Open Access Article
Open Access ArticleTotal and bioaccessible heavy metals in cabbage from major producing cities in Southwest China: health risk assessment and cytotoxicity
Mengying Lia,
Yishu Qina,
Chengchen Wanga,
Kun Wanga,
Zhihua Denga,
Wumei Xuc,
Ping Xiang *a and
Lena Q. Ma*b
*a and
Lena Q. Ma*b
aInstitute of Environmental Remediation and Human Health, School of Ecology and Environment, Southwest Forestry University, Kunming, 650224, China. E-mail: ping_xiang@126.com
bInstitute of Soil and Water Resources and Environmental Science, College of Environmental and Resource Sciences, Zhejiang University, Hangzhou, 310058, China. E-mail: lqma@zju.edu.cn
cYunnan Provincial Observation and Research Station of Soil Degradation and Restoration for Cultivating Plateau Traditional Chinese Medicinal Plants, Yunnan Normal University, Kunming 650500, China
First published on 29th March 2021
Abstract
Green leafy vegetables are economical and nutritious, but they may be contaminated with heavy metals. In this study, we assessed the total and bioaccessible concentrations of As, Cd, Pb and Cr in a popular vegetable cabbage (Brassica oleracea) from four major producing cities in Yunnan, Southwest China. With the mean concentrations of As, Cd, Pb and Cr being 0.24, 0.20, 0.32 and 1.28 mg kg−1, the As, Cd and Pb concentrations were within the limits of 0.2–0.5 mg kg−1 based on Chinese National Standards and the WHO/FAO, but Cr concentration was 2.6-times greater than the limit of 0.5 mg kg−1. Based on an in vitro bioaccessibility assay of the Solubility Bioaccessibility Research Consortium (SBRC), As bioaccessibility was the lowest at 11% while those of Cd, Pb and Cr were much greater at 68–87%. The estimated daily intake (EDI) of metals through cabbage ingestion was similar for children and adults. Among the four metals, only Cr's EDI at 2.29–1.87 exceeded 1 based on total and bioaccessible concentrations. The high Cr concentration at 1.28 mg kg−1 coupled with its high bioaccessibility at 67.5% makes Cr of concern in cabbage. However, human gastrointestinal cells exposed to the gastric digesta with high bioaccessible heavy metals and risky EDI, showed no obvious cytotoxicity, indicating that existing models based on total or bioaccessible heavy metals may overestimate their human health risk. Taken together, to accurately assess the human health risk of heavy metals in cabbage, both total/bioaccessible concentrations and the gastrointestinal cell responses should be considered.
1. Introduction
Given their nutritional values, vegetables are an important part of human diet. However, anthropogenic sources including agricultural and industrial activities have elevated their metal concentrations.1 As such, there is an increasing concern of food safety in recent years.2 Due to metals' toxicity, contamination by heavy metals is a serious health and ecological problem and heavy metal contamination in vegetables represents a threat to the sustainability of human health.3 Factors influencing the concentration of heavy metals in vegetables include climate, environmental pollution, the soil properties, the vegetable species, and the nature of the pollutant.4 Fertilizers also contain heavy metals, thereby becoming an additional source of metal pollution in vegetables.5,6 Metals accumulated in vegetables can easily enter human body through the food chain, adversely impacting human health.7 In China, vegetables are the most important crop besides grain, with production reaching 22 million tons in 2018.8 Thus, it is important to evaluate metal concentrations in vegetables.9Heavy metals are often present in vegetables, with As, Cd and Pb often exceeding the WHO standards.10 However, their risks vary with vegetables and metals.11 Total concentrations have often been used to evaluate the health risks of heavy metals in vegetables, with little information available regarding their bioaccessibility. However, total metal concentration may overestimate their health risk as not all metals are available for human absorption. As such, bioaccessible metal has been used for more accurate risk assessment. Bioaccessible metal refers to the fraction of a metal that is released from its matrix in a simulated gastrointestinal system, which may become available for human absorption.12
To measure metal bioaccessibility, various in vitro methods have been developed. Four common in vitro assays include physiologically-based extraction test (PBET), Solubility Bioaccessibility Research Consortium (SBRC), in vitro gastrointestinal (IVG), and Unified Bioaccessibility Method (UBM), with SBRC method being the easiest to use and being most popular.13 The average bioaccessible metals in vegetables is 20–95%.14,15 However, metal bioaccessibility depends on both food matrix and the method used. Food rich in animal protein showed higher Cd bioaccessibility and foods rich in plant protein had higher Cu bioaccessibility.16 Fu and Cui17 demonstrated that cooking and mineral nutrients (i.e., Ca and Fe) also affected Cd and Pb bioaccessibility. Metal bioaccessibility reflects its soluble fraction in simulated gastrointestinal fluid, but its toxicity is unclear. As such, it is important to determine the cytotoxicity using human cells.
Although in vitro gastrointestinal simulation method is widely accepted in assessing human health risk, its accuracy is recently concerned by scientific community since it is difficult to simulate the physiological function of the gastrointestinal tract and obtain the accurate toxicological data due to its lack of human gastrointestinal cell components. The stomach is an important part of the gastrointestinal tract, and gastric epithelial cells have the function to produce and secrete pepsinogen for gastric digestion.18 Heavy metal damage on human gastric epithelial cells usually results in gastric diseases including gastric cancer.19,20 As such, it is important to validate the bioaccessibility-based health risk assessment results and analyzed the potential cytotoxicity via human cellular experiments.
Among vegetables, cabbage (Brassica oleracea) is popular due to its nutritional values.21 According to FAO,22 China dominates its production in the world, with almost half of the production being from China.23 Yunnan in Southwest China is a major vegetable producer including cabbage.24 As it borders with Myanmar, Vietnam, Laos, and Thailand, ∼70% of vegetables are sold overseas.25 However, Yunnan is also well-known as a major producer of non-ferrous metals, which has elevated metal concentrations in soils including As, Cd, Pb and Cr.26 Therefore, it is important to accurately evaluate metal concentrations in cabbage produced in Yunnan to ensure food safety.
In this study, cabbage from four major producing cities in Yunnan were assessed. The objectives were to: (1) determine total concentrations of As, Cd, Pb and Cr in cabbage; (2) measure their bioaccessible concentrations using the SBRC method; (3) calculate the estimated daily intake of As, Cd, Pb and Cr in cabbage based on total and bioaccessible concentrations; and (4) combined with gastric cell SGC-7901 to explore whether it has the toxicity of cabbage following human consumption. The data may provide insight into metal contamination in cabbage to help improve food safety.
2. Materials and methods
2.1. Sample collection and preparation
Four major producing cities of cabbage including Wuding, Huize, Kunming, and Yimen in Yunnan, Southwest China, were selected (Fig. 1). For each city, cabbage was purchased from three different agricultural trade markets in July–August, 2018. For each sample, ∼0.5 kg of cabbage was collected to make a composite. Following transport to the lab, they were washed with distilled water three times. They were freeze-dried, crushed with a porcelain mortar and pestle, and stored in a refrigerator at −20 °C. Fresh and dry mass of vegetable was weighed using analytical balance. All chemicals were of reagent grade and obtained from Sigma-Aldrich (MO, USA).2.2. Total and bioaccessible metals in cabbage
The samples (0.10 g, dry weight) of the vegetables were digested with concentrated HNO3 (Guaranteed Reagent, GR) and 30% H2O2 (Guaranteed Reagent, AR) according to USEPA Method 3050B and analyzed for heavy metals concentration using inductively coupled plasma mass spectrometry (ICP-MS, ICAPQR, Thermo Fisher Scientific, USA). Besides total concentrations, we also determined the bioaccessible metals in cabbage. The content of analyzed metals in the samples of vegetables was calculated on the fresh weight (fw) by dry weight (dw).Among the four bioaccessibility methods, SBRC is easier to use, which was utilized in this study based on its gastric phase, which contains 30.0 g L−1 glycine at pH 1.5.13 Gastric solution of 20 mL was added to ∼1 g (dw) of sample in a 50 mL centrifuge tube. The mixtures were shaken at 150 rpm and 37 °C in an oscillator for 1 h. After that, samples were centrifuged for 10 min at 4000 rpm, with the supernatant being collected.
The digested solutions and gastric supernatants filtered through 0.45 μm filters were analyzed for As, Cd, Pb and Cr concentrations using ICP-MS, with each sample having three replicates. The detection limits of As, Cd, Pb and Cr were 0.05 mg kg−1. Metal bioaccessibility (%) was defined as the ratio of bioaccessible to total concentration.
2.3. Health risk assessment
The estimated daily intake (EDI: mg kg−1 d−1) of metals from cabbage consumption was calculated based on metal concentrations in cabbage and its consumption rate, which was determined based on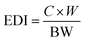 , where C is metal concentrations in cabbage (mg kg−1, fw), W is the average daily consumption of cabbage at 100 and 55 g d−1 for adults and children of age 18–65 and 7–14,27 and BW is the average body weight (kg) at 55.9 and 32.7 kg for adults and children. If the EDI value is higher than 1.0, then there may be adverse health effects on human health.
, where C is metal concentrations in cabbage (mg kg−1, fw), W is the average daily consumption of cabbage at 100 and 55 g d−1 for adults and children of age 18–65 and 7–14,27 and BW is the average body weight (kg) at 55.9 and 32.7 kg for adults and children. If the EDI value is higher than 1.0, then there may be adverse health effects on human health.
2.4. Cell culture, exposure, morphology and viability
SGC-7901 cells (human gastric adenocarcinoma cell) were from China Center for Type Culture Collection, China. The cells were maintained in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin–streptomycin solution in an incubator with humidified atmosphere of 5% CO2 and 95% air at 37 °C. Before cell treatment, SGC-7901 cells were reseeded into 96-well plates or Petri dishes at 2.5 × 104 cells per cm2 density overnight to allow cell attachment.The gastric digesta was then placed in a 90 °C water bath for 5 min to inactivate the enzyme and it were mixed with basic culture medium 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to make exposure solution. The solutions were sterilized by 0.22 μm filter. To determine the cytotoxicity of exposure solution, SGC-7901 cells were seeded into a 96-well plate at the density of 8 × 103 cells per 100 μL per well. After overnight culture, the medium was replaced by 100 μL exposure medium with exposure solution for different time periods treatment as a preliminary experiment. After exposure, cellular morphology was observed and photographed by inverted microscope (TS-100, Nikon, Japan). Subsequently, a CCK-8 cell viability assay kit was employed to measure cell viability according to the manufacturer's instruction.
1 to make exposure solution. The solutions were sterilized by 0.22 μm filter. To determine the cytotoxicity of exposure solution, SGC-7901 cells were seeded into a 96-well plate at the density of 8 × 103 cells per 100 μL per well. After overnight culture, the medium was replaced by 100 μL exposure medium with exposure solution for different time periods treatment as a preliminary experiment. After exposure, cellular morphology was observed and photographed by inverted microscope (TS-100, Nikon, Japan). Subsequently, a CCK-8 cell viability assay kit was employed to measure cell viability according to the manufacturer's instruction.
2.5. Quality controls and statistical analysis
To ensure quality, a blank and a certified reference material (GBW10048, celery GSB-26, Institute of Geophysical & Geochemical Exploration, China) were included. The gastric solution was also included for quality assurance. The experiments were conducted with three replicates. Data were summarized using descriptive statistics based on mean values and standard deviations, with all data being analyzed using Microsoft Excel (Version 2018) and GraphPad Prism Version 8.0 software (GraphPad Software LLC, CA, USA). The significant difference among treatments was assessed at P = 0.05, with Origin 2018 being used for graph (Origin Lab Corp., MA, USA).3. Results and discussions
Cabbage is the leading leafy vegetable in the world, with almost half of the world's production from China.23 High vegetable consumption has been linked to improve human health, as such increased vegetable intake has been widely recommended. However, metal contents in vegetables vary among producing areas, which can be of health concerns. Therefore, there is a need to measure metal contents as well as their bioaccessibility to properly asses their impacts on human health. In this study, we determined total and bioaccessible concentrations of four important metals including As, Cd, Pb and Cr in cabbage from four major producing cities in Yunnan, Southwest China. The allowable limits of As, Cd, Pb, and Cr in cabbage based on Chinese National Standards (2017)28 and WHO/FAO (2019)29 are 0.5, 0.2, 0.3, and 0.5 mg kg−1 (Table 1).| Region | As | Cd | Pb | Cr | Reference |
|---|---|---|---|---|---|
| Yunnan, China | 0.24 | 0.20 | 0.32 | 1.28 | This study |
| Guangdong, China | 0.01 | 0.01 | 0.02 | 0.03 | 57 |
| Hong Kong, China | — | 0.04 | 0.08 | 0.1 | 30 |
| Serbia | <0.03 | <0.01 | 0.05 | — | 31 |
| United Kingdom | — | <0.01 | 0.01 | — | 32 |
| Ghana | — | 0.05 | <0.01 | 0.35 | 58 |
| Uganda | — | 0.13 | 0.35 | 0.99 | 33 |
| Zimbabwe | 0.93 | 0.08 | 11.7 | 19.5 | 59 |
| Allowable limit | 0.5 | 0.2 | 0.3 | 0.5 | China (2017); WHO/FAO (2019) |
3.1. Total As, Cd, Pb and Cr in cabbage
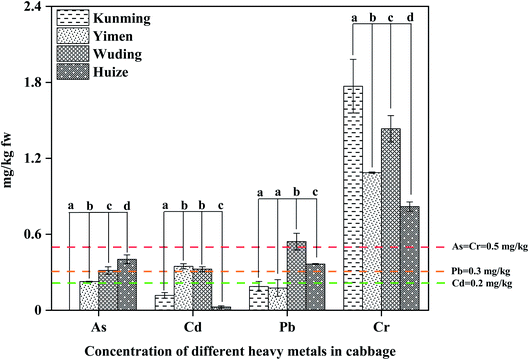
The mean concentrations of As, Cd, Pb and Cr in the cabbage from four main producing cities were 0.24, 0.20, 0.32, and 1.28 mg kg−1 (Table 1). Among the four metals, the Cr concentrations in the cabbage were the highest at 0.82–1.77 mg kg−1 (Fig. 2), exceeding the 0.2 mg kg−1 limit. On the other hand, the As concentrations were 0.05–0.40 mg kg−1, within the 0.5 mg kg−1 limit.28 Among the four cities, the cabbage from Kunming, the capital city of Yunnan, had the lowest metal concentrations, with only Cr exceeding the 0.5 mg kg−1 limit (Fig. 2). In comparison, the cabbage from Wuding had the highest metal concentrations, with only As within the limit (Fig. 2). For example, the Cd concentration at 0.35 mg kg−1 exceeded 0.2 mg kg−1 limit, and the Pb concentration at 0.54 mg kg−1 exceeded 0.3 mg kg−1 limit.29To put metal concentrations in this study into perspectives, we compared our data with the literature (Table 1). The concentrations of As, Cd, Pb and Cr in the cabbage from Guangdong and Hong Kong, China are all low, below 0.1 mg kg−1 (Table 1).30 Similarly, those from Serbia and United Kingdom are also low at <0.05 mg kg−1.31,32 Nabulo et al.33 determined the metal concentrations in the cabbage from contaminated urban soils, which are similar to our data, with Pb and Cr exceeding the limit (Table 1). Similarly, those from Zimbabwe are high, with As, Pb and Cr, exceeding the limit.
The relatively high metal concentrations in the cabbage from Yunnan may be due to the high background metal concentrations in the soils (Table 2). Due to its karst topography with high content of CaCO3, the metal concentrations in soils from Yunnan are naturally high.34 Based on Chen et al.,35 the mean concentrations of As, Cd, Pb and Cr in soils in Yunnan are 19.2, 0.22, 57.6, and 106 mg kg−1 (Table 2), which are 1.2–14 times higher than other regions (except for Cd).36–39 In fact, they are higher than the Chinese soil guidelines for Grade I, which are 15, 0.2, 35, and 90 mg kg−1 (Table 2). So, the high metal concentrations in cabbage may contribute to the high metal concentrations in the soils. Besides high background metal concentrations in soils, application of fertilizers may also contribute to high metal concentrations in vegetables.40
| Region | As | Cd | Pb | Cr | Reference |
|---|---|---|---|---|---|
| Yunnan, China | 19.2 | 0.22 | 57.6 | 106 | 35 |
| Agricultural soils, China | 10.7 | 0.24 | 32.1 | 62.2 | 39 |
| Background value of China | 11.2 | 0.10 | 26.0 | 61.0 | 34 |
| São Paulo, Brazil | 1.38 | 0.1 | 10.1 | 36.6 | 38 |
| USA | 5.20 | 1.60 | 37.0 | 16.0 | |
| England and Wales | 15.0 | 0.33 | 49.0 | 68.0 | 36 |
| Australia | 3.00 | 0.04 | 13.0 | 48.0 | 37 |
| Chinese soil guidelines (Grade I) | 15 | 0.2 | 35 | 90 | 35 |
Among the 4 metals, Cr was the highest in cabbage, averaging 1.28 mg kg−1 (Table 1). Plants in the Brassicaceae family tend to accumulate high levels of Cr.41 Soil–plant transfer of Cr is controlled by the activities of numerous external and endogenous factors.42 Like soil redox potential is one of the important factors affecting plants uptake Cr. Cr exists in two oxidation states of which the reduced form i.e. Cr(III) is quite insoluble in water while the oxidized form (Cr-VI) is highly soluble and readily available in the soil solution to the plants.43 Apart from that, vegetables type can influence Cr absorption as well. The transfer of metals from root to stem and then to fruit during the transpiration and translocation process is longer in nonleafy vegetables and results in lower accumulation.43 Because of their large surface areas, leafy vegetables can readily assimilate atmospheric particles containing heavy metals through their stomatal pores and the cuticle.44 In majority of the cases, Cr is mainly accumulated in roots.2 However, some plant can uptake and translocate high Cr levels in the aerial parts.45 This may have some relation to valences and it has been suggested that trivalent chromium accumulates in the roots, whereas hexavalent chromium is translocated to shoot tissues.46 Of which structural likeness of Cr(VI) with nutrient salts because of it can by way of phosphate, etc. transporter.43,47 Cr uses channels of nutrient elements for up translocation, which causes competition among its. The Brassicaceae family are reported to accumulate high levels of Cr, thereby signifying that Cr is translocated from root to shoot via nutrient elements-uptake and translocation mechanism carriers.41,48 For example, India mustard (Brassica juncea) can tolerate high Cr concentrations because of their ability to sequester Cr in the roots and leaves.49 Some researchers reported that enhanced Cr uptake by plants may be due to its ability to sequestrate it in the vacuoles.41 Others also corroborated that the Brassicaceae species displayed high Cr tolerance.50 Compared to Cr, the concentrations of As, Cd and Pb in cabbage were relatively low. It is may be because the high pH in Karst areas reduce the Cd and Pb availability to plants, leading to low absorption.51 However, it is unclear why As availability to plants in this study was low. Given that, more possible measures like soil remediation should be considered to prevent the excess accumulation of heavy metals in the vegetables.
3.2. Bioaccessible As, Cd, Pb and Cr in cabbage
Besides total metal concentrations, we also determined bioaccessible metals in the cabbage based on the SBRC method (Fig. 3). The mean bioaccessibility of As, Cd, Pb and Cr were 11.3, 87.3, 78.1, and 67.5% based on the gastric phase. Among the four metals, As showed the lowest bioaccessibility at 11.3%, which was inconsistent with Pizarro et al.52 that As in beets and carrots showed high bioavailability at 90 and 98%. This difference may be attributed to differences in plants and As species. Studies showed that inorganic As was easier to be released from the food matrix during gastrointestinal digestion than organic As.53 The acidic environment in the simulated stomach will result in a more efficient breakdown of the food protein fraction and it has been found that inorganic As species are more likely to bind to the protein matrix of endosperm cells more particularly by complexing to thiol containing amino acids.54 However, cabbage is abundant in glucosinolates and it exists in plant vacuoles. When plant cells are affected by the external environment, glucosinolates will be degraded and production of secondary sulphur metabolites. More probably, arsenic bound to proteins through cysteine and other sulfur complexes that could not be extracted in the conditions of the stomach and small intestine.55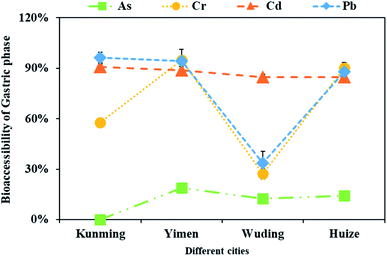 | ||
| Fig. 3 The bioaccessibility of As, Cd, Pb and Cr in the cabbage from four cities of Yunnan, Southwest China based on SBRC method. | ||
While Cd showed the highest 87.3%. The data are similar to Hu et al.30 who showed Cd bioaccessibility at 71% in various cabbages from Hong Kong. Similarly, Fu and Cui17 showed Cd bioaccessibility at 65% in Pakchoi cabbage (Brassica rapa). Similarly, Hu et al.30 who showed Cd bioaccessibility at 71% in various cabbages from Hong Kong. However, the concentration of Cd in cabbages from Hong Kong was 0.03 mg kg−1. In this study, the concentration of Cd is 0.20 mg kg−1, and its bioaccessibility at 87.3%. Irrespective of the concentration of Cd in cabbage, it had high bioaccessibility in the gastric phase. Studies have reported that most Cd accumulates in the vacuoles of plant cells, except that absorbed by cell wall, so Cd is easily released from plant tissues.17 In addition to this, it was may be related to the pH value of gastric juice in gastric phase. The low pH of the gastric juice may result in the breakdown of chemical bonds between metals with carbohydrates and proteins which can lead to substantial release of Cd in the gastric phases.16
3.3. Health risk assessment of As, Cd, Pb and Cr in cabbage and cellular validation
The health risk assessment formula is a method to evaluate human health based on pollutants, which can predict the possibility of harmful effects of pollutants on human health. The evaluation formula is also the most direct way to predict the human health risk of heavy metals in food. The estimated daily intake (EDI) of As, Cd, Pb and Cr from cabbage consumption is presented in Table 3. Similar to their total concentrations, Cr showed the highest risk (2.29–2.15), with As, Cd and Pb (0.57–0.02) being below one (Fig. 2). Among four metals, only Cr EDI was great than 1 for both adults and children, indicating potential health risk to human health. The data suggest that excessive consumption of cabbage may contribute to Cr accumulation in humans, which deserves more attention. It had been suggested that the bioaccessible fraction of metals rather than total heavy metals levels was needed to assess the level of health risk. Thus, a health risk assessment based on heavy metal concentration of total in cabbages will bring a large uncertainty when assessing health risk. To accurately assess the risk associated with cabbage consumption, we estimated the EDI based on their bioaccessible metals at 11.3–87.3% (Table 3). The risks based on bioaccessibility were lower, with only Cr showing >1 (1.99–1.87; Table 3). Although the high risk of B-EDI, the value has declined. The estimation of metal bioaccessibility in cabbages is helpful for human health risk assessment. Given that, bioaccessibility is therefore considered viable as a risk standard of the detection of human health by scientific community.| Metal | Total | Bioaccessible | ||
|---|---|---|---|---|
| Adult | Child | Adult | Child | |
| As | 0.43 | 0.40 | 0.05 | 0.04 |
| Cd | 0.36 | 0.34 | 0.03 | 0.02 |
| Pb | 0.57 | 0.54 | 0.45 | 0.42 |
| Cr | 2.29 | 2.15 | 1.99 | 1.87 |
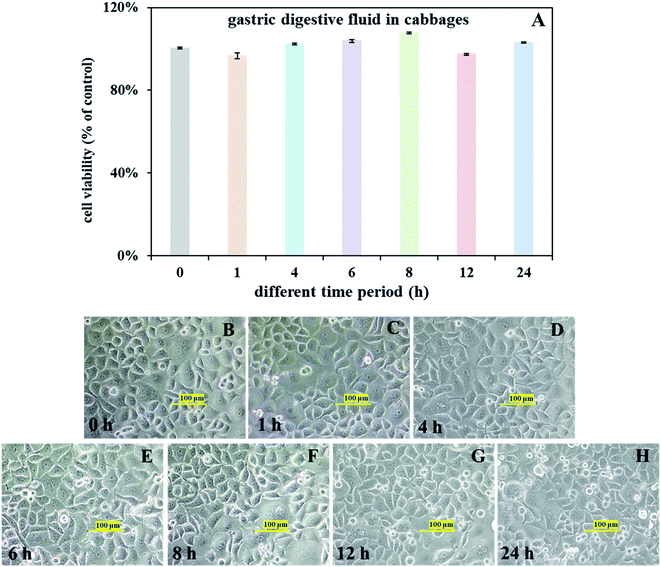
Although in vitro gastrointestinal simulation method is acceptable in calculating health risk, it is difficult to simulate the physiological function of the gastrointestinal tract and obtain the accurate toxicological data due to its lack of human gastrointestinal cell components. The stomach is an important part of the gastrointestinal tract, and gastric epithelial cells have the function to produce and secrete pepsinogen for gastric digestion.18 Heavy metal damage on human gastric epithelial cells usually results in gastric diseases including gastric cancer.19,20 As such, it is important to evaluate the bioaccessible heavy metal in cabbages on gastric epithelial cells. To overcome the shortcomings of in vitro gastrointestinal simulation method and validate above-mentioned results of bioaccessibility-based health risk assessment, human gastric epithelial cells (SGC7901) were firstly employed in present study to validate the high risk of bioaccessibility-based assessments and analyzed the potential cytotoxicity. Cellular responses were well-known to be a sensitive and accurate way to evaluate the human health risks of xenobiotic pollutants.19,56 Cell viability is an important indicator to reflect cellular toxicity to heavy metals.56 To further validate the result of human health risk assessment and study its potential gastric cytotoxicity, SGC-7901 cells were employed and exposed to the gastric digesta with high bioaccessible heavy metals and risky EDI for 0–24 h. Interestingly, cell viability and morphology were not affected (Fig. 4) even treated with samples with highest risk (Cr EDI value >2.15). That's maybe the fact that most of the current health risk assessment models depend on stoichiometric calculation of the concentrations and types of contaminants, but ignored the cellular ability of adaptation and defense (e.g., antioxidation system) to environmental stress. Given that, existing models based on total or bioaccessible heavy metals may overestimate their human health risk. Taken together, to accurately assess the human health risk of heavy metals in cabbage, cellular responses also should be considered.
4. Conclusion
This study revealed the presence of As, Cd, Pb, and Cr concentration and bioaccessibility in indigenous cabbage from the agricultural trade market in Yunnan as well as metals from cabbage by adults and children for their health risk implications from consuming cabbage in terms of EDI. The concentration of As was within the Chinese National Standards, but the average concentration of Cr, Pb and Cd were 2.55, 1.02 and 1.07 times higher than standards, respectively. The average bioaccessibility of the four heavy metals from cabbage varied within 4.26–96.2% using an SBRC method. Taken together, these results suggest that the heavy metal concentrations changed among cabbages from different cities, owing to their different cumulation capabilities. In particular, the cabbage possesses a stronger ability to absorb Cr and high bioaccessibility of Cd in cabbage. An implication of this is the possibility that the edible those vegetables may be injurious to human health. From the risk assessment methods (EDI) applied in this study, it can be concluded that the associated risks are almost equal for children than they are for adults for Cr concentration in the vegetable. However, the health risk of EDI associated with the ingestion of these metal-contaminated vegetables is high (Cr > 1), for both children and adults. The bioaccessibility of heavy metals in vegetables was used to correct the EDI formula and the reduction of B-EDI values. Despite that, the potential for local residents through cabbage consumption should not be overlooked. In addition, human gastrointestinal cells didn't show any adverse effects after exposure to the gastric digesta with high bioaccessible heavy metals and risky EDI, indicating existing models based on total or bioaccessible heavy metals may overestimate their human health risk. To accurately assess the human health risk of heavy metals in cabbage, cellular responses should be considered.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was partially supported by the Yunnan Innovative Research Team (202005AE160017), the Yunnan Agricultural Joint Foundation Projects (2018FG001-048), the National Natural Science Foundation of China (41967026), the Scientific and Technological Innovation Top Young Talents Project of National Forestry and Grassland Administration (2020132613), the Yunnan Thousand Youth Talent Program (YNQR-QNRC-2018-049), the Open Project of Beijing Key Laboratory of Toxicological Research and Risk Assessment for Food Safety (KF-2020-01) and the National College Students Innovation Training Program (202010677006).References
- S. Gallego, L. Pena, R. Barcia, C. Azpilicueta, F. Iannone, E. Rosales, M. Zawoznik, M. Groppa and M. P. Benavides, Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms, Environ. Exp. Bot., 2012, 83, 33–46 CrossRef CAS.
- S. Jaison and T. Muthukumar, Chromium accumulation in medicinal plants growing naturally on tannery contaminated and non-contaminated soils, Biol. Trace Elem. Res., 2016, 1–13 Search PubMed.
- H. Han, X. Sheng, J. Hu, L. He and Q. Wang, Metal-immobilizing Serratia liquefaciens CL-1 and Bacillus thuringiensis X30 increase biomass and reduce heavy metal accumulation of radish under field conditions, Ecotoxicol. Environ. Saf., 2018, 161, 526–533 CrossRef CAS PubMed.
- G. Qin, Z. Niu, J. Yu, Z. Li and P. Xiang, Soil heavy metal pollution and food safety in china: effects, sources and removing technology, Chemosphere, 2021, 267, 129205 CrossRef CAS PubMed.
- T. Xiong, C. Dumat, A. Pierart, M. Shahid, Y. Kang, N. Li, G. Bertoni and C. Laplanche, Measurement of metal bioaccessibility in vegetables to improve human exposure assessments: field study of soil–plant–atmosphere transfers in urban areas, South China, Environ. Geochem. Health, 2016, 38(6), 1283–1301 CrossRef CAS PubMed.
- B. Lei, K. Lu, F. Ding, K. Zhang, Y. Chen, H. Zhao, L. Zhang, Z. Ren, C. Qu, W. Guo, J. Wang and W. Pan, RNA sequencing analysis reveals transcriptomic variations in tobacco (Nicotiana tabacum) leaves affected by climate, soil, and tillage factors, Int. J. Mol. Sci., 2014, 15(4), 6137–6160 CrossRef CAS PubMed.
- K. S. Mohammed Abdul, S. S. Jayasinghe, E. P. S. Chandana, C. Jayasumana and P. M. C. S. De Silva, Arsenic and human health effects: A review, Environ. Toxicol. Pharmacol., 2015, 40(3), 828–846 CrossRef CAS PubMed.
- Statistics N. B., National Bureau of Statistics of China, 2018.
- S. Kumar, S. Prasad, K. K. Yadav, M. Shrivastava, N. Gupta, S. Nagar, Q. Bach, H. Kamyab, S. A. Khan, S. Yadav and L. C. Malav, Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches-A review, Environ. Res., 2019, 179, 108792 CrossRef CAS PubMed.
- P. Wachirawongsakorn, Health risk assessment via consumption of Pb and Cd contaminated vegetables collected from fresh markets in the lower north of Thailand, Human and Ecological Risk Assessment: An International Journal, 2016, 22(3), 611–622 CrossRef CAS.
- V. Antoniadis, J. Boersch, T. Frohne, G. Du Laing and J. Rinklebe, Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in Germany, J. Environ. Manage., 2017, 186, 192–200 CrossRef CAS PubMed.
- J. R. Dean, Heavy metal bioavailability and bioaccessibility in soil, in Bioremediation, Humana Press, 2010, pp. 15–36 Search PubMed.
- H. B. Li, M. Y. Li, D. Zhao, J. Li, S. W. Li, P. Xiang, A. L. Juhasz and L. Q. Ma, Arsenic, lead, and cadmium bioaccessibility in contaminated soils: Measurements and validations, Crit. Rev. Environ. Sci. Technol., 2020, 50(1), 1303–1338 CrossRef CAS.
- S. M. Praveena and N. A. Omar, Heavy metal exposure from cooked rice grain ingestion and its potential health risks to humans from total and bioavailable forms analysis, Food Chem., 2017, 235(15), 203–211 CrossRef CAS PubMed.
- G. M. Chiocchetti, T. Latorre, M. J. Clemente, C. Jadán-Piedra, V. Devesa and D. Vélez, Toxic trace elements in dried mushrooms: Effects of cooking and gastrointestinal digestion on food safety, Food Chem., 2020, 306, 125478 CrossRef CAS PubMed.
- K. Sharafi, R. N. Nodehi, A. H. Mahvi, M. Pirsaheb, S. Nazmara, B. Mahmoudi and M. Yunesian, Bioaccessibility analysis of toxic metals in consumed rice through an in vitro human digestion model – Comparison of calculated human health risk from raw, cooked and digested rice, Food Chem., 2019, 299(30), 125126 CrossRef CAS PubMed.
- J. Fu and Y. Cui, In vitro digestion/Caco-2 cell model to estimate cadmium and lead bioaccessibility/bioavailability in two vegetables: the influence of cooking and additives, Food Chem. Toxicol., 2013, 59, 215–221 CrossRef CAS PubMed.
- X. T. Hu, C. Ding, N. Zhou and C. Xu, Quercetin protects gastric epithelial cell from oxidative damage in vitro and in vivo, Eur. J. Pharmacol., 2015, 754, 115–124 CrossRef CAS PubMed.
- K. Wang, J. Y. Ma, M. Y. Li, Y. S. Qin, X. C. Bao, C. C. Wang, D. L. Cui, P. Xiang and L. Q. Ma, Mechanisms of Cd and Cu induced toxicity in human gastric epithelial cells: Oxidative stress, cell cycle arrest and apoptosis, Sci. Total Environ., 2021, 756, 143951 CrossRef CAS PubMed.
- W. Yuan, N. Yang and X. Li, Advances in understanding how heavy metal pollution triggers gastric cancer, BioMed Res. Int., 2016, 1–10 Search PubMed.
- H. Nawaz, M. A. Shad and A. Rauf, Optimization of extraction yield and antioxidant properties of Brassica oleracea Convar Capitata Var L. leaf extracts, Food Chem., 2018, 242, 182–187 CrossRef CAS PubMed.
- FAO, Food and Agriculture Organization of the United Nations, 2017.
- N. Aramrueang, S. Asavasanti and A. Khanunthong, Leafy Vegetables, in Integrated Processing Technologies for Food and Agricultural By-Products, ed. Z. Pan, R. Zhang and S. Zicari, Academic Press, 2019, ch. 10, pp. 245–272 Search PubMed.
- F. Zhu, L. Qu, W. Fan, M. Qiao, H. Hao and X. Wang, Assessment of heavy metals in some wild edible mushrooms collected from Yunnan Province, China, Environ. Monit. Assess., 2011, 179(1–4), 191–199 CrossRef CAS PubMed.
- W. Yang and P. Kang, Research on Yunnan Vegetable Export Industry in the Context of “Belt and Road Initiative”, in 4th International Symposium on Business Corporation and Development in South-East and South Asia under B&R Initiative (ISBCD 2019), Atlantis Press, 2020, pp. 214–218 Search PubMed.
- J. Qi, H. Zhang, X. Li, J. Lu and G. Zhang, Concentrations, spatial distribution, and risk assessment of soil heavy metals in a Zn–Pb mine district in southern China, Environ. Monit. Assess., 2016, 188(7), 1–11 CrossRef CAS PubMed.
- Dietary guidelines for Chinese residents (in Chinese), ed. Nutrition C., People's Medical Publishing House, 2016 Search PubMed.
- China, Maximum levels of contaminants in food, National Food Safety Standard GB 2762-2017, Ministry of Health of the People's Republic of China, 2017 Search PubMed.
- WHO, General Standard for Contaminants and Toxins in Food and Feed CXS 193-1995, Codex Alimentarius International Food Standards, 2019 Search PubMed.
- J. Hu, F. Wu, S. Wu, Z. Cao, X. Lin and M. H. Wong, Bioaccessibility, dietary exposure and human risk assessment of heavy metals from market vegetables in Hong Kong revealed with an in vitro gastrointestinal model, Chemosphere, 2013, 91, 455–461 CrossRef CAS PubMed.
- B. Škrbić, J. Živančev and N. Mrmoš, Concentrations of arsenic, cadmium and lead in selected foodstuffs from Serbian market basket: Estimated intake by the population from the Serbia, Food Chem. Toxicol., 2013, 58, 440–448 CrossRef PubMed.
- G. J. Norton, C. M. Deacon, A. Mestrot, J. Feldmann, P. Jenkins, C. Baskaran and A. A. Meharg, Cadmium and lead in vegetable and fruit produce selected from specific regional areas of the UK, Sci. Total Environ., 2015, 533, 520–527 CrossRef CAS PubMed.
- G. Nabulo, S. D. Young and C. R. Black, Assessing risk to human health from tropical leafy vegetables grown on contaminated urban soils, Sci. Total Environ., 2010, 408, 5338–5351 CrossRef CAS PubMed.
- Y. Wen, W. Li, Z. Yang, Q. Zhang and J. Ji, Enrichment and source identification of Cd and other heavy metals in soils with high geochemical background in the karst region, Southwestern China, Chemosphere, 2020, 245, 125620 CrossRef CAS PubMed.
- H. Chen, Y. Teng, S. Lu, Y. Wang and J. Wang, Contamination features and health risk of soil heavy metals in China, Sci. Total Environ., 2015, 512, 143–153 CrossRef PubMed.
- B. G. Rawlins, S. P. McGrath, A. J. Scheib, N. Breward, M. Cave, T. R. Lister, M. Ingham, C. Gowing and S. Carter, The advanced soil geochemical atlas of England and Wales, British Geological Survey, 2012 Search PubMed.
- C. Reimann and P. de Caritat, New soil composition data for Europe and Australia: Demonstrating comparability, identifying continental-scale processes and learning lessons for global geochemical mapping, Sci. Total Environ., 2012, 416, 239–252 CrossRef CAS PubMed.
- T. A. R. Nogueira, C. H. Abreu-Junior, L. R. F. Alleoni, Z. He, M. R. Soares, C. D. Santos Vieira, L. G. F. Lessa and G. F. Capra, Background concentrations and quality reference values for some potentially toxic elements in soils of São Paulo State, Brazil, J. Environ. Manage., 2018, 221, 10–19 CrossRef CAS PubMed.
- Y. Huang, L. Wang, W. Wang, T. Li, Z. He and X. Yang, Current status of agricultural soil pollution by heavy metals in China: A meta-analysis, Sci. Total Environ., 2019, 651, 3034–3042 CrossRef CAS PubMed.
- C. Vogel, M. Radtke, U. Reinholz, F. Schäfers and C. Adam, Chemical state of chromium, sulfur, and iron in sewage sludge ash based phosphorus fertilizers, ACS Sustainable Chem. Eng., 2015, 3(10), 2376–2380 CrossRef CAS.
- H. P. Singh, P. Mahajan, S. Kaur, D. R. Batish and R. K. Kohli, Chromium toxicity and tolerance in plants, Environ. Chem. Lett., 2013, 11(3), 229–254 CrossRef CAS.
- L. M. Sandalio, M. Rodríguez-Serrano, D. K. Gupta, A. Archilla, M. C. Romero-Puertas and A. Luis, Reactive oxygen species and nitric oxide in plants under cadmium stress: from toxicity to signaling, in Environmental adaptations and stress tolerance of plants in the era of climate change, Springer, New York, NY, 2012, pp. 199-215 Search PubMed.
- N. Gupta, K. K. Yadav, V. Kumar, S. Kumar, R. P. Chadd and A. Kumar, Trace elements in soil–vegetables interface: Translocation, bioaccumulation, toxicity and amelioration – A review, Sci. Total Environ., 2019, 651, 2927–2942 CrossRef CAS PubMed.
- G. Guo, D. Zhang and Y. Wang, Probabilistic Human Health Risk Assessment of Heavy Metal Intake via Vegetable Consumption around Pb/Zn Smelters in Southwest China, Int. J. Environ. Res. Public Health, 2019, 16(18), 3267 CrossRef CAS PubMed.
- L. M. de Oliveira, J. Gress, J. De, B. Rathinasabapath, G. Marchi, Y. Chen and L. Q. Ma, Sulfate and chromate increased each other's uptake and translocation in As-hyperaccumulator Pteris vittata, Chemosphere, 2016, 147, 36–43 CrossRef CAS PubMed.
- A. E. M. Rebhi, H. Lounici, M. B. Lahrech and J. L. Morel, Response of Artemisia herba alba to hexavalent chromium pollution under arid and semi-arid conditions, Int. J. Phytorem., 2019, 21(3), 224–229 CrossRef CAS PubMed.
- L. M. de Oliveira, L. Q. Ma, J. A. Santos, L. R. Guilherme and J. T. Lessl, Effects of arsenate, chromate, and sulfate on arsenic and chromium uptake and translocation by arsenic hyperaccumulator Pteris vittata L., Environ. Pollut., 2014, 184, 187–192 CrossRef CAS PubMed.
- C. Cervantes, J. Campos-García, S. Devars, F. Gutiérrez-Corona, H. Loza-Tavera, I. C. Torres-Guzmán and R. Moreno-Sánchez, Interactions of chromium with microorganisms and plants, FEMS Microbiol. Rev., 2001, 25(3), 335–347 CrossRef CAS PubMed.
- H. Chen, J. M. Arocena, J. Li, R. W. Thring and J. Zhou, Assessments of chromium (and other metals) in vegetables and potential bio-accumulations in humans living in areas affected by tannery wastes, Chemosphere, 2014, 112, 412–419 CrossRef CAS PubMed.
- X. Cao, X. Wang, W. Tong, H. K. Gurajala, M. Lu, Y. Hamid, Y. Feng, Z. He and X. Yang, Distribution, availability and translocation of heavy metals in soil-oilseed rape (Brassica napus L.) system related to soil properties, Environ. Pollut., 2019, 252, 733–741 CrossRef CAS PubMed.
- Y. Wen, W. Li, Z. Yang, X. Zhuo, D. Guan, Y. Song, C. Guo and J. Ji, Evaluation of various approaches to predict cadmium bioavailability to rice grown in soils with high geochemical background in the karst region, Southwestern China, Environ. Pollut., 2020, 258, 113645 CrossRef CAS PubMed.
- I. Pizarro, M. Gómez-Gómez, J. León, D. Román and M. A. Palacios, Bioaccessibility and arsenic speciation in carrots, beets and quinoa from a contaminated area of Chile, Sci. Total Environ., 2016, 565, 557–563 CrossRef CAS PubMed.
- E. Lombi, K. G. Scheckel and J. Pallon, Speciation and distribution of arsenic and localization of nutrients in rice grains, New Phytol., 2009, 184, 193–201 CrossRef CAS PubMed.
- P. Alava, G. Du Laing, F. Tack, T. De Ryck and T. Van De Wiele, Westernized diets lower arsenic gastrointestinal bioaccessibility but increase microbial arsenic speciation changes in the colon, Chemosphere, 2015, 119, 757–762 CrossRef CAS PubMed.
- T. Chávez-Capilla, M. Beshai, W. Maher, T. Kelly and S. Foster, Bioaccessibility and degradation of naturally occurring arsenic species from food in the human gastrointestinal tract, Food Chem., 2016, 212, 189–197 CrossRef PubMed.
- P. Xiang, K. Wang, J. Bi, M. Y. Li, R. W. He, D. L. Cui and L. Q. Ma, Organic extract of indoor dust induces estrogen-like effects in human breast cancer cells, Sci. Total Environ., 2020, 726, 138505 CrossRef CAS PubMed.
- Y. Liang, X. Yi, Z. Dang, Q. Wang, H. Luo and J. Tang, Heavy Metal Contamination and Health Risk Assessment in the Vicinity of a Tailing Pond in Guangdong, China, Int. J. Environ. Res. Public Health, 2017, 14, 1557 CrossRef PubMed.
- S. T. Ametepey, S. J. Cobbina, F. J. Akpabey, A. B. Duwiejuah and Z. N. Abuntori, Health risk assessment and heavy metal contamination levels in vegetables from Tamale Metropolis, Ghana, International Journal of Food Contamination, 2108, 5(1), 1–8 Search PubMed.
- M. L. Meck, D. Mudimbu and T. C. Davies, Accumulation of potentially harmful elements in edible parts of vegetables grown on two different geological substrates in Zimbabwe, J. Geochem. Explor., 2020, 208, 106392 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |