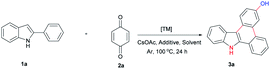Open Access Article
Open Access ArticleDual C–H activation: Rh(III)-catalyzed cascade π-extended annulation of 2-arylindole with benzoquinone†
Qijing Zhang‡
,
Qianrong Li‡ and
Chengming Wang *
*
Department of Chemistry, College of Chemistry and Materials Science, Jinan University, Guangzhou, 511443, China. E-mail: cmwang2019@jnu.edu.cn
First published on 7th April 2021
Abstract
A rhodium-catalyzed, N–H free indole directed cyclization reaction of benzoquinone via a dual C–H activation strategy is disclosed. This protocol has a good functional group tolerance and affords useful indole-fused heterocylces. Besides, it is insensitive to moisture, commercially available solvent can be directly used and work quite well for this transformation.
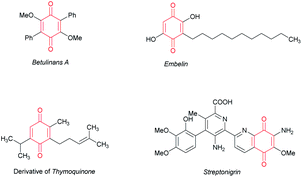
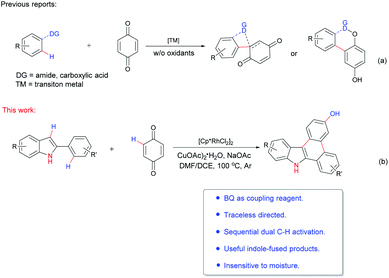
Quinones are widely distributed in nature, and commonly occur in bacteria, flowering plants and arthropods (Fig. 1). They have a wide range of applications, including diverse important pharmacological properties, involvement in redox reactions and development for advanced electrochemical energy storage.1 Among varied reported quinones, benzoquinone (BQ) is the simplest and most important one. It has been well reported that BQ has a significant and unique role in oxidative palladium(II)-catalyzed coupling reactions.2 The chemistry of benzoquinone has been extensively explored in detail, including nucleophilic addition and cycloaddition reactions, photochemistry and oxidative coupling.1b,c,2 Although great achievements have been obtained, only a few examples are disclosed about BQ as a reactant applying to transition-metal catalyzed C–H functionalization.1e Among the examples reported, cyclization or BQ direct functionalization products were mainly afforded (Scheme 1a).
Transition-metal catalyzed C–H functionalization has undergone great progresses in the past two decades.3 In order to get a better reactivity and controlled selectivity, a directing group is usually needed for this process. Therefore, various directing groups have been developed.4 However, many of them (e.g. various nitrogen-containing heterocycles) remained parts of products after reaction, therefore increasing the procedures and difficulty for structure further modification and manipulation.5 As a result, it is highly demanded to explore traceless or easily removable directing groups.6 In this context, N–H free indole moiety has gradually emerged as a versatile functionalizable directing group in transition-metal catalyzed cyclization reaction.7
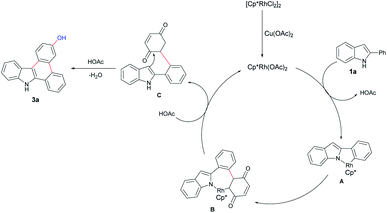
On the other hand, although the above-mentioned great breakthrough obtained in C–H functionalization, there are few examples reported for dual C–H activation reactions.8 During our research program exploring transition-metal catalysis and heterocyclic synthesis,9 we intended to prepare the indole-containing heterocycles based on the consideration of their potential biological activity. Herein, we report a rhodium-catalyzed N–H free indole directed annulation reaction with BQ through dual C–H activation strategy (Scheme 1b).
Our initial study was carried out by examining 2-phenyl indole 1a and benzoquinone 2a in the presence of [{Cp*RhCl2}2] and Cu(OAc)2·H2O in commercial available N,N-dimethylformamide under argon atmosphere. To our delight, the desired 9H-dibenzo[a,c]carbazol-3-ol product 3a was isolated in 55% yield (Table 1, entry 1). Further investigation showed the reaction did not occur in the absence of copper additive (Table 1, entry 2). DMF appears to be the best solvent for this transformation, other solvents such as DMAc, DMSO and t-Amyl-OH did not participate in this transformation (Table 1, entry 4–6) [{Cp*RhCl2}2] proved to be crucial to this reaction, other catalysts only gave trace product (Table 1, entry 3, 7–9). Several other additives were tested, all of them shut down this transformation (Table 1, 10–12). The optimized conditions were eventually identified as (Table 1, entry 13): 1.5 equiv. 2-phenyl indole, 1.0 equiv. BQ, 5 mol% [{Cp*RhCl2}2], 2 equiv. NaOAc, and 2.1 equiv. Cu(OAc)2·H2O.
| Entry | Solvent | Catalyst | Additive | Yield |
|---|---|---|---|---|
| a Reaction on a 0.2 mmol scale, using 1a (1.0 equiv.), 2a (1.0 equiv.), additive (2.0 equiv.), CsOAc (2.0 equiv.), [TM] (5 mol%), solvent (1.0 mL), under N2, isolated yield.b 1a (1.5 equiv.), solvent (0.3 M).c NaOAc was used instead of CsOAc. | ||||
| 1 | DMF | [Cp*RhCl2]2 | Cu(OAc)2·H2O | 55% |
| 2 | DMF | [Cp*RhCl2]2 | — | <5% |
| 3 | DMF | — | Cu(OAc)2·H2O | — |
| 4 | t-Amyl-OH | [Cp*RhCl2]2 | Cu(OAc)2·H2O | — |
| 5 | DMAc | [Cp*RhCl2]2 | Cu(OAc)2·H2O | <5% |
| 6 | DMSO | [Cp*RhCl2]2 | Cu(OAc)2·H2O | Trace |
| 7 | DMF | [RuCl2(p-cymene)]2 | Cu(OAc)2·H2O | — |
| 8 | DMF | Pd(OAc)2 | Cu(OAc)2·H2O | — |
| 9 | DMF | RhCl(PPh3)3 | Cu(OAc)2·H2O | <5% |
| 10 | DMF | [Cp*RhCl2]2 | AgOAc | — |
| 11 | DMF | [Cp*RhCl2]2 | Ag2O | — |
| 12 | DMF | [Cp*RhCl2]2 | Cu(acac)2 | Trace |
| 13b,c | DMF/DCE | [Cp*RhCl2]2 | Cu(OAc)2·H2O | 84% |
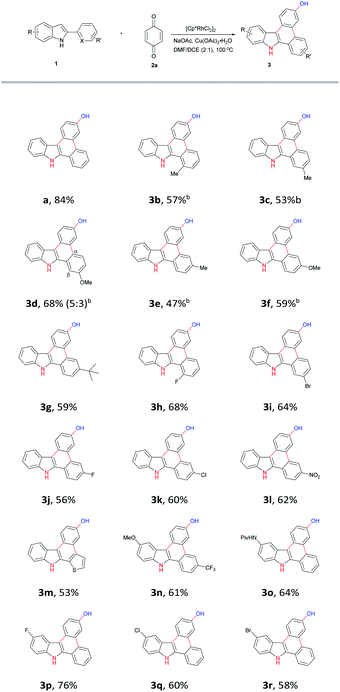
With the optimized conditions in hand, we next tend to examine the substrates scope of this reaction. Various 2-aryl indoles with electron-rich substituted groups were tested and worked well for this reaction (Table 2, 3b–g); in some cases, the reaction temperature could even be lowered to 60 °C. Halogens did not interfere with this transition-metal catalyzed process, affording the desired products smoothly (Table 2, 3h–k, 3p–r). Substrates with strong electron-withdrawing groups (3l, 3n), such as nitro-, trifluoromethyl, also proceeded regularly in this transformation. Interestingly, substrate containing other directing group such as amide group could also produce the related product 3n in 64% yield, with quite excellent regioselectivity.10 Finally, an interesting S, N-fused heterocycle 3m was obtained when 2-thienyl indole was employed. Other derivatives of benzoquinone such as 1,4-naphthaquinone or methyl-p-benzoquinone currently failed to produce the related cyclization products with proper yields.
a Condition A: 2-aryl indole (1.5 equiv.), BQ (1.0 equiv.), [Rh] (5 mol%), Cu(OAc)2·H2O (2.1 equiv.), NaOAc (2.0 equiv.), DMF/DCE(1.5 mL, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 °C.b Condiiton B: 2-aryl indole (1.0 equiv.), BQ (2.0 equiv.), [Rh] (5 mol%), Cu(OAc)2·H2O (2.1 equiv.), NaOAc (2.0 equiv.), DMF/DCE(1.5 mL, 2 1), 100 °C.b Condiiton B: 2-aryl indole (1.0 equiv.), BQ (2.0 equiv.), [Rh] (5 mol%), Cu(OAc)2·H2O (2.1 equiv.), NaOAc (2.0 equiv.), DMF/DCE(1.5 mL, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 60 °C. 1), 60 °C. |
|---|
 |
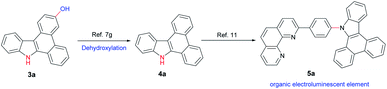
In addition, this method allows quick access to a number of functional heterocycles (Scheme 2).7g,11 For example, the hydroxyl group can be easily removed to afford 9H-dibenzo[a,c]carbazole 4a which can be further converted into organic electroluminescent element 5a via reported methods.11
Finally, we proposed a mechanism for this transformation (Scheme 3) based on reported literatures.7,9a–c,12 First, [{Cp*RhCl2}2] dissociates and delivers the active catalyst monomer [Cp*Rh(OAc)2] with the assistance of copper acetate and sodium acetate.9a–c C–H activation of 2-phenyl indole by Rh(III) produces rhodacyclic intermediate A,7 followed by insertion of benzoquinone affording intermediate B, which can be transformed into C via two folds protonation and fulfills the catalytic cycle. The final product 3a can be easily accessed via intramolecular condensation of C.7g
In conclusion, we have developed a Rh(III)-catalyzed traceless directed dual C–H activation of 2-aryl indole and annulation with benzoquinone affording indole-fused heterocycles. The protocol is applicable to a wide range of indole derivatives, affording related products in middle to good yields. Further exploration of the synthetic utilities of this chemistry and detailed mechanistic study are currently in progress in our lab and will be reported in due course.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by “the Fundamental Research Funds for the Central Universities” (21620318, 2019QNGG22). We thank the Jinan University (start-up fund) for additional support.Notes and references
- For related reviews, see: (a) P. R. Dandawate, A. C. Vyas, S. B. Padhye, M. W. Singh and J. B. Baruah, Mini-Rev. Med. Chem., 2010, 10, 436 CrossRef CAS; (b) I. Abraham, R. Joshi, P. Pardasani and R. T. Pardasani, J. Braz. Chem. Soc., 2011, 22, 385 CrossRef CAS; (c) V. Nair, R. S. Menon, A. T. Biju and K. G. Abhilash, Chem. Soc. Rev., 2012, 41, 1050 RSC; (d) C. Han, H. Li, R. Shi, T. Zhang, J. Tong, J. Lia and B. Li, J. Mater. Chem. A, 2019, 7, 23378 RSC; (e) J. M. Wood, R. L. de Carvalho and E. N. d. S. Júnior, Chem. Rec., 2021, 21, 1 CrossRef.
- (a) A. Vasseur, J. Muzart and J. L. Bras, Eur. J. Org. Chem., 2015, 4053 CrossRef CAS; (b) B. Yang, Y. Qiu and J.-E. Bäckvall, Acc. Chem. Res., 2018, 51, 1520 CrossRef CAS PubMed.
- For selected reviews, see: (a) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (b) T. Gensch, M. N. Hopkinson, F. Glorius and J. Wencel-Delord, Chem. Soc. Rev., 2016, 45, 2900 RSC; (c) O. Eisenstein, J. Milani and R. N. Perutz, Chem. Rev., 2017, 117, 8710 CrossRef CAS; (d) Z. Dong, Z. Ren, S. J. Thompson, Y. Xu and G. Dong, Chem. Rev., 2017, 117, 9333 CrossRef CAS PubMed; (e) P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz and L. Ackermann, Chem. Rev., 2019, 119, 2192 CrossRef CAS.
- Z. Chen, B. Wang, J. Zhang, W. Yu, Z. Liu and Y. Zhang, Org. Chem. Front., 2015, 2, 1107 RSC.
- R. L. Carvalho, R. G. Almeida, K. Murali, L. A. Machado, L. F. Pedrosa, P. Dolui, D. Maiti and E. N. d. S. Júnior, Org. Biomol. Chem., 2021, 19, 525 RSC.
- (a) G. Rousseau and B. Breit, Angew. Chem., Int. Ed., 2011, 50, 2450 CrossRef CAS PubMed; (b) C. Wang and Y. Huang, Synlett, 2013, 24, 145 CAS; (c) G. Rani, V. Luxami and K. Paul, Chem. Commun., 2020, 56, 12479 RSC.
- (a) K. Morimoto, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2010, 12, 2068 CrossRef CAS PubMed; (b) L. Ackermann, L. Wang and A. V. Lygin, Chem. Sci., 2012, 3, 177 RSC; (c) B. Li, B. Zhang, X. Zhang and X. Fan, Chem. Commun., 2017, 53, 1297 RSC; (d) Y.-Q. Xia and L. Dong, Org. Lett., 2017, 19, 2258 CrossRef CAS PubMed; (e) X. Yang, Y. Li, L. Kong and X. Li, Org. Lett., 2018, 20, 1957 CrossRef CAS PubMed; (f) S. Guo, Y. Liu, Y. Wang, X. Zhang and X. Fan, J. Org. Chem., 2020, 85, 8910 CrossRef CAS PubMed. During the preparation of this manuscript, an example of Rh(III)-catalyzed oxidative annulation of 2-arylindole with BQ under high temperature (at 140 °C) using chlorobenzene as solvent was reported, see: (g) S. Guo, Y. Liu, L. Zhao, X. Zhang and X. Fan, Org. Lett., 2019, 21, 6437 CrossRef CAS PubMed.
- For selected recent examples, see: (a) L. Zhang, Y. Wang, Y. Shi, Y. Wu, J. Lan, W. Ma and J. You, ACS Catal., 2019, 9, 5358 CrossRef CAS; (b) M. Uryu, T. Hiraga, Y. Koga, Y. Saito, K. Murakami and K. Itami, Angew. Chem., Int. Ed., 2020, 59, 6551 CrossRef CAS PubMed; (c) K. Ghosh, Y. Nishii and M. Miura, Org. Lett., 2020, 22, 3547 CrossRef CAS PubMed; (d) A. D. Streit, A. J. Zoll, G. L. Hoang and J. A. Ellman, Org. Lett., 2020, 22, 1217 CrossRef CAS PubMed.
- (a) C. Wang, H. Chen, Z. Wang, J. Chen and Y. Huang, Angew. Chem., Int. Ed., 2012, 51, 7242 CrossRef CAS PubMed; (b) C. Wang and Y. Huang, Org. Lett., 2013, 15, 5294 CrossRef CAS PubMed; (c) C. Wang, H. Sun, Y. Fang and Y. Huang, Angew. Chem., Int. Ed., 2013, 52, 5795 CrossRef CAS PubMed; (d) C. Wang, A. Wang and M. Rueping, Angew. Chem., Int. Ed., 2017, 56, 9935 CrossRef CAS PubMed; (e) C. Wang and M. Rueping, ChemCatChem, 2018, 10, 2681 CrossRef CAS; (f) C. Wang, B. Maity, L. Cavallo and M. Rueping, Org. Lett., 2018, 20, 3105 CrossRef CAS PubMed; (g) W. Zhang, C. Wang and Q. Wang, ACS Catal., 2020, 10, 13179 CrossRef CAS; (h) C. Wang and L. Liu, Org. Chem. Front., 2021 10.1039/d0qo01508c.
- Amide has been reported to act as a directing group, see: R.-Y. Zhu, M. E. Farmer, Y.-Q. Chen and J.-Q. Yu, Angew. Chem., Int. Ed., 2016, 55, 10578 CrossRef CAS PubMed.
- J. R. Lee, K. Park, J. S. Yu, S. G. Lee, E. Cho and J. Lee, PCT Int. Appl., 2018, WO2018092927A1 Search PubMed.
- T. Satoh and M. Miura, Chem.–Eur. J., 2010, 16, 11212 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01779a |
| ‡ Contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |