Open Access Article
Open Access ArticleAccess to 3,3-disubstituted oxindoles via microwave-assisted Cannizzaro and aldol reactions of formaldehyde with isatins and their imines†
Xuan Huang‡
,
Hongling Wang‡,
Qingxiang Cao,
Yong Li and
Junmin Zhang *
*
International Joint Research Centre for Molecular Science, College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen, 518060, P. R. China. E-mail: zhangjm@szu.edu.cn
First published on 11th May 2021
Abstract
3,3-Disubstituted oxindoles are important structure motifs in natural products and pharmaceutical agents. Here we disclose a simple and direct access to this class of molecules by using readily available formaldehyde and isatins (and their imines) as the substrates. The reaction proceeds with the assistance of microwave heating in the presence of a mild base. Formaldehyde behaves as both a reductant (via a Cannizzaro process with isatin) and an electrophile.
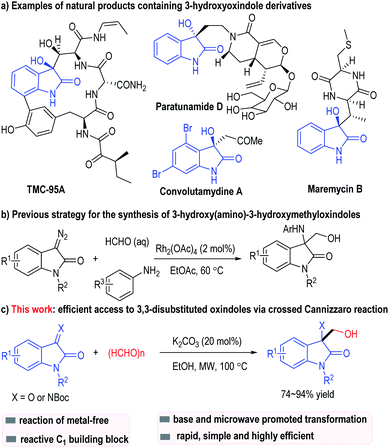
3,3-Disubstituted oxindoles are valuable structure motifs that have widely existed in biologically active natural products, alkaloids and pharmaceutical agents.1,2 For example, TMC-95A and its analogues are isolated from Apoispora montagnei. They can be used as specific proteasome inhibitors.3 Paratunamide D from paratude or Cinnamodendron axillare (Canellaceae) has shown moderate cytotoxicity against human epidermoid carcinoma KB cells (IC50 = 6 μg mL−1).4 Convolutamydine A, which is isolated from the Floridian marine bryozoan Amathia convoluta, has exhibited significant antinociceptive effects.5 Maremycin B is another oxindole-containing natural product molecule that has been isolated from the Streptomyces species B 9173.6 The above natural products have shown proven biological activities such as antioxidant, antimicrobial or antitumor activities (Fig. 1a).7 Therefore, the synthesis of 3-hydroxyoxindole derivatives has long been interesting and attractive.8
3-Hydroxyoxindole derivatives are one class of the most basic functional molecules containing oxindole scaffolds. Traditionally, 3-hydroxyoxindoles are obtained through aldol reactions, Friedel–Crafts reactions, Morita–Baylis–Hillman reactions and Hosomi–Sakurai allylation reactions.9 In 2014, Hu and co-workers10 developed a rhodium(II)-catalyzed three-component reaction for the synthesis of substituted 3-hydroxy(amino)-3-hydroxymethyloxindoles (Fig. 1b). Nobel metal catalysts and pre-functionalized diazo substrates are needed in this protocol.11
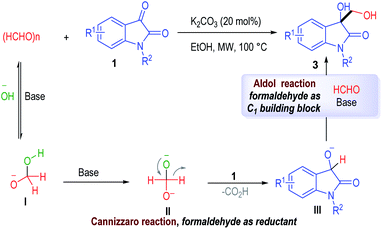
Herein, we describe a microwave assisted Cannizzaro/aldol reaction of paraformaldehyde and isatins (or its imine derivatives). Paraformaldehyde behaves as both a reductant12 (via a Cannizzaro process with isatin) and an electrophile (Fig. 1c). Simple and inexpensive inorganic bases are applied as the only catalysts for this process, with the functionalized 3-hydroxymethyl-oxindole products afforded in good to excellent yields.
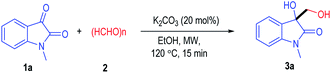
N-Methyl isatin 1a and paraformaldehyde 2 were selected as the model substrates to evaluate the reaction condition for the synthesis of 3-hydroxy-3-hydroxymethyloxindole 3a (Table 1). The target product 3a could be obtain in 29% yield under the catalysis of K2CO3 at 120 °C in EtOH (Table 1 entry 1). Importantly, no desired product was detected under the absence of base (entry 2). To our delight, the yield of 3a could be dramatically improved to 94% with the assistance of the microwave irradiation at 120 °C (entry 3). Switching the base catalyst to other organic or inorganic base cannot further increase the reaction yield (entries 4–8). The reaction can also be carried out in various organic solvent but can only be afforded in moderate yields (entries 9–11). Decreasing the reaction temperature to 100 °C led to little erosion on the product yield (entry 12). However, the product yield dropped significantly with a less amount of paraformaldehyde used as the starting material (entry 13).
| Entry | Base | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction condition: a mixture of 0.1 mmol of 1a, 1.5 mmol of 2, 20 mol% of base and 1.5 mL of solvent was irradiated in a microwave reactor at 120 °C for 15 min.b Yields are of isolated products based on 1a.c Performed at 100 °C.d The reaction mixture of 2 is 0.1 mmol in 100 °C microwave reactor. n.d. = no detected. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene. | |||
| 1 | K2CO3 | EtOH (w/o MW) | 29 |
| 2 | — | EtOH | n.d. |
| 3 | K2CO3 | EtOH | 94 |
| 4 | Na2CO3 | EtOH | 46 |
| 5 | KOAc | EtOH | 78 |
| 6 | t-BuOK | EtOH | 65 |
| 7 | DBU | EtOH | 70 |
| 8 | Et3N | EtOH | 29 |
| 9 | K2CO3 | CH3CN | 47 |
| 10 | K2CO3 | Toluene | 53 |
| 11 | K2CO3 | DMF | 49 |
| 12c | K2CO3 | EtOH | 92 |
| 13d | K2CO3 | EtOH | 34 |
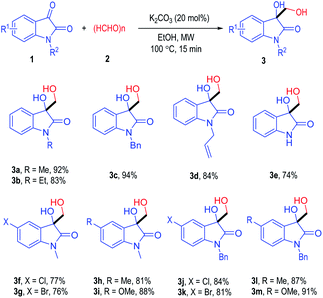
Having established an optimal condition for the reaction, the scope of the isatin derivatives was examined (Table 2). The N-methyl group isatin 1a could be switched to various alkyl groups, with the corresponding products afforded in good to excellent yields (3b to 3d). Interesting, the N protecting groups on the isatin substrates could even be removed and give the free oxindole derivatives 3e in a 74% yield. Both electron-withdrawing and electron-donating groups were well tolerated on the benzene rings of the isatin substrates, with the substituted 3-hydroxy-3-hydroxymethyloxindole products formed in good to excellent yields (3f to 3m).
| a Reaction condition: a mixture of 0.2 mmol of 1, 3.0 mmol of 2, 20 mol% of base and 1.5 mL of solvent was irradiated in a 100 °C microwave reactor with 15 min and yields are of isolated products based on 1. |
|---|
 |
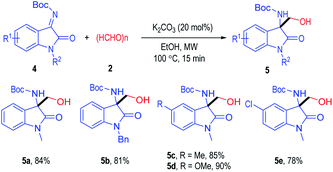
It is worth to note that the isatin-derived ketimine substrates 4 could also be used as suitable reactants for this microwave assisted Cannizzaro/aldol reaction process (Table 3). Ketimine substrate 4 bearing an N-Me or an N-Bn group could give the target product in a good yield (5a & 5b). Substituents could also be installed on the 5-position of the ketimine substrate regardless of their electron properties, with the desired products afforded in good to excellent yields (5c to 5e).
| a Reaction condition: a mixture of 0.2 mmol of 4, 3.0 mmol of 2, 20 mol% of base and 1.5 mL of solvent was irradiated in a 100 °C microwave reactor with 15 min and yields are of isolated products based on 4. |
|---|
 |
A postulated reaction mechanism is depicted in Fig. 2. The paraformaldehyde can react with the hydroxyl group under basic condition to give the intermediate I, which can be further deprotonated to give the dianion intermediate II. A hydride shift between the intermediate II and isatin substrate 1 leads to the formation of the reduced oxindole intermediate III with the elimination of the formic acid as the byproduct. An aldol reaction between the intermediate III and the formaldehyde gives the desired product 3. To gain more insight into this mechanism, several control experiments were conducted. The 3-hydroxy-1-methylindolin-2-one instead of the isatin with K2CO3 as the catalyst gave desired product in 90% yield. Based on this control experiment, we proposed the mechanism is reasonable.
In summary, we have developed an efficient Cannizzaro/aldol reaction with the assistance of microwave irradiation. Paraformaldehyde and isatin derivatives are successfully used as the reaction substrates. Functionalized 3-hydroxymethyloxindole derivatives bearing various substituents and substitution patterns are afforded as the reaction products in good to excellent yields. Inexpensive and the readily available K2CO3 is applied as the only catalyst in this protocol. Further application of the 3-hydroxymethyloxindole derivatives obtained through this method in synthetic chemistry and investigations into novel microwave assisted transformation are currently in progress in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Miss Qiongji Yao for assistance. We acknowledge financial supports from the National Natural Science Foundation of China (No. 22001173), Basic and Applied Research Foundation of Guangdong (No. 2019A1515110906), China Postdoctoral Science Foundation Funded Project (No. 2020M672767) and the Project of Department of Education of Guangdong Province (No. 2020KTSCX116), the Shenzhen Science and Technology Foundation (No. KQJSCX20180328100401788) and the Principal Foundation of Shenzhen University (No. 8570700000307). We gratefully acknowledge the support from the Instrumental Analysis Centre of Shenzhen University.Notes and references
- For the synthesis of 3,3-disubstituted oxindoles review, see: (a) Z.-Y. Cao, F. Zhou and J. Zhou, Development of synthetic methodologies via catalytic enantioselective synthesis of 3,3-disubstituted oxindoles, Acc. Chem. Res., 2018, 51, 1443–1454 CrossRef CAS; (b) J.-R. Chen, X.-Y. Yu and W.-J. Xiao, Tandem radical cyclization of N-arylacrylamides: an emerging platform for the construction of 3,3-disubstituted oxindoles, Synthesis, 2015, 47, 604–629 CrossRef CAS; (c) A. Moyano and X. Companyò, Catalytic asymmetric strategies for the synthesis of 3,3-disubstituted oxindoles, Studies in Natural Products Chemistry, 2013, ch. 4, vol. 40, pp. 71–132 Search PubMed; (d) B. M. Trost and M. K. Brennan, Asymmetric syntheses of oxindole and indole spirocyclic alkaloid natural products, Synthesis, 2009, 18, 3003–3025 CrossRef; (e) C. Marti and E. M. Carreira, Construction of spiro[pyrrolidine-3,3′-oxindoles] – recent applications to the synthesis of oxindole alkaloids, Eur. J. Org. Chem., 2003, 2945–2963 Search PubMed.
- For bioactive examples, see: (a) D. L. Silverio, S. Torker, T. Pilyugina, E. M. Vieira, M. L. Snapper, F. Haeffner and A. H. Hoveyda, Simple organic molecules as catalysts for enantioselective synthesis of amines and alcohols, Nature, 2013, 494, 216–221 CrossRef CAS PubMed; (b) A. P. Antonchick, C. G. Reimers, M. Catarinella, M. Schürmann, H. Preut, S. Ziegler, D. Rauh and H. Waldmann, Highly enantioselective synthesis and cellular evaluation of spirooxindoles inspired by natural products, Nat. Chem., 2010, 2, 735–740 CrossRef CAS PubMed; (c) F. Zhou, Y.-L. Liu and J. Zhou, Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3 position, Adv. Synth. Catal., 2010, 352, 1281–1407 Search PubMed; (d) S. Peddibhotla, 3-Substituted-3-hydroxy-2-oxindole, an emerging new scaffold for drug discovery with potential anti-cancer and other biological activities, Curr. Bioact. Compd., 2009, 5, 20–80 CrossRef CAS; (e) C. V. Galliford and K. A. Scheidt, Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 CrossRef CAS PubMed.
- (a) J. Kohno, Y. Koguchi, M. Nishio, K. Nakao, M. Kuroda, R. Shimizu, T. Ohnuki and S. Komatsubara, Structures of TMC-95A-D: novel proteasome inhibitors from Apiospora montagnei Sacc. TC 1093, J. Org. Chem., 2000, 65, 990–995 CrossRef CAS PubMed; (b) Y. Koguchi, J. Kohno, M. Nishio, K. Takahashi, T. Okuda, T. Ohnuki and S. Komatsubara, TMC-95A, B, C, and D, novel proteasome inhibitors produced by Apiospora montagnei Sacc. TC 1093 taxonomy, production, isolation, and biological activities, J. Antibiot., 2000, 53, 105–109 CrossRef CAS PubMed; (c) M. Groll, Y. Koguchi, R. Huber and J. Kohno, Crystal structure of the 20 S proteasome: TMC-95A complex: a non-covalent proteasome inhibitor, J. Mol. Biol., 2001, 311, 543–548 CrossRef CAS PubMed; (d) Z.-Q. Yang, B. H. B. Kwok, S. Lin, M. A. Koldobskiy, C. M. Crews and S. J. Danishefsky, Simplified synthetic TMC-95A/B analogues retain the potency of proteasome inhibitory activity, ChemBioChem, 2003, 4, 508–531 CrossRef CAS PubMed; (e) A. K. L. Yuen and C. A. Hutton, Preparation of cyclic peptide alkaloids containing functionalized tryptophan residues, Nat. Prod. Commun., 2006, 1, 907–919 CrossRef CAS.
- T. Kagata, S. Saito, H. Shigemori, A. Ohsaki, H. Ishiyama, T. Kubota and J. Kobayashi, Paratunamides a-d, oxindole alkaloids from cinnamodendron axillare, J. Nat. Prod., 2006, 69, 1517–1521 CrossRef CAS PubMed.
- (a) G. S. M. Figueiredo, R. S. Zardo, B. V. Silva, F. A. Violante, A. C. Pinto and P. D. Fernandes, Convolutamydine A and Synthetic Analogues have Antinociceptive Properties in Mice, Pharmacol. Biochem. Behav., 2013, 103, 431–439 CrossRef CAS PubMed; (b) P. D. Fernandes, R. S. Zardoa, G. S. M. Figueiredo, B. V. Silva and A. C. Pinto, Anti-inflammatory properties of convolutamydine A and two structural analogues, Life Sci., 2014, 116, 16–24 CrossRef CAS PubMed; (c) T. B. S. Giorno, Y. L. L. Ballard, M. S. Cordeiro, B. V. Silva, A. C. Pinto and P. D. Fernandes, Central and peripheral antinociceptive activity of 3-(2-oxopropyl)-3-hydroxy-2-oxindoles, Pharmacol., Biochem. Behav., 2015, 135, 13–19 CrossRef CAS PubMed.
- (a) W. Balk-Bindsei, E. Helmke, H. Weyland and H. Laatsch, Maremycin A and B, new diketopiperazines from a marine Streptomyces sp, Liebigs Ann., 1995, 1291–1294 CrossRef; (b) Y. Liu, L. Zhang and Y. Jia, Total synthesis of Maremycins A, B, C1/C2, D1, and D2, Tetrahedron Lett., 2012, 53, 684–687 CrossRef CAS; (c) G. Bergonzini and P. Melchiorre, Dioxindole in asymmetric catalytic synthesis: routes to enantioenriched 3-substituted 3-hydroxyoxindoles and the preparation of Maremycin A, Angew. Chem., Int. Ed., 2012, 51, 971–974 CrossRef CAS PubMed.
- See ref. 3–6 and (a) Y. Kamano, H.-P. Zhang, Y. Ichihara, H. Kizu, K. Komiyama and G. R. Pettit, Convolutamydine A, a novel bioactive hydroxyoxindole alkaloid from marine bryozoan Amathia convoluta, Tetrahedron Lett., 1995, 36, 2783–2784 CrossRef CAS; (b) M. Grolla and R. Huber, Inhibitors of the eukaryotic 20S proteasome core particle: a structural approach, Biochim. Biophys. Acta, 2004, 1695, 33–44 CrossRef PubMed; (c) S. Lin, Z.-Q. Yang, B. H. B. Kwok, M. Koldobskiy, C. M. Crews and S. J. Danishefsky, Total synthesis of TMC-95A and -B via a new reaction leading to Z-enamides. Some preliminary findings as to SAR, J. Am. Chem. Soc., 2004, 126, 6347–6355 CrossRef CAS PubMed.
- (a) G. M. Ziarani, R. Moradi and N. Lashgari, Asymmetric synthesis of chiral 3,3-disubstituted oxindoles using isatin as starting material, Tetrahedron: Asymmetry, 2015, 26, 517–541 CrossRef; (b) S. Mohammadi, R. Heiran, R. P. Herrera and E. Marqués-López, Isatin as a strategic motif for asymmetric catalysis, ChemCatChem, 2013, 5, 2131–2148 CrossRef CAS.
- See ref. 8 and (a) X. Wang, J. Liu, L. Xu, Z. Hao, L. Wang and J. Xiao, Friedel–Crafts alkylation of heteroarenes and arenes with indolyl alcohols for construction of 3,3-disubstituted oxindoles, RSC Adv., 2015, 5, 101713–101717 RSC; (b) B. Dounay and L. E. Overman, The asymmetric intramolecular heck reaction in natural product total synthesis, Chem. Rev., 2003, 103, 2945–2964 CrossRef PubMed.
- C. Wang, D. Xing, D. Wang, X. Wu and W. Hu, Synthesis of 3-amino-3-hydroxymethyloxindoles and 3-hydroxy-3-hydroxymethyloxindoles by Rh2(OAc)4-catalyzed three-component reactions of 3-diazooxindoles with formaldehyde and anilines or water, J. Org. Chem., 2014, 79, 3908–3916 CrossRef CAS PubMed.
- (a) Z.-Y. Cao, Y.-H. Wang, X.-P. Zeng and J. Zhou, Catalytic asymmetric synthesis of 3,3-disubstituted oxindoles: diazooxindole joins the field, Tetrahedron Lett., 2014, 55, 2571–2584 CrossRef CAS; (b) C. Wang, S. Liu, D. Xing, X. Wang, X. Wu and W. Hu, Efficient synthesis of α-aryl serine derivatives via three-component reactions of aryldiazoacetates, anilines and formaldehyde, Tetrahedron, 2013, 69, 11203–11208 CrossRef CAS; (c) C. Jing, D. Xing, C. Wang and W. Hu, Synthesis of 3-(hydroxymethyl)-3-indol-3′-yloxindoles via Rh(II)-catalyzed three-component reaction of 3-diazooxindoles, indoles and formalin, Tetrahedron, 2015, 71, 3597–3602 CrossRef CAS.
- S. Desmons, R. Fauré and S. Bontemps, Formaldehyde as a promising C1 source: the instrumental role of biocatalysis for stereocontrolled reactions, ACS Catal., 2019, 9, 9575–9588 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02150h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |