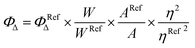Open Access Article
Open Access ArticleBSA-encapsulated cyclometalated iridium complexes as nano-photosensitizers for photodynamic therapy of tumor cells†
Yao Xu,
Xiang Wang,
Kang Song,
Jun Du,
Jinliang Liu,
Yuqing Miao and
Yuhao Li*
Institute of Bismuth Science, College of Science, University of Shanghai for Science and Technology, Shanghai 200093, China. E-mail: yhli@usst.edu.cn
First published on 23rd April 2021
Abstract
Photodynamic therapy is a promising treatment method. The development of suitable photosensitizers can improve therapeutic efficacy. Herein, we report three iridium complexes (Ir1, Ir2, and Ir3), and encapsulate them within bovine serum albumin (BSA) to form nano-photosensitizers (Ir1@BSA, Ir2@BSA, and Ir3@BSA) for photodynamic therapy (PDT) of tumor cells. In the structures of Ir(III) complexes, we use the pyrazine heterocycle as part of the C^N ligands and explore the effect of different ligands on the ability to generate singlet oxygen (1O2) by changing the conjugation length of the ligand and increasing the coplanarity of the ligand. Besides, the fabricated nano-photosensitizers are beneficial to improve water dispersibility and increase cellular uptake ability. Through studying photophysical properties, 1O2 generation capacity, and cellular uptake performance, the results show that Ir1@BSA has the best photodynamic therapeutic effect on 4T1 tumor cells. This study provides an effective research basis for the further design of new nano-photosensitizers.
Introduction
Photodynamic therapy (PDT) is a noninvasive, photo-controlled treatment method.1–4 Based on the generation of photo-induced reactive oxygen species (ROS), this method has been widely utilized in cancer treatment such as for bladder cancer, esophageal cancer, lung cancer, and brain cancer, exhibiting some superiorities over traditional cancer treatments including operative treatment, radiotherapy, and chemotherapy.5–9 Three factors influence the effectiveness of PDT including photosensitizer, oxygen, and light.10 Through producing more ROS to obtain a better therapeutic effect, the researchers have started from these three factors and designed multiple methods to enhance PDT. For example, the nanomaterial is used to carry oxygen to supply raw materials for PDT.11–14 For another example, the structure of the photosensitizer is adjusted to increase singlet oxygen quantum yield.15–18 Besides, the excitation light is regulated to increase the penetration depth of the light into the tissue for internal tumor treatment.19,20Numerous studies have shown that among the three factors, designing novel photosensitizers through structural optimization strategies is a feasible and effective way to enhance the therapeutic effect.21–24 Benefit from the development of chemical synthesis, many types of photosensitizers have been developed. Among them, noble metal complex photosensitizers have attracted great attention, such as ruthenium complexes,25,26 platinum complexes,27,28 iridium complexes,29,30 etc. Iridium Ir(III) complexes possess higher intersystem crossing efficiency and better photobleaching resistance than some organic photosensitizers. More specifically, Ir(III) complexes show a triplet excited-state lifetime from nanosecond to microsecond, beneficial for the energy transfer to react with 3O2 to generate 1O2.31 For heteroleptic cationic Ir(III) complexes, through adjusting their cyclometalated ligands (C^N ligand) and ancillary ligands (N^N ligand), the ground state absorption and excited-state absorption of the complexes are regulated to achieve effective triplet energy transfer, and ultimately increase singlet oxygen quantum yield.32,33 Although some studies have been focused on the relationship between ligand and singlet oxygen quantum yield, and some Ir(III) complexes have been synthesized based on the modification of C^N ligand and N^N ligand, there is still no clear enunciation for the structure–activity relationship between them.
Besides, most Ir(III) complexes are water-insoluble. To make these Ir(III) complexes become photosensitizers for biological application, it is necessary to overcome the problem of insufficient water solubility. There are two methods to solve this problem. One way is to introduce hydrophilic groups or water-soluble polymers into an Ir(III) complex by covalent bonding to improve the solubility.34–36 Another way is to utilize biocompatible polymers or protein to encapsulate the Ir(III) complex to improve the water dispersibility.37,38 These two strategies provide strategies for the further application of Ir(III) complexes as photosensitizers.
Herein, we reported a series of three bovine serum albumin (BSA)-encapsulated Ir(III)-based nano-photosensitizers (Ir1@BSA, Ir2@BSA, and Ir3@BSA) for photodynamic therapy of tumor cells. The three iridium complexes (Ir1 to Ir3) possess different C^N ligands. Based on the 2,3-diphenylquinoxaline structure in Ir1, introduce a benzene ring to extend the conjugation length of C^N ligand in Ir2 or connect the two phenyl groups to construct a more plane-rigid C^N ligand in Ir3 to explore the relationship between these influencing factors and singlet oxygen generation ability. Also, to increase the water dispersibility, we encapsulated BSA on the surface of the synthesized complexes by self-assembly to construct nano-photosensitizers. Introducing two long alkyl chains into the N^N ligand would make an Ir(III) complex that could be better embedded in the hydrophobic cavity of BSA during self-assembly to achieve a tighter combination. The study of three protein-encapsulated nano-photosensitizers could provide a research foundation with exploring Ir(III) complexes for photodynamic therapy (Scheme 1).
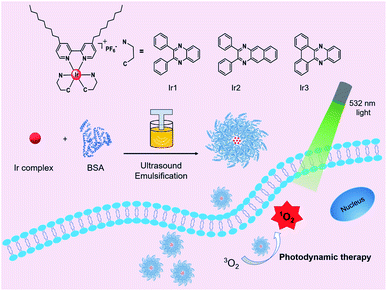 | ||
| Scheme 1 Molecular structures of Ir1 to Ir3 complexes and schematic of Ir1@BSA to Ir3@BSA, fabrication, and application for photodynamic therapy. | ||
Results and discussion
Synthesis
The synthesis route from Ir1 to Ir3 was shown in Fig. S1.† These iridium complexes were synthesized in a three-step method. To begin with, three pyrazine derivatives (dpqx, dpbq, and dbpz) were synthesized through the reaction of dehydration between diketones and diamine compounds. Subsequently, the dimeric iridium complexes were obtained from the reaction between these ligands and IrCl3, and the Ir1 to Ir3 complexes were obtained from the reaction between the dimeric iridium complexes and the alkyl chain decorated bipyridine (N^N) ligand. The structures of some ligands and final products were characterized by 1H-nuclear magnetic resonance (1H-NMR) and high resolution-mass spectrometry (HR-MS) (Fig. S2–S9†), and the result showed that the synthesized structures were in line with its design.Optical properties
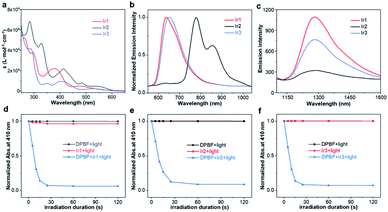
The absorption spectra of Ir1, Ir2, Ir3 in CH2Cl2 were shown in Fig. 1a. Variable concentration experiments confirmed that no ground state aggregation occurred in the concentration range from 5 to 50 μM (Fig. S10–S12†). Three Ir(III) complexes exhibited a wide absorption band from UV to visible region. The absorption band below 350 nm was due to the 1π,π* transition of C^N and N^N ligands. The absorption band from 350 to 450 nm was due to the 1π,π* transition and spin allowed singlet metal to ligand charge transfer (1MLCT) mixed with some singlet ligand to ligand charge transfer (1LLCT) and singlet intra-ligand charge transfer (1ILCT). The absorption band larger than 450 nm was due to the 1MLCT/1LLCT. Especially, three Ir(III) complexes had obvious absorption at 532 nm, the molar absorption coefficients were 2225, 4686, and 2425 L mol−1 cm−1, for Ir1 to Ir3 respectively, beneficiating to the PDT induced by green light (532 nm).The emission spectra of Ir1, Ir2, Ir3 in CH2Cl2 were shown in Fig. 1b. Variable concentration experiments confirmed that no quenching emission intensity occurred from 5 to 50 μM (Fig. S10–S12†). Upon excitation at 532 nm, Ir1 exhibited major photoluminescence peaks at 636 nm, Ir2 exhibited major photoluminescence peaks at 778 nm and 855 nm, and Ir3 exhibited major photoluminescence peaks at 650 nm. Compared to Ir1, the Ir2 showed a red-shifted emission peak and the emission peak located in the near-infrared (NIR) region, which indicated that increasing the conjugation length of the C^N ligand can significantly redshift the position of the emission peak. This result was in line with the reported phenomenon that the degree of π-conjugation of the C^N ligands impacts the location of emission.39,40 Further, compared to Ir1, Ir3 exhibited a similar emission location, indicating that increasing the rigidity of the C^N ligand cannot change the location of the Ir(III) complex emission. Due to the long linear alkyl substituents on the N^N ligand, three complexes presented good solubility and could be dissolved in different organic solvents such as toluene, tetrahydrofuran (THF), CH2Cl2, CH3CN, and CH3OH (Fig. S10–S12†).
Taking the air-sensitive emission, luminous lifetime from nanosecond to microsecond, and large Stokes shift into consideration (Fig. S13–S15, Table S1†), we confirmed that the emission of these complexes was phosphorescence. Then, the position of the phosphorescence emission peak and the luminescence quantum efficiency did not show regular changes with the increase of solvent polarity. Based on these considerations, the emission of Ir1 to Ir3 was ascribed to the triplet metal to ligand charge transfer (3MLCT) mixed with triplet ligand to ligand charge transfer (3LLCT). The study of these optical properties showed that the luminescence of these complexes could be applied for cell phosphorescence imaging (Table 1).
| λabsa/nm (ε/104 L mol−1 cm−1) | λemb/nm (τem/ns, Φem) | λT1–Tnc/nm (τTA/ns) | ΦΔd | |
|---|---|---|---|---|
| a Absorption band maxima and molar extinction coefficients in CH2Cl2 at room temperature.b Room temperature emission band maxima, lifetime, and quantum yield in CH2Cl2 (1 × 10−5 mol L−1).c Triplet transient absorption band maxima and lifetime in degassed CH3CN.d Singlet oxygen quantum yield in CH3CN.e Too weak to be measured. | ||||
| Ir1 | 295 (3.8), 382 (2.2), 480 (0.49) | 636 (817, 0.176) | 430 (336) | 0.84 |
| Ir2 | 283 (6.8), 327 (4.7), 413 (2.2), 485 (0.79) | 778, 855 (—e, —) | 510 (144) | 0.27 |
| Ir3 | 285 (4.2), 350 (0.74), 400 (0.97), 530 (0.23) | 650 (737, 0.182) | 460 (309) | 0.64 |
Singlet oxygen generation evaluation
Based on the ROS generation mechanism, the triplet excited state lifetime (τT) of the Ir(III) complex is a key factor in the generation of 1O2. The longer the triply excited-state lifetime, the more beneficial it is to increase the probability of the complex colliding with oxygen. The transient absorption (TA) spectra of Ir1, Ir2, and Ir3 in degassed CH3CN were shown in Fig. S16.† The τT of Ir1 to Ir3 were 336 ns, 144 ns, and 309 ns at the maximum triple excited absorption wavelength of 430 nm, 510 nm, and 460 nm, respectively, indicating Ir1 qualified the longest τT. Then, two ways were utilized to evaluate the 1O2 generation ability of three complexes.First, 1O2 presented the unique emission at 1270 nm. In Fig. 1c, we monitored the unique emission of 1O2 for three complexes. Through 405 nm light excitation, the 1O2 emission at 1270 nm of Ir1, Ir2, and Ir3 in CH3CN was observed, and the luminescence intensity trend of 1O2 was Ir1 > Ir3 > Ir2, indicating three complexes could catalyze the production of 1O2, and Ir1 had the highest catalytic efficiency. Subsequently, 1,3-disphenylisobenzofuran (DPBF) as a 1O2 trap could observe the rate of 1O2 production and calculate the singlet oxygen quantum yield (ΦΔ). In Fig. 1d–f, under the irradiation of 532 nm light, when DPBF was mixed with the solutions of the three complexes, the absorption at 410 nm decreased rapidly with the extension of the irradiation time, and the absorption decreased dramatically within 30 s. After the DPBF solution and the complex solution alone were irradiated with light, almost no decrease in absorbance was observed. This showed that the three complexes could quickly catalyze oxygen to produce 1O2, and the catalytic rate was fast. Furthermore, the singlet oxygen quantum yields of Ir1 to Ir3 complexes were calculated to be 0.84, 0.27, and 0.64 respectively (Fig. S17†). Similarly, Ir1 showed the highest singlet oxygen quantum yield. All of these indicated that Ir1 qualified the remarkable 1O2 generation performance, and also revealed that increasing the conjugation length of the C^N ligand or increasing its planar rigidity cannot effectively increase the singlet oxygen quantum yield.
Fabrication and characterization of Ir1@BSA to Ir3@BSA
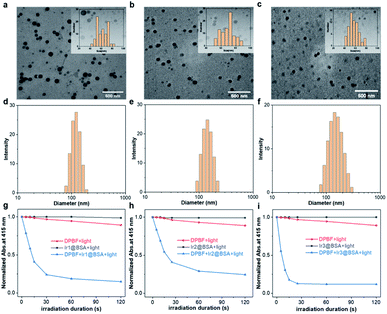
Next, to improve biocompatibility, these iridium complexes were self-assembled with BSA to form nano-photosensitizers by an emulsify method. To weaken the electrostatic interaction by adjusting the pH of the aqueous solution to make BSA around the isoelectric point, these iridium complexes could be well encapsulated with BSA through hydrophobic–hydrophobic interaction. The structures of Ir1@BSA to Ir3@BSA were characterized by transmission electron microscope (TEM). In Fig. 2a–c, many monodisperse balls were observed in the TEM images. The particle size statistics showed that the particle sizes of the three nano-photosensitizers were 61.1 ± 9.7 nm, 68.5 ± 9.1 nm, and 56.6 ± 8.7 nm for Ir1@BSA to Ir3@BSA, respectively. This showed that the emulsification method was a general encapsulation method, and the prepared nanomaterials have similar particle sizes. In Fig. 2d–f, dynamic light scattering (DLS) results showed larger hydration particle sizes and the sizes were 135.7 nm, 148.6 nm, and 151.2 nm for Ir1@BSA to Ir3@BSA, respectively, which was due to the formation of a hydration layer. Furthermore, we analyzed the stability of Ir1@BSA to Ir3@BSA by hydrated particle size. The results showed that the hydrated particle size of them did not change significantly after 15 days, which maintained a good nano-shape and showed good nano-stability (Fig. S18†). At last, DPBF was also utilized to measure the 1O2 generation ability of Ir1@BSA to Ir3@BSA in H2O. In Fig. 2g–i, similar to the result of Ir1 to Ir3, the absorption of DPBF was sharply decreased when it was mixed with Ir1@BSA to Ir3@BSA. The calculated 1O2 quantum yields were 0.20, 0.06, and 0.23 in H2O (Fig. S19†), and the rate of singlet oxygen generation has a linear relationship with the excitation light power and the concentration of the Ir1@BSA to Ir3@BSA (Fig. S20†). These results showed that the three Ir(III) complexes could still show good 1O2 generation ability after being wrapped by BSA to form nano-photosensitizers.Cellular uptake and cytotoxicity assay
Benefits to the luminescence properties of iridium complexes, the uptake of nano-photosensitizers by cells could be observed through phosphorescence imaging. The emission of Ir2@BSA located at the NIR region, which exceeded the detection capability of the microscope camera, so we only used cell imaging for Ir1@BSA and Ir3@BSA to observe their uptake. In Fig. 3, Ir1@BSA showed a strong phosphorescence signal in the cytoplasm, and the signal further increased with time, which showed that the uptake of Ir1@BSA by cells was good. In comparison, the uptake of Ir3@BSA by cells was poor, that is, as the incubation time increased, the fluorescent signal observed in the cells was significantly weaker than Ir1@BSA. Further by detecting the content of Ir3+ ion in cells, we used the inductively coupled plasma-mass spectrometry (ICP-MS) to detect the uptake of the three nano-photosensitizers in tumor cells. ICP-MS result indicated that the contents of Ir3+ ion in cells were 0.95, 0.67, and 0.66 μg g−1 for Ir1@BSA, Ir2@BSA and Ir3@BSA respectively, indicating cells had a high uptake rate of Ir1@BSA. The difference in the uptake of photosensitizers by cells has a potential impact on the effect of PDT on cells. The mechanism behind this phenomenon is still unclear, and we will study it in our following research.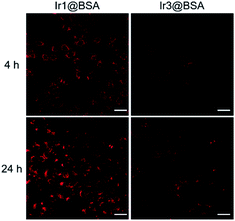 | ||
| Fig. 3 Luminescence images of 4T1 cells incubated with Ir1@BSA or Ir3@BSA at 4 h and 24 h. Scale bar, 40 μm. | ||
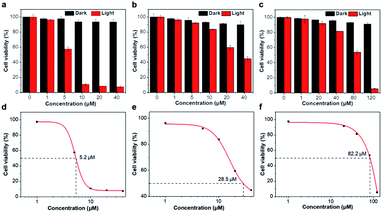
Afterward, to assess the intracellular light-induced 1O2 production, cell viability was analyzed by Cell Counting Kit-8 (CCK-8) assay in Fig. 4. At a concentration of 40 μM, the cell viability was greater than 90% after cells were incubated with Ir1@BSA and Ir2@BSA for 24 h, and even at a concentration of 120 μM, the dark toxicity of Ir3@BSA was weak, indicating that the dark toxicity of the nano-photosensitizers to cells could be ignored. After exposure to light, we observed different degrees of phototoxicity of cells. As the concentration of nano-photosensitizer increases, Ir1@BSA exhibited greater phototoxicity, that is, it has a significant inhibitory effect on cell viability at a lower concentration. The calculated 50% effective concentration (EC50) values of Ir1@BSA, Ir2@BSA, and Ir3@BSA were 5.2 μM, 28.5 μM, and 82.2 μM respectively. The results presented that the efficiency of PDT was Ir1@BSA > Ir2@BSA > Ir3@BSA. The difference in therapeutic effect could be explained by the above-mentioned cellular uptake and singlet oxygen quantum yield. The uptake of Ir1@BSA by cells was relatively high, and Ir1@BSA showed a remarkable singlet oxygen production rate. Therefore, we believe that the dual roles of Ir1@BSA resulted in the best PDT treatment effect.
Live/dead cell staining
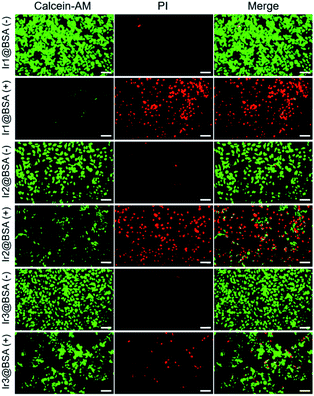
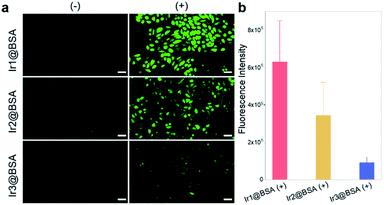
The calcein O,O′-diacetate tetrakis(acetoxymethyl) ester/propidium iodide (calcein-AM/PI) double stain kit was used to directly differentiate the living and dead cells after treatment. In Fig. 5, when mouse breast cancer (4T1) cells were incubated with three nano-photosensitizers without 532 light irradiation, strong green fluorescence emitted from calcein-AM was monitored and the observed red fluorescence emitted from PI was negligible, indicating almost all cells were alive. Further, after the cells were incubated with photosensitizers and irradiated with light, the green fluorescence decreased and the red fluorescence increased, indicating that many cells were dead cells and the three nano-photosensitizers caused different amounts of cell death. The live and dead cell staining consolidated that PDT was effective for 4T1 tumor cell therapy, and Ir1@BSA presented the best treatment effect.Intracellular ROS detection
To verify that the production of ROS in cells, 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) as a cellular ROS indicator could be used to evaluate the 1O2 generation ability of Ir1@BSA to Ir3@BSA in 4T1 cells. The generated 1O2 could induce DCFH-DA to convert to 2,7-dichlorofluorescein (DCF), which could emit green fluorescence. In Fig. 6, when cells were incubated with Ir1@BSA to Ir3@BSA respectively, green fluorescence was observed in three group cells after 532 nm light irradiation, while no green fluorescence was monitored in the cells without light irradiation. Through the analysis of the fluorescence intensity, among the three groups of cells exposed to light, the cells incubated with Ir1@BSA showed the strongest green fluorescence, indicating that a large amount of ROS was produced in the cells. This further showed that Ir1@BSA was an excellent nano-photosensitizer.Experimental
General
All solvents and reagents used to synthesis were acquired from chemical reagent suppliers, such as Alfa-Aesar, Adamas, Aladdin, and TCI. 1H-NMR spectra were obtained by using a Bruker 400 MHz NMR spectrometer, and the chloroform-d (CDCl3) was used as a solvent. HR-MS spectra were obtained by using an AB SCIEX X500B QTOF electrospray ionization time-of-flight mass spectrometer.Synthesis procedures
dpqx. Raw materials: benzil (420.5 mg, 2 mmol) and benzene-1,2-diamine (216.3 mg, 2 mmol); product: white solid (536 mg, 95%).
dpbq. Raw materials: benzil (420.5 mg, 2 mmol) and naphthalene-2,3-diamine (316.4 mg, 2 mmol); product: bright yellow solid (565 mg, 85%).
dbpz. Raw materials: 9,10-phenanthraquinone (416.4 mg, 2 mmol) and benzene-1,2-diamine (216.3 mg, 2 mmol); product: light yellow solid (527 mg, 94%). 1H-NMR (400 MHz, CDCl3) δ 9.40 (d, J = 7.9 Hz, 2H), 8.56 (d, J = 7.9 Hz, 2H), 8.37–8.28 (m, 2H), 7.90–7.83 (m, 2H), 7.82–7.71 (m, 4H). HR-MS: m/z calculated for [C20H12N2 + H]+: 281.1073; found: 281.0991.
[Ir(dpqx)2]2Cl2. Raw materials: dpqx (124 mg, 0.44 mmol) and IrCl3·xH2O (70 mg, 0.2 mmol); product: red solid (120 mg, 76%).
[Ir(dpbq)2]2Cl2. Raw materials: dpbq (146 mg, 0.44 mmol) and IrCl3·xH2O (70 mg, 0.2 mmol); product: dark red solid (128 mg, 72%).
[Ir(dbpz)2]2Cl2. Raw materials: dbpz (123 mg, 0.44 mmol) and IrCl3·xH2O (70 mg, 0.2 mmol); product: dark red solid (80 mg, 51%).
Ir1. Raw materials: [Ir(dpqx)2]2Cl2 (158 mg, 0.1 mmol), dnbpy (81.6 mg, 0.2 mmol), and F3CSO3Ag (51.4 mg, 0.2 mmol); column chromatography (100/1, v/v); product: red solid (117 mg, 44.7%). 1H-NMR (400 MHz, CDCl3) δ 8.47 (d, J = 5.7 Hz, 2H), 8.21 (s, 2H), 8.04 (d, J = 0.9 Hz, 1H), 8.02 (d, J = 0.9 Hz, 1H), 7.83 (d, J = 3.9 Hz, 4H), 7.68–7.65 (m, 6H), 7.60 (dd, J = 11.2, 3.9 Hz, 2H), 7.49 (dd, J = 5.7, 1.1 Hz, 2H), 7.36 (s, 1H), 7.34 (s, 1H), 7.18–7.11 (m, 4H), 6.78–6.73 (m, 2H), 6.68 (td, J = 7.5, 1.1 Hz, 2H), 6.47 (d, J = 7.3 Hz, 2H), 2.82 (dd, J = 14.5, 7.3 Hz, 4H), 1.61 (dd, J = 13.6, 7.3 Hz, 4H), 1.26–1.20 (m, 24H), 0.86 (t, J = 6.9 Hz, 6H). HR-MS: m/z calculated for [C68H70F6IrN6P − PF6]+: 1163.5286; found: 1163.5194.
Ir2. Raw materials: [Ir(dpbq)2]2Cl2 (178 mg, 0.1 mmol), dnbpy (81.6 mg, 0.2 mmol), and F3CSO3Ag (51.4 mg, 0.2 mmol); column chromatography (50/1, v/v); product: dark red solid (174 mg, 61.7%). 1H-NMR (400 MHz, CDCl3) δ 8.63–8.58 (m, 4H), 8.17 (s, 2H), 7.99 (d, J = 8.4 Hz, 2H), 7.91–7.87 (m, 6H), 7.72–7.68 (m, 6H), 7.64 (dd, J = 5.4, 0.8 Hz, 2H), 7.51 (dd, J = 9.7, 4.7 Hz, 2H), 7.42–7.37 (m, 2H), 7.23 (s, 2H), 7.12 (d, J = 8.3 Hz, 2H), 6.78–6.73 (m, 2H), 6.68–6.63 (m, 2H), 6.56 (d, J = 7.1 Hz, 2H), 2.80 (dq, J = 20.6, 6.8 Hz, 4H), 1.64–1.56 (m, 4H), 1.27–1.18 (m, 24H), 0.85 (t, J = 7.0 Hz, 6H). HR-MS: m/z calculated for [C76H74F6IrN6P − PF6]+: 1263.5599; found: 1263.5569.
Ir3. Raw materials: [Ir(dbpz)2]2Cl2 (78.6 mg, 0.05 mmol), dnbpy (40.8 mg, 0.1 mmol), and F3CSO3Ag (25.7 mg, 0.1 mmol); column chromatography (30/1, v/v); product: dark red solid (53 mg, 40.6%). 1H-NMR (400 MHz, CDCl3) δ 9.45 (d, J = 9.0 Hz, 1H), 8.60 (d, J = 5.1 Hz, 3H), 8.56 (d, J = 8.1 Hz, 1H), 8.39 (d, J = 9.1 Hz, 2H), 8.33 (s, 3H), 8.12 (d, J = 7.9 Hz, 1H), 07.91 (t, J = 7.0 Hz, 2H), 7.86–7.76 (m, 3H), 7.62 (d, J = 5.7 Hz, 1H), 7.29 (d, J = 5.6 Hz, 3H), 7.22 (d, J = 4.4 Hz, 4H), 7.10 (t, J = 7.7 Hz, 2H), 7.02 (d, J = 5.7 Hz, 1H), 6.45 (d, J = 7.5 Hz, 1H), 2.76–2.70 (m, 4H), 1.73–1.69 (m, 4H), 1.33–1.21 (m, 24H), 0.88–0.85 (m, 6H). HR-MS: m/z calculated for [C68H66F6IrN6P − PF6]+: 1159.4973; found: 1159.4961.
Photophysical studies
The UV-vis absorption spectra of Ir1, Ir2, and Ir3 in different solvents including toluene, THF, CH2Cl2, CH3CN, and CH3OH were measured by a UV-1900 spectrometer (Shimadzu, Japan) in a 1 cm quartz cuvette. The emission spectra (λex = 436 nm) of Ir1 and Ir3 in different solvents were recorded by a fluorescence spectrometer (Edinburgh FS5, UK). The emission spectra of Ir2 in several solvents were recorded by a fiber optic spectrometer (NOVA-EX, Ideaoptics, China) with a 532 nm laser (China) as an excitation light source. The quantum yields of Ir1 and Ir3 were obtained by an FS5 fluorescence spectrometer with an integrating sphere. The emission lifetime was obtained by a flash photolysis spectrometer (Edinburgh LP980, UK) with a 355 nm Nd:YAG laser or a fluorescence spectrometer (FS5) with a 365 nm picosecond laser (Edinburgh, UK).Singlet oxygen detection
The generated singlet oxygen catalyzed by Ir1, Ir2, and Ir3 in CH3CN was measured by an FS5 fluorescence spectrometer with an InGaAs detector (λex = 405 nm). The emission range was recorded from 1100 nm to 1600 nm.Fabrication and characterization of Ir1@BSA to Ir3@BSA
Ir1 (1.3 mg), Ir2 (1.4 mg), or Ir3 (1.3 mg) was dissolved in 1 mL CH2Cl2 as an oil phase and BSA (7 mg) was dissolved in 5 mL water (pH = 5) as an aqueous phase. Two phases were emulsified by using an ultrasonic cell crusher (SCIENTZ-950E, China). Afterward, CH2Cl2 in the emulsion was removed to obtain a pale orange transparent solution. Finally, the Ir1@BSA to Ir3@BSA was obtained by filtrating with a mixed cellulose ester membrane filter (pore = 1 μm, Titan, China). The morphology of Ir1@BSA to Ir3@BSA was observed by a transmission electron microscope (HT7800, Hitachi, Japan). Hydrate particle sizes of Ir1@BSA to Ir3@BSA in aqueous solution were measured by a laser particle sizer (Malvern Zetasizer Nano ZS90, UK).Singlet oxygen quantum yield measurement
Singlet oxygen quantum yields (ΦΔ) of Ir1 to Ir3 and Ir1@BSA to Ir3@BSA were calculated by using a reference method. DPBF was utilized to capture 1O2, and [Ru(bpy)3]Cl2 was used as a reference (ΦΔ = 0.56 in CH3CN).41 The ΦΔ of materials depended linearly upon the absorbance degradation rate of DPBF at 410 nm (in CH3CN) or 415 nm (in H2O) under the same conditions. In general, DPBF (30 μM) was mixed with Ir1 to Ir3 (in CH3CN) or Ir1@BSA to Ir3@BSA (in 95% H2O containing 5% ethanol) in various concentration, then the mixture was irradiated with 532 nm laser. The absorbance at 410 nm or 415 nm was recorded and the ΦΔ was calculated using the following equation.W is the DPBF degradation rate caused by 1O2, A = 1 − 10−OD, A and OD are the absorptivity and absorbance of Ir1 to Ir3 or Ir1@BSA to Ir3@BSA at 532 nm, and η is the refractive index of solvent.42,43
Cellular uptake
4T1 cells were seeded in 24-well plates at the density of 1.2 × 104 cells per well and cultured for 24 h (37 °C, 5% CO2). Ir1@BSA or Ir3@BSA (20 μM) were added and further incubated for 4 or 24 h. All cells were washed with PBS (pH = 7.4). For imaging method, phosphorescence imaging of cells was recorded by using a CKX41 inverted microscope (Olympus, Japan) with an Olympus U-RFLT50 mercury lamp. A blue light was utilized as an excitation source and the emission was collected in a red channel. For ICP-MS method, after incubation for 4 h, the cells were collected and digested, and then the content of Ir3+ ion was detected by the ICP-MS spectrometer (iCAP™ RQ ICP-MS, Thermo Fisher, USA).Cytotoxicity assay
4T1 cells were seeded in 96-well plates at the density of 5 × 103 cells per well and cultured for 24 h. Various concentrations of Ir1@BSA to Ir3@BSA were added into cells and further incubated for 24 h. For dark-toxicity, the cells were washed with PBS. CCK-8 was utilized to evaluate cell viability. The absorbance at 450 nm was recorded by a BioTek Cytation 3 microplate reader (USA). For phototoxicity, after incubation, the cells were irradiated with 532 nm light (100 mW cm−2) for 5 min. After another 24 h incubation, the cells were stained with CCK-8 and the absorbance at 450 nm was measured. The EC50 values of phototoxicity were calculated by a sigmoidal fitting of the concentration–cell viability curve.Live/dead cell staining
Furthermore, a calcein-AM/PI double stain kit was used to stain live and dead cells respectively. The pre-seeded 4T1 cells were incubated with Ir1@BSA to Ir3@BSA (40 μM) for 24 h, and then cells were irradiated with or without 532 nm irradiation (100 mW cm−2) for 5 min. After another 24 h irradiation, all cells were co-stained with calcein-AM (10 μM) and PI (5 μM) for 30 min and washed with PBS. The fluorescence cell images were collected by an inverted fluorescence microscope (calcein-AM, blue light excitation, green light channel emission; PI, green light excitation, red light channel emission).Intracellular ROS detection
The fluorescence probe DCFH-DA (Beyotime, China) was used to detect the generation of intracellular ROS. 4T1 cells were seeded in 24-well plates at the density of 1.2 × 104 cells per well and cultured for 24 h. Ir1@BSA to Ir3@BSA (40 μM) was added and further incubated with cells for 24 h. Subsequently, the cells were washed with PBS and further incubated with DCFH-DA (10 μM) for 30 min. After washing with PBS, cells were irradiated with a 532 nm light (100 mW cm−2) for 5 min. The intracellular fluorescence was recorded immediately by using a CKX41 inverted microscope. The fluorescence intensity was calculated by the cell fluorescence quantitative method via ImageJ software.Conclusions
In summary, we have synthesized and characterized three cyclometalated Ir(III) complexes Ir1, Ir2, and Ir3, and fabricated them with BSA to form nano-photosensitizers Ir1@BSA, Ir2@BSA, and Ir3@BSA for PDT of tumor cells. The results of photophysical studies show that the three iridium complexes have absorption in the UV to the visible light region and phosphorescent emission in the visible light region to the NIR region. Singlet oxygen can be generated after 532 nm light irradiation, and the ability to generate singlet oxygen is Ir1 > Ir3 > Ir2. Structure–activity relationship studies have shown that extending the conjugation length or increasing the plane rigidity of C^N ligands cannot effectively increase the production of singlet oxygen. After further encapsulation by BSA, Ir1 to Ir3 can self-assemble to form nanostructures, which are spherical-like and well dispersed. The diameters are between 50–70 nm and have a stable nano-morphology. After forming the nano-photosensitizers, the singlet oxygen quantum yields of the Ir1@BSA, Ir2@BSA, and Ir3@BSA were 0.20, 0.06, and 0.23 in H2O, respectively. According to CCK-8 analysis, the three nano-photosensitizers have lower dark toxicity and stronger phototoxicity. The EC50 values are 5.2 μM, 28.5 μM, and 82.2 μM for Ir1@BSA, Ir2@BSA, and Ir3@BSA respectively. We attribute this to the dual factors of the difference of cell uptake ability and singlet oxygen generation ability. We furtherly verified the PDT effects of the three nano-photosensitizers with the live/dead cell staining method and the intracellular reactive oxygen staining method, which are consistent with the cell phototoxicity results by the measurement of CCK-8. Therefore, we believe that nano-photosensitizers are different from traditional small-molecule photosensitizers in PDT, which have important significance and value for PDT to increase tumor uptake and biocompatibility in tumor treatment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Natural Science Foundation of Shanghai (19ZR1434700), the National Natural Science Foundation of China (21501121).Notes and references
- S. S. Lucky, K. C. Soo and Y. Zhang, Nanoparticles in Photodynamic Therapy, Chem. Rev., 2015, 115, 1990–2042 CrossRef CAS PubMed
.
- K. K. Ng and G. Zheng, Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications, Chem. Rev., 2015, 115, 11012–11042 CrossRef CAS PubMed
.
- M. Wainwright, The problem with dyes in infection control, Dyes Pigm., 2017, 146, 402–407 CrossRef CAS
.
- C. P. Sabino, M. Wainwright, C. dos Anjos, F. P. Sellera, M. S. Baptista, N. Lincopan and M. S. Ribeiro, Inactivation kinetics and lethal dose analysis of antimicrobial blue light and photodynamic therapy, Photodiagn. Photodyn. Ther., 2019, 28, 186–191 CrossRef CAS PubMed
.
- D. Dolmans, D. Fukumura and R. K. Jain, Photodynamic therapy for cancer, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS PubMed
.
- P. Agostinis, K. Berg, K. A. Cengel, T. H. Foster, A. W. Girotti, S. O. Gollnick, S. M. Hahn, M. R. Hamblin, A. Juzeniene, D. Kessel, M. Korbelik, J. Moan, P. Mroz, D. Nowis, J. Piette, B. C. Wilson and J. Golab, Photodynamic Therapy of Cancer: An Update, Ca-Cancer J. Clin., 2011, 61, 250–281 CrossRef PubMed
.
- J. Jiang, Y. Qian, Z. Xu, Z. Lv, P. Tao, M. Xie, S. Liu, W. Huang and Q. Zhao, Enhancing singlet oxygen generation in semiconducting polymer nanoparticles through fluorescence resonance energy transfer for tumor treatment, Chem. Sci., 2019, 10, 5085–5094 RSC
.
- L. K. McKenzie, H. E. Bryant and J. A. Weinstein, Transition metal complexes as photosensitisers in one- and two-photon photodynamic therapy, Coord. Chem. Rev., 2019, 379, 2–29 CrossRef CAS
.
- L. Zhang, Y. Li, W. Che, D. Zhu, G. Li, Z. Xie, N. Song, S. Liu, B. Z. Tang, X. Liu, Z. Su and M. R. Bryce, AIE Multinuclear Ir(III) Complexes for Biocompatible Organic Nanoparticles with Highly Enhanced Photodynamic Performance, Adv. Sci., 2019, 6, 1802050 CrossRef PubMed
.
- J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization, Chem. Rev., 2010, 110, 2795–2838 CrossRef CAS PubMed
.
- S. Ma, J. Zhou, Y. Zhang, B. Yang, Y. He, C. Tian, X. Xu and Z. Gu, An Oxygen Self-sufficient Fluorinated Nanoplatform for Relieved Tumor Hypoxia and Enhanced Photodynamic Therapy of Cancers, ACS Appl. Mater. Interfaces, 2019, 11, 7731–7742 CrossRef CAS PubMed
.
- Q. Yu, T. Huang, C. Liu, M. Zhao, M. Xie, G. Li, S. Liu, W. Huang and Q. Zhao, Oxygen self-sufficient NIR-activatable liposomes for tumor hypoxia regulation and photodynamic therapy, Chem. Sci., 2019, 10, 9091–9098 RSC
.
- R. Kv, T.-I. Liu, I. L. Lu, C.-C. Liu, H.-H. Chen, T.-Y. Lu, W.-H. Chiang and H.-C. Chiu, Tumor microenvironment-responsive and oxygen self-sufficient oil droplet nanoparticles for enhanced photothermal/photodynamic combination therapy against hypoxic tumors, J. Control. Release, 2020, 328, 87–99 CrossRef CAS PubMed
.
- S.-Z. Ren, B. Wang, X.-H. Zhu, D. Zhu, M. Liu, S.-K. Li, Y.-S. Yang, Z.-C. Wang and H.-L. Zhu, Oxygen Self-Sufficient Core-Shell Metal-Organic Framework-Based Smart Nanoplatform for Enhanced Synergistic Chemotherapy and Photodynamic Therapy, ACS Appl. Mater.
Interfaces, 2020, 12, 24662–24674 CrossRef CAS PubMed
.
- K. Qiu, M. Ouyang, Y. Liu, H. Huang, C. Liu, Y. Chen, L. Ji and H. Chao, Two-photon photodynamic ablation of tumor cells by mitochondria-targeted iridium(III) complexes in aggregate states, J. Mater. Chem. B, 2017, 5, 5488–5498 RSC
.
- L. Wang, H. Yin, P. Cui, M. Hetu, C. Wang, S. Monro, R. D. Schaller, C. G. Cameron, B. Liu, S. Kilina, S. A. McFarland and W. Sun, Near-infrared-emitting heteroleptic cationic iridium complexes derived from 2,3-diphenylbenzo[g]quinoxaline as in vitro theranostic photodynamic therapy agents, Dalton Trans., 2017, 46, 8091–8103 RSC
.
- L. Wang, S. Monro, P. Cui, H. Yin, B. Liu, C. G. Cameron, W. Xu, M. Hetu, A. Fuller, S. Kilina, S. A. McFarland and W. Sun, Heteroleptic Ir(III)N6 Complexes with Long-Lived Triplet Excited States and in Vitro Photobiological Activities, ACS Appl. Mater. Interfaces, 2019, 11, 3629–3644 CrossRef CAS PubMed
.
- L. C. Lee, A. W. Tsang, H. W. Liu and K. K. Lo, Photofunctional Cyclometalated Iridium(III) Polypyridine Complexes Bearing a Perfluorobiphenyl Moiety for Bioconjugation, Bioimaging, and Phototherapeutic Applications, Inorg. Chem., 2020, 59, 14796–14806 CrossRef CAS PubMed
.
- V. Novohradsky, A. Rovira, C. Hally, A. Galindo, G. Vigueras, A. Gandioso, M. Svitelova, R. Bresoli-Obach, H. Kostrhunova, L. Markova, J. Kasparkova, S. Nonell, J. Ruiz, V. Brabec and V. Marchan, Towards Novel Photodynamic Anticancer Agents Generating Superoxide Anion Radicals: A Cyclometalated Ir(III) Complex Conjugated to a Far-Red Emitting Coumarin, Angew. Chem., Int. Ed. Engl., 2019, 58, 6311–6315 CrossRef CAS PubMed
.
- Q. Yang, H. Jin, Y. Gao, J. Lin, H. Yang and S. Yang, Photostable Iridium(III)-Cyanine Complex Nanoparticles for Photoacoustic Imaging Guided Near-Infrared Photodynamic Therapy in Vivo, ACS Appl. Mater. Interfaces, 2019, 11, 15417–15425 CrossRef CAS PubMed
.
- R. Guan, Y. Chen, L. Zeng, T. W. Rees, C. Jin, J. Huang, Z. S. Chen, L. Ji and H. Chao, Oncosis-inducing cyclometalated iridium(III) complexes, Chem. Sci., 2018, 9, 5183–5190 RSC
.
- M. Lan, S. Zhao, W. Liu, C. S. Lee, W. Zhang and P. Wang, Photosensitizers for Photodynamic Therapy, Adv. Healthc. Mater., 2019, 8, 1900132 CrossRef PubMed
.
- Y. Deng, S. Pan, J. Zheng, Y. Hong, J. Liu, H. Chang, Y. Miao, Y. Sun and Y. Li, Electrostatic self-assembled Iridium(III) nano-photosensitizer for selectively disintegrated and mitochondria targeted photodynamic therapy, Dyes Pigm., 2020, 175, 108105 CrossRef CAS
.
- H. Lu, X. Jiang, Y. Chen, K. Peng, Y. Huang, H. Zhao, Q. Chen, F. Lv, L. Liu, S. Wang and Y. Ma, Cyclometalated iridium(III) complex nanoparticles for mitochondria-targeted photodynamic therapy, Nanoscale, 2020, 12, 14061–14067 RSC
.
- X. Liu, G. Li, M. J. Xie, S. Guo, W. L. Zhao, F. Y. Li, S. J. Liu and Q. Zhao, Rational design of type I photosensitizers based on Ru(II) complexes for effective photodynamic therapy under hypoxia, Dalton Trans., 2020, 49, 11192–11200 RSC
.
- K. Y. Zhang, L. N. Song, T. H. Gu, H. Wang, C. Yang, H. C. Zhou, P. L. Gao, S. J. Liu and Q. Zhao, Cell-Membrane Staining Properties and Photocytotoxicity of a Ruthenium(II) Photosensitizer, Eur. J. Inorg. Chem., 2020, 42, 3996–4001 CrossRef
.
- H. Su, S. Q. Zhu, M. X. Qu, R. Liu, G. L. Song and H. J. Zhu, 1,3,5-Triazine-Based Pt(II) Metallogel Material: Synthesis, Photophysical Properties, and Optical Power-Limiting Performance, J. Phys. Chem. C, 2019, 123, 15685–15692 CrossRef CAS
.
- S. Q. Zhu, H. Liu, K. Y. Wang, Q. Cheng, Z. X. Ma, R. Liu, G. L. Song and H. J. Zhu, The effects of extended pi-conjugation in bipyridyl ligands on the tunable photophysics, triplet excited state and optical limiting properties of Pt(II) naphthalimidyl acetylide complexes, Dalton Trans., 2019, 48, 15105–15113 RSC
.
- L. H. Zhou, F. F. Wei, J. J. Xiang, H. F. Li, C. B. Li, P. F. Zhang, C. J. Liu, P. Gong, L. T. Cai and K. M. C. Wong, Enhancing the ROS generation ability of a rhodamine-decorated iridium(III) complex by ligand regulation for endoplasmic reticulum-targeted photodynamic therapy, Chem. Sci., 2020, 11, 12212–12220 RSC
.
- J. He, Z. Q. Bai, P. F. Yuan, L. Z. Wu and Q. Liu, Highly Efficient Iridium-Based Photosensitizers for Thia-Paterno-Buchi Reaction and Aza-Photocyclization, ACS Catal., 2021, 11, 446–455 CrossRef CAS
.
- Y. You and W. Nam, Photofunctional triplet excited states of cyclometalated Ir(III) complexes: beyond electroluminescence, Chem. Soc. Rev., 2012, 41, 7061–7084 RSC
.
- Y. H. Li, N. Dandu, R. Liu, L. Hu, S. Kilina and W. F. Sun, Nonlinear Absorbing Cationic Iridium(III) Complexes Bearing Benzothiazolylfluorene Motif on the Bipyridine (N^N) Ligand: Synthesis, Photophysics and Reverse Saturable Absorption, ACS Appl. Mater. Interfaces, 2013, 5, 6556–6570 CrossRef CAS PubMed
.
- Y. H. Li, N. Dandu, R. Liu, Z. J. Li, S. Kilina and W. F. Sun, Effects of Extended π-Conjugation in Phenanthroline (N∧N) and Phenylpyridine (C∧N) Ligands on the Photophysics and Reverse Saturable Absorption of Cationic Heteroleptic Iridium(III) Complexes, J. Phys. Chem. C, 2014, 118, 6372–6384 CrossRef CAS
.
- Q. Yang, M. Shi, H. Zhao, J. M. Lin, L. An, L. L. Cui, H. Yang, Z. G. Zhou, Q. W. Tian and S. P. Yang, Water-Soluble Polymer Nanoparticles Constructed by Three-Component Self-Assembly: An Efficient Theranostic Agent for Phosphorescent Imaging and Photodynamic Therapy, Chem.–Eur. J., 2017, 23, 3728–3734 CrossRef CAS PubMed
.
- Y. Deng, F. Huang, J. Zhang, J. Liu, B. Li, R. Z. Ouyang, Y. Q. Miao, Y. Sun and Y. H. Li, Dual-light triggered metabolizable nano-micelles for selective tumor-targeted photodynamic/hyperthermia therapy, Dyes Pigm., 2020, 182, 108105 CrossRef
.
- Y. Deng, X. Wang, Y. T. Liu, Y. Xu, J. Zhang, F. Huang, B. Li, Y. Q. Miao, Y. Sun and Y. H. Li, Dual-light triggered metabolizable nano-micelles for selective tumor-targeted photodynamic/hyperthermia therapy, Acta Biomater., 2021, 119, 323–336 CrossRef CAS PubMed
.
- F. F. Xue, M. Shi, Y. P. Yan, H. Yang, Z. G. Zhou and S. P. Yang, Iridium complex loaded polypyrrole nanoparticles for NIR laser induced photothermal effect and generation of singlet oxygen, RSC Adv., 2016, 6, 15509–15512 RSC
.
- H. J. Xiang, H. Z. Chen, H. J. P. Tham, S. Z. F. Phua, J. G. Liu and Y. L. Zhao, Cyclometalated Iridium(III)-Complex-Based Micelles for Glutathione-Responsive Targeted Chemotherapy and Photodynamic Therapy, ACS Appl. Mater. Interfaces, 2017, 9, 27553–27562 CrossRef CAS PubMed
.
- Z. J. Li, P. Cui, C. Z. Wang, S. Kilina and W. F. Sun, Nonlinear Absorbing Cationic Bipyridyl Iridium(III) Complexes Bearing Cyclometalating Ligands with Different Degrees of π-Conjugation: Synthesis, Photophysics, and Reverse Saturable Absorption, J. Phys. Chem. C, 2014, 118, 28764–28775 CrossRef CAS
.
- L. Wang, P. Cui, S. Kilina and W. F. Sun, Toward Broadband Reverse Saturable Absorption: Investigating the Impact of Cyclometalating Ligand π-Conjugation on the Photophysics and Reverse Saturable Absorption of Cationic Heteroleptic Iridium Complexes, J. Phys. Chem. C, 2017, 121, 5719–5730 CrossRef CAS
.
- M. C. DeRosa and R. J. Crutchley, Photosensitized singlet oxygen and its applications, Coord. Chem. Rev., 2002, 233, 351–371 CrossRef
.
- B. Q. Liu, S. Monro, L. Lystrom, C. G. Cameron, K. Colon, H. M. Yin, S. Kilina, S. A. McFarland and W. F. Sun, Photophysical and Photobiological Properties of Dinuclear Iridium(III) Bis-tridentate Complexes, Inorg. Chem., 2018, 57, 9859–9872 CrossRef CAS PubMed
.
- H. He, S. Ji, Y. He, A. Zhu, Y. Zou, Y. Deng, H. Ke, H. Yang, Y. Zhao, Z. Guo and H. Chen, Photoconversion-Tunable Fluorophore Vesicles for Wavelength-Dependent Photoinduced Cancer Therapy, Adv. Mater., 2017, 29, 1606690 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01740c |
| This journal is © The Royal Society of Chemistry 2021 |