Open Access Article
Open Access ArticleRed, green, and blue light-emitting carbon dots prepared from o-phenylenediamine†
Yulong An a,
Xu Lin*a,
Yuxi Zhoua,
Yan Lia,
Yunwu Zheng
a,
Xu Lin*a,
Yuxi Zhoua,
Yan Lia,
Yunwu Zheng b,
Chunhua Wub,
Kaimeng Xub,
Xijuan Chaib and
Can Liu*ab
b,
Chunhua Wub,
Kaimeng Xub,
Xijuan Chaib and
Can Liu*ab
aKey Laboratory for Forest Resources Conservation and Utilization in the Southwest Mountains of China, College of Material Science & Engineering, Southwest Forestry University, Kunming, China. E-mail: liucanswfu@163.com
bKey Laboratory of State Forestry Administration for Highly-Efficient Utilization of Forestry Biomass Resources in Southwest China, Southwest Forestry University, Kunming, China
First published on 5th August 2021
Abstract
Carbon dots (CDs), as the most important type of carbon-based material, have been widely used in many fields because of their excellent properties. In particular, multicolor fluorescent CDs with high photoluminescence quantum yield are the focus of active research. Herein, red, green and blue CDs (RGB CDs) were successfully synthesized by a solvothermal method from o-phenylenediamine under different reaction conditions. The RGB-CDs have stable optical properties and significant photoluminescence characteristics. Structural and elemental analyses propose a conjugated structure and the surface state of the CDs as the main causes for the different color emission of RGB-CDs. In addition, a white fluorescent CD solution was prepared by mixing these multicolor fluorescent CDs in appropriate proportions.
Introduction
Since the discovery of Carbon Dots (CDs) in 2004, great progress has been made in their applications including in solar cells, photocatalysis, sensing detection, and biomedical applications.1,2 Recently, to construct intelligent photoelectric systems, there has been an increasing demand for improving the functional characteristics of CDs in photoelectric devices.3 For instance, applying CDs in optoelectronic devices results in tunable PL emissions, modified surface structure, chemical doping, and solid–liquid PL.4,5 To obtain superior CDs, efforts should be devoted to the design and synthesis of multicolour fluorescent CDs.Trichromatic luminescence and high PLQYs are essential to realize full-color display. The CDs with visible spectral emission can be obtained through a one pot pyrolysis method by adjusting the precursor or reaction conditions.6–10 Lin et al.11 developed a variety of colored fluorescent CDs using different precursors, and differences in particle size and nitrogen content were proposed to result in red-shifted emission. The emission wavelength of CDs changed from 426 to 603 nm by changing the precursors from (o-, m-, p-) phenylenediamine, and tricolour CDs were prepared. Bai et al.12 used citric acid and urea as precursors, and the luminescence of CDs changed from blue and green to red when the solvent was used water, ethanol, and dimethylformamide (DMF), respectively. Detailed characterization has shown that the eigenstates and surface states associated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N are derived from the multistate absorptions and blue, green, and red emission of CDs. Xiong and Yang's team13 used 3,5-diaminobenzoic acid and 3,4-diaminobenzoic acid to obtain red, green, and blue (RGB) color-emitting CDs (RGB-CDs) via a one-pot solvothermal route. The conjugated graphite structure of the carbon cores and the presence of oxygen on the surface states were the main causes for the RGB color emissions of CDs. However, at present, there are still many problems, such as the synthesis method is relatively complex and the source of raw materials is limited. Therefore, developing simple methods to prepare polychromatic CDs is still a great challenge.
N are derived from the multistate absorptions and blue, green, and red emission of CDs. Xiong and Yang's team13 used 3,5-diaminobenzoic acid and 3,4-diaminobenzoic acid to obtain red, green, and blue (RGB) color-emitting CDs (RGB-CDs) via a one-pot solvothermal route. The conjugated graphite structure of the carbon cores and the presence of oxygen on the surface states were the main causes for the RGB color emissions of CDs. However, at present, there are still many problems, such as the synthesis method is relatively complex and the source of raw materials is limited. Therefore, developing simple methods to prepare polychromatic CDs is still a great challenge.
In this study, we report the preparation of multicolour luminescent CDs using a simple one-pot pyrolysis method. By using o-phenylenediamine as the reaction precursor, adding reaction auxiliaries, and changing the reaction conditions, red, green, and blue luminescent CDs were obtained. The low cost of the precursor is of great significance for further developing the electro-optical application and the batch synthesis of CDs. The three CDs showed excellent and stable PL performance in solutions. Furthermore, a stable white fluorescent CDs solution was prepared by mixing the three CD solutions in appropriate proportions.
Materials and methods
Materials
o-Phenylenediamines (99%), ethanol (99.7%), potassium hydrogen sulfate (99.0%) and methylene chloride (99%) were provided by Shanghai Titan Scientific Co., Ltd (Shanghai, China). All reagents were used as received without further purification unless otherwise specified. Deionized (DI) water was used throughout this study.Methods
Transmission electron microscopy (TEM) images was carried out using a FEI Tecnai G2 F20 operating at an acceleration voltage of 200 kV. UV-Vis spectra were recorded with a Shimadzu UV-2600 spectrometer. Fluorescence measurements were collected using a Shimadzu fluorescence spectrophotometer RF-6000. Nanosecond fluorescence lifetime experiments were performed by the time correlated single-photon counting (TCSPC) system (HORIBA Scientific iHR 320). A 290 nm (<1 ns) and a 485 nm (<200 ps) nano-LED light source were used to excite the samples. CIE chromaticity coordinate was measured by KONICA MINOLTA CS-150 colorimeter. The Fourier transform infrared (FT-IR) spectra were obtained in transmission mode on a Thermo Scientific Nicolet iS5 spectrometer (Waltham, MA, USA) with the KBr pellet technique, and 8 scans at a resolution of 1 cm−1 were accumulated to obtain one spectrum. X-ray photoelectron spectroscopy (XPS) was investigated by using K-Alpha spectrometer with a mono X-ray source Al Kα excitation (1486.6 eV). Binding energy calibration was based on C 1s at 284.8 eV.Quantum yield measurements
Absolute PLQY measurements were performed in a FLS1000 spectrometer equipped with a calibrated integrating sphere. We conducted the test light from a FLS1000 spectrometer to the sphere. The PLQY was determined by the ratio between photons emitted and absorbed by CDs. The aqueous solution of CDs was placed in a cuvette to measure its QY, while the solvent water was used as a blank sample for the reference measurement.Synthesis of R-CDs, G-CDs and B-CDs
o-Phenylenediamine (0.5 g or 2.0 g) was dissolved in 10 mL ethanol, and then the solutions was transferred into poly(tetrafluoroethylene)-lined autoclaves. After heating at 180 °C or 230 °C in oven for 12 h and cooling down to room temperature naturally, green and blue fluorescent suspensions were obtained from carbonized at different concentration and temperatures, respectively. In addition, potassium bisulfate (0.1 g) mixed with o-phenylenediamine (0.5 g) was dissolved in 10 mL ethanol and the solution was transferred to a poly(tetrafluoroethylene)-lined autoclaves. After heating at 180 °C in oven for 12 h and cooling down to room temperature naturally, red suspensions were obtained. The crude products were then purified with a silica column chromatography using mixtures of methylene chloride and ethanol as eluents. The process was repeated three times to remove excess impurities and unreacted precursors. After removing solvents and further drying under vacuum, the three purified R-CDs, G-CDs and B-CDs could be finally obtained in 10–20 wt% yields.Results and discussion
The multicolor CDs were prepared via the solvothermal route based on the reaction of only o-phenylenediamine under different reaction conditions (Fig. 1). Although Jiang et al. reported RGB emitting CDs by carbonizing different substituted phenylenediamine (o-, m-, p-) as precursors at 180 °C, we tried to find a catalyst that can activate phenylenediamine, to obtain different fluorescent color CDs more conveniently.14 We have tried to use strong acid such as hydrochloric acid as catalyst to active amino group for (o-, m-, p-) phenylenediamine. The o- and m-phenylenediamine showed red light-emitting after carbonization, but after adjusting the pH value to 7, the fluorescence color changed from red to green. After trying a lot of catalysts (such as copper sulfate, titanium isopropylate, pyrolidine, potassium hydrogen sulfate), we found that o-phenylenediamine can be used to prepare CDs with stable red light-emitting at 180 °C under potassium hydrogen sulfate as the catalysis. In the optimization of concentration of precursors and temperature of carbonization, we found that blue fluorescent CDs could be obtained at higher concentration and reaction temperature without catalyst. At relatively low concentration, o-phenylenediamine can only produce green fluorescent CDs, which is consistent with the research of Jiang et al. The resulting CDs can be dispersed in various common organic solvents to obtain clear solutions, but they are insoluble in water. For example, clear RGB emitting solutions were formed when the CDs were dispersed in ethanol under UV irradiation (λex = 365 nm; Fig. 1).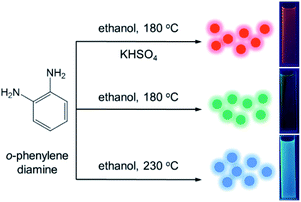 | ||
| Fig. 1 Preparation of R-CD, G-CD, and B-CD tricolor CDs from o-phenylenediamine using different conditions. Photographs of tricolor dispersed in ethanol under λ = 365 nm UV irradiation. | ||
Transmission electron microscopy (TEM) was performed to examine the morphologies of the as-prepared samples. As shown in Fig. 2a–c, TEM images demonstrate that the R-CDs, G-CDs, and B-CDs were monodispersed and had similar sizes of ca. 2.5 nm. The high-resolution TEM images (Fig. 2d–f) of the samples show well-resolved lattice fringes of graphite carbon with an interplanar spacing of 0.19 nm.15,16 Obviously, the similar particle size of the three samples suggests that the quantum size effect was not the dominant mechanism responsible for the occurrence of chromatic emissions.17
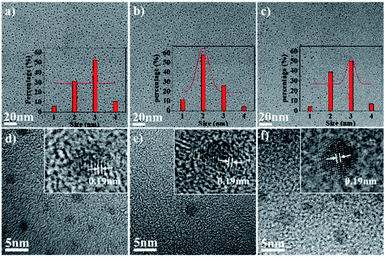 | ||
| Fig. 2 (a–c) TEM images of R-CD, G-CD, and B-CD. Inset: histograms and Gauss fittings of particle size distribution and (d–f) enlarged TEM images of R-CD, G-CD, and B-CD. | ||
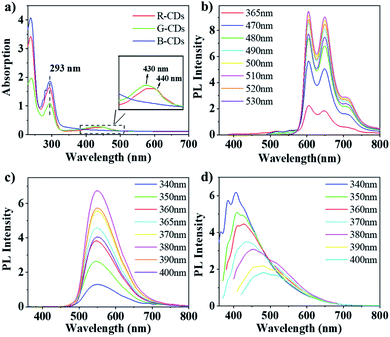
The UV/Vis absorption spectra of the CDs were measured in ethanol, as shown in Fig. 3a. In the UV region, significant absorption peak is observed at 293 nm for three CDs, can be assigned to the intrinsic state (n–π*) transition from the aromatic sp2 domains (C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).18 However, in the lower-energy region, three CDs show distinct absorption behavior. The absorption peak of 440 nm is shown for G-CDs, which is consistent with the literature.19 The absorption peak of R-CDs red-shift by 10 nm compared to that of G-CDs, which means that a longer conjugation system is formed. In contrast, B-CDs not show absorption in the lower-energy region, which indicate that there is no large conjugation structure.
O).18 However, in the lower-energy region, three CDs show distinct absorption behavior. The absorption peak of 440 nm is shown for G-CDs, which is consistent with the literature.19 The absorption peak of R-CDs red-shift by 10 nm compared to that of G-CDs, which means that a longer conjugation system is formed. In contrast, B-CDs not show absorption in the lower-energy region, which indicate that there is no large conjugation structure.
Fig. 3b–d show the PL emission (PL) spectra of the three CDs recorded in ethanol solution, in which the emission maxima of R-CDs, G-CDs, and B-CDs can be observed at λ = 600, 541, and 406 nm, respectively. The emission spectra of B-CDs exhibit classical excitation-dependent properties, namely, red-shifting of the emission peaks with increasing excitation wavelength (Fig. 3d). According to previous reports, this shifting can be assigned to surface-defect emission.20 According to previous reports, this shifting can be assigned to intrinsic and defect emission the spectra of B-CDs present an obvious blue-light emission under UV excitation (320–380 nm), with a peak at 406 nm and a PLQY of 10.0% under excitation at 340 nm. In contrast, the emission of G-CDs and R-CDs exhibit an excitation-independent feature. For G-CDs (Fig. 3c), intense green emissions are observed, forming a wide emission band cantered at 541 nm under excitations at 380–440 nm, and a PLQY of 10.5% was obtained at an excitation of 400 nm. Meanwhile, the R-CDs excited at 490–560 nm show two fluorescence peaks at 600 and 650 nm, as can be seen from the corresponding PL spectrum (Fig. 3b). A PLQY of 9.5% for the red-light emission was achieved under an excitation of 500 nm. The emission peak remained unchanged when changing the excitation wavelength, which differs from B-CDs emission, this can be attributed to nitrogen organic fluorophores, the so-called molecular states emission.20–22
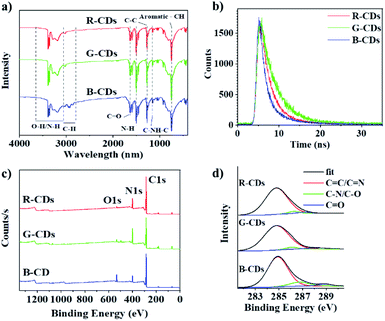
Fourier transform infrared (FT-IR) spectroscopies were conducted to characterize the surface functional groups of the samples. As shown in Fig. 4a, the three CDs exhibit similar FT-IR spectra, indicating their similar chemical compositions. R-CD, G-CD, and B-CD possess abundant hydrophilic groups according to the O–H and N–H stretching vibrations at 3050–3650 cm−1, ensuring good solubility in polar organic solvents.16,23 Other peaks corresponding to the functional groups, such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1630 cm−1), H–N (1500 cm−1), C
O (1630 cm−1), H–N (1500 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1270 cm−1), C–NH–C (1160 cm−1), C–C (1030–1060 cm−1), were also observed, which can be partially attributed to the stretching vibrations of the bonds between C, N, and O in the aliphatic group. Moreover, the origin of the distinct chromatic emissions was confirmed by determining the PL lifetimes (Fig. 4b). Average lifetimes of 3.6, 4.4, and 2.6 ns were obtained in ethanol for the red, green, and blue emissions. The corresponding error bars of these CDs are shown in Fig. S1.† The PL lifetime of B-CDs is much shorter than that of RG-CDs, indicating that they have different PL mechanisms between B-CDs and RG-CDs.25
C (1270 cm−1), C–NH–C (1160 cm−1), C–C (1030–1060 cm−1), were also observed, which can be partially attributed to the stretching vibrations of the bonds between C, N, and O in the aliphatic group. Moreover, the origin of the distinct chromatic emissions was confirmed by determining the PL lifetimes (Fig. 4b). Average lifetimes of 3.6, 4.4, and 2.6 ns were obtained in ethanol for the red, green, and blue emissions. The corresponding error bars of these CDs are shown in Fig. S1.† The PL lifetime of B-CDs is much shorter than that of RG-CDs, indicating that they have different PL mechanisms between B-CDs and RG-CDs.25
X-ray photoelectron spectroscopy (XPS) measurements were performed to gain more insight into the chemical bond composition. As shown in Fig. 4c, the XPS survey spectra of different CD samples demonstrate three main peaks at 285.0, 399.0, and 532.0 eV, respectively, corresponding to C 1s, N 1s, and O 1s, indicating that all the CDs mainly comprise C, N, and O. Table 1 shows the proportions of the three elements in R-CD, G-CD, and B-CD. As can be seen, the element proportions differ appreciably between different samples with B-CD having the highest O 1s intensity, this may be attributed to the fact that B-CD are prepared at relatively higher carbonization temperatures. The high-resolution XPS spectra (Fig. 4d and Table 1) of C 1s confirm the existence of sp2 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C
C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds (284.8 eV), C–N/C–O bonds (286.3 eV), and C
N bonds (284.8 eV), C–N/C–O bonds (286.3 eV), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds (288.2 eV). The proportion of the C
O bonds (288.2 eV). The proportion of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C
C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N components increased from B-CDs to G-CDs to R-CDs, which signify more sp2 carbon structure are formed.
N components increased from B-CDs to G-CDs to R-CDs, which signify more sp2 carbon structure are formed.
| R-CD | G-CD | B-CD | |
|---|---|---|---|
| C 1s | 81.5% | 75.2% | 79.7% |
| O 1s | 2.6% | 2.9% | 9.7% |
| N 1s | 15.9% | 21.9% | 10.6% |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
93.0% | 92.0% | 85.0% |
| C–N/C–O | 4.0% | 4.0% | 10.0% |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
3.0% | 4.0% | 5.0% |
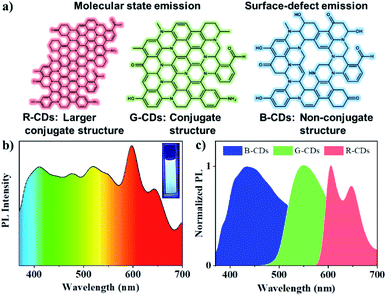
According to previous reports, the solvothermal route of phenylenediamine can form polyaniline fluorophore generally,24 like as formation of G-CDs in this study. The addition of potassium hydrogen sulfate can activate the amino group and enhance the solvothermal reaction activity, which may form a phenylenediamine with larger conjugate domain than that of G-CDs, like as formation of R-CD. However, at higher reactant concentration and reaction temperature, phenylenediamine may not be able to slowly form a thermodynamically stable polyaniline, can only form a kinetically stable molecule, resulting in the lack of conjugated structure in the interiors of the CDs. In B-CDs, high degrees of oxidation leading to rich surface-defect density, electrons and holes radiatively recombine under illumination by excitation light, causing blue photoluminescence (Fig. 5a). The oxygen-containing functional group such as C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and O
O, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH in B-CDs surface produce many distinct energy levels, with the fluorescent emission then depending on excitation wavelength.25,26 Therefore, we speculate that the fluorescence of B-CDs originates from the surface-defect state, and the fluorescence of R- and G-CDs originate the molecular state.
C–OH in B-CDs surface produce many distinct energy levels, with the fluorescent emission then depending on excitation wavelength.25,26 Therefore, we speculate that the fluorescence of B-CDs originates from the surface-defect state, and the fluorescence of R- and G-CDs originate the molecular state.
In order to explore the stability of the fluorescence of CDs, we studied its spectral changes in different visible and ultraviolet light continuous irradiation time (Fig. S2†), different temperatures (Fig. S3†) and different polar solvent environment (Fig. S4†). The maximum attenuation rate of the fluorescence intensity of the CDs under visible light for 7 days is less than 15%. Meanwhile, the attenuation rate of the fluorescence intensity of the CDs is less than 20% after 8 hours of continuous ultraviolet light (λ = 365 nm) (Fig. S5a and b†). When the temperature of the CDs reaches 80 °C the fluorescence attenuation rate of the CDs also has not more than 20% (Fig. S5c†). The CDs show some stability in different polar solvents, especially B-CDs, whose emission peak ranges within 10 nm, and the fluorescence ranges of G-CDs and R-CDs are 30 nm and 50 nm. The results show that RGB-CDs have good stability which makes them have great potential for sustainable applications in lighting equipment.
One application of RGB-CDs is to achieve white glow. Considering the liquid fluorescence stability of CDs, we prepared 1 mg mL−1 solutions of the tricolored CDs, and a stable white fluorescent CDs solution was obtained by mixing the solutions in a R-CDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G-CDs
G-CDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B-CDs ratio of 1
B-CDs ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Fig. 5b). Under UV light of 365 nm, their emission maxima locate at 415, 550, and 605 nm, respectively, almost the same with those of the original CDs (Fig. 5c). The 1931 CIE chromaticity coordinate of the white fluorescent CDs was (0.38, 0.37) (Fig. S6†).
2 (Fig. 5b). Under UV light of 365 nm, their emission maxima locate at 415, 550, and 605 nm, respectively, almost the same with those of the original CDs (Fig. 5c). The 1931 CIE chromaticity coordinate of the white fluorescent CDs was (0.38, 0.37) (Fig. S6†).
Conclusions
In summary, we developed a simple method to synthesize red, green, and blue luminescent CDs via the solvothermal method by changing the reaction conditions. The three CDs showed bright RGB luminescence in a solution. Under the optimal excitation wavelength, the fluorescence QY of R-CDs, G-CDs, and B-CDs reached 9.5%, 10.5%, and 10.0%, respectively. The different PL emissions of RGB-CDs can be attributed to the differences in the conjugate structure and surface state. This study provides a new method for the preparation of RGB-emitting CDs and a new way of thinking for developing low-cost, environmentally friendly, and high-performance white LEDs.Author contributions
Conceptualization, Y. A. and C. L.; methodology, Y. A. and X. L.; writing—original draft preparation, Y. A.; writing—review and editing, K. X., X. C., Y. L., and Y. Z.; visualization, X. L.; supervision, C. W.; project administration, Y. Z.; funding acquisition, C. L. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. There are no conflicts to declare.Acknowledgements
This work was partially supported by the National Natural Science Foundation (No. 21961036, 31660179, and 31960297). The study also was supported by the project of Yunnan Province (No. 202101AT070041 and 202002AA10007) and Key Laboratory for Forest Resources Conservation and Utilization in the Southwest Mountains of China No. KLESWFU-201908.Notes and references
- S. Diao, X. Zhang, Z. Shao, K. Ding, J. Jie and X. Zhang, Nano Energy, 2017, 31, 359–366 CrossRef CAS.
- F. Guo, X. Huang, Z. Chen, H. Sun and W. Shi, Sep. Purif. Technol., 2020, 253, 117518 CrossRef CAS.
- Z. Zhang, Y. Lei, X. Yang, N. Shi, L. Geng, S. Wang, J. Zhang and S. Shi, J. Mater. Chem. B, 2019, 7, 2130–2137 RSC.
- J. Xu, Y. Miao, J. Zheng, H. Wang, Y. Yang and X. Liu, Nanoscale, 2018, 10, 11211–11221 RSC.
- H. Wang, C. Sun, X. Chen, Y. Zhang, V. L. Colvin, Q. Rice, J. Seo, S. Feng, S. Wang and W. Y. William, Nanoscale, 2017, 9, 1909–1915 RSC.
- J. R. Adsetts, R. Zhang, L. Yang, K. Chu, J. M. Wong, D. A. Love and Z. Ding, Front. Chem., 2020, 8, 580022 CrossRef CAS.
- J. Zhu, X. Bai, J. Bai, G. Pan, Y. Zhu, Y. Zhai, H. Shao, X. Chen, B. Dong and H. Zhang, Nanotechnology, 2018, 29, 085705 CrossRef.
- T. Zhang, F. Zhao, L. Li, B. Qi, D. Zhu, J. Lu and C. Lü, ACS Appl. Mater. Interfaces, 2018, 10, 19796–19805 CrossRef CAS PubMed.
- C. Sun, Y. Zhang, Y. Wang, W. Liu, S. Kalytchuk, S. V. Kershaw, T. Zhang, X. Zhang, J. Zhao, W. W. Yu and A. L. Rogach, Appl. Phys. Lett., 2014, 104, 261106 CrossRef.
- Y. F. Yuan, Z. Wang, X. Li, Y. Li, Z. A. Tan, L. Fan and S. Yang, Adv. Mater., 2017, 29, 1604436 CrossRef PubMed.
- K. Jiang, S. Sun, L. Zhang, Y. Lu, A. Wu, C. Cai and H. Lin, Angew. Chem., 2015, 127, 5450–5453 CrossRef.
- J. Zhu, X. Bai, X. Chen, Z. Xie, Y. Zhu, G. Pan, Y. Zhai, H. Zhang, B. Dong and H. Song, Dalton Trans., 2018, 47, 3811–3818 RSC.
- K. Zhao, X. Zheng, H. Zhang, M. Xu, S. Wang, Q. Yang and C. Xiong, J. Alloys Compd., 2019, 793, 613–619 CrossRef CAS.
- T. Guo, B. H. Yuan and W. J. Liu, Org. Biomol. Chem., 2018, 16, 57–61 RSC.
- X. Chen, X. Bai, C. Sun, L. Su, Y. Wang, Y. Zhang and W. Y. William, RSC Adv., 2016, 6, 96798–96802 RSC.
- S. Qu, D. Zhou, D. Li, W. Ji, P. Jing, D. Han, L. Liu, H. Zeng and D. Shen, Adv. Mater., 2016, 28, 3516–3521 CrossRef CAS.
- H. Ding, S. B. Yu, J. S. Wei and H. M. Xiong, ACS Nano, 2016, 10, 484–491 CrossRef CAS PubMed.
- X. Wang, Y. Feng, P. Dong and J. Huang, Front. Chem., 2019, 7, 671 CrossRef CAS.
- X. T. Zheng, A. Ananthanarayanan, K. Q. Luo and P. Chen, Small, 2015, 11, 1620–1636 CrossRef CAS.
- T. Zhang, J. Zhu, Y. Zhai, H. Wang, X. Bai, B. Dong, B. Dong, H. Wang and H. Song, Nanoscale, 2017, 9, 13042–13051 RSC.
- S. Zhu, Y. Song, X. Zhao, J. Shao, J. Zhang and B. Yang, Nano Res., 2015, 8, 355–381 CrossRef CAS.
- J. Schneider, C. J. Reckmeier, Y. Xiong, M. von Seckendorff, A. S. Susha, P. Kasák and A. L. Rogach, J. Phys. Chem. C, 2017, 121, 2014–2022 CrossRef CAS.
- Y. Zhai, X. Bai, H. Cui, J. Zhu, W. Liu, T. Zhang, B. Dong, G. Pan, L. Xu and S. Zhang, Nanotechnology, 2017, 29, 025706 CrossRef PubMed.
- C. Tan, X. Su, C. Zhou, B. Wang, Q. Zhan and S. He, RSC Adv., 2017, 7, 40952–40956 RSC.
- L. Ai, Y. Yang, B. Wang, J. Chang, Z. Tang, B. Yang and S. Lu, Sci. Bull., 2021, 66, 839–856 CrossRef.
- J. Douda, C. R. González-Vargas, I. I. Mota-Díaz, E. V. Basiuk, X. A. Hernández-Contreras, J. A. Fuentes-García, J. Bornacelli and C. Torres-Torres, Nano Express, 2020, 1, 030009 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02298a |
| This journal is © The Royal Society of Chemistry 2021 |