Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The preparation of three-dimensional flower-like TiO2/TiOF2 photocatalyst and its efficient degradation of tetracycline hydrochloride
Chentao Hou *,
Huayang Liu* and
Yijie Li
*,
Huayang Liu* and
Yijie Li
College of Geology and Environment, Xi'an University of Science and Technology, Xi'an 710054, China. E-mail: 807484470@qq.com
First published on 21st April 2021
Abstract
A kind of high-efficiency photocatalyst of the three-dimensional flower-like TiO2/TiOF2 was synthesized by a one-step hydrothermal method. XRD, FE-SEM, EDS, HTEM, BET, XPS, PL, and UV-Vis-DRS were utilized to characterize the photocatalyst. The photocatalyst of TiO2/TiOF2 shows a narrow band gap of 2.8 eV. The generation of Ti3+ and an oxygen vacancy (Ov) in the photocatalyst are helpful to increase the absorption of visible light, and to inhibit faster charge recombination by capturing photogenerated carriers. Through the degradation of tetracycline hydrochloride (TCH) under simulated sunlight, the photocatalytic activity and stability of the synthesized samples were investigated. The results showed that the removal rate of tetracycline hydrochloride was 59% only in 0.5 h of dark reaction and 85% in 0.5 h of simulated sunlight. The removal efficiency of the photocatalyst for the adsorption and photocatalytic degradation of TCH is higher than that of the single TiO2, TiOF2, and Degussa P25. The synthesized three-dimensional flower-like TiO2/TiOF2 has great application potential in the treatment of antibiotic wastewater.
1. Introduction
Drugs are a new kind of water pollutant, which is closely monitored by researchers in the aspect of ecology and human health.1–3 Antibiotics, as a kind of medicine for the treatment and prevention of bacterial infection, have been widely used in the world, but their risks have been gradually revealed.4–6 Over the years, and until now, almost all known symbiotic bacteria and pathogenic bacteria have acquired the ability to produce resistance to one or more antibiotics.7,8 Compared with aerosols and soil, water phase transport is considered to be the main way for antibiotics and antibiotic resistance to spread to remote areas and society. Therefore, the treatment of antibiotic wastewater is urgent.Tetracycline hydrochloride (TCH) is a typical tetracycline broad-spectrum antibiotic, which is widely used as a growth promoter in the treatment of human diseases and animal feeding.9 However, because the naphthol ring in the tetracycline hydrochloride structure cannot be completely metabolized by humans and animals, a large amount of absorbed TCH is excreted into various water bodies through feces and urine.10 At present, they can be detected in soil, sediment, groundwater, surface water and even drinking water, thus posing a significant risk to the ecosystem and human health. Therefore, the removal of TCH residues from the water environment is very important for the ecological environment and human health.
In recent years, several methods for treating wastewater-containing TCH have been studied, such as microbial degradation,11,12 adsorption,2,5,9 electrochemical method,13 and photocatalytic degradation.6,7 Although these methods have a certain removal effect on TCH in wastewater, most of them have some problems. Due to the antibacterial property of TCH, microorganisms do not easily survive in the process of biodegradation, and traditional microbial degradation methods (such as the activated sludge method) cannot effectively remove TCH residues in wastewater.14 Although adsorption is one of the most effective methods to remove antibiotics in water, it can only transfer antibiotics from the liquid phase to solid phase, and cannot completely degrade antibiotics.15,16 Due to its high energy consumption and poor circulation ability, electrochemical degradation greatly limits its application.17 Among these methods, photocatalytic water purification technology is considered to be the most promising water treatment technology due to its cost-effectiveness, efficiency and environment friendliness.18–20
TiO2, as the most commonly used semiconductor photocatalyst, is nontoxic and chemically stable.5,7 However, it has a wide energy band gap (3.0–3.2 eV, which means it only reacts to UV) and a high carrier recombination rate.21,22 So far, much effort has been made to make up for these shortcomings, such as metal doping,23 non-metal doping,24,25 and building a heterogeneous semiconductor composite.26–28 However, there are some problems in metal doping, such as thermal instability, toxicity, and high cost.29,30 Nonmetallic doping and heterogeneous semiconductor composite materials can effectively improve the separation efficiency of the catalyst carrier, which is recognized as an effective method to improve the performance of the TiO2 catalyst.31 At present, the construction of a heterojunction mostly needs to prepare the two semiconductors separately, and then form the heterostructure in various ways. This preparation method not only consumes time, but also produces high-energy consumption, which is not conducive to practical production and environmental protection. Therefore, it is very important to find a simple method to prepare a TiO2-based heterojunction.
The existence of fluorine could induce Ti3+ and Ti–F–Ti bonds to improve the photocatalytic property.29,32 The appearance of Ti3+ is usually accompanied by oxygen vacancies, which form a continuous vacancy band of electronic states below the conduction band of TiO2.33 This effectively reduces the recombination of carriers, and makes TiO2 respond to visible light. Interestingly, when TiO2 is in a high concentration F-ion environment, TiOF2 can be formed.34
More and more researchers are paying attention to TiOF2 photocatalytic materials. According to previous reports, TiOF2 has two crystal forms, corresponding to no. 08-0060 and no. 01-0190 in the JCPDS database. The TiOF2 corresponding to JCPDS no. 08-0060 shows regular cubic morphology, while the TiOF2 corresponding to JCPDS no. 01-0490 shows irregular morphology of nanoparticles. In previous studies, large cubic TiOF2 has been widely studied. However, few people have explored the smaller size TiOF2 nanoparticles. For example, Dong et al.35 synthesized the Ag3PO4/TiOF2 composite with cubic TiOF2, which improved the photocatalytic activity of Ag3PO4. Chen et al.36 used cubic TiOF2 as a precursor, through the solid calcination strategy and one-step topological transformation, constructing TiO2 hollow nanocubes, and reduced graphene oxide hybrids with a 3D/2D heterojunction to degrade rhodamine B efficiently. Liu et al.37 found that different hydrothermal synthesis temperatures have a significant effect on the physical and chemical properties, and the photocatalytic activity of TiO2/TiOF2 nanocomposites. In our previous studies, we used cubic TiOF2 as a precursor and synthesized it with Cu2O to synthesize the Cu2O@TiOF2/TiO2 composite, which can degrade TCH under simulated sunlight.38 However, in our previous research, we did not pay attention to the influence of the different amounts of HF on the formation of the TiO2/TiOF2 composite. In the preparation process, we need to prepare the cubic TiOF2 and Cu2O photocatalysts, and then composite them, which greatly increases the manufacturing cost of the photocatalyst. Wang et al.39–41 explored how different reaction times, different temperatures, and different hydrofluoric acid contents have different effects on the photocatalytic hydrogen production of the {001} surface TiO2. They found that TiO2 and TiOF2 seem to have a synergistic effect. However, they did not continue to explore what reaction conditions could enhance this synergy. Therefore, it is necessary to further reveal the effect of different amounts of HF on TiO2, and by controlling the dosage of HF, it is expected that a TiO2/TiOF2 photocatalyst with Ti3+/Ov can be synthesized in a one-step hydrothermal method.
In this paper, we discussed the influence of different hydrofluoric acid dosages on the formation of TiO2/TiOF2, and synthesized a kind of three-dimensional flower-like TiO2/TiOF2 nanocomposite by one-step hydrothermal method at low temperature for the first time. The flower-like TiO2/TiOF2 composite has partial oxygen vacancies and a narrow band gap of 2.8 eV, which can respond well to the sun. The photocatalytic activity of the photocatalyst was tested by degradation of tetracycline hydrochloride. The results show that the photocatalyst has a strong adsorption capacity for tetracycline hydrochloride, and shows good photocatalytic activity. Besides, the photocatalyst has good stability, and is expected to be used in the treatment of antibiotic wastewater.
2. Experimental section
2.1. Reagents and chemicals
Tetrabutyl titanate (TBOT, A. R.), anhydrous ethanol (C2H5OH, A. R.), p-benzoquinone (PBQ), methanol (MT), 1,4-terephthalic acid (PTA), and hydrofluoric acid (HF, A. R.) were purchased from Fuchen Chemical Reagent Factory, Tianjin, China. Tetracycline hydrochloride (TCH) was purchased from Aladdin Industrial Corporation (Shanghai, China). All reagents were used without purification. Ultra-pure water was used as experimental water.2.2. Preparation of the photocatalysts
The TiO2/TiOF2 heterostructure semiconductor composite was synthesized by a one-step hydrothermal method. 40 mL C2H5OH was added to 100 mL polytetrafluoroethylene liner, and then the required amount of HF (4 mL, 6 mL, 8 mL, 10 mL) was slowly add under low-speed magnetic stirring at 25 °C. The mixture was mixed at 25 °C for 10 min, and recorded as solution A. 20 mL TBOT was added to solution A under stirring at the rate of 2 drops per second to form a white suspension, and the mixture was stirred at 25 °C for 2 h. Then, the inner liner of polytetrafluoroethylene was placed in a high-pressure reactor and reacted at 150 °C for 15 h. After the system was naturally cooled to room temperature, the solid products were collected by centrifugation, and washed with ethanol and ultra-pure water three times. The samples were dried at 60 °C and named x-TF (x = 4, 6, 8, 10). For comparative study, we added 4 mL ultra-pure water instead of hydrofluoric acid to prepare pure TiO2, and prepared pure TiOF2 according to previous reports.422.3. Characterization
The crystal structure of the sample was analyzed by X-ray diffraction (XRD, GD-XD-2, China) equipped with Cu-Kα X-ray source (λ = 0.15418 nm). SEM and EDS (JSM7500F, Japan) were used to record the surface morphology and element distribution of the samples. Transmission electron microscopy (TEM) images were achieved with an FEI Tecnai G2 F20, USA. The absorption characteristics of the samples were determined by UV-visible diffuse absorption spectroscopy (UV-Vis DRS, Shimadzu UV-2600, Japan). The photoluminescence spectrum of the photocatalyst was measured by a fluorescence spectrometer (Shimadzu-RF-6000, Japan) with an excitation wavelength of 300 nm. The chemical valence states of the samples were analyzed by X-ray photoelectron spectroscopy (XPS). The specific surface area and porosity of the samples were measured by the N2 adsorption–desorption specific surface analyzer (Bet, Micrometrics ASAP 2020, USA).2.4. Photocatalytic tests
The photocatalytic activity of the catalyst was tested by the degradation of TCH. 100 mL of TCH solution (10 mg L−1) in a measuring cylinder was put into a 150 mL photocatalytic reaction tube, and then 30 mg of photocatalyst was weighed and added to the TCH solution. The photocatalytic reaction tube was placed into the photocatalytic reactor, and a 500 W xenon lamp was used to simulate the degradation of TCH by sunlight. Before the xenon lamp was turned on, the solution was magnetically stirred in the dark for 30 min to ensure the adsorption–desorption balance. Then, the xenon lamp was turned on to start the photodegradation test. An aliquot of 7 mL suspension was taken out every 5 min, and centrifugated at high speed to remove the catalyst. Then, the remaining TCH concentration was analyzed with a UV-Vis spectrophotometer at the maximum absorption wavelength of 357 nm. To explore the degradation ability of 8-TF to TCH under visible light, a 420 nm filter was added to the photocatalytic reactor, and the above photocatalytic experiment was repeated. Different scavengers (PBQ, PTA, MT) were used to trap the active components (˙O2−, ˙OH, h+) in the photocatalytic process. This test is similar to the photocatalytic degradation test, except that a certain amount of scavenger was added to the TCH solution, and then photocatalyst was added. Finally, the photocatalyst was separated from the TCH solution, and then the next operation was started to study the durability of the catalyst.3. Results and discussion
3.1. Crystal structure analysis
Fig. 1a clearly shows the crystal structure of the prepared sample. The diffraction peaks of the samples are very sharp, which shows that the crystal of the material is good. For pure TiOF2, the diffraction peaks at 2θ = 13.59°, 23.44°, 27.42°, 33.86°, 47.8°, 53.92°, 59.6°, 69.6°, and 74.7° match with those of TiOF2 (JCPDS no. 01-0490), indicating that we successfully prepared hexagonal TiOF2.42 For pure TiO2, the diffraction peaks at 2θ = 25°, 37.76°, 47.8°, 54.86°, 56.6°, 70.3°, 75°, and 82.22° are attributed to the {101}, {004}, {200}, {105}, {211}, {220}, {215}, {224} planes of anatase TiO2 (JCPDS no. 21-1272), respectively.10 However, the XRD patterns of 4-TF and 6-TF do not show the diffraction peak of TiOF2, which may be due to the low concentration of the F atom. The XRD patterns of 8-TF and 10-TF show the combination characteristics of anatase TiO2 and TiOF2, which show that we have successfully prepared the TiO2/TiOF2 composite and the peak intensity of TiOF2 increases with the increase of HF. Moreover, no additional crystal phase was observed in all of the spectra, indicating that no impurity was produced in the preparation process.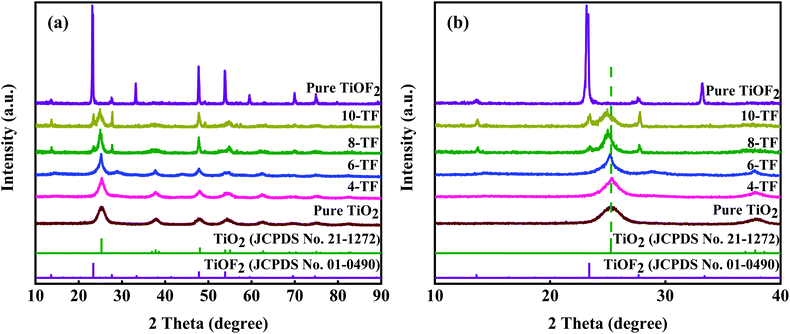 | ||
| Fig. 1 (a) XRD patterns of the prepared samples, and (b) the enlarged XRD patterns of the prepared samples. | ||
Fig. 1b shows the amplification comparison of the X-ray diffraction patterns of the prepared samples. It is worth noting that the diffraction peaks on the surface of the TiO2 anatase {101} crystals distributed in 8-TF and 10-TF deviate slightly to the low diffraction angle. According to Bragg's law, the distance between D and the diffraction angle is inversely proportional to 2θ. Therefore, the negative displacement of the TiO2 anatase {101} peaks in 8-TF and 10-TF can be called the broadening of the D-gap, which reveals the interaction between TiO2 and TiOF2.43 The average grain sizes of 4-TF, 6-TF, 8-TF and 10-TF are 18, 15, 13, and 9 nm, respectively, by Scherrer formula
D = Kλ/βχ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, θ,
| (1) |
3.2. Morphology analysis of the photocatalyst
The morphology and composition of the material were analyzed by SEM and EDS. As shown in Fig. 2a, pure TiO2 is composed of nanospheres with a size of 100–500 nm and a relatively rough surface. Fig. 2b shows an image of the product formed by adding 4 mL HF. Under the etching of a small amount of HF, irregular TiO2 nanoflakes of 300–500 nm were formed. It can be seen that these nanoflakes are thick and relatively smooth. With the increase of the hydrofluoric acid content, the etching effect is more obvious.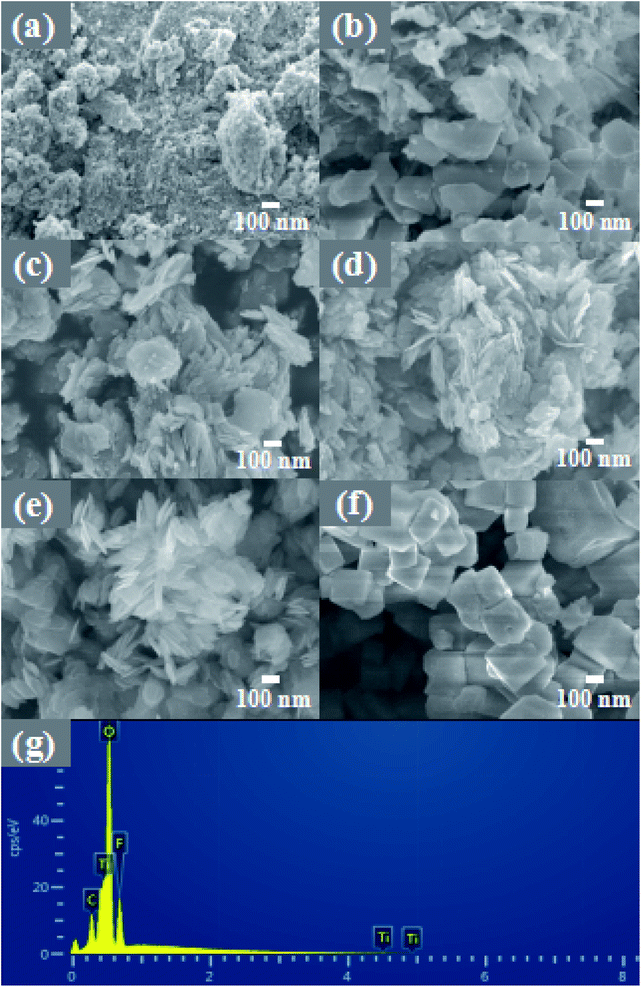 | ||
| Fig. 2 The high-magnification FE-SEM images of (a) TiO2, (b) 4-TF, (c) 6-TF, (d) 8-TF, (e) 10-TF and (f). TiOF2; EDS images of 8-TF (g). | ||
As shown in Fig. 2c, with the further increase of the HF content, most of the thicker and some thinner nanoflakes are generated, which are closely stacked, making the surface somewhat rough. As shown in Fig. 2d, when the HF content reaches 8 mL, the nanoflakes formed are thinner than before, and the nanoflakes are more aggregated, forming a flower-like structure, and obvious mesopores can be seen. This unique morphology with a large specific surface area can provide a large number of reactive sites for pollutants. The measured higher BET specific surface area (67.74 m2 g−1) is consistent with SEM observation. Interestingly, the XRD pattern of 8-TF has an obvious diffraction peak of TiOF2, but there is no image of TiOF2 in the SEM pattern. We speculate that the content of 8 mL HF is not enough to react to form a large amount of TiOF2, and part of the generated TiOF2 has close interface contact with TiO2, so it does not appear. Fig. 2e shows the image of the product generated by adding 12 mL HF to the reaction. At this time, compared with the previous nanoflakes, the size is smaller and thinner, the nanoflakes are more dispersed, and the surface tends to be smooth. Fig. 2f shows the image of pure TiOF2, showing a small irregular hexagonal structure. Fig. 2g is the EDS spectrum of the 8-TF. The weight percentages of Ti, O, and F in the 8-TF are 66.24%, 27.27%, and 6.49%, respectively.
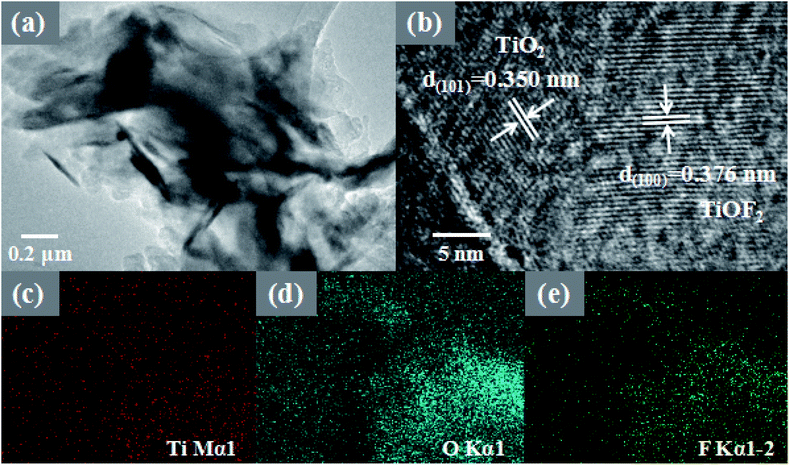
More morphological details on 8-TF were obtained using transmission electron microscopy (TEM) images. It can be seen from Fig. 3a that 8-TF is composed of irregularly shaped nanoflakes, which is consistent with the results observed by SEM. The HR-TEM image of 8-TF showed the intimate contact between TiO2 and TiOF2 (Fig. 3b). The lattice spacing of ca. 0.350 nm can be assigned to the (101) plane of anatase TiO2, and the lattice spacing of ca. 0.376 nm can be assigned to the (100) plane of TiOF2.40,44 To further describe the element distribution of 8-TF, elemental mapping analysis (Fig. 3c–e) of 8-TF was performed. The distribution of Ti, O, and F is uniform. These results confirm that we have successfully synthesized a three-dimensional flower-like TiO2/TiOF2 nanocomposite.
3.3. BET specific surface area and pore structure
The specific surface area and pore size distribution of 8-TF, pure TiO2, and pure TiOF2 were analyzed by nitrogen adsorption–desorption technology. As shown in Fig. 4, according to the classification of IUPAC, all samples show a typical IV type adsorption desorption isotherm with H3 type hysteresis ring, which represents that the sample has a mesoporous formation and matches the image presented by SEM.38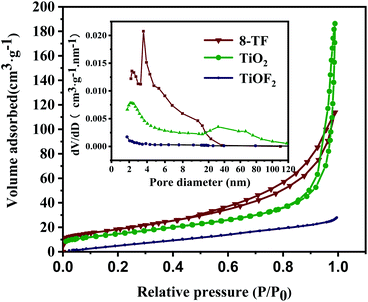 | ||
| Fig. 4 Nitrogen adsorption and desorption isotherms of 8-TF, pure TiOF2, and pure TiO2. The inset is the corresponding pore size distribution. | ||
According to the adsorption–desorption isotherm, the specific surface areas of pure TiOF2, TiO2, and 8-TF are 4.8, 53.6, 67.74 m2 g−1, respectively. The specific surface area of the composite photocatalyst is significantly higher than that of TiOF2 and TiO2. The larger specific surface area is beneficial to provide more active sites during photocatalysis, and promotes the degradation of TCH on the surface of the photocatalyst. Table 1 shows the specific surface area, pore-volume, and average pore size for the tested samples. The average pore size of pure TiOF2, TiO2, and 8-TF is 15.95, 18.69, and 8.56 nm, respectively.
| Samples | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Average pore size (nm) |
|---|---|---|---|
| TiO2 | 53.6 | 0.28713 | 18.69 |
| TiOF2 | 4.8 | 0.023123 | 15.95 |
| 8-TF | 67.7 | 0.18628 | 8.56 |
According to the corresponding BJH pore size distribution diagram (Fig. 4), the pore size distribution curve of the 8-TF composite is sharper than that of TiOF2 and TiO2 alone, indicating that the pore size distribution in the sample is more uniform. Considering the size of the TCH molecule (1.41 nm in length, 0.46 nm in width, and 0.82 nm in height), we think that the 8-TF composite has good adsorption performance. This is consistent with the strong removal rate of the dark reaction stage in the subsequent photocatalytic degradation experiment. Moreover, these uniform and small pore size mesopores are conducive to the absorption of light and multiple reflections inside the material, and provide an effective transmission path for photogenerated carriers.45
3.4. XPS analysis
The surface compositions and chemical states of pure TiO2, TiOF2, and 8-TF are displayed in Fig. 5. The survey spectra reveal signals of Ti, O, F, and C on 8-TF that match their respective signals from the individual spectra of TiOF2 and TiO2 (Fig. 5a), which follows the above EDS results. The existence of the peaks of element C at 284.8 eV can be ascribed to surface adventitious reference carbon, which is unavoidable during the XPS measurement.44 From the high-resolution XPS spectrum of Ti 2p in the samples (Fig. 5b), the shape of all of the spectra, being similar to those reported by others, featured a strong peak at about 458 eV and a weak peak at about 464 eV, which were marked as Ti 2p3/2 and Ti 2p1/2, respectively.42 Through Gauss fitting, only 458.68 and 464.38 eV can be observed for pure TiO2, corresponding to Ti4+ 2p3/2 and Ti4+ 2p1/2, respectively. The distance between the two signal peaks is 5.7 eV, which is strong evidence for the existence of Ti4+, indicating that a part of Ti in TiO2/TiOF2 exists in the form of Ti4+.46 It is worth noting that the two main peaks of Ti 2p3/2 and Ti 2p1/2 could be fitted to four peaks after the Gauss fitting of 8-TF and TiOF2. For 8-TF, the peaks of 459.2 and 464.2 eV are attributed to Ti3+ 2p3/2 and Ti3+ 2p1/2, the peaks of 459.3 and 464.99 eV are attributed to Ti4+ 2p3/2 and Ti4+ 2p1/2, respectively, which indicate that Ti3+ has formed in the reduction process. The distance between the two signal peaks is 5.0 eV, which is strong evidence for the existence of Ti3+, indicating that a part of Ti in TiO2/TiOF2 exists in the form of Ti3+. For TiOF2, the peaks of 460.2 and 465.2 eV are attributed to Ti3+ 2p3/2 and Ti3+ 2p1/2, the peaks of 460.08 and 465.78 eV are attributed to Ti4+ 2p3/2 and Ti4+ 2p1/2, respectively. The binding energies of Ti 2p3/2 and Ti 2p1/2 of 8-TF and TiOF2 move to the high energy region, respectively, compared with the binding energies of TiO2, which may be attributed to the fact that the strength of the Ti–F bond is greater than that of the Ti–O bond.47 The O 1s spectra of the samples are shown in Fig. 5c. After Gauss fitting of all samples, it can be seen that the O 1s spectrum of TiO2 shows two peaks located at 530.6 and 531.28 eV, which are attributed to the Ti–O bond, and the oxygen of the surface hydroxyl.47 Interestingly, for 8-TF and TiOF2, the asymmetric O 1s spectra were fitted to four different types of O. The photoelectric peaks at 530.35, 531.28, 532.39, and 533.55 eV corresponds to lattice oxygen (Ti–O), hydroxyl oxygen (Ti–OH), oxygen vacancy (Ov) and oxygen in adsorbed water (H2O), respectively, which can be considered as convincing evidence for the existence of Ti3+.48 Compared with TiO2, the binding energy of TiOF2 and 8-TF O 1s moves to the low energy region, which may be attributed to the Ov in TiOF2 and 8-TF. It has been proved in the photo-induced superhydrophilic TiO2 that H2O molecules are more easily adsorbed onto the Ov, which explains why the catalyst surfaces are capable of forming more adsorbed H2O.24,32 It is well accepted that the presence of Ti3+/Ov can suppress recombination of photo-generated electron–hole pairs, improve the formation rate of photo-induced hydroxyl radicals, and enhance the visible light response. This is consistent with the solid UV spectrum, PL spectrum, and capture experiment. Fig. 5d shows the F 1s XPS spectra. The binding energy of the F 1s electrons shifted from 684.84 eV for 8-TF to 685.06 eV for TiOF2 particles. The slight increase in the F 1s binding energy indicated shifting of the surface Ti–F species on the 8-TF nanoparticles to the Ti–F bonds in the bulk TiOF2 particles.41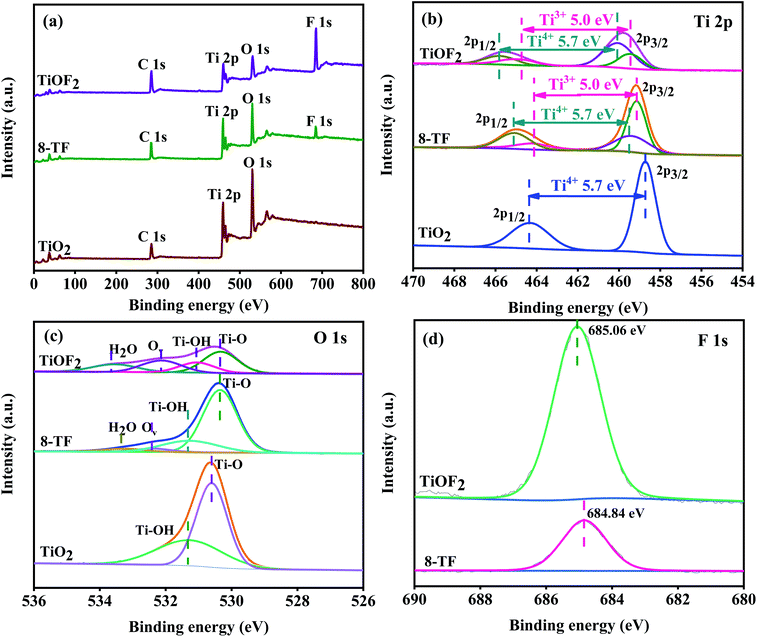 | ||
| Fig. 5 (a) Survey XPS spectra of the samples; (b–d) high-resolution XPS data of Ti 2p, O 1s, and F 1s for the samples, respectively. | ||
3.5. Light absorption performance
The diffuse UV-Vis absorption spectra of pure TiO2, pure TiOF2, and x-TF composites are shown in Fig. 6a. It can be seen that pure TiO2 and TiOF2 show obvious adsorption in the UV region, but show limited absorption in the visible region. Compared with pure TiO2 and pure TiOF2, the absorption of the x-TF (x = 4, 6, 8, 10) composite in the visible light region is significantly enhanced, the absorption threshold is significantly red-shifted, and the absorption edge in the visible light region is significantly increased, which may be due to its special flower cluster structure, close interface contact between TiO2 and TiOF2, and partial oxygen vacancy in the photocatalyst.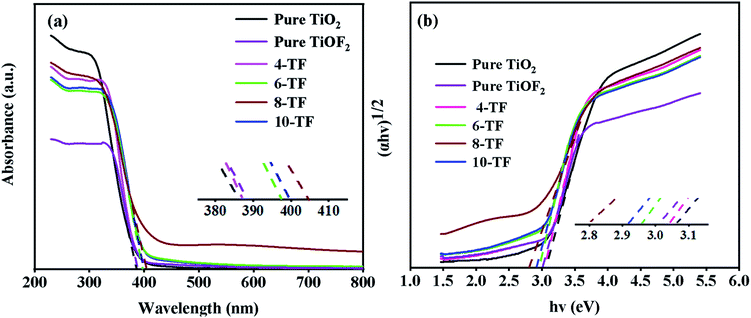 | ||
| Fig. 6 (a) UV-Vis absorbance spectra of the samples, and (b) the corresponding plots of (αhv)1/2 versus hv. | ||
Interestingly, when the dosage of HF is increased from 4 mL to 8 mL, the absorption of visible light by the composite is continuously enhanced. However, when the dosage of HF is increased to 10 mL, the absorption of visible light by the composite is weakened, indicating that the content of TiOF2 is too much, which may produce a light-shielding effect. Because 8-TF has strong absorption in the UV region and can absorb part of the visible light, the composite can make greater use of sunlight, which can improve the overall quantum efficiency of pollutant oxidation.49 This is consistent with the subsequent degradation test.
The band gap energy (Eg) of pure TiO2, TiOF2, and x-TF was calculated according to the Tauc curve. The results are shown in Fig. 6b. The band gap energies of TiO2 and TiOF2 are 3.08 and 3.01 eV, respectively, which are close to those reported in the literature.42,49 Because of the heterojunction between TiO2 and TiOF2, the band gap of the x-TF composite is narrowed to 3.04 (4-TF), 2.97 (6-TF), 2.8 (8-TF), and 2.92 eV (10-TF), respectively.
3.6. Photocatalytic activity
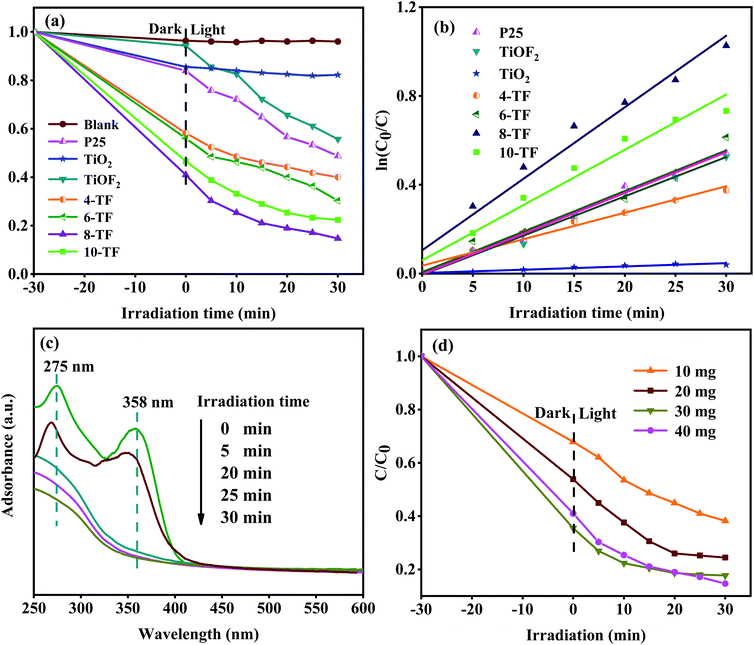
To study the photocatalytic performance of our samples, tetracycline hydrochloride (TCH) was used as a typical antibiotic pollutant in water to simulate the solar photocatalytic degradation test. The results are shown in Fig. 7a. The photocatalysis system reacts in the dark for 30 min to reach the adsorption–desorption equilibrium before the sunlight exposure. Because TCH is also sensitive to light, for comparison, we carried out a blank experiment without any photocatalyst. TCH has little self-degradation under sunlight. Moreover, the removal rate of pure TiO2 for TCH is only 14.5%, which may be attributed to its low specific surface area. This makes it unable to provide more active sites for TCH. This explanation also applies to the pure TiOF2 nanoparticles with a low adsorption degradation rate (8%). Due to the low utilization rate of sunlight and high surface charge recombination rate of TiO2, the total removal rate of TCH after 0.5 h of sunlight irradiation is only 17.8%. The photodegradation efficiency of TCH (about 44%) by pure TiOF2 is also not satisfactory.We also investigated the photocatalytic activity of commercial titanium dioxide (Degusse P25). The adsorption and degradation of p25 to TCH are consistent with that of pure TiO2. After 30 min of photocatalytic action, the total degradation rate of TCH is 51%. The results show that the photocatalytic activity of p25 is higher than that of pure TiO2 synthesized in this study. Although the two TiO2 particles are similar in morphology and structure, the commercial p25 particles are composed of two crystal types: anatase type and rutile type. Compared with single-phase TiO2, P25 is more conducive to the use of visible light.
All of the x-TF (x = 4, 6, 8, and 10) samples showed significantly enhanced adsorption and photocatalysis performance compared with pure TiO2 and TiOF2. The adsorption and removal rates of TCH were 42%, 44%, 59%, and 53.4%. The total removal rates were 60%, 70%, 85%, and 77% after 30 min of reaction under sunlight. Among them, 8-TF has the highest total removal rate of TCH. This may be attributed to the fact that 8-TF can easily reach the surface of the catalyst due to its unique flower-like morphology, and its large specific surface area provides more active sites for the photocatalyst, which make it have strong adsorption. Due to the higher absorption of UV and visible light, 8-TF has high electron separation efficiency. The smaller particle size makes its active center density larger. Furthermore, the close heterogeneous interface and the presence of Ti3+/Ov slow down the electron–hole recombination rate, thus showing good photocatalytic activity.
The degradation kinetic model was studied and the photocatalytic properties of the samples were further analyzed. Fig. 7b shows that the linear relationship between the degradation time (t) and ln(C0/C) is almost a straight line, which proves that the degradation process of TCH conforms to the pseudo-first-order reaction.50 The rate constants k of pure TiO2, TiOF2, P25, and x-TF (x = 4, 6, 8, 10) were 0.00149, 0.01771, 0.01837, 0.01192, 0.01829, 0.03222, and 0.0249 min−1, respectively. The results show that the rate constants of 8-TF are 21, 1.8, and 1.7 times higher than those of TiO2, TiOF2, and P25, respectively. The results show that the addition of 8 mL HF is conducive to the formation of the TiO2/TiOF2 composite with high photocatalytic activity. The absorption spectrum of TCH degraded by 8-TF is shown in Fig. 7c. TCH has two main absorption peaks at 275 and 358 nm. The absorption peak at 275 nm may be related to the hydroxyl and acylamino produced in the reduction process.51 The absorption peak at 358 nm can be attributed to the aromatic ring B–D. The absorption peak decreased rapidly after sunlight irradiation. After 30 min of irradiation, the absorption peak almost disappeared, which indicated that the ring structure of TCH was destroyed after the light source was added.
To study the effect of catalyst loading on the photocatalytic degradation system, the experiment was carried out by changing the amount of catalyst. Fig. 7d shows the degradation curve of TCH solution with different 8-TF dosages (0.1 g L−1, 0.2 g L−1, 0.3 g L−1, 0.4 g L−1). When the amount of catalyst is 0.3 g L−1, the degradation effect of TCH is the best. Although the degradation rate of the 0.4 g L−1 catalyst is the same as that of the 0.3 g L−1 catalyst, the photocatalytic rate of the 0.4 g L−1 catalyst is lower than that of the 0.3 g L−1 catalyst, which may be due to the aggregation of excessive catalyst. This makes the solution turbid and leads to photon scattering, thus reducing the photocatalytic rate.52
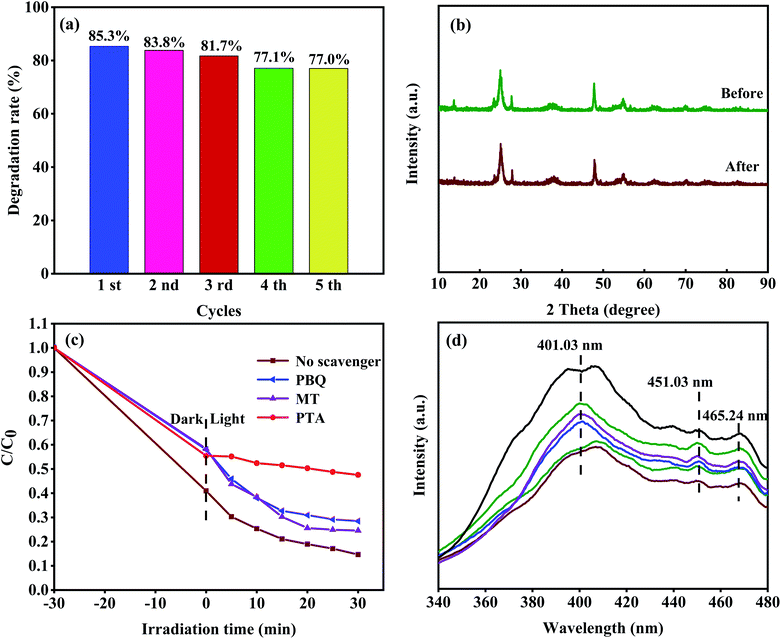
Moreover, we used a 420 nm filter to filter the ultraviolet light in simulated sunlight, and tested the photocatalytic activity of 8-TF under visible light (Fig. 8). The results showed that 8-TF still had a good degradation effect on TCH under 30 min visible light irradiation (85%). The stability and reusability of the photocatalyst are important factors affecting its practical application. Therefore, five consecutive cyclic photodegradation tests were carried out on sample 8-TF, and the results are shown in Fig. 9a.
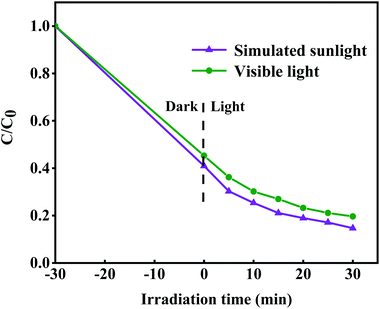 | ||
| Fig. 8 Photodegradation efficiency of TCH by 8-TF under simulated sunlight and visible light (>420 nm) irradiations. | ||
After five cycles, the degradation rate of sample 8-TF in simulated sunlight decreased from 85.3% to 77.0%, indicating that sample 8-TF has a high reuse rate and photocatalytic performance. As shown in Fig. 9b, after five cycles, the peak intensity of the 8-TF photocatalyst decreased, but the position of the diffraction peak was the same as that before the reaction, indicating that the 8-TF heterojunction has high chemical stability.
3.7 Photocatalysis mechanism
In the process of photodegradation, the photo-induced electron–hole pair can be separated and transferred to the surface of the photocatalyst, directly participating in the photocatalytic reaction or producing active radicals.53 In general, hydroxyl radicals (˙OH), superoxide radicals (˙O2−), and holes (h+) can be found in the photocatalytic degradation process.For this reason, many scavengers, such as p-benzoquinone (PBQ, ˙O2−), 1,4-terephthalic acid (PTA, ˙OH), methanol (MT, h+) and others, were introduced to carry out free radical trapping experiments. It can be seen from Fig. 9c that in the dark reaction stage, the scavenger has a slight influence on the adsorption of the system. We speculate that the photocatalyst has a different degree of adsorption on different scavengers, and the scavengers and TCH have formed a competitive relationship. The combination of different scavenger and catalyst surfaces may also be different, so different catalysts show different adsorption effects in the dark reaction stage. After adding PTA, the degradation efficiency of TCH decreased from 85.3% (no scavenger) to 52.4% (with PTA), indicating that the ˙OH radical is the main active species in the photocatalytic degradation process. When PBQ entered the reaction system, the degradation rate of TCH decreased from 85.3% (no scavenger) to 71.5% (PBQ existed), indicating that ˙O2− played a secondary role in the degradation system. When MT was added to the reaction system, the degradation rate of TCH decreased from 85.3% (no scavenger) to 75.4% (MT existed), indicating that h+ played a secondary role in the reaction system. Therefore, the contribution order of the active species in the photocatalytic degradation process is ˙OH > ˙O2− > h+.
The migration of photoexcited e− and h+ in the photocatalyst was studied by PL analysis. It is well known that the photoluminescence intensity is closely related to the carrier recombination rate. The PL spectrum (Fig. 9d) of the sample was obtained by the excitation wavelength of 300 nm. The broad emission band at 401.03 nm is considered to be the emission boundary formed by the capture of free excitons by the titanium group near the defect.44 The peaks of 451.03 and 465.24 nm can be attributed to oxygen vacancy and electron capture, respectively. It can be seen that the luminous intensity of 8-TF is significantly lower than that of other samples, which indicates that the composite rate of e− and h+ is the lowest. This is consistent with the results of the photocatalytic degradation test. This may be due to the close interface contact between TiO2 and TiOF2, which promotes the transmission of electrons.
The position of the band gap plays an important role in the flow direction of photoexcited electrons and holes. This plays an important role in the production of active species and the performance of photocatalysis. We calculated the location of the conduction band (CB) and valence band (VB) for TiO2 and TiOF2 using the following empirical formula:
| Ec = χ − E0 − 0.5Eg | (2) |
| Ev = Ec + Eg | (3) |
Based on the experimental results and band gap position, we proposed the possible mechanism of outstanding photocatalytic activity of 8-TF (Fig. 10). First, the specific surface area of the catalyst is greatly increased due to its unique flower cluster morphology. A large specific surface area not only increases the contact area of the reactant adsorption, but also provides more active sites for the photocatalytic reaction.10 This unique morphology also makes it have strong light absorption characteristics, which is conducive to improving the quantum efficiency of photons. Due to the existence of Ti3+/Ov, there are local states below the conduction band of TiO2 and TiOF2, which makes TiO2 and TiOF2 respond well to simulated sunlight. Moreover, the heterostructure formed by TiO2 and TiOF2 reduces the recombination efficiency of the electrons and holes, which makes the electrons and holes more likely to interact with O2 and H2O to generate an active species capable of degrading TCH. Due to the CB potential (−0.24 eV) of TiO2 being more negative than that of TiOF2 (1.295 eV), a strong driving force will be generated between the close heterogeneous interfaces, which will promote the electron transfer from the CB of TiO2 to the CB of TiOF2. Due to the existence of some oxygen vacancies in the material, some electrons can be captured. It is obvious that the directional transfer of electrons and the capture of oxygen vacancies effectively reduce the recombination rate of electron–hole pairs, which is the main reason for the excellent photocatalytic activity of the photocatalyst. Because the CB potential (−0.24 eV) of TiO2 is more negative than that of O2/˙O (−0.046 eV),54 the adsorbed O2 on the surface of the photocatalyst is more likely to capture the electrons on TiO2 CB and form ˙O2−. ˙O2− can highly oxidize TCH or other pollutants. Moreover, because the material has some oxygen vacancies, these oxygen vacancies can better adsorb water, which plays an important role in the formation of hydroxyl radicals. Because the VB potential (4.305 eV) of TiOF2 is higher than that of TiO2 (2.84 eV), the h+ on TiOF2 VB is easier to transfer to the VB of TiO2, and the VB potential of both is higher than the standard redox potential (1.99 eV) of ˙OH/OH−.55 This allows the adsorbed water on the surface of the photocatalyst to more easily form ˙OH with strong oxidation and can mineralize TCH well.
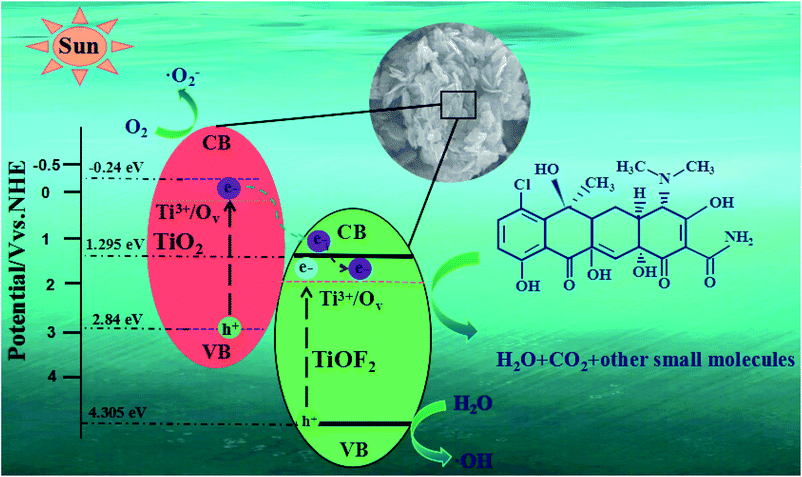 | ||
| Fig. 10 Schematic illustration of the photocatalytic mechanism for TCH removal over the 8-TF photocatalyst. | ||
4. Conclusions
To sum up, a new type of three-dimensional flower-like TiO2/TiOF2 heterostructure semiconductor composite was successfully prepared by a one-step hydrothermal method. Compared with Degusse P25, TiOF2, and pure TiO2, the degradation of TCH on the prepared photocatalyst was improved, and the rate constants were 1.7, 1.8, and 21 times higher than that for P25, TiOF2 and TiO2 respectively. The unique morphology provides a large specific surface area for the adsorption of reactants, and the close heterogeneous interface and Ti3+/Ov are conducive to charge transfer and separation, reducing the carrier recombination rate. It is worth noting that there are many interference factors in the actual TCH wastewater. Therefore, the interference factors in the actual antibiotic wastewater should be evaluated and tested in the follow-up study.Conflicts of interest
There is no conflict of interests existing in the manuscript submission, and it is approved by all of the authors for publication. All the authors listed have approved the manuscript to be enclosed.Acknowledgements
This work was supported by Chongqing Special Financial Funds Project (19511).References
- I. C. Iakovides, I. Michael-Kordatou, N. F. F. Moreira, A. R. Ribeiro, T. Fernandes, M. F. R. Pereira, O. C. Nunes, C. M. Manaia, A. M. T. Silva and D. Fatta-Kassinos, Water Res., 2019, 159, 333–347 CrossRef CAS PubMed.
- H. Liu, G. Xu and G. Li, J. Colloid Interface Sci., 2021, 587, 271–278 CrossRef CAS PubMed.
- X. Liu, Y. Yang, H. Li, Z. Yang and Y. Fang, Chem. Eng. J., 2021, 408, 127259 CrossRef CAS.
- C. Li, R. Hu, X. Lu, S. Bashir and J. B. L. Liu, Catal. Today, 2020, 350, 171–183 CrossRef CAS.
- N. N. Tri, D. Q. Ho, A. J. P. Carvalho, M. T. Nguyen and N. T. Trung, Surf. Sci., 2021, 703, 121723 CrossRef CAS.
- B. Yu, F. M. Meng, M. W. Khan, R. Qin and X. B. Liu, Ceram. Int., 2020, 46, 13133–13143 CrossRef CAS.
- M. Pudukudy, Q. Jia, J. Yuan, S. Megala, R. Rajendran and S. Shan, Mater. Sci. Semicond. Process., 2020, 108, 104891 CrossRef CAS.
- Y. Chen, J. Li, F. Wang, H. Yang and L. Liu, Chemosphere, 2021, 265, 129133 CrossRef CAS PubMed.
- D. Wan, L. Wu, Y. Liu, J. Chen, H. Zhao and S. Xiao, Langmuir, 2019, 35, 3925–3936 CrossRef CAS.
- C. Lv, X. Lan, L. Wang, X. Dai, M. Zhang, J. Cui, S. Yuan, S. Wang and J. Shi, Environ. Technol., 2021, 42, 377–387 CrossRef CAS PubMed.
- X. Zhao, X. Li, Y. Li, X. Zhang, F. Zhai, T. Ren and Y. Li, J. Hazard. Mater., 2021, 408, 124880 CrossRef CAS PubMed.
- Y. Ma, H. Xiong, Z. Zhao, Y. Yu, D. Zhou and S. Dong, Chem. Eng. J., 2018, 351, 967–975 CrossRef CAS.
- J. Wang, L. Jiang, F. Liu, M. Jia, M. Liu, J. Li and Y. Lai, Chem. Eng. J., 2021, 407, 127195 CrossRef CAS.
- B. Li and T. Zhang, Environ. Sci. Pollut. Res., 2013, 20, 3024–3033 CrossRef CAS PubMed.
- Z. Zhang, Y. Chen, Z. Wang, C. Hu, D. Ma, W. Chen and T. Ao, Appl. Surf. Sci., 2021, 542, 148662 CrossRef CAS.
- H. Wo, X. Liu, J. Wen, Y. Liu and X. Zheng, Colloids Surf., A, 2021, 610, 125933 CrossRef.
- Y. Xu, H. Li, B. Sun, P. Qiao, L. Ren, G. Tian, B. Jiang, K. Pan and W. Zhou, Chem. Eng. J., 2020, 379, 122295 CrossRef CAS.
- W. Tang, J. Chen, Z. Yin, W. Sheng, F. Lin, H. Xu and S. Cao, Chin. J. Catal., 2021, 42, 347–355 CrossRef CAS.
- J. Ma, M. Xing, L. Yin, K. S. Hui and K. N. Hui, Appl. Surf. Sci., 2021, 536, 147735 CrossRef CAS.
- N. M. Tran, Q. T. H. Ta and J. S. Noh, Appl. Surf. Sci., 2021, 538, 148023 CrossRef.
- D. F. F. Brossault, T. M. McCoy and A. F. Routh, J. Colloid Interface Sci., 2021, 584, 779–788 CrossRef CAS PubMed.
- E. B. Ertus, C. Vakifahmetoglu and A. Ozturk, J. Eur. Ceram. Soc., 2021, 41, 1530–1536 CrossRef CAS.
- Q. Chen, K. Wang, G. Gao, J. Ren, R. Duan, Y. Fang and X. Hu, Appl. Surf. Sci., 2021, 538, 147944 CrossRef CAS.
- Y. Yu, L. Zhu, G. Liu, M. Qiu, P. Chen and Z. Chang, Appl. Surf. Sci., 2021, 540, 148239 CrossRef CAS.
- X. He, M. Wu, Z. Ao, B. Lai, Y. Zhou, T. An and S. Wang, J. Hazard. Mater., 2021, 403, 124048 CrossRef CAS PubMed.
- K. Xu, Z. Liu, S. Qi, Z. Yin, S. Deng, M. Zheng and Z. Sun, Appl. Surf. Sci., 2021, 538, 148044 CrossRef CAS.
- W. Zhu, J. Mi, Y. Fu, D. Cui and C. Lu, Appl. Surf. Sci., 2021, 538, 148087 CrossRef CAS.
- Y. Yang, M. Liu, S. Han, H. Xi, C. Xu, R. Yuan, J. Long and Z. Li, Appl. Surf. Sci., 2021, 537, 147991 CrossRef CAS.
- Y. Jian, H. Liu, J. Zhu, Y. Zeng, Z. Liu, C. Hou and S. Pu, RSC Adv., 2021, 10, 42860–42873 RSC.
- Y. Zhu, Y. Pan, E. Zhang and W. Dai, New J. Chem., 2020, 44, 11076–11084 RSC.
- C. P. Sajan, S. Wageh, A. A. Al-Ghamdi, J. G. Yu and S. Cao, Nano Res., 2016, 9, 3–27 CrossRef CAS.
- J. Zhang, H. Jiang, Y. Liu and R. Chen, Appl. Surf. Sci., 2019, 488, 555–564 CrossRef CAS.
- J. Wang, Y. Wang, W. Wang, T. Peng, J. Liang, P. Li, D. Pan, Q. Fan and W. Wu, Environ. Pollut., 2020, 262, 114373 CrossRef CAS PubMed.
- M. Jung, Y. Kim and Y. S. Lee, J. Ind. Eng. Chem., 2017, 47, 187–193 CrossRef CAS.
- P. Dong, E. T. Cui, G. Hou, R. Guan and Q. Zhang, Mater. Lett., 2015, 143, 20–23 CrossRef CAS.
- L. Chen, L. Tian, J. Xie, C. Zhang, J. Chen, Y. Wang, Q. Li, K. Lv and K. Deng, Appl. Surf. Sci., 2020, 504, 144353 CrossRef CAS.
- Z. Liu, X. Liu, Q. Lu, Q. Wang and Z. Ma, J. Taiwan Inst. Chem. Eng., 2019, 96, 214–222 CrossRef CAS.
- C. Hou, J. Xie, H. Yang, S. Chen and H. Liu, RSC Adv., 2019, 9, 37911–37918 RSC.
- Y. Zhang, T. Xia, M. Shang, P. Wallenmeyer, D. Katelyn, A. Peterson, J. Murowchick, L. Dong and X. chen, RSC Adv., 2014, 4, 16146–16152 RSC.
- Y. Zhang, Q. Zhang, T. Xia, D. Zhu, Y. Chen and X. Chen, ChemNanoMat, 2015, 1, 270–275 CrossRef CAS.
- Y. Zhang, M. Shang, Y. Mi, T. Xia, P. Wallenmeyer, J. Murowchick, L. Dong, Q. Zhang and X. Chen, ChemNanoMat, 2014, 79, 1159–1166 CAS.
- C. Hou, W. Liu and J. Zhu, Catalysts, 2017, 7, 243 CrossRef.
- J. C. C. Yu, V. H. Nguyen, J. Lasek and J. C. S. Wu, Appl. Catal., B, 2017, 219, 391–400 CrossRef CAS.
- C. Hou and W. Liu, R. Soc. Open Sci., 2018, 5, 172005 CrossRef PubMed.
- A. C. Martins, A. L. Cazetta, O. Pezoti, J. R. B. Souza, T. Zhang, E. J. Pilau, T. Asefa and V. C. Almeida, Ceram. Int., 2017, 43, 4411–4418 CrossRef CAS.
- C. Zhao, J. Guo, C. Yu, Z. Zhang, Z. Sun and X. Piao, Mater. Chem. Phys., 2020, 248, 122873 CrossRef CAS.
- T. Zhao, Z. Xing, Z. Xiu, Z. Li, L. Shen, Y. Cao, M. Hu, S. Yang and W. Zhou, Mater. Res. Bull., 2018, 103, 114–121 CrossRef CAS.
- J. Pan, Z. Dong, B. Wang, Z. Jiang, C. Zhao, J. Wang, C. Song, Y. Zheng and C. Li, Appl. Catal., B, 2019, 242, 92–99 CrossRef CAS.
- Y. Jiang, X. Jing, K. Zhu, Z. Peng, J. Zhang, Y. Liu, W. Zhang, L. Ni and Z. Liu, Dalton Trans., 2018, 47, 13113–13125 RSC.
- Y. Shi, Z. Yang, B. Wang, H. An, Z. Chen and H. Cui, Appl. Clay Sci., 2016, 119, 311–320 CrossRef CAS.
- G. H. Safari, M. Hoseini, M. Seyedsalehi, H. Kamani, J. Jaafari and A. H. Mahvi, Int. J. Environ. Sci. Technol., 2015, 12, 603–616 CrossRef CAS.
- E. O. Oseghe and A. E. Ofomaja, J. Photochem. Photobiol., A, 2018, 360, 242–248 CrossRef CAS.
- Y. Jin, D. Jiang and M. Chen, Catal. Sci. Technol., 2017, 7, 2308–2317 RSC.
- Y. Jiang, Z. Peng, S. Zhang, F. Li, Z. Liu, J. Zhang, Y. Liu and K. Wang, Ceram. Int., 2018, 44, 6115–6126 CrossRef CAS.
- P. Ning, H. Chen, J. Pan, J. Liang, L. Qin, D. Chen and Y. Huang, Catal. Sci. Technol., 2020, 10, 8295–8304 RSC.
| This journal is © The Royal Society of Chemistry 2021 |