@Pr-Cl 19 was prepared and dispersed in dry DMF, and then reacted with ciprofloxacin 104 to give Fe3O4@SiO2@Pr-ciprofloxacin 105 (Scheme 38).66
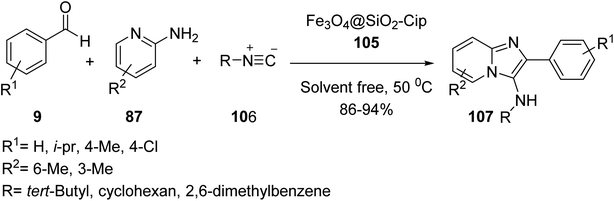
Fe3O4@SiO2@Pr-Cip 105 was then investigated in the synthesis of imidazo[1,2-a]pyridines 107 through the three-component reaction of various benzaldehyde 9, 2-aminopyridine 87, and cyclohexyl isocyanide 106 (Scheme 39).66
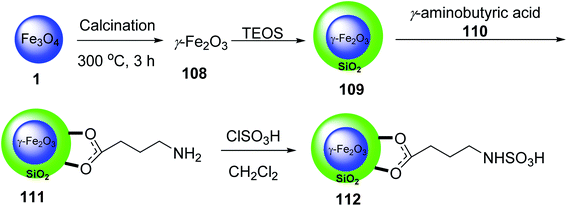
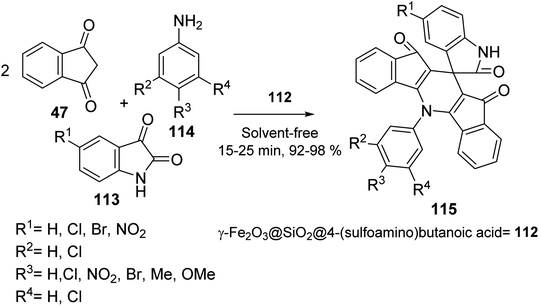
Mohammadi et al. synthesized Fe2O3 nanoparticles 1 according to a previously reported method.67 Calcination of Fe2O3 provided γ-Fe2O3 108, which was convered to γ-Fe2O3@SiO2 MNPs 109 by the reaction with tetraethyl orthosilicate (TEOS) 3, followed by the functionalization with γ-aminobutyric acid 110 to yield γ-Fe2O3@SiO2-aminobutyric acid nanoparticles 111. Then, it was dispersed in chloroform and reacted with chlorosulfonic acid to provide γ-Fe2O3@SiO2 γ-aminobutyric acid-SO3H 112 (Scheme 40).68
γ-Fe2O3@SiO2@4-(sulfoamino)butanoic acid-SO3H 112 was utilized in the synthesis of 5-(aryl)-5H-spiro[diindeno[1,2-b:2′,1′-e]pyridine-11,30-indoline]-2′,10,12-trione derivatives 115 through the pseudo four-component reaction of 1,3-indandione 47, isatins 113 with various aromatic amines 114 (Scheme 41).68
 | ||
| Scheme 41 Synthesis of 5-(aryl)-5H-spiro[diindeno[1,2-b:2′,1′-e] pyridine-11,30-indoline]-2′,10,12-trione derivatives 115. | ||
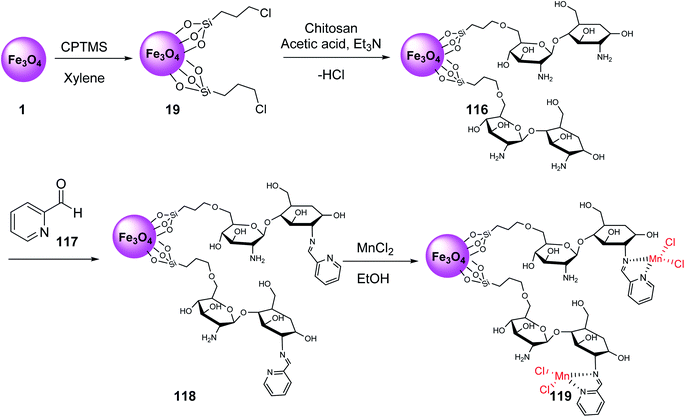
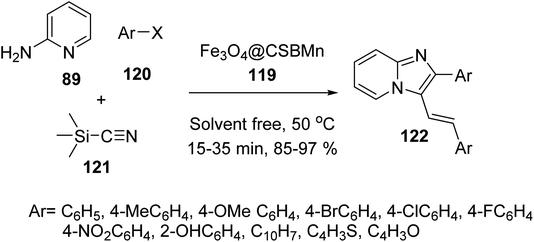
Fe3O4@Si-Pr-Cl 19 was reacted with chitosan and acetic acid solutions to provide chitosan-coated MNPs 116, which were modified with 2-formylpyridine 117 to give compound 118, followed by the reaction with manganese chloride to provide manganese Schiff-base complex Fe3O4@CSBMn 119 (Scheme 42).69,70
Fe3O4@CSBMn 119 was employed in the synthesis of 3-iminoaryl-imidazo[1,2-a]pyridine (IAIP) derivatives 122 through the three-component reaction of aryl halide derivatives 120, trimethylsilyl cyanide 121, and 2-aminopyridine 89 (Scheme 43). According to the results, the aldehydes with an electron-withdrawing group provided higher yields in comparison with electron-donating groups.70
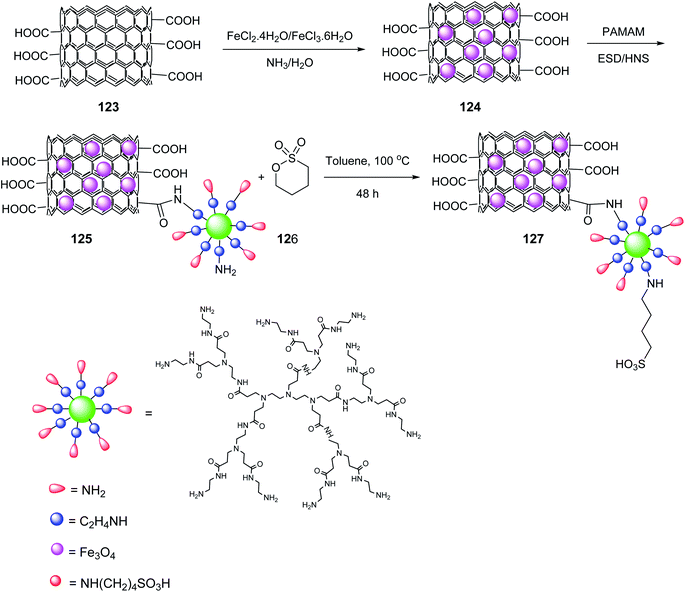
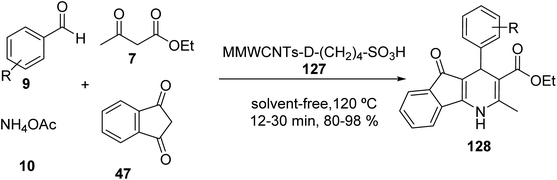
Multi-walled carbon nanotubes systems MWCNTs-COOH 123 (ref. 71) were synthesized according to the literature. A mixture of FeCl3·6H2O and FeCl2·4H2O was added to MWCNTs-COOH 123 in distilled water and stirred at 50 °C to give the magnetic multi-walled carbon nanotubes (MMWCNTs) 124, which were subsequently reacted with 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride (EDC·HCl) and N-hydroxysuccinimide (NHS) to obtain MMWCNTs-D-NH2 125 followed by reaction with 1,4-butanesultone 126 to yield MMWCNTs-D–(CH2)4–SO3H 127 (Scheme 44).72
MMWCNTs-D–(CH2)4–SO3H 127 was employed in the synthesis of dihydro-1H-Indeno[1,2-b] Pyridines 128 by the reaction of various aldehydes 9, 1,3-indandione 47, ethyl acetoacetate 7, and ammonium acetate 10 (Scheme 45).72
3. Conclusions
Due to the high importance of magnetic nano-catalysts, featuring non-toxic nature, high surface area, simple preparation, easy surface modification, and simple separation, such systems have relevant applications in organic synthesis and catalysis. In this contribution, the synthesis methods of magnetic nano-catalysts have been disclosed in view of their applications in the synthesis of pyridine derivatives. According to most studies, these catalysts have excellent activities to target products, also featuring high reusability with the possibility to be recycled several times without reducing their catalytic activities.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We are grateful for the Research Council support of Alzahra University. R. Luque gratefully acknowledges MINECO for funding under project PID2019-109953GB-I00. This paper has been supported by RUDN University Strategic Academic Leadership Program (R. Luque).References
- A. Gulati, J. Malik and R. Kakkar, Peanut shell biotemplate to fabricate porous magnetic Co3O4 coral reef and its catalytic properties for p-nitrophenol reduction and oxidative dye degradation, Colloids Surf., A, 2020, 604, 125328, DOI:10.1016/j.colsurfa.2020.125328.
- R. Taheri-Ledari, J. Rahimi and A. Maleki, Method screening for conjugation of the small molecules onto the vinyl-coated Fe3O4/silica nanoparticles: highlighting the efficiency of ultrasonication, Mater. Res. Express, 2020, 7, 015067, DOI:10.1088/2053-1591/ab69cc.
- W. Zhang, R. Taheri-Ledari, Z. Hajizadeh, E. Zolfaghari, M. R. Ahghari, A. Maleki, M. R. Hamblin and Y. Tian, Enhanced activity of vancomycin by encapsulation in hybrid magnetic nanoparticles conjugated to a cell-penetrating peptide, Nanoscale, 2020, 12, 3855–3870, 10.1039/C9NR09687F.
- N. Kang, D. Xu, Y. Han, X. Lv, Z. Chen, T. Zhou, L. Ren and X. Zhou, Magnetic targeting core/shell Fe3O4/Au nanoparticles for magnetic resonance/photoacoustic dual-modal imaging, Mater. Sci. Eng., C, 2019, 98, 545–549, DOI:10.1016/j.msec.2019.01.013.
- Z. Hajizadeh, K. Valadi, R. Taheri-Ledari and A. Maleki, Convenient Cr (VI) removal from aqueous samples: executed by a promising clay-based catalytic system, magnetized by Fe3O4 nanoparticles and functionalized with humic acid, ChemistrySelect, 2020, 5, 2441–2448, DOI:10.1002/slct.201904672.
- R. Taheri-Ledari, W. Zhang, M. Radmanesh, S. S. Mirmohammadi, A. Maleki, N. Cathcart and V. Kitaev, Multi-Stimuli Nanocomposite Therapeutic: Docetaxel Targeted Delivery and Synergies in Treatment of Human Breast Cancer Tumor, Small, 2020, 16, 2002733 CrossRef CAS.
- A. Maleki, F. Hassanzadeh-Afruzi, Z. Varzi and M. S. Esmaeili, Magnetic dextrin nanobiomaterial: an organic-inorganic hybrid catalyst for the synthesis of biologically active polyhydroquinoline derivatives by asymmetric Hantzsch reaction, Mater. Sci. Eng., C, 2020, 109, 110502, DOI:10.1002/slct.201904672.
- R. A. Sheldon, Green solvents for sustainable organic synthesis: state of the art, Green Chem., 2005, 7, 267–278, 10.1039/B418069K.
- Q.-H. Xia, H.-Q. Ge, C.-P. Ye, Z.-M. Liu and K.-X. Su, Advances in homogeneous and heterogeneous catalytic asymmetric epoxidation, Chem. Rev., 2005, 105, 1603–1662, DOI:10.1021/cr0406458.
- D. Astruc, F. Lu and J. R. Aranzaes, Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis, Angew. Chem., Int. Ed., 2005, 44, 7852–7872, DOI:10.1002/anie.200500766.
- S. Panigrahi, S. Basu, S. Praharaj, S. Pande, S. Jana, A. Pal, S. K. Ghosh and T. Pal, Synthesis and size-selective catalysis by supported gold nanoparticles: study on heterogeneous and homogeneous catalytic process, J. Phys. Chem. C, 2007, 111, 4596–4605, DOI:10.1021/jp067554u.
- C.-J. Zhong and M. M. Maye, Core–shell assembled nanoparticles as catalysts, Adv. Mater., 2001, 13, 1507–1511, DOI:10.1002/1521-4095.
- O. Veiseh, J. W. Gunn and M. Zhang, Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging, Adv. Drug Delivery Rev., 2010, 62, 284–304, DOI:10.1016/j.addr.2009.11.002.
- R. N. Baig and R. S. Varma, Magnetically retrievable catalysts for organic synthesis, Chem. Commun., 2013, 49, 752–770, 10.1039/C2CC35663E.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J.-M. Basset, Magnetically recoverable nano-catalysts, Chem. Rev., 2011, 111, 3036–3075, DOI:10.1021/cr100230z.
- S. Zhang, X. Zhao, H. Niu, Y. Shi, Y. Cai and G. Jiang, Superparamagnetic Fe3O4 nanoparticles as catalysts for the catalytic oxidation of phenolic and aniline compounds, J. Hazard. Mater., 2009, 167, 560–566, DOI:10.1016/j.jhazmat.2009.01.024.
- P. Tartaj and C. J. Serna, Synthesis of monodisperse superparamagnetic Fe/silica nanospherical composites, J. Am. Chem. Soc., 2003, 125, 15754–15755, DOI:10.1021/ja0380594.
- Y. Lu, X. Lu, B. T. Mayers, T. Herricks and Y. Xia, Synthesis and characterization of magnetic Co nanoparticles: a comparison study of three different capping surfactants, J. Solid State Chem., 2008, 181, 1530–1538, DOI:10.1016/j.jssc.2008.02.016.
- A. El Harrak, G. Carrot, J. Oberdisse, C. Eychenne-Baron and F. Boué, Surface- atom transfer radical polymerization from silica nanoparticles with controlled colloidal stability, Macromolecules, 2004, 37, 6376–6384, DOI:10.1021/ma035959w.
- S. Azad and B. B. F. Mirjalili, Fe3O4@ nano-cellulose/TiCl: a bio-based and magnetically recoverable nano-catalyst for the synthesis of pyrimido [2, 1-b] benzothiazole derivatives, RSC Adv., 2016, 6, 96928–96934, 10.1039/C6RA13566H.
- J. Safari and L. Javadian, Ultrasound assisted the green synthesis of 2-amino-4H-chromene derivatives catalyzed by Fe3O4-functionalized nanoparticles with chitosan as a novel and reusable magnetic catalyst, Ultrason. Sonochem., 2015, 22, 341–348, DOI:10.1016/j.ultsonch.2014.02.002.
- R. Mohammadi, E. Eidi, M. Ghavami and M. Z. Kassaee, Chitosan synergistically enhanced by successive Fe3O4 and silver nanoparticles as a novel green catalyst in one-pot, three-component synthesis of tetrahydrobenzo [α] xanthene-11-ones, J. Mol. Catal. A: Chem., 2014, 393, 309–316, DOI:10.1016/j.molcata.2014.06.005.
- J. Tang, L. Wang, Y. Yao, L. Zhang and W. Wang, One-pot synthesis of 2-amino-3-cyanopyridine derivatives catalyzed by ytterbium perfluorooctanoate [Yb (PFO)3], Tetrahedron Lett., 2011, 52, 509–511, DOI:10.1016/j.tetlet.2010.11.102.
- T. Murata, M. Shimada, S. Sakakibara, T. Yoshino, K. Kadono, T. Masuda, M. Shimazaki, T. Shintani, K. Fuchikami and K. Sakai, Erratum to Discovery of novel and selective IKK-b serine-threonine protein kinase inhibitors. Part 1, Bioorg. Med. Chem. Lett., 2003, 13, 3627, DOI:10.1016/S0960-894X(03)00701-7.
- F. Mohajer, G. Mohammadi Ziarani and A. Badiei, New advances on Au–magnetic organic hybrid core–shells in MRI, CT imaging, and drug delivery, RSC Adv., 2021, 12, 6517–6525, 10.1039/D1RA00415H.
- G. Mohammadi Ziarani, M. Malmir, N. Lashgari and A. Badiei, The role of hollow magnetic nanoparticles in drug delivery, RSC Adv., 2019, 43, 25094–25106, 10.1039/C9RA01589B.
- Z. Kheilkordi, G. Mohammadi Ziarani and A. Badiei, Fe3O4@ SiO2@(BuSO3H)3 synthesis as a new efficient nanocatalyst and its application in the synthesis of heterocyclic [3.3. 3] propellane derivatives, Polyhedron, 2020, 178, 114343, DOI:10.1016/j.poly.2019.114343.
- Z. Kheilkordi, G. Mohammadi Ziarani, N. Lashgari and A. Badiei, An efficient method for the synthesis of functionalized 4H-chromenes as optical sensor for detection of Fe3+ in ethanol, Polyhedron, 2019, 166, 203–209, DOI:10.1016/j.poly.2019.03.042.
- G. Mohammadi Ziarani, P. Mofatehnia, F. Mohajer and A. Badiei, Rational design of yolk–shell nanostructures for drug delivery, RSC Adv., 2020, 10, 30094–30109, 10.1039/D0RA03611K.
- S. Azizi, J. Soleymani and M. Hasanzadeh, Iron oxide magnetic nanoparticles supported on amino propyl-functionalized KCC-1 as robust recyclable catalyst for one pot and green synthesis of tetrahydrodipyrazolopyridines and cytotoxicity evaluation, Appl. Organomet. Chem., 2020, 34, e5440, DOI:10.1002/aoc.5440.
- K. Can, M. Ozmen and M. Ersoz, Immobilization of albumin on aminosilane modified superparamagnetic magnetite nanoparticles and its characterization, Colloids Surf., B, 2009, 71, 154–159, DOI:10.1016/j.colsurfb.2009.01.021.
- T. Zeng, L. Yang, R. Hudson, G. Song, A. R. Moores and C.-J. Li, Fe3O4 nanoparticle-supported copper (I) pybox catalyst: magnetically recoverable catalyst for enantioselective direct-addition of terminal alkynes to imines, Org. Lett., 2011, 13, 442–445, DOI:10.1021/ol102759w.
- S. Asadbegi, M. A. Bodaghifard and A. Mobinikhaledi, Poly N, N-dimethylaniline-formaldehyde supported on silica-coated magnetic nanoparticles: a novel and retrievable catalyst for green synthesis of 2-amino-3-cyanopyridines, Res. Chem. Intermed., 2020, 46, 1629–1643, DOI:10.1007/s11164-017-3200-4.
- S. Qu, H. Yang, D. Ren, S. Kan, G. Zou, D. Li and M. Li, Magnetite nanoparticles prepared by precipitation from partially reduced ferric chloride aqueous solutions, J. Colloid Interface Sci., 1999, 215, 190–192, DOI:10.1006/jcis.1999.6185.
- S. Kalhor, M. Yarie, M. Rezaeivala and M. A. Zolfigol, Novel magnetic nanoparticles with morpholine tags as multirole catalyst for synthesis of hexahydroquinolines and 2-amino-4, 6-diphenylnicotinonitriles through vinylogous anomeric-based oxidation, Res. Chem. Intermed., 2019, 45, 3453–3480, DOI:10.1007/s11164-019-03802-7.
- M. Ashouri, H. Kefayati and S. Shariati, Synthesis, characterization, and catalytic application of Fe3O4-Si-[CH2]3-N= CH-aryl for the efficient synthesis of novel poly-substituted pyridines, J. Chin. Chem. Soc., 2019, 66, 355–362, DOI:10.1002/jccs.201800255.
- H. Ebrahimiasl, D. Azarifar, J. Rakhtshah, H. Keypour and M. Mahmoudabadi, Application of novel and reusable Fe3O4@ CoII (macrocyclic Schiff base ligand) for multicomponent reactions of highly substituted thiopyridine and 4H-chromene derivatives, Appl. Organomet. Chem., 2020, 34, e5769, DOI:10.1002/aoc.5769.
- F. Majidi Arlan, R. Javahershenas and J. Khalafy, An efficient one-pot, four-component synthesis of a series of pyrazolo [3, 4-b] pyridines in the presence of magnetic LDH as a nano-catalyst, Asian J. Nanosci. Mater., 2020, 3, 238–250, DOI:10.26655/AJNANOMAT.2020.3.7.
- F. Sadri, A. Ramazani, A. Massoudi, M. Khoobi, V. Azizkhani, R. Tarasi, L. Dolatyari and B.-K. Min, Magnetic CoFe2O4 nanoparticles as an efficient catalyst for the oxidation of alcohols to carbonyl compounds in the presence of oxone as an oxidant, Bull. Korean Chem. Soc., 2014, 35, 2029, DOI:10.5012/bkcs.2014.35.7.2029.
- W. Stöber, A. Fink and E. Bohn, Controlled growth of monodisperse silica spheres in the micron size range, J. Colloid Interface Sci., 1968, 26, 62–69, DOI:10.1016/0021-9797(68)90272-5.
- Z. Hosseinzadeh, A. Ramazani, H. Ahankar, K. Ślepokura and T. Lis, Synthesis of 2-amino-4,6-diarylnicotinonitrile in the presence of CoFe2O4@SiO2-SO3H as a reusable solid acid nano-catalyst under microwave irradiation in solvent-free conditions, Silicon, 2019, 11, 2169–2176 CrossRef CAS.
- S. Forouzandehdel, M. Meskini and M. R. Rami, Design and application of (Fe3O4)-GOTfOH based AgNPs doped starch/PEG-poly (acrylic acid) nanocomposite as the magnetic nano-catalyst and the wound dress, J. Mol. Struct., 2020, 1214, 128142, DOI:10.1016/j.molstruc.2020.128142.
- Z. Kheilkordi, G. Mohammadi Ziarani, A. Badiei and H. Vojoudi, A green method for the synthesis of indeno [1, 2-b] pyridines using Fe3O4@ SiO2@ PrSO3H as a nanomagnetic catalyst, Iran, J. Catal., 2020, 10, 65–70 CAS.
- Z. Hosseinzadeh, N. Razzaghi-Asl, A. Ramazani and H. Aghahosseini, Synthesis, cytotoxic assessment, and molecular docking studies of 2, 6-diaryl-substituted pyridine and 3, 4-dihydropyrimidine-2 (1H)-one scaffolds, Turk. J. Chem., 2020, 44, 194–213, DOI:10.3906/kim-1903-72.
- A. Maleki, Z. Hajizadeh and P. Salehi, Mesoporous halloysite nanotubes modified by CuFe2O4 spinel ferrite nanoparticles and study of its application as a novel and efficient heterogeneous catalyst in the synthesis of pyrazolopyridine derivatives, Sci. Rep., 2019, 9, 5552, DOI:10.1038/s41598-019-42126-9.
- B. Maleki, H. Natheghi, R. Tayebee, H. Alinezhad, A. Amiri, S. A. Hossieni and S. M. M. Nouri, Synthesis and characterization of nanorod magnetic Co–Fe mixed oxides and its catalytic behavior towards one-pot synthesis of polysubstituted pyridine derivatives, Polycyclic Aromat. Compd., 2020, 40, 633–643, DOI:10.1080/10406638.2018.1469519.
- Z. Kheilkordi, G. Mohammadi Ziarani, S. Bahar and A. Badiei, The green synthesis of 2-amino-3-cyanopyridines using SrFe12O19 magnetic nanoparticles as efficient catalyst and their application in complexation with Hg2+ ions, J. Iran. Chem. Soc., 2019, 16, 365–372, DOI:10.1007/s13738-018-1514-9.
- S. Mahmoudi-GomYek, D. Azarifar, M. Ghaemi, H. Keypour and M. Mahmoudabadi, Fe3O4-supported Schiff-base copper (II) complex: A valuable heterogeneous nano-catalyst for one-pot synthesis of new pyrano [2, 3-b] pyridine-3-carboxamide derivatives, Appl. Organomet. Chem., 2019, 33(6), e4918 CrossRef.
- A. Ghorbani-Choghamarani, B. Tahmasbi, N. Noori and R. Ghafouri-nejad, A new palladium complex supported on magnetic nanoparticles and applied as an catalyst in amination of aryl halides, Heck and Suzuki reactions, J. Iran. Chem. Soc., 2017, 14, 681–693, DOI:10.1007/s13738-016-1020-x.
- M. Nikoorazm, N. Noori, B. Tahmasbi and S. Faryadi, A palladium complex immobilized onto mesoporous silica: a highly efficient and reusable catalytic system for carbon–carbon bond formation and anilines synthesis, Transition Met. Chem., 2017, 42, 469–481, DOI:10.1007/s11243-017-0151-y.
- D. Azarifar, S. Mahmoudi-GomYek and M. Ghaemi, Immobilized Cu (II) Schiff base complex supported on Fe3O4 magnetic nanoparticles: a highly efficient and reusable new catalyst for the synthesis of pyranopyridine derivatives, Appl. Organomet. Chem., 2018, 32, e4541, DOI:10.1002/aoc.4541.
- S. Gajaganti, D. Kumar, S. Singh, V. Srivastava and B. K. Allam, A New Avenue to the Synthesis of Symmetrically Substituted Pyridines Catalyzed by Magnetic Nano–Fe3O4: Methyl Arenes as Sustainable Surrogates of Aryl Aldehydes, ChemistrySelect, 2019, 4, 9241–9246, DOI:10.1002/slct.201900289.
- N. Basavegowda, K. Mishra and Y. R. Lee, Fe3O4-decorated MWCNTs as an efficient and sustainable heterogeneous nano-catalyst for the synthesis of polyfunctionalised pyridines in water, Materials and Technology, 2019, 34, 558–569, DOI:10.1080/10667857.2019.1593291.
- T. Akbarpoor, A. Khazaei, J. Y. Seyf, N. Sarmasti and M. M. Gilan, One-pot synthesis of 2-amino-3-cyanopyridines and hexahydroquinolines using eggshell-based nano-magnetic solid acid catalyst via anomeric-based oxidation, Res. Chem. Intermed., 2020, 46, 1539–1554, DOI:10.1007/s11164-019-04049-y.
- M. Aghayee, M. A. Zolfigol, H. Keypour, M. Yarie and L. Mohammadi, Synthesis and characterization of a novel magnetic nano-palladium Schiff base complex: application in cross-coupling reactions, Appl. Organomet. Chem., 2016, 30, 612–618, DOI:10.1002/aoc.3477.
- F. Karimi, M. A. Zolfigol and M. Yarie, A novel and reusable ionically tagged nanomagnetic catalyst: Application for the preparation of 2-amino-6-(2-oxo-2H-chromen-3-yl)-4-arylnicotinonitriles via vinylogous anomeric based oxidation, Mol. Catal., 2019, 463, 20–29, DOI:10.1016/j.mcat.2018.11.009.
- F. Karimi, M. Yarie and M. A. Zolfigol, A convenient method for synthesis of terpyridines via a cooperative vinylogous anomeric based oxidation, RSC Adv., 2020, 10, 25828–25835, 10.1039/D0RA04461J.
- S. Azizi, N. Shadjou and J. Soleymani, CuI/Fe3O4 NPs@ Biimidazole IL-KCC-1 as a leach proof nano-catalyst for the synthesis of imidazo [1, 2-a] pyridines in aqueous medium, Appl. Organomet. Chem., 2021, 35, e6031, DOI:10.1002/aoc.6031.
- S. Azizi, N. Shadjou and J. Soleymani, CuI/Fe3O4 NPs@ Biimidazole IL-KCC-1 as a leach proof nano-catalyst for the synthesis of imidazo [1, 2-a] pyridines in aqueous medium, Appl. Organomet. Chem., 2020, e6031, DOI:10.1002/aoc.6031.
- R. Shojaei, M. Zahedifar, P. Mohammadi, K. Saidi and H. Sheibani, Novel magnetic nanoparticle supported ionic liquid as an efficient catalyst for the synthesis of spiro [pyrazole-pyrazolo [3, 4-b] pyridine]-dione derivatives under solvent free conditions, J. Mol. Struct., 2019, 1178, 401–407, DOI:10.1016/j.molstruc.2018.10.052.
- R. N. Baig, S. Verma, M. N. Nadagouda and R. S. Varma, Room temperature synthesis of biodiesel using sulfonated graphitic carbon nitride, Sci. Rep., 2016, 6, 39387, DOI:10.1038/srep39387.
- A. S. Krishna Kumar, J.-G. You, W.-B. Tseng, G. Dwivedi, N. Rajesh, S.-J. Jiang and W.-L. Tseng, Magnetically Separable Nanospherical g-C3N4@ Fe3O4 as a Recyclable Material for Chromium Adsorption and Visible-Light-Driven Catalytic Reduction of Aromatic Nitro Compounds, ACS Sustainable Chem. Eng., 2019, 7, 6662–6671, DOI:10.1021/acssuschemeng.8b05727.
- M. Edrisi and N. Azizi, Sulfonic acid-functionalized graphitic carbon nitride composite: a novel and reusable catalyst for the one-pot synthesis of polysubstituted pyridine in water under sonication, J. Iran. Chem. Soc., 2020, 17, 901–910, DOI:10.1007/s13738-019-01820-1.
- M. Torabi, M. Yarie and M. A. Zolfigol, Synthesis of a novel and reusable biological urea based acidic nanomagnetic catalyst: Application for the synthesis of 2-amino-3-cyano pyridines via cooperative vinylogous anomeric based oxidation, Appl. Organomet. Chem., 2019, 33, e4933, DOI:10.1002/aoc.4933.
- E. Soleimani, M. Naderi Namivandi and H. Sepahvand, ZnCl2 supported on Fe3O4@SiO2 core–shell nanocatalyst for the synthesis of quinolines via Friedländer synthesis under solvent-free condition, Appl. Organomet. Chem., 2017, 31, e3566, DOI:10.1002/aoc.3566.
- E. Soleimani, S. Torkaman, H. Sepahvand and S. Ghorbani, Ciprofloxacin-functionalized magnetic silica nanoparticles: as a reusable catalyst for the synthesis of 1H-chromeno [2, 3-d] pyrimidine-5-carboxamides and imidazo [1, 2-a] pyridines, Mol. Diversity, 2019, 23, 739–749, DOI:10.1007/s11030-018-9907-3.
- K. M. Ho and P. Li, Design and synthesis of novel magnetic core-shell polymeric particles, Langmuir, 2008, 24, 1801–1807, DOI:10.1021/la702887m.
- H. Mohammadi and H. R. Shaterian, γ-Fe2O3@SiO2@ 4-(sulfoamino) butanoic acid as a novel superparamagnetic nanocatalyst promoted green synthesis of 5-(aryl)-5 H-spiro [diindeno [1, 2-b: 2′, 1′-e] pyridine-11, 3′-indoline]-2′, 10, 12-trione derivatives, Res. Chem. Intermed., 2018, 44, 7519–7538, DOI:10.1007/s11164-018-3571-1.
- M. Ma, Y. Zhang, W. Yu, H.-y. Shen, H.-q. Zhang and N. Gu, Preparation and characterization of magnetite nanoparticles coated by amino silane, Colloids Surf., A, 2003, 212, 219–226, DOI:10.1016/S0927-7757(02)00305-9.
- J. Rakhtshah and F. Yaghoobi, Catalytic application of new manganese Schiff-base complex immobilized on chitosan-coated magnetic nanoparticles for one-pot synthesis of 3-iminoaryl-imidazo [1, 2-a] pyridines, Int. J. Biol. Macromol., 2019, 139, 904–916, DOI:10.1016/j.ijbiomac.2019.08.054.
- H. Alinezhad, M. Tarahomi, B. Maleki and A. Amiri, SO3H-functionalized nano-MGO-D-NH2: Synthesis, characterization and application for one-pot synthesis of pyrano [2, 3-d] pyrimidinone and tetrahydrobenzo [b] pyran derivatives in aqueous media, Appl. Organomet. Chem., 2019, 33, e4661, DOI:10.1002/aoc.4661.
- F. Adibian, A. R. Pourali, B. Maleki, M. Baghayeri and A. Amiri, One-pot synthesis of dihydro-1H-indeno [1, 2-b] pyridines and tetrahydrobenzo [b] pyran derivatives using a new and efficient nanocomposite catalyst based on N-butylsulfonate-functionalized MMWCNTs-D-NH2, Polyhedron, 2020, 175, 114179, DOI:10.1016/j.poly.2019.114179.
| This journal is © The Royal Society of Chemistry 2021 |