Open Access Article
Open Access ArticleDivergent synthesis of flavones and flavanones from 2′-hydroxydihydrochalcones via palladium(II)-catalyzed oxidative cyclization†
Seung Hwan Son‡
a,
Yang Yil Cho‡a,
Hyung-Seok Yooa,
Soo Jin Leea,
Young Min Kima,
Hyu Jeong Janga,
Dong Hwan Kima,
Jeong-Won Shinb and
Nam-Jung Kim *ab
*ab
aCollege of Pharmacy, Kyung Hee University, Seoul 02447, Republic of Korea. E-mail: kimnj@khu.ac.kr
bDepartment of Life and Nanopharmaceutical Sciences, Graduate School, Kyung Hee University, Seoul 02447, Republic of Korea
First published on 13th April 2021
Abstract
Divergent and versatile synthetic routes to flavones and flavanones via efficient Pd(II) catalysis are disclosed. These Pd(II) catalyses expediently provide a variety of flavones and flavanones from 2′-hydroxydihydrochalcones as common intermediates, depending on oxidants and additives, via discriminate oxidative cyclization sequences involving dehydrogenation, respectively, in a highly atom-economic manner.
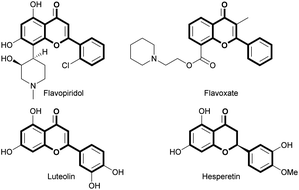
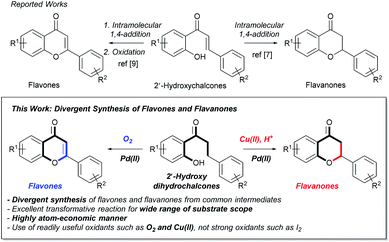
Flavones and flavanones are widely occurring natural flavonoids produced by various medicinal plants and their synthetic derivatives, featuring a main 15-carbon skeleton possessing 2 phenyl rings and 1 oxacycle.1 In recent decades, they have been considered privileged structures exhibiting various biological activities,2 such as anti-inflammatory,3 anti-cancer,4 neuroprotective,5 and estrogen-related functions6 (Fig. 1). As routes towards such privileged flavonoids, many strategies, including the Allan–Robinson reaction for flavones and intramolecular conjugate addition of 2′-hydroxychalcones for flavanones, have been reported.7 Among these, intramolecular cyclization of 2′-hydroxychalcone intermediates was conventionally used for the synthesis of flavanones due to the readily available properties of the substrates through the condensation of 2′-hydroxy-acetophenones and corresponding aldehydes.8 In addition, such flavanones can be readily converted into flavones via further oxidation processes (Scheme 1).9 Thus, these types of flavonoid synthesis involving intramolecular conjugate addition have been generally used for flavonoid synthesis,10 but they generally have drawbacks of requiring harsh conditions such as acidic or basic reflux conditions for cyclization, indicating that chemically labile compounds may in some cases not be tolerable in reaction conditions.11 Additional oxidation steps in which flavanones are transformed into flavones often require the use of strong oxidants such as I2 that enable side reactions to occur.12 In this regard, novel synthetic routes featuring transformative and compatible reaction conditions toward flavonoids have been pursued in recent decades. In particular, given the increasing importance of constructing a privileged chemical library for probing biological systems, efficient and divergent synthesis of flavonoids is necessary.13
Taken together, it is important to develop a novel and versatile synthetic transformation of common substrates into flavones and flavanones under mild conditions and such a transformation is also expected to enable straightforward reactions for the construction of a privileged flavonoid library. As mentioned above, 2′-hydroxychalcones have been conventionally used as main precursors in flavonoid synthesis, but they are reactive and unmanageable in some cases due to their reactive α,β-unsaturated carbonyl and enol ether moieties. In addition, their use is generally involved with several disadvantages, as mentioned above.14 Thus, we think that the use of common intermediates instead of 2′-hydroxychalcones that is traditionally used but somewhat problematic is required. Recently, direct β-functionalization of simple ketones via several catalysts using transition metals and light was reported,15 indicating that simple ketones can be useful and potential surrogates of α,β-unsaturated ketones for further oxidative catalysis. In addition, we reported that chromanones, simple ketones, can be converted into flavones and flavanones via palladium(II)-catalyzed dehydrogenation-mediated coupling sequences with arylboronic esters and acids, respectively, and also recently reported that N-substituted azaflavanone can be synthesized from N-substituted aminodihydrochalcones via Pd(II)-catalyzed oxidative cyclization.16 Inspired by these previous works, we sought to find novel and divergent synthetic routes to a variety of privileged flavonoids, particularly flavones and flavanones using common starting materials.
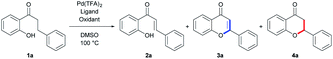
Herein, we report palladium(II)-catalyzed oxidative cyclization of 2′-hydroxydihydroxychalcones that are novel and tolerable isosteres of 2′-hydroxychalcones as an efficient and divergent route for the synthesis of flavones and flavanones. To find an optimal reaction condition where flavone and flavanone were synthesized via Pd(II)-catalyzed oxidative cyclization from the common intermediate in independent manners, simple 2′-hydroxydihydrochalcone was selected as the model compound to screen the reaction conditions (Table 1). Based on the speculation that the dehydrogenation process from 2′-hydroxydihydrochalcone to 2′-hydroxychalcone might proceed further reactions into flavone and flavanone, we initially tested the reaction conditions which are known to enable Pd(II)-catalyzed dehydrogenation to occur with Pd(TFA)2 and DMSO under an O2 atmosphere, which is eco-friendly oxidant.17 The use of this reaction condition resulted in the formation of flavanone 4a as the major product (31% yield, entry 1) and flavone 3a with 12% yield, along with a small amount of 2′-hydroxychalcone 2a (14%). This result implied that 2′-hydroxydihydrochalcone could be dehydrogenated and slightly transformed into desired flavonoids under the reaction conditions featuring Pd(II) catalysis. Based on the results, we tried to optimize the reaction conditions by using additives such as heterocyclic amines or inorganic bases as Pd(II) ligands. In the presence of most of the heterocyclic amines and K2CO3, the overall yields of the reactions for the synthesis of flavonoids were significantly increased (entries 2–5). Interestingly, the catalytic system featuring 2,2′-bipyridine (bpy) as a ligand provided flavone 3a in 55% yield as a major product, along with flavanone 4a in 10% yield, indicating that bidentate amine was better than the other monodentate amines, including pyridine as a Pd(II) ligand, under incorporating conditions (entry 6). Notably, the use of 5-nitro-1,10-phenanthroline as a ligand enables the reaction to exclusively yield flavone 3a with the highest yield of 81% and flavanone 4a in 2% yield (entry 7).
| Entry | Additive | Oxidant | Yieldb [%] | ||
|---|---|---|---|---|---|
| 2a | 3a | 4a | |||
| a Reactions were carried out in the presence of 0.3 mmol of 1a, 10 mol% Pd(TFA)2, 20 mol% additive, molecular oxygen or 1.0 equiv. oxidant and DMSO 1 mL at 100 °C for 15–48 h.b Isolated yield.c DMSO 3 mL.d Addition of 2 N HCl 20 mL and ethyl acetate 10 mL for 24 h after 1a was consumed.e Cu(OAc)2 2 equiv. | |||||
| 1 | — | O2 | 14 | 12 | 31 |
| 2 | K2CO3 | O2 | 8 | 28 | 20 |
| 3 | DMAP | O2 | 6 | 29 | 36 |
| 4 | Pyridine | O2 | 5 | 41 | 37 |
| 5 | Pyrimidine | O2 | 7 | 48 | 32 |
| 6 | bpy | O2 | 5 | 55 | 10 |
| 7 | 5-NO2 phen | O2 | 3 | 81 | 2 |
| 8 | — | Benzoquinone | 7 | 1 | 12 |
| 9 | — | K2S2O8 | 11 | 4 | 19 |
| 10 | — | AgOAc | 12 | 13 | 29 |
| 11 | — | Cu(OAc)2 | 33 | 6 | 44 |
| 12 | Phenanthroline | Cu(OAc)2 | 23 | 27 | 34 |
| 13 | Pyridine | Cu(OAc)2 | 14 | 13 | 33 |
| 14 | DMAP | Cu(OAc)2 | 25 | 22 | 35 |
| 15 | K2CO3 | Cu(OAc)2 | 7 | 1 | 16 |
| 16 | AcOH | Cu(OAc)2 | 20 | 11 | 42 |
| 17 | HCO2H | Cu(OAc)2 | 9 | 6 | 43 |
| 18 | p-TsOH | Cu(OAc)2 | 15 | 21 | 27 |
| 19c | — | Cu(OAc)2 | 30 | 3 | 55 |
| 20c,d | — | Cu(OAc)2 | 8 | 10 | 79 |
| 21c,e | — | Cu(OAc)2 | — | 16 | — |
Next, we focused on optimizing the conditions where flavanone, rather than flavone, is converted from 2′-hydroxydihydrochalcone that is a common starting material. In the view of the oxidation process, we supposed that the use of a stoichiometric amount of oxidant rather than molecular oxygen can result in a stepwise conversion of 2′-hydroxydihydrochalcone into 2′-hydroxychalcone, leading to the flavanone through the concurrent intramolecular 1,4-addition, rather than to the flavone for which an additional oxidation process may be needed. With this insight, we tested stoichiometric amounts of various oxidants instead of molecular oxygen for obtaining flavanone exclusively based on the entry 1 conditions (entries 8–11). As anticipated, in most of the conditions, flavanone was synthesized as a major product, but the yields were generally poor. When Cu(OAc)2 was used as the oxidant, the yield of flavanone was increased to 44%, along with the formation of flavone at a yield of 6% (entry 11). In addition, we screened various bases and acids as additives or potential ligands for improving the reaction under the conditions where Cu(OAc)2 was used as the oxidant.
Heterocyclic ligands, including monodentates and bidentates, slightly decrease the yields of the reaction for providing flavanones (entries 12–14). This trend was also observed for the addition of potassium carbonate or acids (entries 15–18). Thus, unlike for flavone synthesis, additives did not significantly improve the reaction efficiency. With the entry 11 condition (no additive), the concentration was diluted to 0.1 M for facilitating intramolecular cyclization, and the reaction afforded flavanone 4a in 55% yield (entry 19). Next, with the conditions of entry 19, an additional 2 N HCl was added to cyclize the remaining chalcone to flavanone under acidic conditions when 2′-hydroxydihydrochalcone 1a was completely consumed. Under these conditions, flavanone 4a was successfully obtained with the highest yield of 79% (entry 20). The use of 2 equivalents of Cu(OAc)2 generated flavone 3a as a detectable major product in lower yield (16%) than expected, indicating that excess Cu(OAc)2 is less effective than molecular oxygen for flavonoid synthesis.
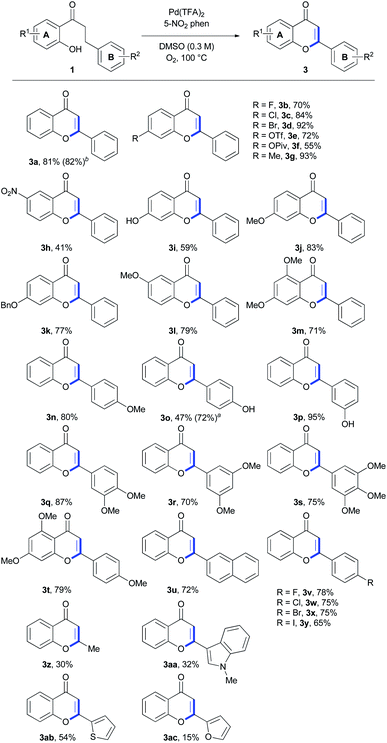
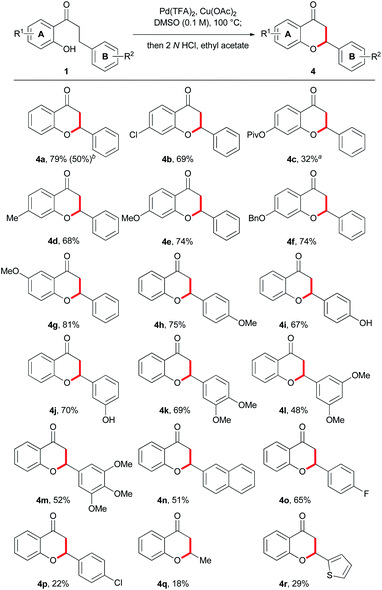
With the optimized conditions in hand (Table 1, entry 7), we applied our methodology for the synthesis of flavones with various substituents from the corresponding 2′-hydroxydihydrochalcone (Scheme 2). A variety of aryl A ring derivatives were successfully synthesized under these conditions. The reaction was well-tolerated and proceeded with the 2′-hydroxydihydrochalcone derivatives with electron-withdrawing groups such as halogens, triflate, pivalate and nitro groups (3b–f, 3h, and 3v–y), regardless of the A or B ring. In the case of using the substrates possessing electron-donating substituents such as methyl (3g), hydroxy (3i, 3o, and 3p), methoxy (3j, 3l and 3n), benzyloxy (3k), dimethoxy (3 m, 3q and 3r) and trimethoxy groups (3s and 3t), the reactions progressed well in moderate to good yields. In particular, the highest yield of 95% was obtained for 3′-hydroxyflavone 3p. 2-(Naphthalen-2-yl)-4H-chromen-4-one 3u was formed in 72% yield. However, 3-methylchromenone 3z was obtained from a corresponding 2′-hydroxydihydrochalcone in a relatively lower yield (30%). The substrates with heterocycles such as N-methyl indol-3-yl, thiophen-2-yl, and furan-2-yl, as B ring, were also converted to corresponding flavones 3aa, 3ab, and 3ac in 32%, 54%, and 15% yields.
Next, under the optimized conditions (Table 1, entry 20), we performed the reaction for the synthesis of flavanones using various derivatives of 2′-hydroxydihydrochalcone 1 that is the common substrate for the synthesis of flavones (Scheme 3). The desired flavanone products possessing electron-deficient substituents such as halogens and ester groups (4b–c, and 4o) as well as electron-rich substituents such as hydroxy and alkoxy moieties regardless of the A or B ring (4d–m) were readily obtained in moderate to good yields. In particular, flavanones with naturally abundant oxygen-containing moieties (4e and 4g–m) were generated effectively in the reaction. Flavanone 4n with a naphthyl moiety was also formed well in 72% yield. A substrate containing a thiophene-2-yl group as B ring was also converted to flavanone 4r in 29% yield. 3-Methylchromanone 4q was obtained from a corresponding 2′-hydroxydihydrochalcone in a lower yield (18%) compared to the other flavanones, which is similar to the case of 3-methylchromenone 3z. In the case of reactions for preparing 3z and 4q, lots of 1-(2-hydroxyphenyl)butan-1-one, a starting material, remained not to be reacted. A few flavones such as 3ac and flavanones such as 4p were not obtained in good yield, along with several side products. Collectively, these results indicated that 2′-hydroxydihydrochalcones that are chemically compatible substrates can be obviously transformed into flavones and flavanones in divergent and efficient manners, respectively.
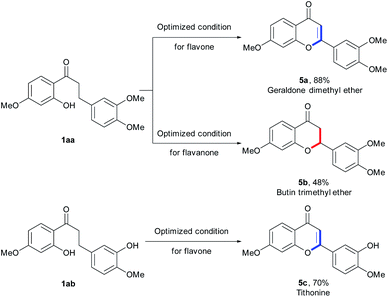
To further investigate the divergent utility of our synthetic methodology, we applied it to the synthesis of natural flavonoids (Scheme 4). Under the optimal conditions, geraldone dimethyl ether 5a (ref. 18) and butin trimethyl ether 5b,19 the biologically active natural flavonoids, were successfully transformed from common substrate 1aa in moderate yields (88% and 48%, respectively, Scheme 4). Tithonine 5c, an anti-inflammatory flavone known as a selective COX (cyclooxygenase)-1 inhibitor,20 was also synthesized well under our reaction conditions.
Next, to investigate the mechanism of our synthetic methodology, we carried out kinetic analysis of the reaction using high-pressure liquid chromatography (HPLC) to examine the time-dependent conversion of 2′-hydroxy-4-methoxydihydrochalcone 1n to the corresponding flavone 3n or flavanone 4h under optimized reaction conditions. For flavone synthesis, it was observed that the amount of 2′-hydroxy-4- methoxydihydrochalcone 1n gradually decreased over time, and 2′-hydroxy-4-methoxychalcone 2n was increasingly formed in a dramatic manner with the concurrent formation of flavone 3n within 5 h (Scheme 5a). Then, 2n was gradually diminished, and 3n was significantly formed, indicating that 2n may be consumed for the synthesis of the desired flavone 3n. In addition, in the conditions of flavanone synthesis (Scheme 5b), it was observed that the reaction proceeded rapidly at first (Fig. S1†), resulting in the drastic formation of 2′-hydroxy-4-methoxychalcone 2n with the concurrent formation of flavonoids. After 15 h, the reaction was observed to reach the plateau state. Upon adding 2 N aqueous HCl, flavanone 4h was immediately formed with a significant disappearance of 2n, indicating that 2n may also be consumed for the synthesis of the desired flavanone 4h.
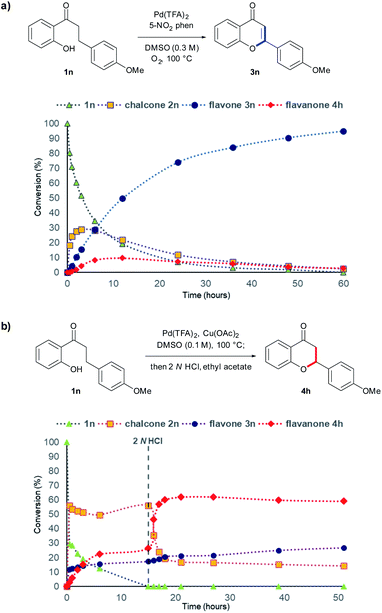 | ||
| Scheme 5 Kinetic experiments for flavone and flavanone synthesis from 2′-hydroxy-4-methoxydihydrochalcone 1n. (a) Flavone synthesis condition. (b) Flavanone synthesis condition. | ||
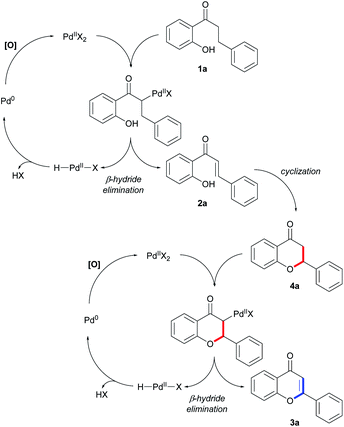
Based on the kinetic results, a plausible mechanism for this divergent synthesis is proposed, as shown in Fig. 2. First, Pd(II)-catalyzed dehydrogenation may result in the oxidative conversion of 2′-hydroxydihydrochalcone 1a into 2′-hydroxychalcone 2a.16 Then, common intermediate 2a can be transformed into the desired flavanone 4a through Michael addition and flavone 3a through oxidative Heck coupling, respectively, depending on the optimized reaction conditions that are used in the synthesis. During oxidation processes such as dehydrogenation and oxidative cyclization, Pd(0) species regenerated in the reaction would be reoxidized into Pd(II) via the [O] process, where O2 or Cu(OAc)2 acts as the main oxidant.21
Conclusions
We reported novel and versatile approaches for the synthesis of flavones and flavanones from common intermediates via Pd(II)-catalyzed oxidative cyclization. These methodologies greatly facilitate the construction of chemical libraries of flavones and flavanones in moderate to high yields using easily accessible reagents and offer good compatibilities with various functional groups under mild conditions in atom-economic manner. Further studies on the biological evaluation of a wide variety of flavonoids synthesized by our methodology are ongoing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Basic Research Laboratory Program (BRL) of the Korean National Research Foundation funded by the Korean Ministry of Science, ICT and Future Planning (NRF-2020R1A4A1016142), and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2019R1H1A2079819).Notes and references
- (a) R. J. Nijveldt, E. van Nood, D. E. van Hoorn, P. G. Boelens, K. van Norren and P. A. van Leeuwen, Flavonoids: a review of probable mechanisms of action and potential applications, Am. J. Clin. Nutr., 2001, 74, 418 CrossRef CAS PubMed; (b) D. Barreca, G. Gattuso, E. Bellocco, A. Calderaro, D. Trombetta, A. Smeriglio, G. Laganà, M. Daglia, S. Meneghini and S. M. Nabavi, Flavanones: Citrus phytochemical with health-promoting properties, BioFactors, 2017, 43, 495 CrossRef CAS.
- (a) U. Raju, E. Nakata, K. A. Mason, K. K. Ang and L. Milas, Flavopiridol, a Cyclin-dependent Kinase Inhibitor, Enhances Radiosensitivity of Ovarian Carcinoma Cells, Cancer Res., 2003, 63, 3263 CAS; (b) D. Arcaniolo, S. Conquy and T. Tarcan, Flavoxate: present and future, Eur. Rev. Med. Pharmacol. Sci., 2015, 19, 719 CAS; (c) Y. Kim and Y. Je, Flavonoid intake and mortality from cardiovascular disease and all causes: A meta-analysis of prospective cohort studies, Clin. Nutr., 2017, 20, 68 Search PubMed; (d) A. Ghorbani, Mechanisms of antidiabetic effects of flavonoid rutin, Biomed. Pharmacother., 2017, 96, 305 CrossRef CAS; (e) J. Alvarez-Collazo, A. López-Requena, L. Galán, A. Talavera, J. L. Alvarez and K. Talavera, The citrus flavanone hesperetin preferentially inhibits slow-inactivating currents of a long QT syndrome type 3 syndrome Na+ channel mutation, Br. J. Pharmacol., 2019, 176, 1090 CrossRef CAS.
- (a) K. W. Choy, D. Murugan, X.-F. Leong, R. Abas, A. Alias and M. R. Mustafa, Flavonoids as Natural Anti-Inflammatory Agents Targeting Nuclear Factor-Kappa B (NFκB) Signaling in Cardiovascular Diseases: A Mini Review, Front. Pharmacol., 2019, 10, 1295 CrossRef CAS; (b) R. Ginwala, R. Bhavsar, D. G. I. Chigbu, P. Jain and Z. K. Khan, Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin, Antioxidants, 2019, 8, 35 CrossRef PubMed; (c) F. Ali and Y. H. Siddique, Bioavailability and Pharmaco-therapeutic Potential of Luteolin in Overcoming Alzheimer's Disease, CNS Neurol. Disord.: Drug Targets, 2019, 18, 352 CrossRef CAS PubMed.
- (a) L. Yong, S. Ranxin, W. Xia and S. Han-Ming, Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy, Curr. Cancer Drug Targets, 2008, 8, 634 CrossRef PubMed; (b) M. Imran, A. Rauf, T. Abu-Izneid, M. Nadeem, M. A. Shariati, I. A. Khan, A. Imran, I. E. Orhan, M. Rizwan, M. Atif, T. A. Gondal and M. S. Mubarak, Luteolin, a flavonoid, as an anticancer agent: A review, Biomed. Pharmacother., 2019, 112, 108612 CrossRef CAS PubMed.
- (a) K. Sowndhararajan, P. Deepa, M. Kim, S. J. Park and S. Kim, Baicalein as a potent neuroprotective agent: A review, Biomed. Pharmacother., 2017, 95, 1021 CrossRef CAS PubMed; (b) S. Antoni, C. Xavier and T. Silvia, Neuroprotective Effects of Flavonoid Compounds on Neuronal Death Associated to Alzheimer's Disease, Curr. Med. Chem., 2019, 26, 5124 CrossRef PubMed.
- E. Wielogorska, K. Blaszczyk, O. Chevallier and L. Connolly, The origin of in-vitro estrogen-like activity in oregano herb extracts, Toxicol. In Vitro, 2019, 56, 101 CrossRef CAS PubMed.
- (a) J. Allan and R. Robinson, CCXC.—An accessible derivative of chromonol, J. Chem. Soc., Trans., 1924, 125, 2192 RSC; (b) G. J. Sagrera and G. A. Seoane, Microwave accelerated solvent-free synthesis of flavanones, J. Braz. Chem. Soc., 2005, 16, 851 CrossRef CAS; (c) H. Jiang, X. Zheng, Z. Yin and J. Xie, An Efficient Catalytic Synthesis of Flavanones under Green Conditions, J. Chem. Res., 2011, 35, 220 CrossRef CAS.
- (a) C. Zhuang, W. Zhang, C. Sheng, W. Zhang, C. Xing and Z. Miao, Chalcone: A Privileged Structure in Medicinal Chemistry, Chem. Rev., 2017, 117, 7762 CrossRef CAS PubMed; (b) A. S. Kumar, R. A. Kumar, V. Satyanarayana, E. P. Reddy, B. J. M. Reddy, D. N. Kumar, A. Khurana, G. Chandraiah and J. S. Yadav, Catalyst-Free Synthesis of Novel 6-Phenyl-6H-chromeno [4, 3-b] quinoline Derivatives at RT: Their Further Structure Evaluation Leads to Potential Anti-cancer Agents, Nat. Prod. Commun., 2017, 7, 1129 Search PubMed.
- (a) T. Patonay, J. A. S. Cavaleiro, A. Lévai and A. M. S. Silva, Dehydrogenation by iodine/dimethylsulfoxide system: A general route to substituted chromones and thiochromones and thiochromones, Heterocycl. Commun., 1997, 3, 223 CAS; (b) K. Mal, A. Kaur, F. Haque and I. Das, PPh3·HBr–DMSO: A Reagent System for Diverse Chemoselective Transformations, J. Org. Chem., 2015, 80, 6400 CrossRef CAS PubMed; (c) S. U. Dighe, S. Mukhopadhyay, K. Priyanka and S. Batra, Metal-Free Oxidative Nitration of α-Carbon of Carbonyls Leads to One-Pot Synthesis of Thiohydroximic Acids from Acetophenones, Org. Lett., 2016, 18, 4190 CrossRef CAS PubMed; (d) H. A. Agisho, S. Hairat and M. Zaki, An efficient TBHP/TBAI-mediated protocol for the synthesis of 4H-chromen-4-ones from chroman-4-ones via oxidative C–C bond formation, Monatsh. Chem., 2020, 151, 599 CrossRef CAS.
- (a) H. Eshghi, M. Rahimizadeh and S. M. Mousavi, Fe(HSO4)3/SiO2: an efficient and heterogeneous catalyst for one-pot synthesis of 2-aryl-chromene-4-ones (flavanones), Nat. Prod. Res., 2014, 28, 438 CrossRef CAS PubMed; (b) R. Liu, Y. Zhang, K. Xu and G. Tan, Silica-gel-supported Ce(SO4)2·4H2O-mediated cyclization of 2′-amino and 2′-hydroxychalcones under solvent-free conditions, Synth. Commun., 2017, 47, 1 CrossRef CAS; (c) M. Vimal, U. Pathak and A. K. Halve, Water-mediated phosphorylative cyclodehydrogenation: An efficient preparation of flavones and flavanones, Synth. Commun., 2019, 49, 2805 CAS.
- M. Cabrera, M. Simoens, G. Falchi, M. L. Lavaggi, O. E. Piro, E. E. Castellano, A. Vidal, A. Azqueta, A. Monge, A. L. de Ceráin, G. Sagrera, G. Seoane, H. Cerecetto and M. González, Synthetic chalcones, flavanones, and flavones as antitumoral agents: Biological evaluation and structure–activity relationships, Bioorg. Med. Chem., 2007, 15, 3356 CrossRef CAS PubMed.
- (a) M. G. d. Carvalho, V. C. d. Silva, T. M. S. d. Silva, C. A. Camara and R. Braz-Filho, New iodine derivatives of flavonol and isoflavone, An. Acad. Bras. Cienc., 2009, 81, 21 CrossRef PubMed; (b) A. M. Patil, D. A. Kamble and P. D. Lokhande, Iodine-mediated direct synthesis of 3-iodoflavones, Synth. Commun., 2018, 48, 1299 CrossRef CAS.
- (a) L. Jalili-Baleh, E. Babaei, S. Abdpour, S. Nasir Abbas Bukhari, A. Foroumadi, A. Ramazani, M. Sharifzadeh, M. Abdollahi and M. Khoobi, A review on flavonoid-based scaffolds as multi-target-directed ligands (MTDLs) for Alzheimer's disease, Eur. J. Med. Chem., 2018, 152, 570 CrossRef CAS PubMed; (b) N. Min, P. T. Leong, R. C. H. Lee, J. S. E. Khuan and J. J. H. Chu, A flavonoid compound library screen revealed potent antiviral activity of plant-derived flavonoids on human enterovirus A71 replication, Antiviral Res., 2018, 150, 60 CrossRef CAS PubMed.
- (a) T. Ooi, D. Ohara, K. Fukumoto and K. Maruoka, Importance of Chiral Phase-Transfer Catalysts with Dual Functions in Obtaining High Enantioselectivity in the Michael Reaction of Malonates and Chalcone Derivatives, Org. Lett., 2005, 7, 3195 CrossRef CAS PubMed; (b) F. Wu, H. Li, R. Hong and L. Deng, Construction of Quaternary Stereocenters by Efficient and Practical Conjugate Additions to α,β-Unsaturated Ketones with a Chiral Organic Catalyst, Angew. Chem., Int. Ed., 2006, 45, 947 CrossRef CAS PubMed.
- (a) M. T. Pirnot, D. A. Rankic, D. B. C. Martin and D. W. C. MacMillan, Photoredox Activation for the Direct β-Arylation of Ketones and Aldehydes, Science, 2013, 339, 1593 CrossRef CAS PubMed; (b) Z. Huang and G. Dong, Catalytic Direct β-Arylation of Simple Ketones with Aryl Iodides, J. Am. Chem. Soc., 2013, 135, 17747 CrossRef CAS PubMed; (c) Z. Huang, H. N. Lim, F. Mo, M. C. Young and G. Dong, Transition metal-catalyzed ketone-directed or mediated C–H functionalization, Chem. Soc. Rev., 2015, 44, 7764 RSC; (d) C. Wang, N. A. Naren, P. Zheng and G. Dong, Intramolecular β-Alkenylation of Cyclohexanones via Pd-Catalyzed Desaturation-Mediated C(sp3)–H/Alkyne Coupling, J. Am. Chem. Soc., 2020, 142, 8962 CrossRef CAS PubMed; (e) C. Wang and G. Dong, Catalytic β-Functionalization of Carbonyl Compounds Enabled by α,β-Desaturation, ACS Catal., 2020, 10, 6058–6070 CrossRef CAS; (f) W. Xie, D. Kim and S. Chang, Copper-Catalyzed Formal Dehydrogenative Coupling of Carbonyls with Polyfluoroarenes Leading to β-C–H Arylation, J. Am. Chem. Soc., 2020, 142, 20588 CrossRef CAS PubMed.
- (a) J. Muzart and J. P. Pete, Dehydrogenation of cyclohexanones catalyzed by palladium(II) trifluoroacetate, J. Mol. Catal., 1982, 3, 373 CrossRef; (b) J. Muzart, One-Pot Syntheses of α,β-Unsaturated Carbonyl Compounds through Palladium-Mediated Dehydrogenation of Ketones, Aldehydes, Esters, Lactones and Amides, Eur. J. Org. Chem., 2010, 20, 3779 CrossRef; (c) J. Lee, J. Yu, S. H. Son, J. Heo, T. Kim, J.-Y. An, K.-S. Inn and N.-J. Kim, A versatile approach to flavones via a one-pot Pd(II)-catalyzed dehydrogenation/oxidative boron-Heck coupling sequence of chromanones, Org. Biomol. Chem., 2016, 14, 777 RSC; (d) J. L. Bras and J. Muzart, Palladium-Catalyzed Domino Dehydrogenation/Heck-Type Reactions of Carbonyl Compounds, Adv. Synth. Catal., 2018, 13, 2411 CrossRef; (e) H.-S. Yoo, S. H. Son, Y. Y. Cho, S. J. Lee, H. J. Jang, Y. M. Kim, D. H. Kim, N. Y. Kim, B. Y. Park, Y. S. Lee and N.-J. Kim, Synthesis of Flavanones via Palladium(II)-Catalyzed One-Pot β-Arylation of Chromanones with Arylboronic Acids, J. Org. Chem., 2019, 84, 10012 CrossRef CAS PubMed; (f) Y. M. Kim, H.-S. Yoo, S. H. Son, G. Y. Kim, H. J. Jang, D. H. Kim, S. D. Kim, B. Y. Park and N.-J. Kim, A Novel Approach to N-Tf 2-Aryl-2,3-Dihydroquinolin- 4(1H)-ones via a Ligand-Free Pd(II)-Catalyzed Oxidative Aza-Michael Cyclization, Eur. J. Org. Chem., 2021, 4, 618 CrossRef.
- (a) Y. Izawa, D. Pun and S. S. Stahl, Palladium-Catalyzed Aerobic Dehydrogenation of Substituted Cyclohexanones to Phenols, Science, 2011, 333, 209 CrossRef CAS PubMed; (b) T. Diao and S. S. Stahl, Synthesis of Cyclic Enones via Direct Palladium-Catalyzed Aerobic Dehydrogenation of Ketones, J. Am. Chem. Soc., 2011, 133, 14566 CrossRef CAS PubMed; (c) K.-J. Liu, J.-H. Deng, J. Yang, S.-F. Gong, Y.-W. Lin, J.-Y. He, Z. Cao and W.-M. He, Green Chem., 2020, 22, 433 RSC; (d) W.-B. He, L.-Q. Gao, X.-J. Chen, Z.-L. Wu, Y. Huang, Z. Cao, X.-H. Xu and W.-M. He, Chin. Chem. Lett., 2020, 31, 1895 CrossRef CAS; (e) K.-J. Liu, Z. Wang, L.-H. Lu, J.-Y. Chen, F. Zeng, Y.-W. Lin, Z. Cao, X. Yu and W.-M. He, Green Chem., 2021, 23, 496 RSC.
- C. Pouget, C. Fagnere, J.-P. Basly, A.-E. Besson, Y. Champavier, G. Habrioux and A.-J. Chulia, Synthesis and Aromatase Inhibitory Activity of Flavanones, Pharm. Res., 2002, 19, 286 CrossRef CAS PubMed.
- L. M. C. Cursino, N. M. Lima, R. Murillo, C. V. Nunez, I. Merfort and M. Humar, Isolation of Flavonoids from Deguelia duckeana and Their Effect on Cellular Viability, AMPK, eEF2, eIF2 and eIF4E, Molecules, 2016, 21, 192 CrossRef PubMed.
- D. H. S. Silva, Y. Zhang, L. A. Santos, V. S. Bolzani and M. G. Nair, Lipoperoxidation and Cyclooxygenases 1 and 2 Inhibitory Compounds from Iryanthera juruensis, J. Agric. Food Chem., 2007, 55, 2569 CrossRef CAS PubMed.
- (a) M. M. Konnick, N. Decharin, B. V. Popp and S. S. Stahl, O2 insertion into a palladium(II)-hydride bond: Observation of mechanistic crossover between HX-reductive-elimination and hydrogen-atom-abstraction pathways, Chem. Sci., 2011, 2, 326 RSC; (b) D. Wang, A. B. Weinstein, P. B. White and S. S. Stahl, Ligand-Promoted Palladium-Catalyzed Aerobic Oxidation Reactions, Chem. Rev., 2018, 118, 2636 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, characterization data for new compounds. See DOI: 10.1039/d1ra01672e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |