Open Access Article
Open Access ArticleBromotrimethylsilane as a selective reagent for the synthesis of bromohydrins†
Donatella Giomi *ab,
Antonella Salvini
*ab,
Antonella Salvini *ab,
Jacopo Ceccarelli
*ab,
Jacopo Ceccarelli a and
Alberto Brandi
a and
Alberto Brandi ab
ab
aDipartimento di Chimica ‘Ugo Schiff’, Università degli Studi di Firenze, Via della Lastruccia 3-13, I-50019 Sesto Fiorentino (Fi), Italy. E-mail: donatella.giomi@unifi.it; antonella.salvini@unifi.it
bLaboratorio congiunto VALORE, “Valorizzazione di masse algali e sottoprodotti agro-industriali e riduzione di gas serra in atmosfera”, Via Morosini snc, I-50019 Sesto Fiorentino (Fi), Italy
First published on 16th April 2021
Abstract
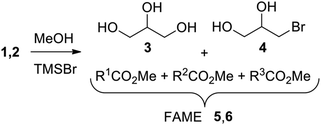
Bromotrimethylsilane (TMSBr) is a very efficient reagent in the solvent-free conversion of glycerol into bromohydrins, useful intermediates in the production of fine chemicals. As glycerol is a relevant by-product in biodiesel production, TMSBr has been also tested as a mediator in transesterification in acidic conditions, providing FAME from castor oil in good yields, along with bromohydrins from glycerol. Subsequently the glycerol conversion was optimized and depending on the reaction conditions, glycerol can be selectively converted into α-monobromohydrin (1-MBH) or α,γ-dibromohydrin (1,3-DBH) in very good yields.
Introduction
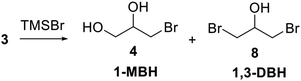
The bromination of glycerol to produce α-monobromohydrin (1-MBH, 4) and α,γ-dibromohydrin (1,3-DBH, 8) is an important process for the importance of these bromoderivatives widely applied as fine chemicals in organic syntheses.1–3 In particular, 1,3-DBH could be oxidized to 1,3-dibromoacetone which is a versatile building block for several compounds important as drugs, antibacterials or inhibitors.1However, few methods to synthesize bromohydrins from glycerol have been reported exploiting bromine, phosphorous and bromine, phosphorous tribromide,4 hydrobromic acid,5 as well as trifluoroacetic anhydride and lithium bromide as brominating agents.6 Multi-step processes have also been used with dichlorohydrins as intermediates.1
Glycerol is an interesting raw material for obtaining products of high added value because it is obtained as by-product of the production of Fatty Acid Methyl Esters (FAME) from vegetable oils for their use as biodiesel. This is a well known process providing a fuel that can be used in combustion plants, or in diesel motorcars without any particular variation of the combustion system. During the transesterification of triglycerides, ca. 10% by weight of the initial mass was transformed in glycerol. In 2020, the global production of crude glycerol has approached 4 billion liters and adequate valorization becomes crucial for the chemical industry. This abundant and important raw material is very extensively applied in pharmaceutical, food, cosmetic, chemical industries. In particular, a great interest has been devoted to its transformation in more valuable chemicals like ethers, esters, organic acids, acrolein, chlorohydrins, dihalo- and dihydroxy-acetones.1,7 The success of the use of glycerol as a raw material for these productions depends on the purity achieved in its production and, therefore, on the biodiesel production process. The low purity of glycerol generally obtained in first-generation biodiesel production makes further conversion more difficult. Moreover, the first-generation process has received in the past much criticism because it utilizes as feed stock materials that belong to the foodstuff supply. Therefore, of particular interest, becomes the production of FAME from exhaust vegetable oils. The use of these oils, however, raises another problem connected with the most common industrial method for the production of FAME, that is the alkaline transesterification of triglycerides in the presence of MeOH.8
In fact, a total free fatty acid content in vegetable oils above 0.5% by weight, because of the formation of soaps (Na or K salts of fatty acids), can provoke their emulsions with water, making difficult the separation of pure FAME. A simple solution to this problem is the switch to acidic transesterification catalysts.
Recently our group has addressed both the tasks, glycerol valorization and exhaust vegetable oils conversion, by showing that trimethylchlorosilane (TMSCl) is an excellent reagent to promote very efficiently the transesterification of vegetable oils and fats to FAME and other fatty acid methyl esters,9,10 and, at the same time, to promote the selective transformation of glycerol to mono- and dichlorohydrins.10,11
Now, the synthesis of bromohydrins from glycerol was studied using trimethylbromosilane (TMSBr) as a reagent, preliminarily also analyzing its effectiveness in the transesterification of triglycerides.
Results and discussion
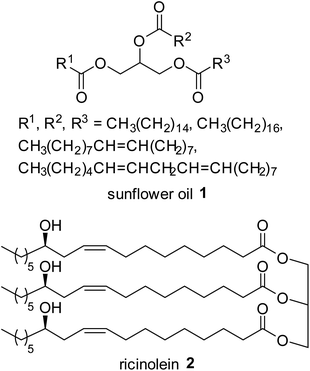
Based on the results obtained with TMSCl,9–11 and taking into account that glycerol is obtained as a co-product of the biodiesel production, it was decided to preliminarily evaluate the effectiveness of TMSBr in transesterification of triglycerides. In fact, despite the higher cost of the new reagent, this system can become interesting if one wishes to produce bromohydrins as a co-product. Furthermore, it seemed interesting to evaluate the different reactivity of the two systems in the presence of Br instead of Cl. Two model triglycerides were chosen for the transesterification studies, i.e. sunflower oil and castor oil (Fig. 1). By analogy with previous studies with TMSCl,9,10 mixtures of oil, MeOH, and TMSBr were heated. The nature of the two oils makes already a difference at this point. Whereas the mixture with castor oil is a homogeneous solution, according to the presence of ricinolein as the major triglyceride (ca. 90%), the mixture with sunflower oil is an emulsion that becomes clear only by increasing the temperature.Under heating a mixture of dried sunflower oil 1, TMSBr and anhydrous MeOH (molar ratios TMSBr/1 2.2, MeOH/1 4.5) at 60 °C a poor conversion of the oil in FAME has been observed after 8 hours through 1H NMR monitoring (Table 1, entry 1).12 Increasing the amount of TMSBr and MeOH (by adding further portions of the starting reagents) the conversion was slightly increased (28%, Table 1, entry 2) after 30 hours at the same temperature. By raising the reaction temperature to 100 °C, after 60 hours the conversion climbed up to 42% (Table 1, entry 3).
The best experimental conditions led to a 60% conversion of 1 into FAME after 72 hours at 100 °C (Table 1, entry 4). 1H NMR analysis (CDCl3) of the reaction mixture after removing under vacuum all the volatile compounds showed the presence of FAME, mono-, di-, and triglycerides besides glycerol 3 (signals in the range 4.35–4.10 ppm), according to a conversion of 60%. No trace of halogenated derivatives of glycerol, like 4, has been observed through 13C NMR analysis of the reaction mixture (DMSO-d6), in contrast to what was observed using TMSCl for the transesterification.9
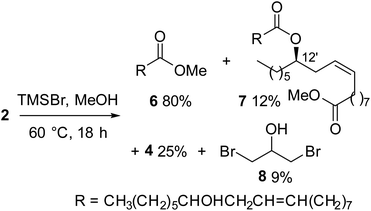
Concerning castor oil, previous data related to the use of TMSCl10 evidenced the need to operate with an excess of MeOH to allow, in addition to transesterification of 2 to give 6, the methanolysis of estolides 7, i.e. dimers obtained by esterification of the free ricinoleic acid with the hydroxy group on C-12 carbon of another molecule of ricinoleic methyl ester (Scheme 1).
Therefore, a mixture of TMSBr, MeOH, and castor oil 2 (molar ratios TMSBr/2 3, MeOH/2 4.5) was heated at 60 °C for 8 hours then added of a further portion of MeOH (final MeOH/2 ratio 35) and heated for additional 6 hours. Delightfully, 1H NMR monitoring of the reaction mixture12 showed the formation of ricinoleic methyl ester with a conversion of 90% of the oil (Table 1, entry 5). A slightly better conversion (93%) was observed by increasing the time of the first step of the reaction from 8 to 12 hours. The 1H NMR analysis of the crude mixture, after removing volatiles under vacuum, showed the presence of ricinoleic methyl ester (6) as the major compound, a small amount of estolide 7 (identified by the characteristic quintet at 4.9 ppm of the H-12′ proton), and glycerol derivatives (signals in the range 4.1–3.5 ppm). The 13C NMR analysis of the reaction crude (in DMSO-d6 to dissolve even glycerol) allowed the identification of monobromohydrin 4 alongside minor amounts of dibromohydrin 8 and traces of glycerol.13
The reaction mixture, after removing under vacuum all the volatile compounds, was subjected to Kugelrhor distillation at reduced pressure giving, in order of decreasing volatility, dibromohydrin 8 (9%), monobromohydrin 4 (25%), and ricinoleic methyl ester (6) (80%), while the estolide 7 was recovered in 12% yield as distillation residue (Scheme 1).
The results of Table 1 show a quite significant difference in the reactivity of the two triglycerides 1 and 2 with TMSBr. Sunflower oil appears much less reactive, likely because of a lower solubility in the reaction medium, hampering the reaching of full conversion, even at higher temperature and longer reaction times, as well as the formation of bromo derivatives of glycerol.
The more polar triglyceride 2, giving rise to a clear solution even at room temperature, can be almost quantitatively converted into FAME at lower temperature, along with bromohydrins 4 and 8.
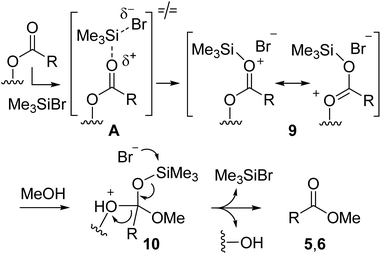
As previously reported for TMSCl,9 the role of TMSBr as mediator for transesterification of triglycerides can be rationalized as shown in Scheme 2.
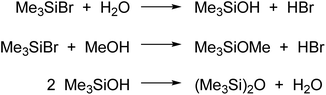
TMSBr activates the ester group by coordination to the carbonyl moiety leading to the electrophilic intermediate 9. Then, nucleophilic attack of methanol affords the tetrahedral intermediate 10 that collapses to FAME by loss of glycerol and TMSBr. The use of TMSBr in higher than catalytic amount can be explained by its decomposition by side processes, like hydrolysis and methanolysis (Scheme 3), as well as bromination of glycerol (see below).
A comparison of the reactivity of TMSBr with TMSCl clearly shows a higher efficiency for TMSCl that requires lower temperatures and reaction times, providing higher conversions of the triglycerides into FAME.9 This different behavior can be justified on the basis of the HSAB theory14 suggesting for the softer TMSBr a poor interaction with the hard oxygen atom in the transition state A (Scheme 2) rendering the formation of 9 less feasible. However, the use of TMSBr in the synthesis of bromohydrins from glycerol appears interesting. Therefore, the reaction of TMSBr and glycerol was studied following the track opened with TMSCl.11
By heating a yellow/orange emulsion of glycerol and TMSBr (2.5 molar ratio) at 60 °C, after 12 hours, under monitoring via 13C NMR analysis (in DMSO-d6 to dissolve even glycerol), the formation of both 1-MBH (4) and 1,3-DBH (8) was observed in roughly 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, besides ca. 29% unreacted glycerol (Table 2, entry 1). However, according to previous observations of a catalytic effect of acetic acid in the reaction of TMSCl with glycerol,11 the experiment was repeated mixing glycerol (3), TMSBr (2.5 molar ratio), and 3% of AcOH (related to 3).
1 ratio, besides ca. 29% unreacted glycerol (Table 2, entry 1). However, according to previous observations of a catalytic effect of acetic acid in the reaction of TMSCl with glycerol,11 the experiment was repeated mixing glycerol (3), TMSBr (2.5 molar ratio), and 3% of AcOH (related to 3).
| Entry | TMSBr/3a | AcOH/3a | T (°C) | t (h) | 3b | 4b | 8b |
|---|---|---|---|---|---|---|---|
| a Molar ratio.b Molar ratios (%) estimated via 13C NMR (DMSO-d6) spectra for semi-quantitative analyses (see ESI). | |||||||
| 1 | 2.5 | — | 60 | 12 | 29 | 59 | 12 |
| 2 | 2.5 | 0.03 | 60 | 6 | 3 | 29 | 68 |
| 3 | 2.5 | 0.03 | 60 | 9 | — | — | ca. 100 |
| 4 | 1.5 | — | 20 | 24 | 82 | 18 | — |
| 5 | 1.5 | 0.03 | 20 | 24 | 46 | 51 | 3 |
| 6 | 1.5 | 0.03 | 20 | 48 | 20 | 60 | 20 |
After heating 6 hours at 60 °C, a roughly 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
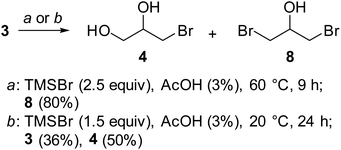
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1,3-DBH and 1-MBH has been obtained, with only traces of unreacted glycerol, and by increasing the reaction times at 9 hours, 1,3-DBH was the almost exclusive product in the reaction crude (Table 2, entries 2 and 3, and Fig. 2a).
1 mixture of 1,3-DBH and 1-MBH has been obtained, with only traces of unreacted glycerol, and by increasing the reaction times at 9 hours, 1,3-DBH was the almost exclusive product in the reaction crude (Table 2, entries 2 and 3, and Fig. 2a).
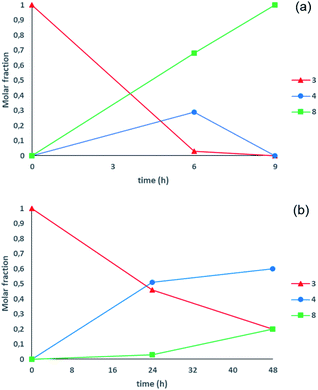 | ||
| Fig. 2 (a) Experiments performed at 60 °C (Table 2, entries 2 and 3). (b) Experiments performed at 20 °C (Table 2, entries 5 and 6). | ||
The reaction mixture obtained after 9 hours heating appeared as a biphasic system: the upper phase containing unreacted TMSBr and (Me3Si)2O (see Scheme 3) was separated and the pale yellow lower one was subjected to distillation under reduced pressure to give 1,3-DBH (8), isolated in 80% yield as a colorless liquid (Scheme 4).
With the aim to produce 1-MBH, the reaction of glycerol (3) was performed at room temperature with a minor amount of TMSBr (1.5 molar ratio). Without acid catalysis the reaction was very slow but in the presence of 3% of AcOH a roughly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of glycerol and 1-MBH was formed after 24 hours, along with traces of 1,3-DBH (Table 2, entries 4 and 5). Increasing reaction times to 48 hours, glycerol was additionally converted with a rise in the amount of 1-MBH (3/4 ratio 1
1 mixture of glycerol and 1-MBH was formed after 24 hours, along with traces of 1,3-DBH (Table 2, entries 4 and 5). Increasing reaction times to 48 hours, glycerol was additionally converted with a rise in the amount of 1-MBH (3/4 ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), but the selectivity of the reaction was reduced as 20% of 1,3-DBH is also formed (Table 2, entry 6). Therefore, to produce 1-MBH the conditions of entry 5 (Table 2 and Fig. 2b) are the most convenient as, after separation of 1-MBH, unreacted glycerol can be recovered and recycled. Distillation at reduced pressure of the reaction crude allowed the isolation of 1-MBH in 50% yield, while unreacted 3 was recovered (36%) from the distillation residue (Scheme 4).
3), but the selectivity of the reaction was reduced as 20% of 1,3-DBH is also formed (Table 2, entry 6). Therefore, to produce 1-MBH the conditions of entry 5 (Table 2 and Fig. 2b) are the most convenient as, after separation of 1-MBH, unreacted glycerol can be recovered and recycled. Distillation at reduced pressure of the reaction crude allowed the isolation of 1-MBH in 50% yield, while unreacted 3 was recovered (36%) from the distillation residue (Scheme 4).
The achieved selectivity in formation of 1-MBH and 1,3-DBH is significant as both compounds can be produced practically as a single product applying different reaction protocols.
An analysis of the literature data shows that chlorination or bromination by TMSCl or TMSBr occurs under the presence of catalysts or co-reagents.15 Only in one case the transformation is reported without using a catalyst, but it concerns exclusively tertiary or benzylic alcohols.16 On the other hand, to our knowledge, glycerol has not been utilized as a substrate in halogenation studies with halogenosilanes. Moreover, it is noteworthy the catalytic role of AcOH in glycerol chlorination with TMSCl, evidenced by our group, and now confirmed with TMSBr.
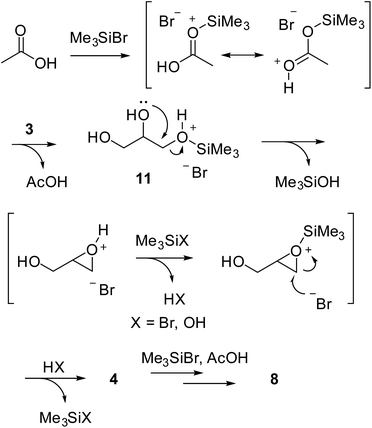
The probable reaction pathway producing bromohydrins from glycerol and TMSBr is described in Scheme 5. The role of catalytic AcOH in accelerating the reaction can be rationalized through activation of TMSBr. The reaction with glycerol likely forms a silyl derivative 11 that undergoes an activated epoxide formation that eventually experiences a selective opening by nucleophilic bromide substitution.
Concerning the reactivity of TMSBr and TMSCl, the experimental results demonstrate a higher reactivity for TMSBr in formation of bromohydrins, performed at lower temperatures and reaction times, with respect to TMSCl. These results can be explained by the better nucleophilic character of bromide anion, compared to chloride, in a polar alcoholic medium as glycerol.
Overall, this methodology appears significant because allows the direct and selective conversion of glycerol into bromohydrins in high yields, operating in mild and solvent-free conditions.
This reactivity appears very attractive because recent results concerning the synthesis of 1,3-DBH (8) still apply a two-step process involving first the transformation of glycerol into 1,3-dichlorohydrin then converted into 1,3-dibromohydrin.1b
Experimental
General information
Chemicals were purchased from commercial suppliers and used as received. 1H and 13C NMR spectra were recorded with Varian Mercury plus 400 operating at 400 MHz and 100 MHz, respectively. Dry methanol was obtained by PureSolv™ Micro 100 Liter solvent purification system.Transesterification of sunflower oil (1)
(A) In a screw-cap Pyrex tube N° 18, dry MeOH (0.164 g, 0.207 mL, 5.13 mmol) and TMSBr (0.384 g, 0.330 mL, 2.51 mmol) were added to sunflower oil (1) [1 g, 1.14 mmol (Mw calc. = 878 g mol−1)] under nitrogen atmosphere. The reaction mixture was heated for 8 hours at 60 °C, under magnetic stirring. After cooling, the separation of two phases was observed. The upper phase, containing FAME, was separated and heated at 50 °C for 2 hours under reduced pressure (5–10 mmHg) to distill off volatiles. 1H NMR (CDCl3) analysis on the residue showed only a poor transesterification of the starting triglycerides (ca. 12%).12,17(B) Operating as above but heating at 100 °C for 72 hours, 1H NMR (CDCl3) analysis on the upper phase, after removal of the volatiles, showed ca. 60% conversion of 1 into biodiesel.
Transesterification of castor oil (2)
In a 15 mL screw-cap Pyrex® glass tube, dry MeOH (0.156 g, 0.197 mL, 4.87 mmol) and TMSBr (0.496 g, 0.428 mL, 3.24 mmol) were added to castor oil (2) [1 g, 1.08 mmol (Mw calc. = 929 g mol−1)] under nitrogen atmosphere. The dull yellow reaction mixture was heated for 12 hours at 60 °C, under magnetic stirring. After cooling to room temperature, a further amount of dry MeOH (1.057 g, 1.33 mL, 33 mmol) was added and the mixture was heated for additional 6 hours. Methanol and the other volatile compounds were removed by heating for 2 hours at 50 °C under reduced pressure (5–10 mmHg) leading to a yellow-brown liquid. NMR analyses, performed both in CDCl3 and DMSO-d6 as solvents, allowed to determine the conversion of castor oil into methyl esters as well as the presence of bromohydrins in the reaction mixture. Distillation under vacuum (3 × 10−2 mmHg) in a Kugelrhor apparatus allowed to recover four fractions. The first fraction collected at 50–55 °C/3 × 10−2 mmHg, was 1,3-DBH (8) (0.021 g, 9%) while 1-MBH (4) (0.042 g, 25%) was recovered at 100 °C/3 × 10−2 mmHg and methyl ricinoleate (6)10 (0.810 g, 80%) between 150–220 °C/3 × 10−2 mmHg. The distillation residue (0.115 g; 12% yield) was a brownish liquid, containing the dimer 7 (ref. 10) as the main product.Synthesis of 1,3-dibromohydrin (1,3-DBH) (8)
In a 15 mL screw-cap Pyrex® glass tube, TMSBr (3.83 g, 3.3 mL, 25 mmol) was added to a mixture of glycerol (0.921 g, 10 mmol) and AcOH (0.02 g, 0.19 mL, 0.33 mmol), under nitrogen atmosphere. The biphasic mixture was heated at 60 °C for 9 hours under magnetic stirring. At the end of the reaction, two phases were formed again: the upper one, containing unreacted TMSBr and hexamethyldisiloxane, was removed by the use of a Pasteur pipette; the lower pale yellow phase, containing bromo derivatives of glycerol, was subjected to distillation under vacuum. While traces of 1-MBH (4) were detected in the distillation residue, 1,3-DBH (8) (1.74 g, 80%) was collected at 75–82 °C/1 mmHg as a colorless liquid; 1H NMR (CDCl3) δ (ppm): 4.02 (quintet, J = 5.3 Hz, 1H), 3.63–3.56 (m, 4H), 2.22 (br s, 1H); 13C-NMR (CDCl3) δ (ppm): 69.5 (d), 35.0 (t).Synthesis of 1-monobromohydrin (1-MBH) (4)
Operating as above, the biphasic mixture composed of glycerol (0.921 g, 10 mmol), TMSBr (2.30 g, 1.98 mL, 15 mmol) and acetic acid (0.02 g, 0.19 mL, 0.33 mmol) was heated at 20 °C for 24 hours, under magnetic stirring. The pale orange lower phase was subjected to evaporation at 50 °C/5–10 mmHg to remove all traces of volatile compounds. Distillation at 50–60 °C/3 × 10−2 mmHg allowed to isolate 1-MBH (4) (0.775 g, 50%) as a colorless liquid; 1H-NMR (CDCl3) δ (ppm): 4.03–3.86 (m, 1H), 3.84–3.64 (m, 2H), 3.57–3.43 (m, 2H), 2.09 (br s, 2H); 13C NMR (CDCl3) δ (ppm): 71.3 (d), 64.2 (t), 35.2 (t).Traces of 1,3-DBH (8) were detected in the head of distillation (1H NMR), while unreacted glycerol (ca. 0.332 g, 36%) was recovered from the distillation residue.
Conclusions
TMSBr is a less efficient mediator than TMSCl for triglycerides transesterification, albeit it is still able to provide transesterification in good yields in acidic conditions with substrates leading to a homogeneous solution.On the other hand, TMSBr is a very efficient reagent, more than TMSCl, in the solvent-free conversion of glycerol into halohydrins. Depending on the reaction conditions, glycerol can be selectively converted into 1-MBH or 1,3-DBH in very good yields. On the whole, we can affirm that while TMSCl is the reagent of choice for the transesterification of triglycerides to give biodiesel, TMSBr is a very efficient reagent for glycerol conversion into bromohydrins.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank MUR (Rome-Italy) and Ente Cassa di Risparmio di Firenze for the financial support and acknowledge Irene Ceccolini for her contribution with Bachelor thesis work.Notes and references
- (a) F. P. N. R. da Silva, P. F. dos Santos, S. R. B. da Silva and V. L. P. Pereira, J. Braz. Chem. Soc., 2020, 31, 1725 CAS; (b) P. F. dos Santos, S. R. B. da Silva, F. P. N. R. da Silva, J. da Silva Costa, J. Sayuri Inada and V. L. P. Pereira, Green Chem. Lett. Rev., 2019, 12, 389 CrossRef CAS.
- For the synthesis of epibromohydrin, see: G. Braun, Org. Synth., 1936, 16, 30 CrossRef CAS.
- For recent applications of bromohydrins, see also: (a) S. Lee and M. H. Seo, J. Nanosci. Nanotechnol., 2017, 17, 7579 CrossRef CAS; (b) M. Mojzych, Z. Bernat, Z. Karczmarzyk, J. Matysiak and A. Fruzinski, Molecules, 2020, 25, 221, DOI:10.3390/molecules25010221; (c) Y. Zhang, G. Chen, L. Wu, K. Liu, H. Zhong, Z. Long, M. Tong, Z. Yang and S. Dai, Chem. Commun., 2020, 56, 3309 RSC.
- G. Braun, Org. Synth., 1934, 14, 42 CrossRef , and references therein.
- A. Bouillaud, M. Dargelos and M.-E. Borredon, Aust. J. Chem., 1994, 47, 2123 CrossRef CAS.
- F. Camps, V. Gasol and A. Guerrero, Synthesis, 1987, 511 CrossRef CAS.
- (a) E. Santacesaria, R. Vitiello, R. Tesser, V. Russo, R. Turco and M. Di Serio, Ind. Eng. Chem. Res., 2014, 53, 8939 CrossRef CAS; (b) X. Fan, R. Burton and Y. Zhou, Open Fuels Energy Sci. J., 2010, 3, 17 CrossRef CAS; (c) F. Yang, M. A. Hanna and R. Sun, Biotechnol. Biofuels, 2012, 5, 1 CrossRef PubMed; (d) R. De Sousa, C. Thurier, C. Len, Y. Pouilloux, J. Barrault and F. Jerome, Green Chem., 2011, 13, 1129 RSC; (e) C. Len and R. Luque, Sustainable Chem. Processes, 2014, 2, 1 CrossRef; (f) H. Saggadi, D. Luart, N. Thiebault, I. Polaert, L. Estel and C. Len, RSC Adv., 2014, 4, 21456 RSC; (g) M. K. Aroua and P. Cognet, Front. Chem., 2020, 8, 69, DOI:10.3389/fchem.2020.00069.
- (a) H. J. Wright, J. B. Segur, H. W. Clark, S. K. Cobum, E. E. Langdon and R. N. DuPuis, J. Am. Oil Chem. Soc., 1944, 21, 145 CAS; (b) U. Schuchardt, R. Sercheli and R. M. Vargas, J. Braz. Chem. Soc., 1998, 9, 199 CrossRef CAS; (c) F. Ma and M. A. Hanna, Bioresour. Technol., 1999, 70, 1 CrossRef CAS; (d) G. Vicente, M. Martínez and J. Aracil, Bioresour. Technol., 2004, 92, 297 CrossRef CAS PubMed; (e) M. Mittelbach and C. Remschmidt, Biodiesel, the Comprehensive Handbook, ed. M. Mittelbach, Graz, 2006, ISBN: 3-200-00249-2 Search PubMed.
- A. Salvini, D. Giomi, G. Cipriani, G. Bartolozzi, R. Alfini and A. Brandi, RSC Adv., 2012, 2, 4864 RSC.
- M. Malavolti, A. Brandi, A. Salvini and D. Giomi, RSC Adv., 2015, 5, 77341 RSC.
- D. Giomi, M. Malavolti, O. Piccolo, A. Salvini and A. Brandi, RSC Adv., 2014, 4, 46319 RSC.
- The yield of FAME has been determined via 1H NMR analyses by comparing the integral of OCH3 protons of FAME (singlet at δ 3.66) produced in the transesterification process with that of –CH2–COO– protons (multiplet at δ 2.40–2.21). See: G. Gelbard, O. Brès, R. M. Vargas, F. Vielfaure and U. F. Schuchardt, J. Am. Oil Chem. Soc., 1995, 72, 1239 CrossRef CAS.
- The reaction crude appeared as a yellow-orange, clear solution (only one phase) confirming the almost total conversion of glycerol into bromohydrins soluble in the reaction medium. The higher solubility of castor oil in MeOH allowed even an easier conversion of glycerol.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533 CrossRef CAS.
- For the use of catalysts and co-reagents such as K2CO3, SeO2, BiCl3, DMSO, and more recently natural sodium montmorillonite, see: (a) M. Lissel and K. Drechsler, Synthesis, 1983, 314 CrossRef CAS; (b) J. G. Lee and K. K. Kang, J. Org. Chem., 1988, 53, 3634 CrossRef CAS; (c) C. Le Roux, H. Gaspard-Iloughmane and J. Dubac, J. Org. Chem., 1994, 59, 2238 CrossRef CAS; (d) D. C. Synder, J. Org. Chem., 1995, 60, 2638 CrossRef; (e) M. A. Tandiary, Y. Masui and M. Onaka, Synlett, 2014, 25, 2639 CrossRef CAS.
- N. Ajvazi and S. Stavber, Tetrahedron Lett., 2016, 57, 2430 CrossRef CAS.
- Operating in the same conditions but with dry sunflower oil [kept on activated molecular sieves (4 Å) under vacuum for 24 hours], no improvements were observed (1H NMR analysis).
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra of reaction mixtures and purified products. See DOI: 10.1039/d1ra01980e |
| This journal is © The Royal Society of Chemistry 2021 |