Open Access Article
Open Access ArticleThermal stability and oxidation characteristics of α-pinene, β-pinene and α-pinene/β-pinene mixture
Pin Liub,
Xiongmin Liu *a,
Tei Saburic,
Shiro Kubotac,
Pinxian Huanga and
Yuji Wadac
*a,
Tei Saburic,
Shiro Kubotac,
Pinxian Huanga and
Yuji Wadac
aCollege of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, China. E-mail: xmliu1@gxu.edu.cn; Fax: +86 771 3237018; Tel: +86 138 77136730
bDepartment of Science and Technology, Guangxi University for Nationalities, Nanning 530006, China
cNational Institute of Advanced Industrial Science and Technology, Tsukuba 3058569, Japan
First published on 8th June 2021
Abstract
Turpentine is a renewable resource, has good combustion performance, and is considered to be a fuel or promising additive to diesel fuel. This is very important for the investigation of thermal stability and energy oxidation characteristics, because evaluation of energy or fuel quality assurance and use safety are necessary. The main components of turpentine are α-pinene and β-pinene, which have unsaturated double bonds and high chemical activity. By investigating their thermal stability and oxidation reaction characteristics, we know the chemical thermal properties and thermal explosion hazard of turpentine. In this present study, the thermal stability and oxidation characteristics of α-pinene, β-pinene and α-pinene/β-pinene mixture were investigated using a high sensitivity accelerating rate calorimeter (ARC) and C80 calorimeter. The important parameters of oxidation reaction and thermal stability were obtained from the temperature, pressure and exothermic behavior in chemical reaction. The results show that α-pinene and β-pinene are thermally stable without chemical reaction under a nitrogen atmosphere even when the temperature reaches 473 K. The initial exothermic temperature of the two pinenes and their mixture is 333–338 K, and the heat release (−ΔH) of their oxidation is 2745–2973 J g−1. The oxidation activation energy (Ea) of α-pinene, β-pinene and α-pinene/β-pinene mixture is 116.25 kJ mol−1, 121.85 kJ mol−1, and 115.95 kJ mol−1, respectively. There are three steps in the oxidation of pinenes: the first is the induction period of the oxidation reaction; the second is the main oxidation stage, and the pressure is reduced; the third is thermal decomposition to produce gas.
1. Introduction
Turpentine is a renewable plant resource, with a productivity of around 330![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year in world, and 220
000 tons per year in world, and 220![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year in China at 2016 (China report network, http://data.chinabaogao.com/jiancai/2018/03S23M92018.html). The main components of turpentine are α-pinene and β-pinene, which are widely used in chemical raw materials, pharmaceutical synthesis, perfume, cosmetics and other fields.1 In recent years, as renewable energy and fuel resources, it has been widely investigated. Saravanan et al. conducted an investigation on performance and exhaust emission of a turpentine oil powered direct injection diesel engine.2 Vallinayagam et al. reported the performance and emission characteristics of pine oil in a diesel engine.3 Mokrani et al. reported the thermal degradation of α-pinene and β-pinene investigated using TGA and GC-MS.4 Kinetic parameters are extracted using three different methods (Kissinger–Akahira–Sunose (KAS), Flynn–Wall–Ozawa (FWO) and Friedman), and activation energies for these α-pinene and β-pinene are given. The main thermal degradation products of α- and β-pinene were analyzed under an air atmosphere. There have been many reports on the effect of turpentine as an additive on fuel,5,6 and the effect of emissions on the environment.7,8 Their research results show that the combustion performance of turpentine is good, and the emissions of HC, CO, PM and NOx are slightly reduced. Reports of turpentine as a high-density fuel are very interesting. For example, the research work of Heather et al. has potential applications as significant components of jet, diesel, and tactical fuels.9 Yuan et al.10 and Nie et al.11 synthesized high energy density fuel from turpentine.
000 tons per year in China at 2016 (China report network, http://data.chinabaogao.com/jiancai/2018/03S23M92018.html). The main components of turpentine are α-pinene and β-pinene, which are widely used in chemical raw materials, pharmaceutical synthesis, perfume, cosmetics and other fields.1 In recent years, as renewable energy and fuel resources, it has been widely investigated. Saravanan et al. conducted an investigation on performance and exhaust emission of a turpentine oil powered direct injection diesel engine.2 Vallinayagam et al. reported the performance and emission characteristics of pine oil in a diesel engine.3 Mokrani et al. reported the thermal degradation of α-pinene and β-pinene investigated using TGA and GC-MS.4 Kinetic parameters are extracted using three different methods (Kissinger–Akahira–Sunose (KAS), Flynn–Wall–Ozawa (FWO) and Friedman), and activation energies for these α-pinene and β-pinene are given. The main thermal degradation products of α- and β-pinene were analyzed under an air atmosphere. There have been many reports on the effect of turpentine as an additive on fuel,5,6 and the effect of emissions on the environment.7,8 Their research results show that the combustion performance of turpentine is good, and the emissions of HC, CO, PM and NOx are slightly reduced. Reports of turpentine as a high-density fuel are very interesting. For example, the research work of Heather et al. has potential applications as significant components of jet, diesel, and tactical fuels.9 Yuan et al.10 and Nie et al.11 synthesized high energy density fuel from turpentine.
The structure of α-pinene and β-pinene in turpentine has double bond, their chemical properties are active, and easy is to occur oxidation reaction. The conversion of α-pinene and β-pinene into perfume by oxidation reaction is one of the important application ways of turpentine.12,13 The oxidation of pinene is of great value in organic synthesis, many scientists have carried out a lot of innovative research. For example, Neuenschwander et al. investigated the properties and mechanism of autoxidation of α-pinene and β-pinene by experimental method and theoretical calculation.14,15 There have been many reports about the oxidation reaction initiated by free radicals,16–20 photooxidation reaction,19,20 ozonation reaction21,22 and oxidation kinetics23,24 of turpentine, α-pinene and β-pinene. It is very important to ensure the quality stability of turpentine when it is stored for a long time. In addition, it has no risk of explosion accidents in production and use.
In this paper, the pressure, temperature and exothermic behavior of α-pinene, β-pinene and α-pinene/β-pinene mixture have been measured by accelerating rate calorimeter (ARC) and C80 calorimeter of high sensitivity thermal analysis instrument. Their thermal stability and oxidation characteristics were investigated. And the hazardous and safety evaluation methods of their oxidation reactions have been explored. Our aim is to acquire knowledge of their reactivity, stability and risk of pinenes and turpentine. This provides a theoretical basis for the safe use of turpentine in energy and fuel.
2. Experimental
2.1. Materials
α-Pinene (purity 97.0 wt%) was obtained from the Wako Pure Chemical Corporation. β-Pinene (purity 95.0 wt%) was obtained from the Wako Pure Chemical Ltd. The O2 and N2 gases were obtained from Tomoe Shokai Co., Ltd.Accelerating rate calorimeter (ARC) and C80 calorimeter are two important instruments for evaluating hazardous materials, ARC is used to simulate the exothermic behavior and pressure behavior of hazardous materials under adiabatic condition, C80 is used to determine the reaction thermal behavior and pressure behavior of hazardous materials during the heating process. Two kinds of thermal analysis instruments were used to evaluate the thermal stability and oxidation hazardous of pinenes.
2.2. Determination of pinene oxidation process by ARC
Accelerating Rate Calorimeter (ARC) (Arthur D. Little, Inc. USA, ARC 2000) has been widely used in the safety evaluation of the hazardous chemicals.25 In recent years, it has also been used to evaluate the safety of oxidation reaction in our work.26,27 Ren et al. reported model-based thermal runaway prediction of lithium-ion batteries from kinetics analysis of cell components.28In this research, the experimental conditions of ARC are as follows: sample mass of α-pinene (or β-pinene, α-pinene/β-pinene mixture) in the test bomb is 0.5–1.0 g. According to the article 2 “high pressure gas” of “high pressure gas safety law” on the initial pressure is less than 1.0 MPa. Therefore the atmospheres are oxygen or nitrogen, and the initial pressure at which the experiments are 0.9 MPa. In order to observe whether pinene has rapid explosion reaction in gas phase, ARC measurement conditions are: mode: ‘heat–wait–search’ mode (HWS mode); start temperature: 25 °C; end temperature: 300 °C; temperature step: 5 °C; slope sensitivity: 0.02 K min−1; wait time: 30 min; search time: 10 min.
2.3. Determination of pinene oxidation process by C80 calorimeter
The C80 calorimeter (SETARAM Scientific & Industrial Equipment Company in France) is a heat flux calorimeter that has merits of high sensitivity. It has been widely used in the safety evaluation of dangerous chemicals,29 energy30,31 and chemical reactions.32 In this research, the experimental conditions of C80 are as follows:Sample mass of α-pinene (or β-pinene, α-pinene/β-pinene mixture) is 0.1 g, the heating rate test was set to limit the temperature increase to 0.2 °C per minute, and the temperature variation scale was set from room temperature to 300 °C. The sample cell is high pressure type, and the pressure of the reaction process is measured.
3. Results and discussion
3.1. Thermal stability of α-pinene and β-pinene under nitrogen atmosphere
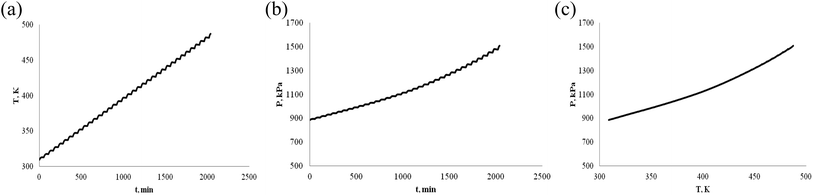
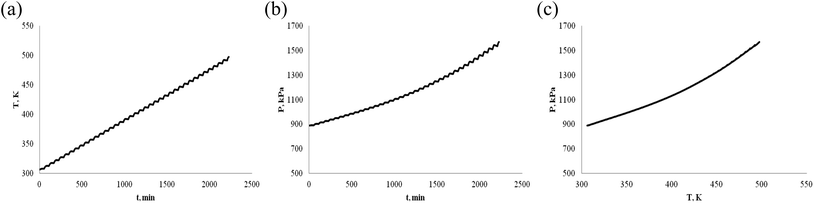
α-Pinene and β-pinene are compounds with double bonds, their structures are shown in Fig. 1. According to the structure of α-pinene and β-pinene, their chemical properties are active. As energy and fuel, it is necessary to investigate their thermal stability.First, we investigate oneself stability of α-pinene and β-pinene. ARC experiment was carried out in nitrogen atmosphere. The temperature vs. time (T–t), pressure vs. time (P–t), and pressure vs. temperature (P–T) plots of α-pinene and β-pinene are shown in Fig. 2 and 3, respectively.
 | ||
| Fig. 2 Pressure and temperature behavior of α-pinene under nitrogen atmosphere. (a) Plot of temperature vs. time. (b) Plot of pressure vs. time. (c) Plot of pressure vs. temperature. | ||
 | ||
| Fig. 3 Pressure and temperature behavior of β-pinene under nitrogen atmosphere. (a) Plot of temperature vs. time. (b) Plot of pressure vs. time. (c) Plot of pressure vs. temperature. | ||
ARC is a highly sensitive experimental device for the determination of exothermic reaction, when chemical exothermic occurs, there will be a mutation point in the thermal effect curve T–t. Fig. 2(a) and 3(a) shows that the relationship between temperature and time is linear, exothermic heat of α-pinene and β-pinene has not been detected by ARC, that is to say, there is no chemical reaction of α-pinene and β-pinene under nitrogen atmosphere.
The P–T curve of ARC is important for investigating the explosion risk of chemical reaction. When hazardous chemicals undergo rapid thermal decomposition reaction, a large number of gases will be produced and the pressure will increase rapidly. Fig. 2(b) and 3(b) shows that none of α-pinene and β-pinene were detected to reduce of pressure or thermally decompose (increase of pressure) the gas produced. That is to say, exothermic reaction of α-pinene and β-pinene was not detected even at temperatures above 230 °C, they are very stable under nitrogen atmosphere. Both Fig. 2 and 3 show that the α-pinene and β-pinene are stable without chemical reaction under nitrogen atmosphere.
Mokrani's results showed that two pinenes were pyrolyzed in nitrogen atmosphere, and their activation energies were calculated. But no exothermic reaction between α-pinene and β-pinene was observed in ARC. The reasons may be as follows: (1) the amount of pinene reaction is small; (2) the isomerization of pinene is the main reaction, and the exothermic amount of pinene isomerization is less.
In Fig. 2(c) and 3(c) (or 2(b) and 3(b)), the relationship between pressure and time (or pressure and temperature) is not very linear, they are slightly curved, because α-pinene and β-pinene have small volatility when there is pressure. The boiling points of α-pinene and β-pinene are 428 K (155 °C) and 438 K (165 °C) at normal pressure, respectively. In the temperature range from room temperature to boiling point, the relationship between vapor pressure and temperature of α-pinene and β-pinene at normal pressure were shown in Fig. 4, and an equation type is straight line: ln(P) = A − B/T, A and B are constants.33 Compared with the P vs. T of N2, the pressure change of α-pinene and β-pinene are small. Therefore, α-pinene and β-pinene did not undergo significant exothermic decomposition reaction under nitrogen atmosphere.
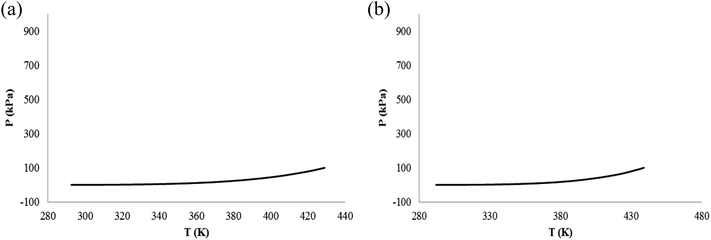 | ||
| Fig. 4 The relationship between vapor pressure and temperature of α-pinene and β-pinene at normal pressure. (a) α-Pinene. (b) β-pinene. | ||
3.2. Oxidation process behavior of α-pinene and β-pinene under an oxygen atmosphere
The thermally stable of α-pinene and β-pinene is very important for energy and fuel in the production process, storage, transportation and use. Therefore, we use ARC to observe α-pinene and β-pinene temperature and pressure behavior of oxidation process. We carried out two experiments of α-pinene (or β-pinene) of different mass, and compared their differences. When the mass of α-pinene is 0.5 g and 1.0 g, the T–t, P–t and P–T plots are shown in Fig. 5 and 6, respectively. Similarly, when the mass of β-pinene is 0.5 g and 1.0 g, the T–t, P–t and P–T plots are shown in Fig. 7 and 8, respectively.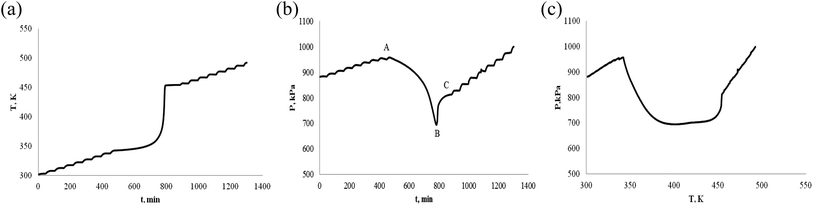 | ||
| Fig. 5 Oxidation procession behavior of α-pinene under an oxygen atmosphere (0.5 g). (a) Plot of temperature vs. time. (b) Plot of pressure vs. time. (c) Plot of pressure vs. temperature. | ||
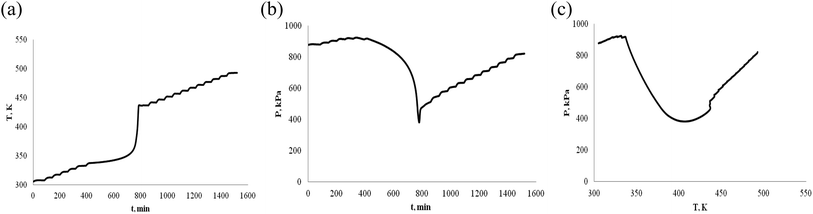 | ||
| Fig. 6 Oxidation procession behavior of α-pinene under an oxygen atmosphere (1.0 g). (a) Plot of temperature vs. time. (b) Plot of pressure vs. time. (c) Plot of pressure vs. temperature. | ||
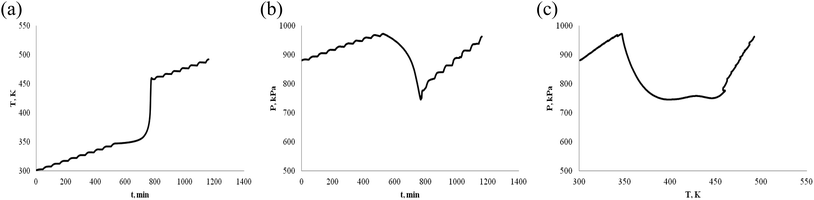 | ||
| Fig. 7 Oxidation procession behavior of β-pinene under an oxygen atmosphere (0.5 g). (a) Plot of temperature vs. time. (b) Plot of pressure vs. time. (c) Plot of pressure vs. temperature. | ||
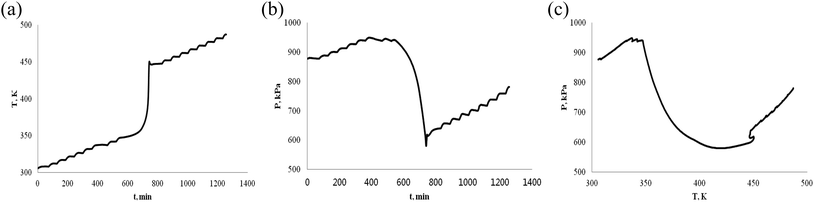 | ||
| Fig. 8 Oxidation procession behavior of β-pinene under an oxygen atmosphere (1.0 g). (a) Plot of temperature vs. time. (b) Plot of pressure vs. time. (c) Plot of pressure vs. temperature. | ||
Fig. 5–8 shows that although samples are different and their masses are different, they all have the following common characteristics:
(1) The relationship between temperature and time is not linear, has a very significant exothermic reaction by Fig. 5–8, (a) T–t plots. In other words, α-pinene (or β-pinene) is oxidized under oxygen atmosphere, because it has no exothermic reaction at room temperature to 230 °C under nitrogen atmosphere. According to Fig. 5–8, (a) we have obtained the initial exothermic temperature (T0) and the end exothermic temperature (Tend) of the oxidation, and the results are shown in Table 1.
| No. | Sample | Mass (g) | Gas atmosphere | (Tabs)0 (K) | (Tabs)end (K) | T0 (K) | Tend (K) |
|---|---|---|---|---|---|---|---|
| a Mass of α-pinene/β-pinene mixture: *0.5014 g = 0.2536 g α-pinene + 0.2478 g β-pinene; **1.0293 g = 0.2536 g α-pinene + 0.2478 g β-pinene. | |||||||
| 1 | α-Pinene | 0.5153 | N2 | No | No | No | No |
| 2 | α-Pinene | 0.5055 | O2 | 341.45 | 399.15 | 342.62 | 454.33 |
| 3 | α-Pinene | 1.0111 | O2 | 336.29 | 405.51 | 337.60 | 436.99 |
| 4 | β-Pinene | 0.4934 | N2 | No | No | No | No |
| 5 | β-Pinene | 0.5059 | O2 | 346.63 | 405.09 | 347.61 | 460.07 |
| 6 | β-Pinene | 0.9876 | O2 | 336.78 | 423.84 | 337.57 | 450.37 |
| 7 | α-Pinene/β-pinene | 0.5014* | O2 | 337.03 | 398.35 | 337.77 | 451.09 |
| 8 | α-Pinene/β-pinene | 1.0293** | O2 | 337.12 | 420.85 | 337.64 | 443.48 |
(2) It is interesting to investigate oxidation reaction by P–t or P–T plot. Compared with the arc experimental results of ether energy,34,35 although the exothermic T–t curves are similar, the pressure behavior curves (P–t) are quite different. The P–t curve of ethers results show that no significant thermal decomposition reaction occurs under nitrogen, while under an oxygen atmosphere, the pressure increases rapidly due to thermal decomposition. That is, the heat decomposition reaction produces a large amount of gas.
The oxidation of α-pinene and β-pinene were studied at 363 K and 0.1 MPa of pure O2 by Hermans.36,37 The results show that the evolution of the a-pinene conversion as a function of time. It can be observed how after an induction period the conversion starts to increase, and the products of oxidation reaction are mainly oxygenated compounds, decomposition into gaseous small molecule products is not the main reaction. Therefore, the decrease of pressure is due to the consumption of oxygen by the reaction of pinenes with oxygen in ARC experiment. The P–t and P–T plots of Fig. 5–8, (b) show that the oxidation of α-pinene (or β-pinene) consumes oxygen and reduces the pressure from A to B. We think that the pinene oxidation is carried out according to r1. From B to C, there is a small increase in pressure, is a small thermal decomposition reaction. But, there is no significant thermal decomposition in the whole oxidation process. The temperature at start of pressure reduction is called the temperature at which oxygen reduction ((Tabs)0). The temperature ((Tab)end) is the temperature at the end of oxygen absorption of pinene. According to Fig. 5–8, (b) and (c), we obtain (Tabs)0 and (Tab)end are shown in Table 1.
(3) The oxidation reaction of pinene may occur in the liquid phase, because the temperature of oxidation reaction is low, and the boiling points of α-pinene and β-pinene are high.
(4) Table 1 shows that the temperature (Tabs)0 of oxygen reduction is lower than the exothermic temperature T0 in oxidation process, that is (Tabs)0 < T0. The possible reason is that oxygen first dissolves into the liquid phase of pinene and then oxidizes. There are three steps in the oxidation of pinenes: the first step is the induction period of the oxidation reaction, pressure is reduced, but there is no exothermic; second step is the main oxidation stage, pressure reduction is significant and exothermic is large; third step is thermal decomposition to produce gas.
3.3. Reaction procession behavior of α-pinene/β-pinene mixture under an oxygen atmosphere
Turpentine is mainly composed of α-pinene and β-pinene, when as energy, it is necessary to investigate the thermal stability of turpentine. In order to reduce the influence of turpentine impurity, we investigated the thermal stability of α-pinene/β-pinene mixture. When the mass of α-pinene/β-pinene is 0.5 g and 1.0 g, the T–t, P–t and P–T plots are shown in Fig. 9 and 10, respectively.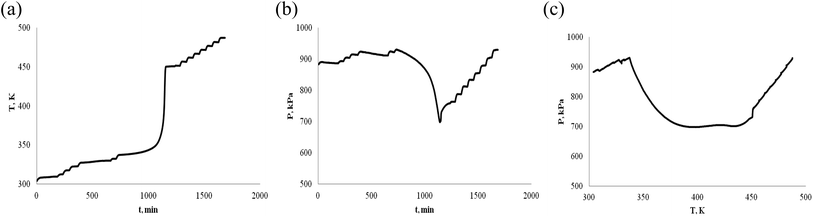 | ||
| Fig. 9 Oxidation procession behavior of α-pinene/β-pinene under an oxygen atmosphere (0.5 g). (a) Plot of temperature vs. time. (b) Plot of pressure vs. time. (c) Plot of pressure vs. temperature. | ||
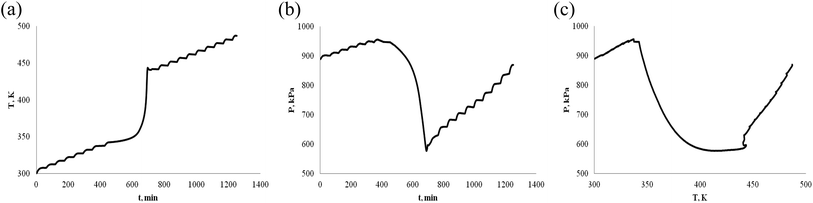 | ||
| Fig. 10 Oxidation procession behavior of α-pinene/β-pinene under an oxygen atmosphere (1.0 g). (a) Plot of temperature vs. time. (b) Plot of pressure vs. time. (c) Plot of pressure vs. temperature. | ||
According to Fig. 9 and 10, we have obtained the T0, Tend, (Tab)0 and (Tab)end are shown in Table 1. The results of Fig. 9 and 10 show that they are the same on the shape of T–t, P–t, P–T plots of α-pinene/β-pinene mixture and the shape of α-pinene (or β-pinene) plots. The data of T0, Tend, (Tab)0 and (Tab)end are not significantly different. It shows that the mixing of α-pinene and β-pinene does not bring about the difference of thermal stability, is also unstable and very prone to oxidation reactions under an oxygen atmosphere.
The initial exothermic temperature T0 is an important parameter to evaluate the safety of hazardous chemicals. When T0 is small, the thermal stability of the material is poor. Similarly, for the oxidation reaction, the initial reaction temperature T0 is low, the stability of the substance is poor, and the chemical activity is high. The oxidation temperature of α-pinene, β-pinene and α-pinene/β-pinene mixture are about 63 °C, which indicates that their oxidation activity is high and their thermal stability is poor under an oxygen atmosphere. Therefore, it is necessary to pay attention to avoid contact temperature (or pinene) with oxygen or air when products are produced, stored and transported.
3.4. The exothermic hazard of oxidation by ARC
Besides the initial exothermic temperature, the exothermic speed, exothermic rise temperature and exothermic time are also very important to evaluate the parameters of hazardous chemicals. Similarly, in order to understand the hazard of pinene oxidation, it is necessary to investigate the self-heat rate of the oxidation process. The self-heat rate (SHR) vs. temperature plots of α-pinene, β-pinene and α-pinene/β-pinene mixture are shown in Fig. 11. In Fig. 11, (a) the sample mass is 0.5 grams, (b) the sample mass is 1.0 grams, and blue dot is α-pinene, red dot is β-pinene, green dot is α-pinene/β-pinene mixture.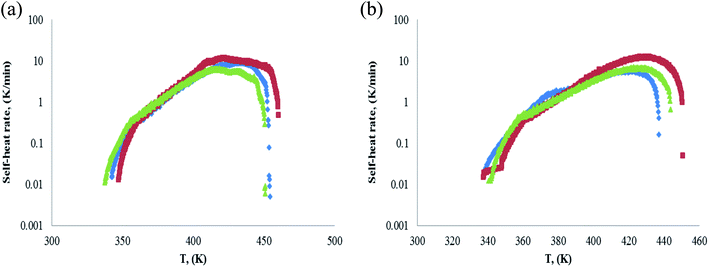 | ||
| Fig. 11 Plot of self-heat rate vs. temperature. Blue dot: α-pinene, red dot: β-pinene; green dot: α-pinene/β-pinene mixture. (a) Sample mass: 0.5 g. (b) Sample mass: 1.0 g. | ||
Fig. 11 shows that there is no significant difference in the curve shape of α-pinene, β-pinene and α-pinene/β-pinene mixture. The parameters of maximum of SHR and temperature rise value of reaction (ΔT) are important when ARC is used to evaluate the risk of hazardous chemicals. SHR is related to reaction rate and ΔT is related to explosion property.
In the experiment, ΔTobs is obtained, the relationship between ΔT and ΔTobs is shown in eqn (1):
| φ = ΔT/ΔTobs = 1 + (Mb × Cb)/(Ms × Cs) | (1) |
| Ea = R(T2 × T1/(T2 − T1)) × ln((t1 × T22)/(t2 × T12)) | (2) |
| No. | Sample | Mass (g) | Cs (J g−1 K−1) | Max. of SHR (K min−1) | Time of SHR Max. (min) | ΔTobs/(K) | φ | ΔT/(K) | Ea/(kJ mol−1) |
|---|---|---|---|---|---|---|---|---|---|
| a Mass of α-pinene/β-pinene mixture: *0.5014 g = 0.2536 g α-pinene + 0.2478 g β-pinene; **1.0293 g = 0.2536 g α-pinene + 0.2478 g β-pinene. | |||||||||
| 1 | α-Pinene | 0.5055 | 1.94 | 9.07 | 162.0 | 111.72 | 2.06 | 242.7 | 111.8 |
| 2 | α-Pinene | 1.0111 | 1.94 | 5.73 | 147.3 | 99.39 | 1.53 | 152.1 | 120.7 |
| 3 | β-Pinene | 0.5059 | 1.93 | 11.52 | 147.9 | 112.46 | 2.07 | 232.2 | 123.2 |
| 4 | β-Pinene | 0.9876 | 1.93 | 12.58 | 157.6 | 112.78 | 1.55 | 174.3 | 120.5 |
| 5 | α-Pinene/β-pinene | 0.5014* | 1.94 | 6.35 | 142.6 | 115.32 | 2.07 | 238.6 | 113.6 |
| 6 | α-Pinene/β-pinene | 1.0293** | 1.94 | 6.93 | 149.6 | 105.84 | 1.52 | 161.0 | 118.3 |
The thermal degradation of pinenes were determined by TGA under air, when calculated by KAS, FWO and Friedman methods, Ea of α-pinene are 60.84 kJ mol−1, 70.83 kJ mol−1 and 64.30 kJ mol−1, Ea of β-pinene are 52.00 kJ mol−1, 57.98 kJ mol−1 and 64.30 kJ mol−1, respectively.4 ARC experiment results show that the average Ea of α-pinene and β-pinene are similar, and there is no significant difference. The Ea value determined by ARC is larger than that determined by TGA. In the determination of TGA, there is less oxygen, and isomerization is the main reaction. In the determination of ARC, oxygen is plentiful, and oxidation is the main reaction. In addition, the activation energy of pinenes in nitrogen atmosphere was not obtained. Therefore, we think that the reaction mechanism of pinenes is different under the conditions of TG and ARC, and the activation energy is also different.
Table 2 shows that their values of exothermic rise temperature are ΔTobs = 99–113 K. The maximum of SHR of β-pinene is higher than that maximum of SHR of α-pinene, it was indicate that the chemical reactivity of β-pinene is high, and oxidation reaction is more violent. From Tables 1 and 2, when the sample mass is 0.5 g and 1.0 g, the effect on T0, maximum of SHR and ΔTobs is not very significant. However, their T0 is a small, and maximum of SHR and ΔTobs are relatively large, therefore, α-pinene, β-pinene and their mixtures are thermally unstable and hazard. The oxidation activation energy of α-pinene is (111.8 + 120.7)/2 = 116.25 kJ mol−1, oxidation activation energy of β-pinene is (123.2 + 120.5)/2 = 121.85 kJ mol−1, and oxidation activation energy of α-pinene/β-pinene is (113.6 + 118.3)/2 = 115.95 kJ mol−1. The Ea of β-pinene is a little bigger than Ea of α-pinene, and there was no significant difference between Ea of α-pinene and Ea of α-pinene/β-pinene.
3.5. Thermal characteristics of α-pinene, β-pinene and α-pinene/β-pinene oxidation by C 80 calorimeter
The results of ARC experiment show that the oxidation of α-pinene and β-pinene is easy to happen, and they are stable under nitrogen atmosphere. In order to further confirm their stability and risk of oxidation, C80 calorimeter of high sensitivity was used to determine the exothermic and pressure behavior of the reaction process. A great number of measurements can be made with C80 calorimeter, such as determination of chemical reaction process and kinetics, hazards evaluation of reactive substance and so on. As a comparative experiment, the oxidation process in nitrogen atmosphere and air atmosphere was investigated. The results of pressure (P) vs. temperature (T) plots and heat flow vs. temperature plots are shown in Fig. 12–17, respectively.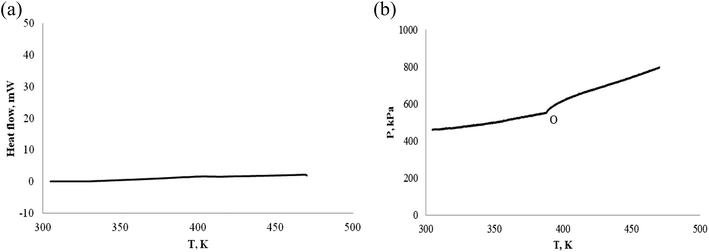 | ||
| Fig. 12 Pressure behavior and heat flow of α-pinene under nitrogen atmosphere. (a) Heat flow vs. T. (b) P vs. T. | ||
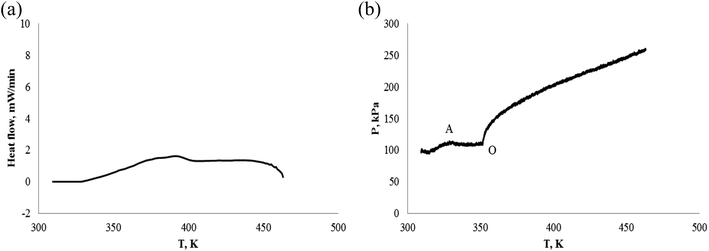
Fig. 12(a) shows that no heat absorption or exothermic of α-pinene was detected, we also did not observe a decrease of pressure from Fig. 12(b), i.e. no chemical reaction, and is stable under nitrogen atmosphere. The pressure change (at O) of Fig. 12(b) may be caused by the volatilization of α-pinene.
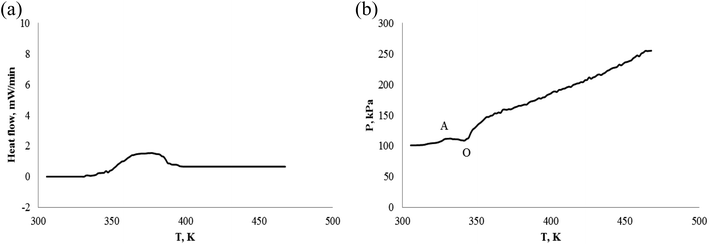
Fig. 13(a) and 15(a) show that the exothermic heat flow of α-pinene and β-pinene is very small, and their figure (b) plot also has a slight pressure reduce (from A to O) in air atmosphere, because the sample cell has less oxygen at 0.1 MPa. That is to say, the oxidation of pinene was observed even though there was little oxygen of sample cell.
Fig. 14, 16 and 17 show that their exothermic peaks are very obvious and the heat flow is very large, their pressure reduction is also very significant under an oxygen atmosphere. Therefore, the oxidation of α-pinene, β-pinene and α-pinene/β-pinene mixture with oxygen has been confirmed again. The main data of C80 are shown in Table 3. Ta or T0 is the starting temperature of exothermic, Te is the ending temperature of exothermic, Tab is the starting temperature of pressure reduction, ΔTae = (Te − Ta) is the temperature range of exothermic reaction, and −ΔH is the heat release of oxidation, Ta, T0 and Te are shown in Fig. 14(a).
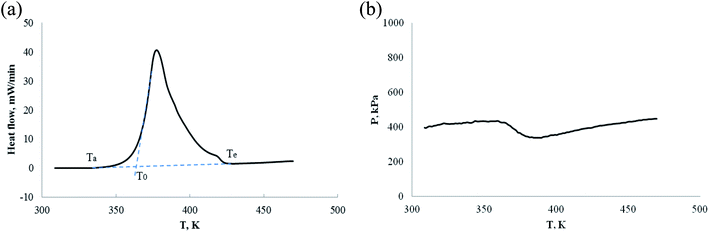 | ||
| Fig. 14 Pressure behavior and heat flow of α-pinene under an oxygen atmosphere. (a) Heat flow vs. T. (b) P vs. T. | ||
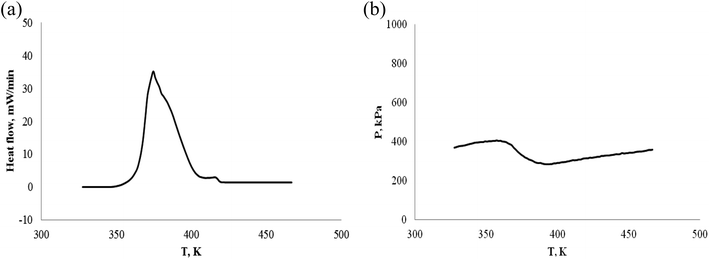 | ||
| Fig. 16 Pressure behavior and heat flow of β-pinene under an oxygen atmosphere. (a) Heat flow vs. T. (b) P vs. T. | ||
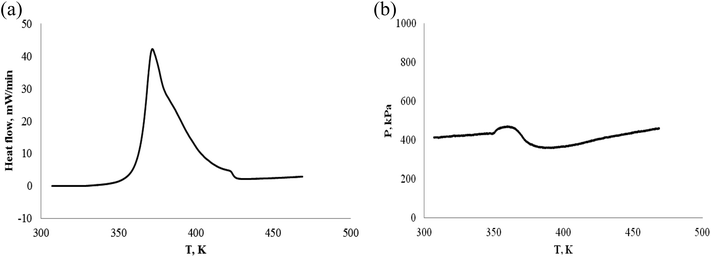 | ||
| Fig. 17 Pressure behavior and heat flow of α-pinene/β-pinene under an oxygen atmosphere. (a) Heat flow vs. T. (b) P vs. T. | ||
| No. | Sample | Mass/(g) | Gas atmosphere | Ta/(K) | Tab/(K) | To/(K) | Te/(K) | ΔTae/(K) | −ΔH/(J g−1) |
|---|---|---|---|---|---|---|---|---|---|
| a (*) Mass of α-pinene/β-pinene: 0.1038 g = 0.0523 g α-pinene + 0.0515 g β-pinene. | |||||||||
| 1 | α-Pinene | 0.1088 | N2 | No | No | No | No | 0 | |
| 2 | α-Pinene | 0.1014 | Air | 336.73 | 331.12 | — | 400.4 | 63.64 | 55.2 |
| 3 | α-Pinene | 0.1012 | O2 | 335.62 | 343.75 | 361.36 | 425.07 | 89.45 | 2940.0 |
| 4 | β-Pinene | 0.9876 | Air | 337.86 | 332.45 | — | 401.42 | 63.56 | 58.7 |
| 5 | β-Pinene | 0.1112 | O2 | 338.24 | 351.87 | 361.11 | 421.43 | 83.19 | 2745.0 |
| 6 | α-Pinene/β-pinene | 0.1038* | O2 | 336.57 | 353.78 | 361.98 | 427.30 | 90.73 | 2973.3 |
Table 3 and Fig. 12–17 shows the following results:
(1) α-Pinene did not react chemically under nitrogen atmosphere.
(2) α-Pinene and β-pinene can react chemically in cell of 0.1 MPa air atmosphere, but the amount of reaction is very small.
(3) Similar to ARC experiment results, the exothermic starting temperatures of α-pinene, β-pinene and α-pinene/β-pinene mixture oxidation are Ta = 335.6–338.2 K (62–65 °C) by C80, their oxidation temperature is low. That is to say, the thermal stability of pinene is poor under oxygen atmosphere.
(4) The three steps of oxidation process are: the first step is the induction period of the oxidation reaction, pinenes combines with oxygen, the pressure is reduced, but no significant exothermic reaction is detected. The second is the main oxidation stage, has significant exothermic reaction, but the pressure is reduced; the third is thermal decomposition to produce gas, which is characterized by exothermic reaction and significant increase of pressure.
(5) In oxygen atmosphere, heat release (−ΔH) of α-pinene, β-pinene and α-pinene/β-pinene mixture oxidation is 2745–2973 J g−1. It is well known that many nitro organic compounds and organic peroxide are hazardous materials. The exothermic value (QDSC) of their thermal decomposition is >1000 J g−1 by differential scanning calorimeter (DSC),42 for example, 2,4-dinitrotoluene QDSC is 3465 J g−1, benzoyl peroxide QDSC is 1830 J g−1. Therefore, from the perspective of exothermic evaluation, α-pinene, β-pinene and α-pinene/β-pinene mixture oxidation is hazardous.
When turpentine is used as energy and fuel, its stability is worthy of attention. The experimental results of ARC and C80 show that, in production and storage, it is important that turpentine or pinenes not come into contact with oxygen (or air) in order to prevent oxidation reactions. This is significant for understanding the chemical activity of pinenes, and the runaway of oxidation process.
3.6. Oxidation reaction and degradation mechanism of pinenes
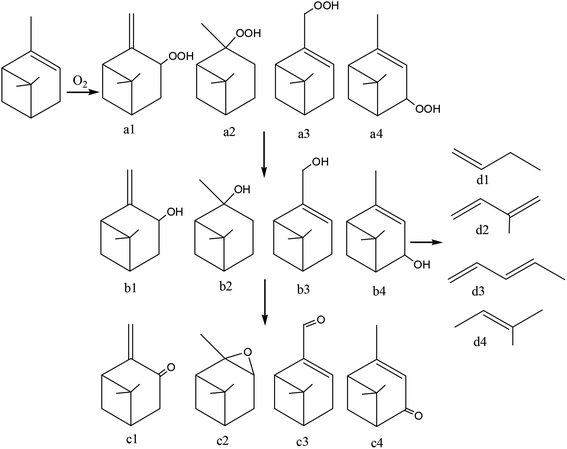
Neuenschwander14,15 reported thermal autoxidation products of α-pinene and β-pinene using a combined experimental and theoretical approach. Main products observed during the thermal oxidation of α-pinene are four types: (a) hydroperoxide: pinocarvyl-hydroperoxide (a1), pinenyl-hydroperoxide (a2), myrtenyl-hydroperoxide (a3) and verbenyl-hydroperoxide (a4); (b) alcohols: pinocarvol (b1), pinenol (b2); myrtenol (b3) and verbenol (b4); (c) ketone compounds: pinocarvone (c1), myrtenal (c3) and verbenone (c4); (d) epoxy compounds: α-pinene oxide (c2). Main products of β-pinene oxidation are: (a) hydroperoxide: pinocarvyl-hydroperoxide and myrtenyl-hydroperoxide; (b) alcohols: pinocarvol and myrtenol; (c) ketone compounds: pinocarvone and myrtenal; (d) epoxy compounds: β-pinene oxide, pinocarveoloxide and myrtenoloxide. Coudour et al. reported the mechanism of α-pinene thermal degradation using a Py-GC/MS. Degradation products are very complex, it includes isomerization, pyrolysis and degradation products, etc. In degradation products, main small molecules have: butane (d1), isoprene (d2), pentadiene (d3), 2-butene-2-methyl (d4), etc.43 Therefore, according to the oxidation reaction products and degradation products, the α-pinene oxidation reaction and degradation mechanism was shown in Fig. 18.Fig. 18 shows only part of α-pinene degradation mechanism, not all of it. The degradation mechanism of β-pinene is similar to that of α-pinene, because α-pinene and β-pinene have similar chemical structure and properties. We describe the oxidation and degradation mechanism of α-pinene and β-pinene with a simple model, which is shown in Fig. 19.
The pressure and exothermic behavior of ARC and C80 thermal analysis can be illustrated by using Fig. 19.
The process of pinene oxidation is divided into three steps as follows.
The first step is the induction period of oxidation reaction. It includes the combination of pinene and oxygen to form peroxide. It is characterized by a decrease in pressure in the reaction vessel, but an obvious exothermic reaction is not detected. The reaction of pinene with oxygen to form peroxide is slow when there is no catalyst. Although a small decrease in pressure can be detected, the amount of reaction is small and exothermic is not detected.
The second step is the main oxidation stage. Peroxides are decomposed into free radicals, and pinene is oxidized to alcohols and ketones under free initiation. The exothermic reaction process is large, and oxygen is consumed more. Therefore, the heat release detected by is large and the pressure reduction is significant ARC or C80.
The third step is thermal degradation. Pinene or pinene oxide undergoes thermal degradation at high temperature to form small molecular compounds (such as butene, etc.), which is characterized by exothermic reaction and significant increase of pressure. The boiling point of butane, isoprene, pentadiene and 2-butene-2-methyl are 266.25 K, 307.25 K, 315.18 K and 311.15 K, respectively. They have a lower boiling point.
4. Conclusions
Turpentine is a very promising renewable energy resource, the main components are α-pinene and β-pinene. In this study, two thermal analysis instruments ARC and C80 were used to investigate the thermal stability and oxidation hazardous of two pinenes. ARC results showed that the oxidation reactions of pinene and ethers were significantly different.α-Pinene and β-pinene are thermally stable under nitrogen atmosphere, and no hazardous exothermic reactions takes place even when the temperature reaches 473 K. The α-pinene, β-pinene and mixture are very thermally unstable under oxygen atmosphere, at 333–338 K, their initial exothermic reaction was observed, and oxygen consumption was detected.
There are three steps in the oxidation of pinene: the first step is the induction period of the oxidation reaction, that is, pinene combines with oxygen, the pressure is reduced, but no significant exothermic reaction is detected. The second step is the main oxidation stage, which is characterized by significant exothermic reaction, but the pressure is reduced; the third step is the stage of thermal decomposition to produce gas, which is characterized by exothermic reaction and significant increase of pressure.
The oxidation reaction of α-pinene, β-pinene and mixtures are violent, and the exothermic energy is very large, they have the danger of thermal explosion. The importance of the research results is that heat runaway is easy to occur in oxidation reaction of turpentine or pinene. In order to avoid safety accidents, the control of oxidation reaction is necessary in organic synthesis and turpentine rectification process.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (21776050; 11762003), Major Science and Technology Special Project in Guangxi (AA17204087-20), and Science and Technology Fund in Guangxi Education Department of China (2019KY0179).References
- A. J. D. Silvestre and A. Gandimi, Monomers, polymers and composites from renewable resources, Terpenes: Major Sources, Properties and Applications, Elsevier Ltd., Holand, 2008, ch. 2, pp. 17–38 Search PubMed.
- C. G. Saravanan, A. B. Prem and S. C. Ananda, Renewable Energy, 2010, 35, 1179–1184 CrossRef.
- R. Vallinayagam, S. Vedharaj, W. M. Yang, P. S. Lee, K. J. E. Chua and S. K. Chou, Energy, 2013, 57, 344–351 CrossRef CAS.
- N. Mokrani, T. Fateh and L. Courty, Fuel, 2020, 267, 117177 CrossRef CAS.
- A. Benk and A. Coban, Renewable Energy, 2020, 147, 1491–1499 CrossRef CAS.
- A. K. Jeevanantham, R. D. Madhusudan, N. Goyal, D. Bansal, G. Kumar, A. Kumar, K. Nanthagopal and B. Ashok, Fuel, 2020, 262, 116551 CrossRef CAS.
- R. Karthikeyan and N. V. Mahalakshmi, Energy, 2004, 32, 1202–1209 CrossRef.
- P. Dubey and R. Gupta, Appl. Therm. Eng., 2017, 115, 1137–1147 CrossRef CAS.
- H. A. Meylemans, R. L. Quintana and B. G. Harvey, Fuel, 2012, 97, 560–568 CrossRef CAS.
- B. Yuan, Z. Wang, X. Yue, F. Yu, C. Xie and S. Yu, Renewable Energy, 2018, 123, 218–226 CrossRef CAS.
- G. Nie, J. Zou, R. Feng, X. Zhang and L. Wang, Catal. Today, 2014, 234, 271–277 CrossRef CAS.
- P. Gallezot, Catal. Today, 2007, 121, 76–91 CrossRef CAS.
- J. M. Castro, P. J. Linares-Palomino and S. Salido, Tetrahedron, 2005, 61(47), 11192–11203 CrossRef CAS.
- U. Neuenschwander, F. Guignard and I. Hermans, ChemSusChem, 2010, 31(1), 75–84 CrossRef PubMed.
- U. Neuenschwander, E. Meier and I. Hermans, ChemSusChem, 2011, 4(11), 1613–1621 CrossRef CAS PubMed.
- T. S. Dibble, J. Am. Chem. Soc., 2001, 123, 4228–4234 CrossRef CAS PubMed.
- L. Xu, K. H. Møller, J. D. Crounse, R. V. Otkjær, H. G. Kjaergaard and P. O. Wennberg, J. Phys. Chem. A, 2019, 123, 1661–1674 CrossRef CAS PubMed.
- S. Iyer, H. Reiman, K. H. Møller, M. P. Rissanen, H. G. Kjaergaard and T. Kurten, J. Phys. Chem. A, 2018, 122, 9542–9552 CrossRef CAS PubMed.
- T. Nah, J. Sanchez, C. M. Boyd and N. Lee, Environ. Sci. Technol., 2016, 50, 222–231 CrossRef CAS PubMed.
- W. Schrader, J. Geiger, D. Klockow and E. H. Korte, Environ. Sci. Technol., 2001, 35, 2717–2720 CrossRef CAS PubMed.
- V. G. Khamaganov and R. A. Hites, J. Phys. Chem. A, 2001, 105(5), 815–822 CrossRef CAS.
- X. Liu, P. Li and H. Liang, Chin. J. Appl. Chem., 2002, 19(7), 711–712 CAS.
- A. Stolle, B. Ondruschka and M. Findeisen, J. Org. Chem., 2008, 73, 8228–8235 CrossRef CAS PubMed.
- B. Chuong, M. Davis, M. Edwards and P. S. Stevens, Int. J. Chem. Kinet., 2002, 34, 300–308 CrossRef CAS.
- K. T. Lua and T. C. Chena, J. Hazard. Mater., 2009, 161, 246–256 CrossRef PubMed.
- X. Liu, S. Ito and Y. Wada, Energy, 2005, 82, 184–192 CrossRef.
- P. Liu, X. Liu, S. Kubota, P. Huang and Y. Wada, J. Therm. Anal. Calorim., 2019, 138, 479–488 CrossRef CAS.
- D. Ren, X. Liu, X. Feng, L. Lu, M. Ouyang, J. Li and X. He, Appl. Energy, 2018, 228, 633–644 CrossRef CAS.
- S. Guo, Q. Wang, J. Sun, X. Liao and Z. Wang, J. Hazard. Mater., 2009, 168, 536–541 CrossRef CAS PubMed.
- Q. Wang, S. Guo and J. Sun, Energy Fuels, 2009, 23, 4871–4876 CrossRef CAS.
- X. Zhai, H. Ge, C. Shu, D. Obracaj, K. Wang and B. Laiwang, Fuel, 2020, 268, 117327 CrossRef CAS.
- Q. Sun, L. Jiang, L. Gong and J. Sun, J. Hazard. Mater., 2016, 314, 230–236 CrossRef CAS PubMed.
- J. E. Hawkins and G. T. Armstrong, J. Am. Chem. Soc., 1954, 76, 3756–3758 CrossRef CAS.
- X. Liu, S. Ito and Y. Wada, Energy, 2015, 82, 184–192 CrossRef CAS.
- X. Liu, Q. Zhang, S. Ito and Y. Wada, Fuel, 2016, 165, 513–525 CrossRef CAS.
- U. Neuenschwander, F. Guignard and I. Hermans, ChemSusChem, 2010, 3, 75–84 CrossRef CAS PubMed.
- U. Neuenschwander, E. Meier and I. Hermans, ChemSusChem, 2011, 4, 1613–1621 CrossRef CAS PubMed.
- E. Langa, A. Palavra, M. Maria Lourenco, C. Nieto de Castro and A. Mainar, J. Chem. Thermodyn., 2013, 57, 493–499 CrossRef CAS.
- J. Sempere, R. Nomen and R. Serra, J. Therm. Anal. Calorim., 1999, 58(1), 215–223 CrossRef CAS.
- G. Hong, Journal of Qujing Teachers College, 2005, 24(3), 1–3 Search PubMed.
- H. Gao, L. Chen, W. Chen and S. Bao, Thermochim. Acta, 2013, 569, 134–138 CrossRef CAS.
- M. Tamura, Security handbook of chemical process, Asakura Asakurasyoten, Printed in Japan, ISBN 4-254-25029-0, 2000 Search PubMed.
- B. Coudour, K. Chetehouna, L. Lemee, P. Bertin and J. P. Garo, J. Therm. Anal. Calorim., 2019, 137, 1315–1328 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |