Open Access Article
Open Access ArticleHigh catalytic performance of Al–Pd–(Ru, Fe) icosahedral approximants for acetylene semi-hydrogenation†
Keishi Abeab,
Ryota Tsukudaab,
Nobuhisa Fujita b and
Satoshi Kameoka
b and
Satoshi Kameoka *b
*b
aDepartment of Materials Processing, Graduate School of Engineering, Tohoku University, Sendai 980-8579, Japan
bInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, Sendai 980-8577, Japan. E-mail: satoshi.kameoka.b4@tohoku.ac.jp
First published on 23rd April 2021
Abstract
Three cubic crystalline icosahedral approximants (C phase: Al72.0Pd16.4Fe11.6, P40 phase: Al72.0Pd16.4Ru11.6, P20 phase: Al70.0Pd22.3Ru7.7) exhibit high ethylene selectivity of over 90% for hydrogenating acetylene at 150 °C. Moreover, the powdered P20 also demonstrates a high catalytic performance under an industry-like ethylene feed containing 0.5% acetylene as an impurity. Overall, icosahedral approximants in the Al–Pd–(Ru, Fe) systems are promising as a novel class of alloy catalysts.
1 Introduction
Intermetallic compounds (IMCs) exhibit a wide range of attractive functions and characteristics that are not attainable in ordinary elemental metals, such as a shape memory effect,1 superconductivity.2 and hydrogen absorption3 They also possess excellent properties for hydrogenation catalysis, such as those demonstrated in Heusler alloys,4,5 Zintl phases,6 and hydrogen absorbing alloys.3,7,8 Quasicrystals9 are complex IMCs with aperiodic structures that often exhibit five-fold symmetry. Crystalline approximants, on the other hand, are periodic crystals having a similar chemical composition and atomic10,11 and electronic structures12 closely related to quasicrystals. The study of approximants is therefore crucial for understanding the structure and physical properties of quasicrystals. Despite earlier studies on quasicrystals as catalyst precursors,13–15 there has been a recent awakening of interest in the ability of quasicrystals and approximants themselves to catalyze useful reactions at their terminated surfaces.Recently, Kovnir and Armbrüster et al. demonstrated that GaPd, an IMC with a B20-type structure, is highly stable and selective for the semi-hydrogenation of acetylene to ethylene.16,17 These authors proposed that the isolation of the catalytic element, Pd, in the Ga matrix as well as an alteration of the electronic structure due to alloying are the keys to the high catalytic performance. This assessment was soon extended to Al13Fe4 (ref. 18) and Al13Co4 (ref. 19) complex IMCs as noble-metal-free semi-hydrogenation catalysts. The fundamental processes in these catalytic reactions has been discussed based on the active site geometry on the surface.20 as well as on the adsorption energies for the reactant molecules21
These studies revealed that the covalently bonded pentagonal TMAl5 (TM = transition metal) complex on the surface provides active sites for both chemisorption of hydrocarbon species and dissociative adsorption of hydrogen,20 and thus the specific surface structure plays a key role in the semi-hydrogenation of acetylene. The complex ensemble effect of TMAl5 pentagons was also shown to be the origin of the activity for butadiene semi-hydrogenation.21
On the other hand, density functional theory (DFT) calculations demonstrated that the (210) surface of an AlPd (or GaPd) IMC with a B20-type crystal structure exposes active sites for the semi-hydrogenation of acetylene with extraordinarily high activity.22,23 These active sites are associated with a triangular atomic configuration consisting of two Al (or Ga) atoms and one Pd atoms called a PdAl2 triplet.
In fact, similar triplets may play a key role in the case of Al13TM4 because the TMAl5 complex can be decomposed into five triangles each with two Al atoms and one TM atom, which is similar to the PdAl2 triplet arrangement. Similar atomic configurations to PdAl2 are frequently encountered in icosahedral quasicrystals and related approximants in Al–Pd–(Ru, Fe) systems. In other words, PdAl2 triplets are involved in the atomic arrangements within the constituent clusters of these compounds.
Moreover, theory has shown that the selectivity for ethylene increases with increasing number of Ga atoms in the 1st coordination shell of each Pd atom,24 and this trend probably also holds for Al–Pd. GaPd (or AlPd) which can be taken as the simplest analogue to the local atomic structure of icosahedral quasicrystals, and has at most four Ga (or Al) atoms adjacent to each Pd atom exposed on the surface. An Al–Pd–(Ru, Fe) approximant, on the other hand, can have up to five Al atoms surrounding the Pd atom, thus it is expected to show more prominent catalytic performance. To date, however, the catalytic performance of the Al–Pd–(Ru, Fe) approximants have rarely been investigated. We hereby present the first catalytic tests of three selected approximants in the Al–Pd–(Ru, Fe) systems for semi-hydrogenation of acetylene to experimentally demonstrate the high catalytic performance of these compounds. A possible origin of their catalytic properties is discussed in terms of their local atomic configurations.
2 Experimental
2.1 Samples
The samples used in this work were cubic crystalline approximants (C phase: Al72.0Pd16.4Fe11.6, P40 phase: Al72.0Pd16.4Ru11.6, P20 phase: Al70.0Pd22.3Ru7.7) related to icosahedral quasicrystals. The pure Al, Pd, and Ru and/or Fe metals were melted in an argon atmosphere by using an arc furnace. Each ingot was typically of about 5 g. Parts of the ingot were annealed under an Ar atmosphere for up to 120 h (see Table S1 of ESI,† for annealing condition of all sample and see Section 1 of ESI,† for more details on sample preparation). The target approximant phases were confirmed by using powder X-ray diffraction (PXRD) patterns. Moreover, firmer evidence of the approximant phases was provided by selected area electron diffraction (SAED) patterns.2.2 Catalytic test
The samples for catalytic test were prepared in a glove box under an Ar atmosphere to prevent surface oxidation. The samples were crushed in an agate mortar and sieved to size in the range of 25–75 μm. About 150 mg of fine-grained samples were packed in a quartz glass plug-flow reactor (inner diameter 7 mm) and plugged both ends by a ball bulb. The C2H2 hydrogenation tests were performed under a gas feed of 2% C2H2/80% H2 in He, 0.1 MPa, with a flow rate of 30 mL min−1. The hydrogenation under industrial like condition was performed with reaction gases 0.5% C2H2/49% C2H4/12.5% H2 in He, 0.1 MPa, with a flow rate of 32 mL min−1 (see Section 3 of ESI,† for calculations of C2H4 selectivity and conversion).3 Results and discussion
The PXRD patterns of C–AlPdFe, P20–AlPdRu, and P40–AlPdRu indexed as approximants were shown in Fig. 1(a). Fig. 1(b) and (c) show SAED patterns for the P20–AlPdRu sample along three-fold and pseudo five-fold axes, respectively. In Fig. 1(b), the periodic array of spots can be indexed to a cubic crystal with a lattice constant of about 20 Å, as reported by Pavlyuchkov et al.28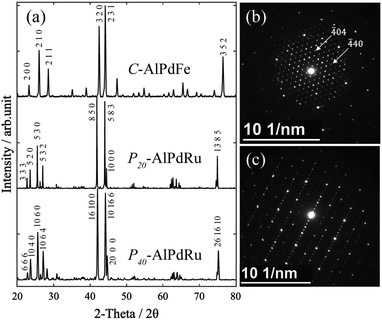 | ||
| Fig. 1 (a) PXRD patterns for Al–Pd–(Ru, Fe) icosahedral approximants. (b and c) SAED patterns for P20–AlPdRu phase, (b) [111], (c) pseudo five-fold zone axis. | ||
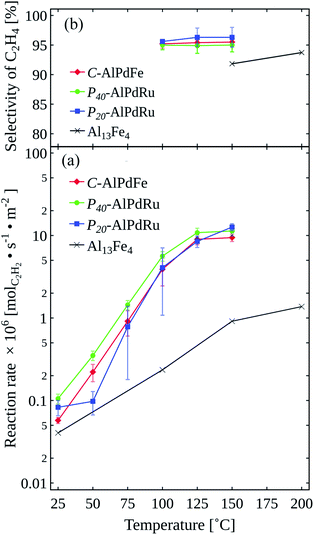
The reaction rates (molC2H2 s−1 m−2) for C2H2 and the C2H4 selectivity (%) are shown in Fig. 2(a) and (b), respectively. All three approximants showed a high ethylene selectivity of above 90% at 100 °C to 150 °C, and the P20–AlPdRu specimen exhibited a maximum value of 98% at 150 °C. All approximants showed roughly the same selectivity, with all three having higher activity than Al13Fe4, which was reported to have high performance for semi-hydrogenation of C2H2, as shown in Fig. 2.
Assuming Langmuir–Hinshelwood (LH) mechanism on a catalytic cycle of the acetylene semi-hydrogenation reaction, reaction steps can be given as follows:
Step 1:
| H2 → [H2(a)] → 2H(a) |
Step 2:
| C2H2 → C2H2(a) |
Step 3:
| C2H2(a) + 2H(a) → [C2H3(a) + H(a)] → C2H4(a) |
Step 4:
| C2H4(a) → C2H4 |
Krajčí and Hafner identified catalytically active sites for C2H2 semi-hydrogenation in a PdAl2 triplet exposed on the pseudo five-fold surface (//210) of B20-type AlPd.22,23 According to their atomistic scenarios via LH mechanism, step (1) occurs on Pd sites that are slightly protruding towards the gas phase. Step (2) and step (3) proceed on PdAl2 triplet sites that again involve a protruding Pd site. At step (2), C atoms in C2H2(a) are di-σ bonded to two Al atoms in the Al–Al bridge position. At step (3), when an H atom is incorporated to from a CH2 group, that end of the molecule is shifted towards and weakly bonded to the Pd atom whereas the bonding of the other end to the Al atom remain rather strong. After another H atom is incorporated, the C atoms are no longer bonded to the Al atoms, so that the molecule C2H4(a) is only weakly π-bonded on top of the Pd atom. The desorption energy of C2H4(a) is reportedly lower than the activation energy of ethylene to ethyl, so that ethylene desorbs at the final step.22,23 As shown in Fig. 3(c) and (d), the present icosahedral approximants in the Al–Pd–(Ru, Fe) systems consist of mini-Bergman clusters (mBCs) and pseudo-Mackay clusters (pMCs).27,29,30 We point out that many PdAl2 triplets in Fig. 3(a) can be observed in the atomic arrangement of these clusters. For instance, five PdAl2 triplets can be readily seen to form a PdAl5 pentagon in the outer shell of pMCs (Fig. 3(b)), while other triplets can be found between the inner and outer shells of mBCs. Hence, the high activity and selectivity of the present approximants for the semi-hydrogenation of C2H2 could be attributed to the presence of similar triplets exposed on the surface.
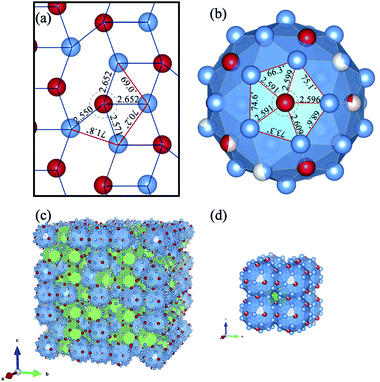 | ||
| Fig. 3 (a) Triangles consisting of two Al atoms and one Pd atom in AlPd (210),25 which are considered to be active for hydrogenation in the calculations. (b) Structure of pMCs constituting P40–AlPdRu.26 (c) Crystal structure of mBCs (shown as green polyhedrons) and pMCs (shown as blue polyhedrons) of P40–AlPdRu.26 Blue, Al; red, Pd; light pink, Ru. (d) Crystal structure of C–AlPdFe.27. | ||
A partial substitution of Ru with Fe in P20–AlPdRu, or Fe with Ru in C–AlPdFe, caused little change in the reaction rate and selectivity, as shown in Section 3 of the ESI,† suggesting that the catalytic performance is generally insensitive to these elemental replacements between Fe and Ru. In other words, these elements seem to contribute much less to the catalytic activity than does Pd. In fact, the crystal structure appear to be much more essential in determining the catalytic properties. The simple binary IMCs, Al3Pd2 (trigonal, P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1) and Al2Ru (orthorhombic, Fddd) (as detailed in Section 1 of the ESI,†), which are both similar in Al composition to the P20–AlPdRu phase but are structurally much simpler, did not compete with the P20–AlPdRu phase in the catalytic activity and selectivity (see Section 4 of the ESI,†). According to Krajčí et al.,22,23 dissociation of hydrogen is possible only at transition metal atoms on the surface, but the Pd atoms need to be isolated because agglomerated transition metal atoms act as strong adsorbent for hydrogen and hydrocarbons, thereby reducing their mobility. Therefore, the high catalytic performance of the present approximants can be attributed to their complex crystal structures.
m1) and Al2Ru (orthorhombic, Fddd) (as detailed in Section 1 of the ESI,†), which are both similar in Al composition to the P20–AlPdRu phase but are structurally much simpler, did not compete with the P20–AlPdRu phase in the catalytic activity and selectivity (see Section 4 of the ESI,†). According to Krajčí et al.,22,23 dissociation of hydrogen is possible only at transition metal atoms on the surface, but the Pd atoms need to be isolated because agglomerated transition metal atoms act as strong adsorbent for hydrogen and hydrocarbons, thereby reducing their mobility. Therefore, the high catalytic performance of the present approximants can be attributed to their complex crystal structures.
The specimens sieved under air did not show the high C2H4 selectivity (see Section 6 of the ESI,†). This is attributed to surface oxidation. Decomposition of the surface structure to Al2O3 and Pd by oxidation seems to trigger Pd aggregation, thus lowering the C2H4 selectivity. This is additional evidence that the surface structure is a key to the high catalytic performance.
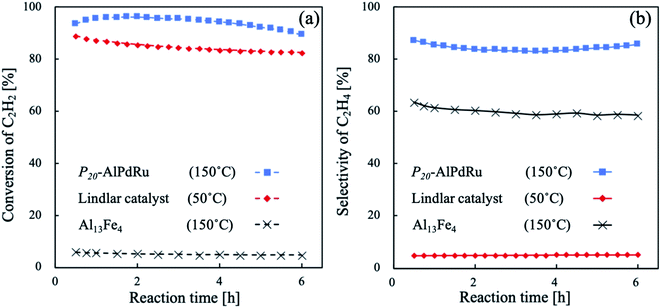
The selective hydrogenation of C2H2 in the C2H4-rich gas is an important reaction for synthesizing polyethylene in the petrochemical industry,31–33 an so we also examined the present samples under a more industry-like C2H4-rich gas feed. In Fig. 4, the catalytic performance of the present P20–AlPdRu powder is compared with those of a commercial Lindlar catalyst and the Al13Fe4 powder under an industry-like C2H4-rich gas containing a 0.5% C2H2 impurity component (0.5% C2H2/49% C2H4/12.5% H2 in He, 0.1 MPa, 32 mL min−1). The P20–AlPdRu phase sample again maintained a high conversion rate of over 80% along with a high ethylene selectivity of more than 83% (for detailed information on how to calculate the conversion and selection rates, see Section 3 of the ESI,†). P20–AlPdRu showed higher selectivity than the commercial Lindlar catalyst and Al13Fe4.
A decrease in the conversion rate from the initial value of 92% was observed up to 6% after 6 h. This can be attributed to the deposition of carbon or higher hydrocarbons, referred to as green oil,34 on the surface of the catalyst as indicated by a carbon-loss rate of about 10% throughout the reaction experiment. Still, it is important to note that the P20–AlPdRu catalyst showed higher selectivity under industry-like conditions without specific optimization of the surface state such as coating with inert elements35,36 or suppressing hydrogenation by CO gas.37,38
4 Conclusion
The quasicrystals and approximants are novel materials with many potential applications that are actively being explored, such as for low friction coefficients, high hardness, brittleness, and low thermal and electrical conductivity.39 However, their crystal structures are often so complex that they are difficult to precisely determine. The structure of P20–AlPdRu, for instance, has yet to be clarified, and those of C–AlPdFe27 and P40–AlPdRu26 are still being argued. Nevertheless, recent crystallographic studies have revealed that their structures include common building blocks involving PdAl2 triplets, which are the key to the semi-hydrogenation reaction. This study showed for the first time that high order Al–Pd–(Ru, Fe) approximants related to icosahedral Al–Pd–Mn quasicrystal are promising as alloy catalysts for the semi-hydrogenation of acetylene, and we expect to see further developments in this respect in the years to come.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by JSPS KAKENHI Grant No. JP19H05819 and JP19H05821.Notes and references
- Shape memory alloys, ed. H. Funakubo, Gordon and Breach Science Publishers, New York, 1987 Search PubMed.
- J. Nagamatsu, N. Nakagawa, T. Muranaka, Y. Zenitani and J. Akimitsu, Nat, 2001, 410, 63–64 CrossRef CAS PubMed.
- R. Tsukuda, R. Yamagishi, S. Kameoka, C. Nishimura and A.-P. Tsai, Sci. Technol. Adv. Mater., 2019, 20, 774–785 CrossRef CAS PubMed.
- T. Kojima, S. Kameoka, S. Fujii, S. Ueda and A.-P. Tsai, Sci. Adv., 2018, 4, eaat6063 CrossRef CAS PubMed.
- T. Kojima, S. Kameoka and A.-P. Tsai, ACS Omega, 2019, 4, 21666–21674 CrossRef CAS PubMed.
- K. L. Hodge and J. E. Goldberger, J. Am. Chem. Soc., 2019, 141, 19969–19972 CrossRef CAS PubMed.
- R. Tsukuda, T. Kojima, D. Okuyama, S. Kameoka, C. Nishimura and A.-P. Tsai, Int. J. Hydrogen Energy, 2020, 45, 19226–19236 CrossRef CAS.
- K. Soga, H. Imamura and S. Ikeda, J. Phys. Chem., 1977, 81, 1762–1766 CrossRef CAS.
- D. Shechtman, I. Blech, D. Gratias and J. W. Cahn, Phys. Rev. Lett., 1984, 53, 1951–1953 CrossRef CAS.
- H. Takakura, C. P. Gómez, A. Yamamoto, M. De Boissieu and A. P. Tsai, Nat. Mater., 2007, 6, 58–63 CrossRef CAS PubMed.
- A. I. Goldman and R. F. Kelton, Rev. Mod. Phys., 1993, 65, 213–230 CrossRef.
- T. Fujiwara and T. Yokokawa, Phys. Rev. Lett., 1991, 66, 333–336 CrossRef CAS PubMed.
- C. J. Jenks and P. A. Thiel, J. Mol. Catal. A: Chem., 1998, 131, 301–306 CrossRef CAS.
- A. Tsai and M. Yoshimura, Appl. Catal., A, 2001, 214, 237–241 CrossRef CAS.
- B. P. Ngoc, C. Geantet, J. A. Dalmon, M. Aouine, G. Bergeret, P. Delichere, S. Raffy and S. Marlin, Catal. Lett., 2009, 131, 59–69 CrossRef CAS.
- K. Kovnir, M. Armbrüster, D. Teschner, T. Venkov, F. Jentoft, A. Knop-Gericke, Y. Grin and R. Schlögl, Sci. Technol. Adv. Mater., 2007, 8, 420–427 CrossRef CAS.
- M. Armbrüster, K. Kovnir, M. Behrens, D. Teschner, Y. Grin and R. Schlögl, J. Am. Chem. Soc., 2010, 132, 14745–14747 CrossRef PubMed.
- M. Armbrüster, K. Kovnir, M. Friedrich, D. Teschner, G. Wowsnick, M. Hahne, P. Gille, L. Szentmiklósi, M. Feuerbacher, M. Heggen, F. Girgsdies, D. Rosenthal, R. Schlögl and Y. Grin, Nat. Mater., 2012, 11, 690–693 CrossRef PubMed.
- M. Armbrüster, K. Kovnir, J. Grin, R. Schlogl, P. Gille, M. Heggen and M. Feuerbacher, European Patent, application No. 09157875A, 2009 Search PubMed.
- M. Krajčí and J. Hafner, J. Catal., 2011, 278, 200–207 CrossRef.
- L. Piccolo, C. Chatelier, M.-C. De Weerd, F. Morfin, J. Ledieu, V. Fournée, P. Gille and E. Gaudry, Sci. Technol. Adv. Mater., 2019, 20, 557–567 CrossRef CAS PubMed.
- M. Krajčí and J. Hafner, J. Phys. Chem. C, 2012, 116, 6307–6319 CrossRef.
- J. Hafner and M. Krají, Acc. Chem. Res., 2014, 47, 3378–3384 CrossRef CAS PubMed.
- M. Krajčí and J. Hafner, J. Phys. Chem. C, 2014, 118, 12285–12301 CrossRef.
- P. Villars, L. D. Calvert and W. B. Pearson, Pearson's handbook of crystallographic data for intermetallic phases, American Society for Metals, Metals Park, Oh, 1985 Search PubMed.
- Y. Hatakeyama, N. Fujita and A. P. Tsai, J. Phys.: Conf. Ser., 2017, 809, 012007 CrossRef.
- L. Hao and F. Changzeng, Crystals, 2019, 9, 526 CrossRef.
- D. Pavlyuchkov, B. Grushko and T. Velikanova, J. Alloys Compd., 2009, 469, 146–151 CrossRef CAS.
- R. Simura, K. Sugiyama, S. Suzuki and T. Kawamata, Mater. Trans., 2017, 58, 1101–1105 CrossRef CAS.
- N. Fujita, H. Takano, A. Yamamoto and A.-P. Tsai, Acta Crystallogr., Sect. A: Found. Crystallogr., 2013, 69, 322–340 CrossRef CAS.
- N. S. Schbib, M. A. García, C. E. Gígola and A. F. Errazu, Ind. Eng. Chem. Res., 1996, 35, 1496–1505 CrossRef CAS.
- A. Borodziński and G. C. Bond, Catal. Rev., 2006, 48, 91–144 CrossRef.
- A. Borodziński and G. C. Bond, Catal. Rev., 2008, 50, 379–469 CrossRef.
- A. Bos and K. Westerterp, Chem. Eng. Process., 1993, 32, 1–7 CrossRef CAS.
- H. Lindlar, Helv. Chim. Acta, 1952, 35, 446–450 CrossRef CAS.
- W. Niu, Y. Gao, W. Zhang, N. Yan and X. Lu, Angew. Chem., Int. Ed., 2015, 54, 8271–8274 CrossRef CAS PubMed.
- D. Pope, J. Catal., 1973, 28, 46–53 CrossRef CAS.
- M. García-Mota, B. Bridier, J. Pérez-Ramírez and N. López, J. Catal., 2010, 273, 92–102 CrossRef.
- J.-M. Dubois, Chem. Soc. Rev., 2012, 41, 6760–6777 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01958a |
| This journal is © The Royal Society of Chemistry 2021 |