 Open Access Article
Open Access ArticleImpacts of octanol and decanol addition on the solubility of methanol/hydrous methanol/diesel/biodiesel/Jet A-1 fuel ternary mixtures
Ahmed I. El-Seesy *ac,
Radwan M. El-Zoheiryc,
Abdelrahman K. Fouadb,
Abdelrahman M. Hussienb,
Salma O. M. Elshabrawyb,
Zhixia He*a and
Alhassan Nasserb
*ac,
Radwan M. El-Zoheiryc,
Abdelrahman K. Fouadb,
Abdelrahman M. Hussienb,
Salma O. M. Elshabrawyb,
Zhixia He*a and
Alhassan Nasserb
aInstitute for Energy Research, Jiangsu University, No. 301, Xuefu Road, Zhenjiang, 212013, China. E-mail: zxhe@ujs.edu.cn
bChemical Engineering Department, Faculty of Engineering, Alexandria University, Alexandria, Egypt
cDepartment of Mechanical Engineering, Benha Faculty of Engineering, Benha University, 13512 Benha, Qalubia, Egypt. E-mail: ahmed.elsysy@bhit.bu.edu.eg
First published on 20th May 2021
Abstract
This study attempts to enhance the mixture instability of methanol/hydrous methanol mixed with diesel fuel, waste cooking oil (WCO) biodiesel, and Jet A-1 fuel using n-octanol and n-decanol as cosolvent at numerous temperatures of 10 °C, 20 °C, and 30 °C. The experiment is divided into two stages: first, blending pure methanol with diesel oil, Jet A-1, and WCO biodiesel individually utilizing n-octanol and n-decanol as cosolvent at various temperatures. Second, combining hydrous methanol (90% methanol + 10 wt% water) with diesel oil, Jet A-1, and WCO biodiesel independently and applying n-octanol and n-decanol as cosolvent at different temperatures. Pure methanol or hydrous methanol is mixed with the base fuels at different mixing proportions varying from 0 to 100 vol% with 10 vol% increments. The co-solvent, mainly n-octanol and n-decanol (titrant), is progressively and separately inserted into the tube with continuous shaking by utilizing a high-precision pipette until the ternary mixtures' phase borders seem. The findings demonstrate phase separation in pure methanol–diesel and pure methanol–Jet A-1 combinations even when the blend temperature increased to 60 °C. The pure methanol/biodiesel combination proves complete solubility without adding an external agent. The results also illustrate that the ambient temperature considerably affects the stability of mixture and amount of cosolvent in the blend. n-Octanol and n-decanol showed promising performance in enhancing the phase stability issue of methanol and hydrous methanol with the base fuels. It can be deduced that the minimum amount of cosolvent is recorded for biodiesel–hydrous methanol, Jet A-1–hydrous methanol, and diesel–hydrous methanol, respectively.
1. Introduction
In recent decades, lower alcohols have gained considerable attention as renewable sources to substitute conventional fuels.1 They are deemed to be encouraging alternative fuels, which have become a critical solution in accomplishing clean combustion for CI engines because of their extensive sources, mass-production ability, and individual physical and chemical properties.2,3 However, it will also bring a series of challenges, such as replacement path, cold start, ignition difficulty under low load conditions, and combustion stability.Methanol is a sole carbon hydroxyl compound, and the leading representative of the alcohol group. It is commercially utilized for a lengthy period efficiently due to its power heightening aspects and its capability to combust with low soot emission.4 It has certain standpoints such as higher ignition delay and smaller cetane number, which obfuscates the direct exploitation of methanol in CI engines. The supplementary limitations of methanol as a substitute for conventional fuels are the low heating value and phase separation challenge when it is combined with conventional fuel or biodiesel.5 To overcome or alleviate these issues, there are primarily two styles to the manipulation of methanol in CI engines, which comprise single injection and binary injection. Single injection implies methanol and diesel blended with an emulsifier or cosolvent, whereas the binary injection implies methanol discretely inserted in the intake line (fumigation).6–8 The crucial advantage of blending methanol and diesel fuel is that methanol is consecutively introduced into the burning chamber and appears in regions, where it effectively diminishes the emissions.
Moreover, higher alcohols (C4 to C10) such as n-butanol, n-pentanol, n-hexanol, n-octanol, and n-decanol are considered entirely mixable with both diesel and biodiesel.9–14 When they are mixed, they augment the individual characteristics, which boost the entire engine performance. It is commonly acknowledged that the higher alcohols possess certain stimulating characteristics that imply their capability of alleviating certain shortcomings of lower alcohols exploited as fuels.15–17 Those highlights result in the higher alcohols being advocated as oxygenated fuel and cosolvent agent to augment the application of methanol in CI engines and other systems.18 Their benefits are high cetane numbers and high calorific values compared to methanol.3,6,19 The mixture stability increased when mixing higher alcohols with diesel fuels, and the latent heat of vaporization of the higher alcohols is smaller than that of lower alcohols. Consequently, they have less scuffle with a low-temperature-ignition issue. However, the modern equipment in the fuel delivery line is normally adept at handling this challenge.
Some efforts have been attempted to use methanol as fuel in the CI engine in the blending mode.20–23 Table 1 summarizes some studies that have utilized higher alcohols as a cosolvent to enhance the stability issue of methanol with diesel fuel. It can be indicated that methanol can be blended up to 20% aiding in the manipulating cosolvents such as n-butanol, n-pentanol, and n-dodecanol.24–31 It can also be seen that the fumigation method is applied to manipulate methanol in intake or exhaust manifold, but this method is quite complicated and needed an extra system to adjust the pilot methanol injection.8
| Base fuel | Alcohol type | Blending method | Percentage | Optimum blends | Reference |
|---|---|---|---|---|---|
| Diesel fuel | Dimethyl ether–methanol | Direct blend | 20% and 30% DME; 10 and 30% methanol | D60 + M10 + 30DME | 20 |
| Diesel fuel | Methanol, 85% ethanol and 15% gasoline | Introduced into the intake line | 20%, 50%, 75% and 90% vol. | — | 37 |
| Diesel fuel | Methanol | Introduced into the intake line | 10, 20, 30, 40 and 50% | — | 21 |
| Diesel fuel | Methanol, water nano emulsion (sorbitan monolaurate emulsifier) | Direct blend using a magnetic stirrer | 10, 20, and 30% vol. | 30% methanol | 22 |
| Diesel fuel | n-Pentanol/methanol | Direct blend | 20 & 15% n-pentanol, 10 & 15% methanol and 70% diesel | — | 38 |
| Diesel fuel | Methanol | Introduced into the intake line | 25, 50 and 75% | — | 39 |
| Diesel fuel | 20% biodiesel, 10% butanol, 10% ethanol, or 10% methanol | Direct blend | 10% | — | 40 |
| Diesel fuel | Methanol, dodecanol | Direct blend | Methanol varied 2.5% to 15%, dodecanol 2.5% and 1% | 10% | 41 |
| Diesel fuel | Polyoxymethylene dimethyl ethers and methanol | Introduced into the intake line | Methanol | 70, 75, 80, 85% | 42 |
| Diesel fuel | Methanol | Introduced into the intake line | Methanol | 0, 50, 100% | 43 |
| Diesel fuel | 10% dodecanol, 1% nitric acid ester | Direct blend | Methanol | 13% | 44 |
| Diesel fuel | Biodiesel (40%), diethyl ether (5% & 10%) | Direct blend | Methanol, ethanol | 20% | 45 |
| Diesel fuel | Methanol; Al2O3 nanoparticles (25, and 100 ppm) | Direct blend | Methanol | 5% & 15% | 46 |
| Diesel fuel – spirulina microalgae (20 & 40%) | Methanol and ethanol | Direct blend | Methanol | 20% | 47 |
In addition, biodiesel and higher alcohols were exploited to heighten the phase separation of ethanol with diesel;5,32,33 for instance, Tongroon et al.,34 Krishna et al.,35 and Paul et al.36 utilized biodiesel as a cosolvent to augment the stability of ethanol/diesel mixtures in a diesel engine. The biodiesel was used in three different ratios, which were 3%, 5%, and 10% by volume. The main findings demonstrated that the biodiesel portion had to be expanded to avert phase separation with the growing ethanol segment in the mixtures. They indicated that the ambient conditions, particularly temperature, substantially influenced the mixture stability, where the phase separation occurred by lowering the ambient temperature. Moreover, Liu et al.5 utilized higher alcohols, principally n-butanol, n-hexanol, n-octanol, and n-dodecanol, to improve the phase separation issues of hydrous ethanol/diesel mixtures at various temperatures (5 °C, 15 °C, and 30 °C). They reported that the higher carbon numbers offer an improvement in the solubility, while n-dodecanol illustrated the gelling of the mixtures due to its low pour point. They also deduced that n-hexanol and n-decanol could be recommended to augment the stability issue of hydrous ethanol/diesel blends.
n-Octanol (C8) and n-decanol (C10) are employed in the existing research because of their appealing highlights. For example, n-octanol has a superior oxygen content of about 12.3% m m−1, nevertheless demonstrates diminished reactivity attributed to its boiling point, which rests at the lower end of diesel's distillation figure.48 Nevertheless, its additional aspects involving superior surface tension, smaller vapor pressure, and greater latent heat of vaporization balance for its low boiling point and certify heightened spray developments. It presents greater kinematic viscosity than diesel, but its adverse control across the A/F combination development is counterbalanced by the mixed impact of its minimal cetane number and boiling point. Compared to other higher alcohols, it exposes non-corrosive aspects attributed to its low hygroscopic nature. Commercially, it is used as additives, emulsifiers, anti-foaming agents, solvents, and frothing agents.9 In addition, it has a lengthy alcohol chain and is manufactured through the oligomerization of ethylene.9 Compared to other higher alcohols, it has acquired more interest from scientific society due to its ecological manufacture procedures such as oxo-synthesis, fatty acid drop, and utilization of bacteria.49,50 It is recognized as a bio-derived fuel owing to its synthesis from biomass sources51 that has persuaded researchers to highlight its burning features.52–54
In addition, n-decanol is a colorless to marginally yellow liquid, holding ten carbon atoms in its straight chain. It is used broadly in food and chemical manufacturing than as a substitute fuel for CI engines.55 Nevertheless, coconut as a source for decanol synthesis is enhanced, which comprises decanoic acid-like Yarrowia lipolytica, and the production cycles are found to be cost-efficient with a soaring yield.56 Hence, the potential of employing vegetable biomass and bio-resources comprising lignocellulosic material, waste protein has persuaded the scientific society to assess decanol as a feasible fuel. Furthermore, it has a higher heating value than most of the existing biodiesels and other alcohol group representatives. Its boiling point is in the assortment of diesel boiling figures and dissimilar diesel, and it does not have any aromatic structures.
1.1 Motivation of the current study
The alcohol group spans from the lone carbon methanol to twenty carbon phytol and higher chain compounds. Fig. 1 illustrates how the properties of the alcohols vary as the carbon group size grows. They are available in plenty and produced by anaerobic fermentation of numerous forestry and cultivated biomass trashes, such as saw-mill, wood-pulp, sugarcane bagasse, and rice straw.9 Fig. 2 demonstrates the common and most favored production approaches for the representatives of the alcohol group. In addition, higher alcohols (from C4 to C10) have hydrogen contributor groups (–OH) connected to molecular functional groups that receive hydrogen, which cooperate further with the polar substances. Moreover, regarding van der Waals forces, the non-polarity or low polarity carbon chains of higher alcohols have a decent similarity with diesel hydrocarbons.57,58 For these benefits, alcohol additives would illustrate considerably superior solubility behavior.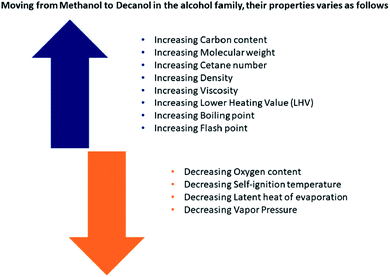 | ||
| Fig. 1 Change in alcohol properties as a function of the number of carbon atoms.9 | ||
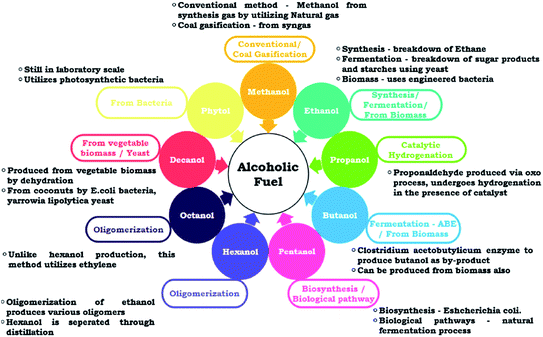 | ||
| Fig. 2 Numerous alcohol fabrication methods.9 | ||
From the above literature survey, it can be deduced that the main problems associated with methanol as a fuel in CI engines are as follows: (i) phase separation or mixture instability (the main reason is the water content in methanol), (ii) low cetane number (difficult to start the combustion, high ignition delay, increased cylinder pressure, and increased NOx content), (iii) high latent heat of vaporization (cooling consequence during the burning), and (iv) low heating value (reduced the BTE and increased bsfc). Moreover, higher alcohols are considered an encouraging cosolvent to improve the instability issue of ethanol with diesel fuel. However, no research has scrutinized the impact of using higher alcohols as a cosolvent with methanol/diesel combination. Thus, to fill this research gap, higher alcohols are used as a cosolvent to alleviate the phase stability issues between methanol/hydrous methanol/diesel/Jet-A1/waste cooking oil (WCO) biodiesel blends. In the primary test, pure methanol was added individually to diesel, biodiesel, and Jet-A1 fuel to assess the miscibility. Also, the primary test was done using different types of higher alcohols starting from C3 (n-propanol) to C10 (n-decanol) as cosolvents with methanol/diesel blends, and it indicated that n-octanol (C8) and n-decanol (C10) are the most effective cosolvents among the utilized alcohols. Thus, this study is divided into three groups of blending fuels: hydrous methanol/diesel, hydrous methanol/WCO biodiesel, and hydrous methanol/Jet-A1. These mixtures are blended using n-octanol and n-decanol as a cosolvent at different temperatures, which are 10 °C, 20 °C, and 30 °C. The mixtures were also kept in a long tube for more than three months to assess their stability.
2. Experimental set-up and procedures
In this study, the base fuel is a conventional commercial diesel from a local gas station, and Jet-A1 was also provided by a local company. The methanol, n-octanol, and n-decanol exploited in this research have a purity of 99.9%. The WCO biodiesel is produced via a transesterification process applying ultrasonic techniques; further information can be obtained in the literature.59 The GC-MS and FTIR techniques were employed to assess the produced WCO biodiesel, as indicated in Fig. 3 and Table 2. The physicochemical properties of diesel, Jet-A1, WCO biodiesel, methanol, n-octanol, and n-decanol are assessed corresponding to the ASTM standard, as illustrated in Table 3. Thermal gravimetric analysis (TGA) for the raw oil and WCO biodiesel is illustrated in Fig. 3b. It can be indicated that the raw oil evaporated in the range of 350–500 °C. This high-temperature scale is attributed to the triglyceride structure of raw oil that makes its decomposition process quite difficult. The WCO biodiesel is evaporated wholly with no residues at a temperature scale of 180–450 °C. The difference in the two curves proves that the raw oil is converted into biodiesel. These results are comparable to those reported in the literature.60,61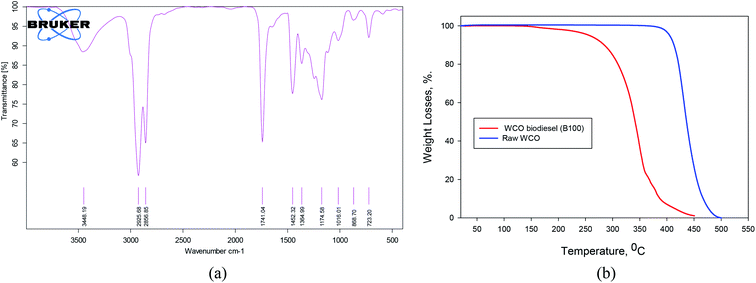 | ||
| Fig. 3 (a) FT-IR band of WCO biodiesel and (b) thermal gravimetric analysis of raw oil and WCO biodiesel. | ||
| RT (min) | Composite | FAME | Formula |
|---|---|---|---|
| 9.75 | Methyl tetradecanoate | C16:1 | C15H30O2, CAS no. 124-10-7 |
| 11.78 | Hexadecanoic acid, methyl ester | C16:1 | C17H34O2, CAS no. 112-39-0 |
| 13.679 | 9-Octadecenoic acid, methyl ester | C18:1 | C19H36O2, CAS no. 2462-84-2 |
| 13.881 | Methyl stearate | C18:1 | C19H38O2, CAS no. 112-61-8 |
| Diesel | Jet A-1 | Methanol | WCO biodiesel | n-Octanol | n-Decanol | |
|---|---|---|---|---|---|---|
| Formula | C12–C25 | — | CH3OH | — | C8H17OH | C10H21OH |
| Molecular weight | 198.4 | 148.02 | 32.04 | 305 | 130.21 | 158.23 |
| CN | 48 | 42 | 3 | 49 | 39 | 50 |
| Oxygen (% wt) | — | — | 50 | 12.6 | 15.7 | — |
| Density (g ml−1) at 20 °C | 0.832 | 0.797 | 0.796 | 0.877 | 0.827 | 0.829 |
| Autoignition temperature (°C) | 210 | — | 470 | — | — | 275 |
| Flash point (°C) | 69 | 38 | 12 | — | 59 | 108 |
| LHV (MJ kg−1) | 42.5 | 43.46 | 19.9 | 37.95 | 52.94 | 41.82 |
| BP (°C) | 280 | 163 | 64.5 | 250 | 195 | 233 |
| Latent heat of vaporization (kJ kg−1) at 25 °C | 270 | 360 | 1109 | — | 486 | 494.8 |
| Viscosity (mm2 s−1) at 40 °C | 3.2 | 1.08 | 0.59 | 4.9 | 5.8 | 6.5 |
The experimental program is divided into two parts, which are the primary test and the main test. The primary one includes the test of pure methanol solubility in diesel, biodiesel, and Jet-A1 fuel. The results of this part showed that there is phase separation in methanol/diesel and methanol/Jet-A1 mixtures even when the temperature of the mixture increased to 60 °C. On the other hand, the pure methanol/biodiesel blend illustrates complete solubility without using an external agent. Thus, n-octanol and n-decanol are used as a cosolvent to enhance the phase separation of pure methanol/diesel and pure methanol/Jet-A1 mixtures at temperatures of 10 °C, 20 °C, and 30 °C.
The second part of the experiment is to blend individually hydrous methanol/biodiesel/biodiesel/Jet-A1 combinations. However, adding water creates immiscibility in the mixture; thus, higher alcohols are used as a cosolvent to improve the phase separation of the mixtures. Therefore, n-octanol and n-decanol are used as a cosolvent to blend hydrous methanol with diesel, waste cooking oil, and Jet-A1 fuel independently. The experimental program is divided into three groups, as revealed in Table 4. It can be spotted that each group has been split into two categories regarding the higher alcohols used. Also, all tests have been performed at three different temperatures, comprising 10 °C, 20 °C, and 30 °C.
| Group 1 | Group 2 | Group 3 | ||||
|---|---|---|---|---|---|---|
| Hydrous methanol (90% methanol + 10 wt% water) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Diesel | ✓ | ✓ | ||||
| WCO biodiesel | ✓ | ✓ | ||||
| Jet-A1 | ✓ | ✓ | ||||
| n-Octanol | ✓ | ✓ | ✓ | |||
| n-Decanol | ✓ | ✓ | ✓ | |||
| Temperature | 10, 20, and 30 °C | 10, 20, and 30 °C | 10, 20, and 30 °C | 10, 20, and 30 °C | 10, 20, and 30 °C | 10, 20, and 30 °C |
2.1 Preparation of ternary mixtures
In order to make the blends, 10 ml samples of fuel-M10 (M10 is 90 wt% methanol and 10 wt% distilled water mixture) binary mixtures were prepared in the composition range 0 to 100 vol% with 10% increments. These combinations were initially in a 2-phase equilibrium due to the immiscibility of M10 in the base fuel. These were then titrated with the alcohol cosolvent (n-octanol or n-decanol) as the titrant using high precision 1 ml pipettes, and the endpoint at which the mixture forms a homogeneous single phase is recorded as a point lying on the boundary of the ternary phase diagram. The endpoints measured for each sample were used to construct the ternary phase diagrams in mass percentage to assess the ternary mixture phase behaviour. The focus of this study was the point of disappearance of the two-phase boundary, and there was no interest in studying the phase behavior at higher cosolvent concentrations, which according to other investigation would form gel phases as such mixtures were not commercially important as fuels. In some cases, the change was not sharp as there was a transition phase between the 2-phase and the single-phase behavior in which a cloudy or hazy phase appeared before a clear solution was obtained on further addition of the cosolvent; this cloudy region was also recorded on the phase diagrams. The phase behavior studies were conducted at temperatures of 10 °C, 20 °C, and 30 °C.3. Results and discussion
3.1 Pure methanol/diesel/Jet-A1/biodiesel blends at different temperatures
This part of the study deals with the impacts of adding n-octanol and n-decanol on the solubility of pure methanol/diesel and pure methanol/Jet-A1 blends at various temperatures of 10 °C, 20 °C, and 30 °C, as illustrated in Fig. 4–6. As mentioned earlier, the results illustrate that pure methanol can be mixed with WCO biodiesel at any concentration, and it was stable at tested temperatures. The alcohol (–OH) group regulates the polarity of the molecule. The electrostatic possibility indicates that oxygen is moderately negative, whereas the carbon and hydrogens are relatively positive. In contrast, diesel (oil) is composed of non-polar molecules, and it has a shell of negative charges or electrons bordering the molecule. Regarding the WCO biodiesel, the functional group of esters is carboxylate (COO), which is polar. Nevertheless, the long carbon chain's impact creates the dominant non-polar characteristic in biodiesel. For this purpose, it is expressed in conditions of comparative non-polarity. Concerning jet fuel, it is recognized that it is primarily non-polar.62 It is acknowledged that the polar molecules only dissolve in polar solvents. Similarly, non-polar molecules only dissolve in non-polar solvents, implying “Like dissolves Like”.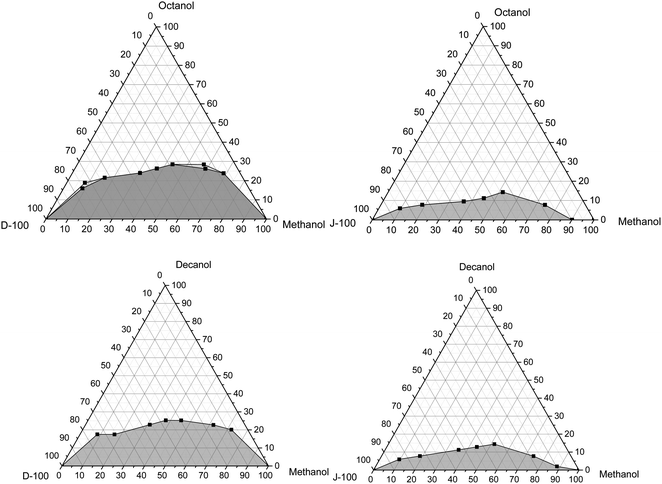 | ||
| Fig. 4 Variation of the solubility of pure methanol with Jet-A1 and diesel using octanol and decanol as the cosolvent at 30 °C. | ||
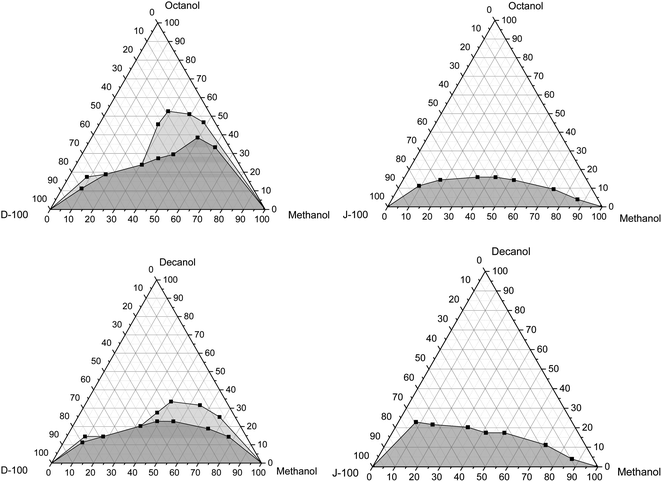 | ||
| Fig. 5 Variation of the solubility of pure methanol with Jet-A1 and diesel using octanol and decanol as the cosolvent at 20 °C. | ||
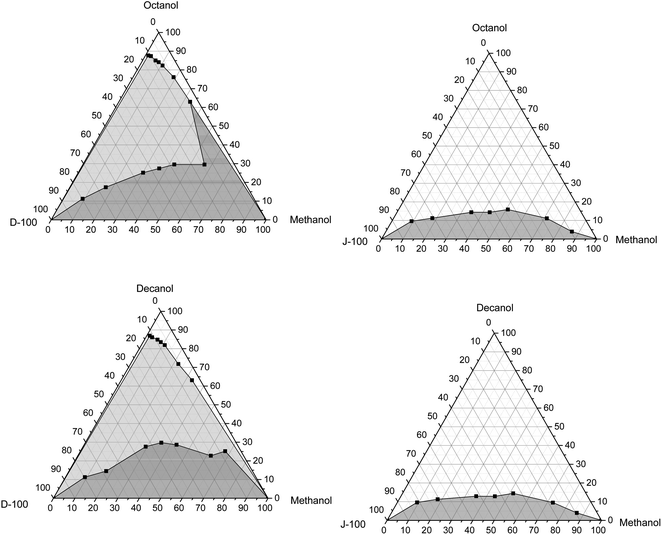 | ||
| Fig. 6 Variation of the solubility of pure methanol with Jet-A1 and diesel using n-octanol and n-decanol as the cosolvent at 10 °C. | ||
In general, it can also be found that the amount of n-octanol and n-decanol is lower for the Jet-A1 fuel than diesel fuel, irrespective of the tested temperatures. This could be attributed to the high polarity of the diesel fuel that could increase the fraction of the cosolvent to stabilize the mixture. Also, there are no cloudy or hazy phases noted in the case of the Jet-A1 fuel, while they appear in the case of the diesel fuel. This cloudy region is enlarged with the decrease in the temperature of the pure methanol/diesel mixtures, as illustrated in Fig. 5 and 6. This could be ascribed to the fact that an increase in temperature leads to an enhancement in the thermal motion that could heighten the diffusion and spreading of molecules, causing a reduction in the cosolvent amount required to reach mixture stability.5 The methanol fraction in the mixture also impacts the solubility trend and the amount of the cosolvent used, where the amount of the cosolvent increases as the methanol percentage increases until it reaches almost 50%, and then it starts to reduce again. It is should be noted that in the case of methanol/diesel blends n-decanol has a decent performance to get the mixtures stable with a small amount compared to n-octanol. This could be because the alcohol with a longer chain has better solubility behavior than that with a shorter chain. Similar results were matched with those cited in the literature.5 These results were comparable to those reported in the literature.63–65
3.2 Hydrous methanol/diesel/Jet-A1/biodiesel blends at different temperatures
This part examines the phase behaviours of hydrous methanol/diesel, hydrous methanol/biodiesel, and hydrous methanol/jet fuel mixtures with utilizing n-octanol and n-decanol as cosolvents at different temperatures of 10 °C, 20 °C, and 30 °C, as shown in Fig. 7–9. The addition of water to methanol made a phase separation when it blended with biodiesel. This trend could be explained by increasing water polarity, which makes the blend unstable and thus needs the cosolvent to be stable. From Fig. 7, it can be seen that n-octanol is more effective as a cosolvent for hydrous methanol than n-decanol with all base fuels. The peak value of n-octanol required with biodiesel is 17 wt% as opposed to 23 wt% for n-decanol. These results match with those cited in the literature.64,66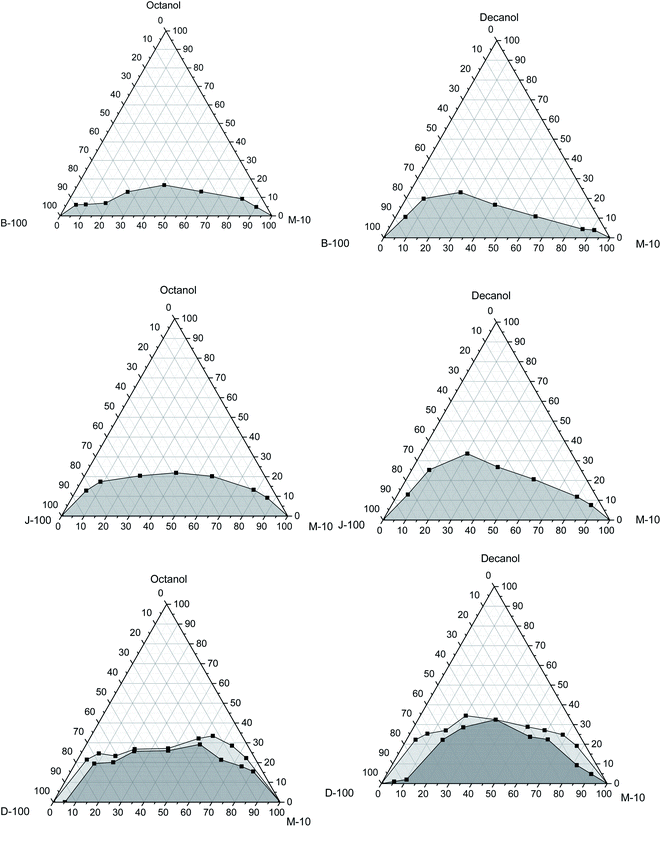 | ||
| Fig. 7 Variation of the solubility of hydrous methanol with biodiesel, Jet-A1, and diesel using octanol and decanol as cosolvent at 30 °C. | ||
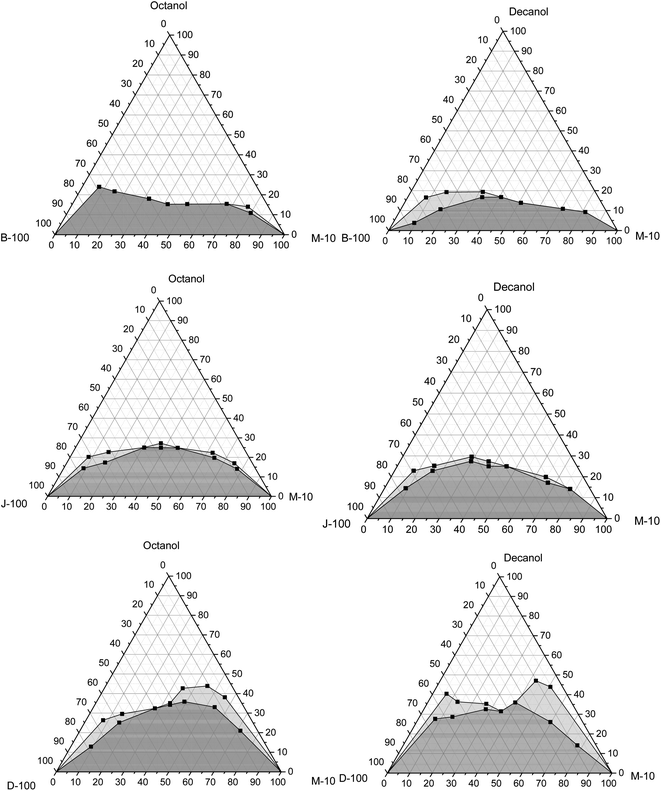 | ||
| Fig. 8 Variation of solubility of hydrous methanol with biodiesel, Jet-A1, and diesel with octanol and decanol as cosolvent at 20 °C. | ||
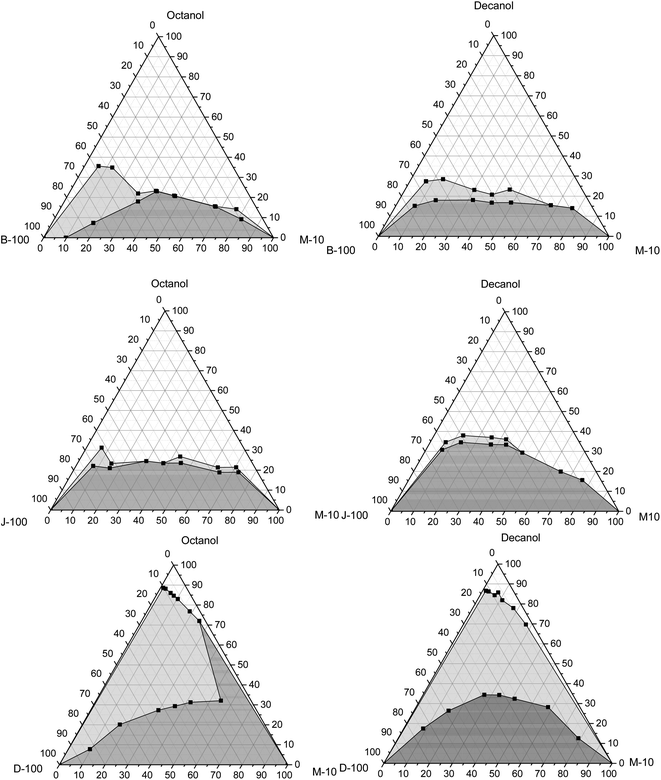 | ||
| Fig. 9 Variation in the solubility of hydrous methanol with biodiesel, Jet-A1, and diesel using octanol and decanol as cosolvent at 10 °C. | ||
Regarding the jet fuel, the maximum amount is 22 wt% with n-octanol and 33.6 wt% with n-decanol. Moreover, it is 33.5 wt% with n-octanol and 34.5 wt% with n-decanol in the case of diesel. This implies that the depth of the 2-phase region was bigger in the case of n-decanol than that with n-octanol. It can also be remarked that the cloudy region appears in the case of the diesel/hydrous methanol blend for both cosolvents. This region is extended with the reduction in the mixture temperatures, which means that more cosolvent is needed to get a stable mixture. From this standpoint, it can also be verified that the high ambient temperature or a comparatively low pour point of the cosolvent acts as the main factor influencing on the inclusive and appropriate adjustment of the phase separation of combinations. It can be realized that the lowest quantity of the cosolvent is verified for biodiesel/hydrous methanol, Jet A-1/hydrous methanol, and diesel/hydrous methanol, respectively. The results match with those reported by Liu et al.,5 who explored the impacts of the ethanol/diesel mixture solubility by applying numerous types of higher alcohols as cosolvent.
4. Conclusion
The major shortcomings of methanol are mixture instability when combined with conventional fuels and elevated latent heat of vaporization. Therefore, this research endeavors to augment the combination separation issues of methanol/hydrous methanol blended with diesel fuel, WCO biodiesel, and Jet A-1 fuel manipulating n-octanol and n-decanol as cosolvent at different temperatures of 10 °C, 20 °C, and 30 °C. The test is performed in two steps: in the first step, the pure methanol is added with diesel oil, Jet A-1, and WCO biodiesel separately, employing n-octanol and n-decanol as cosolvent at tested temperatures. Second, the hydrous methanol is mixed with diesel oil, Jet A-1, and WCO biodiesel individually utilizing n-octanol and n-decanol as cosolvent at tested temperatures. The results of pure methanol/base fuel combinations show that the phase separation in pure methanol/diesel and pure methanol/Jet A-1 combinations is remarked even after the combination temperature increased up to 60 °C.On the other hand, the pure methanol/biodiesel blend confirms complete solubility devoid of an external agent. The findings also demonstrate that the ambient temperature substantially influences the mixture's stability and quantity of the cosolvent in the mixture. The n-octanol and n-decanol appeared an encouraging implementation to boost the phase stability issue of methanol and hydrous methanol with the base-tested fuels. It can be concluded that the smallest fraction of the cosolvent is documented for biodiesel/hydrous methanol, Jet A-1/hydrous methanol, and diesel/hydrous methanol, correspondingly. It can also be inferred that n-octanol gave better solubility characteristics compared to n-decanol. Finally, all combinations were kept in a long tube for more than two months under static conditions to assess their stability, and thus, the observation indicated no phase separation in the blends at tested temperatures.
Regarding the future investigations relating to the current study, the non-random two-liquid (NRTL) method is essential to assess the correlating phase equilibria of the different liquid fuels. Also, the utilization of surfactants with higher alcohols, particularly at low temperatures, is needed in further investigation.
Nomenclature
| ASTM | American Society for Testing and Materials |
| B100 | Biodiesel |
| BTE | Brake thermal efficiency |
| bsfc | Brake specific fuel consumption |
| D100 | Diesel fuel |
| WCO | Waste cooking oil |
| J100 | Jet-A1 fuel |
| M10 | Hydrous methanol containing 90% methanol + 10 wt% water |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. 52050410330).References
- M. Tabatabaei, et al., Acta Innovations, 2020, 36–46 Search PubMed.
- Y. Çelebi and H. Aydın, Fuel, 2019, 236, 890–911 CrossRef.
- S. M. Sarathy, P. Oßwald, N. Hansen and K. Kohse-Höinghaus, Prog. Energy Combust. Sci., 2014, 44, 40–102 CrossRef.
- S. Verhelst, J. W. Turner, L. Sileghem and J. Vancoillie, Prog. Energy Combust. Sci., 2019, 70, 43–88 CrossRef.
- H. Liu, B. Hu and C. Jin, Fuel, 2016, 184, 440–448 CrossRef CAS.
- Y. Çelebi and H. Aydın, Fuel, 2019, 236, 890–911 CrossRef.
- M. A. Ghadikolaei, C. S. Cheung and K. F. Yung, J. Energy Inst., 2018, 92, 1233–1250 CrossRef.
- A. I. El-Seesy, Z. Kayatas, R. Takayama, Z. He, S. Kandasamy and H. Kosaka, Energy Convers. Manage., 2020, 205, 112453 CrossRef CAS.
- K. Nanthagopal, R. S. Kishna, A. E. Atabani, A. H. Al-Muhtaseb, G. Kumar and B. Ashok, Energy Convers. Manage., 2020, 203, 112244 CrossRef CAS.
- V. B. M. Vinod Babu, M. M. K. Madhu Murthy and G. Amba Prasad Rao, Renewable Sustainable Energy Rev., 2017, 78, 1068–1088 CrossRef.
- L. Ning, Q. Duan, Z. Chen, H. Kou, B. Liu, B. Yang and K. Zeng, Fuel, 2020, 266, 117034 CrossRef CAS.
- A. I. El-Seesy, H. Hassan and H. Kosaka, Energy Procedia, 2019, 156, 33–37 CrossRef CAS.
- A. I. El-Seesy and H. Hassan, Energy Procedia, 2019, 162, 48–56 CrossRef CAS.
- L. Razzaq, M. A. Mujtaba, M. E. M. Soudagar, W. Ahmed, H. Fayaz, S. Bashir, I. M. R. Fattah, H. C. Ong, K. Shahapurkar, A. Afzal, S. Wageh, A. Al-Ghamdi, M. S. Ali and A. I. El-Seesy, J. Environ. Manage., 2021, 282, 111917 CrossRef CAS PubMed.
- W. R. d. S. Trindade and R. G. dos Santos, Renewable Sustainable Energy Rev., 2017, 69, 642–651 CrossRef.
- W. Zhong, T. Pachiannan, Z. He, T. Xuan and Q. Wang, Appl. Energy, 2019, 235, 641–652 CrossRef CAS.
- A. I. El-seesy, A. M. A. Attia and H. M. El-batsh, Fuel, 2018, 224, 147–166 CrossRef CAS.
- B. Rajesh Kumar and S. Saravanan, Renewable Sustainable Energy Rev., 2016, 60, 84–115 CrossRef CAS.
- H. Venu, V. D. Raju and L. Subramani, Energy, 2019, 174, 386–406 CrossRef CAS.
- H. Taghavifar, A. Nemati and J. Honore, Energy, 2019, 187, 115951 CrossRef CAS.
- H. Lu, A. Yao, C. Yao, C. Chen and B. Wang, Fuel, 2019, 235, 617–626 CrossRef CAS.
- D. K. Soni and R. Gupta, Energy, 2017, 126, 638–648 CrossRef CAS.
- T. Xuan, Z. Sun, A. I. El-Seesy, Y. Mi, W. Zhong, Z. He, Q. Wang, J. Sun and R. M. El-Zoheiry, Energy, 2021, 289, 120543 CrossRef.
- T. Xuan, Z. Sun, A. I. El-Seesy, Y. Mi, W. Zhong, Z. He, Q. Wang, J. Sun and R. M. El-Zoheiry, Fuel, 2020, 119762 Search PubMed.
- A. I. El-Seesy, M. Nour, H. Hassan, A. Elfasakhany, Z. He and M. A. Mujtaba, Case Stud. Therm. Eng., 2021, 25, 100911 CrossRef.
- A. I. El-Seesy, Z. He and H. Kosaka, Energy, 2021, 214, 118972 CrossRef CAS.
- A. I. El-Seesy, T. Xuan, Z. He and H. Hassan, Energy Convers. Manage., 2020, 226, 113524 CrossRef CAS.
- A. I. El-Seesy, Z. He, H. Hassan and D. Balasubramanian, Fuel, 2020, 279, 118433 CrossRef CAS.
- A. I. El-Seesy, Z. Kayatas, M. Hawi, H. Kosaka and Z. He, Renewable Energy, 2020, 147, 2064–2076 CrossRef CAS.
- A. I. El-Seesy, H. Kosaka, H. Hassan and S. Sato, Energy Convers. Manage., 2019, 196, 370–394 CrossRef CAS.
- A. I. El-Seesy and H. Hassan, Renewable Energy, 2019, 132, 558–574 CrossRef CAS.
- C. Jin, X. Pang, X. Zhang, S. Wu, M. Ma, Y. Xiang, J. Ma, J. Ji, G. Wang and H. Liu, Fuel, 2019, 236, 65–74 CrossRef CAS.
- M. Nour, A. I. El-Seesy, A. K. Abdel-Rahman and M. Bady, Exp. Therm. Fluid Sci., 2018, 98, 634–644 CrossRef CAS.
- M. Tongroon, P. Saisirirat, A. Suebwong, J. Aunchaisri, M. Kananont and N. Chollacoop, Fuel, 2019, 255, 115728 CrossRef CAS.
- S. M. Krishna, P. Abdul Salam, M. Tongroon and N. Chollacoop, Appl. Therm. Eng., 2019, 155, 525–533 CrossRef CAS.
- A. Paul, R. Panua and D. Debroy, Energy, 2017, 141, 839–852 CrossRef CAS.
- W. Tutak, K. Lukács, S. Szwaja and Á. Bereczky, Fuel, 2015, 154, 196–206 CrossRef CAS.
- H. Chen, X. Su, J. He and B. Xie, Energy, 2019, 167, 297–311 CrossRef CAS.
- L. Wei, C. Yao, Q. Wang, W. Pan and G. Han, Fuel, 2015, 140, 156–163 CrossRef CAS.
- A. O. Emiroğlu and M. Şen, Appl. Therm. Eng., 2018, 133, 371–380 CrossRef.
- H. Bayraktar, Fuel, 2008, 87(2), 158–164 CrossRef CAS.
- G. Duraisamy, M. Rangasamy and N. Govindan, Renewable Energy, 2020, 145, 542–556 CrossRef CAS.
- L. Wei, C. Yao, G. Han and W. Pan, Energy, 2016, 95, 223–232 CrossRef CAS.
- C. Fan, C. Song, G. Lv, G. Wang, H. Zhou and X. Jing, Appl. Therm. Eng., 2018, 129, 1382–1391 CrossRef CAS.
- H. Venu and V. Madhavan, Fuel, 2017, 189, 377–390 CrossRef CAS.
- J. Wei, Z. Yin, C. Wang, G. Lv, Y. Zhuang, X. Li and H. Wu, Appl. Therm. Eng., 2020, 116372 Search PubMed.
- T. N. Verma, P. Nashine, P. K. Chaurasiya, U. Rajak, A. Afzal, S. Kumar, D. V. Singh and A. K. Azad, Sustain. Energy Technol. Assess., 2020, 42, 100851 Search PubMed.
- F. Hoppe, B. Heuser, M. Thewes, F. Kremer, S. Pischinger, M. Dahmen, M. Hechinger and W. Marquardt, Int. J. Engine Res., 2016, 17, 16–27 CrossRef CAS.
- T. Vallon, M. Glemser, S. H. Malca, D. Scheps, J. Schmid, M. Siemann-Herzberg, B. Hauer and R. Takors, Chem.-Ing.-Tech., 2013, 85, 841–848 CrossRef CAS.
- M. K. Akhtar, H. Dandapani, K. Thiel and P. R. Jones, Metab. Eng. Commun., 2015, 2, 1–5 CrossRef PubMed.
- J. Julis and W. Leitner, Angew. Chem., Int. Ed., 2012, 51, 8615–8619 CrossRef CAS PubMed.
- J. Preuß, K. Munch and I. Denbratt, Fuel, 2018, 216, 890–897 CrossRef.
- A. E. Atabani and S. Al Kulthoom, Fuel, 2020, 282, 118849 CrossRef CAS.
- M. Nour, A. M. A. Attia and S. A. Nada, Energy Convers. Manage., 2019, 185, 313–329 CrossRef CAS.
- K. L. Wasewar, A. Bert M Heesink, G. F. Versteeg and V. G. Pangarkar, J. Chem. Technol. Biotechnol., 2002, 77, 1068–1075 CrossRef CAS.
- C. D. Rutter and C. V. Rao, Metab. Eng., 2016, 38, 139–147 CrossRef CAS PubMed.
- Y. Reyes, D. A. G. Aranda, L. A. M. Santander, A. Cavado and C. R. P. Belchior, Energy Fuels, 2009, 23, 2731–2735 CrossRef CAS.
- M. Lapuerta, R. García-Contreras, J. Campos-Fernández and M. P. Dorado, Energy Fuels, 2010, 24, 4497–4502 CrossRef CAS.
- M. S. Gad, A. I. El-Seesy, A. Radwan and Z. He, Fuel, 2020, 269, 117466 CrossRef CAS.
- A. M. A. Attia, M. Nour, A. I. El-Seesy and S. A. Nada, Sustain. Energy Technol. Assess., 2020, 42, 100843 Search PubMed.
- M. Nour, A. I. El-Seesy, A. Attia, X. Li and S. Nada, Environ. Prog. Sustainable Energy, 2020, 1–14 CAS.
- M. Commodo, I. Fabris, C. P. T. Groth and Ö. L. Gülder, Energy Fuels, 2011, 25, 2142–2150 CrossRef CAS.
- L. Geng, L. Bi, Q. Li, H. Chen and Y. Xie, Energy Rep., 2021, 7, 904–915 CrossRef.
- M. Nour, A. M. A. Attia and S. A. Nada, Fuel, 2019, 251, 10–22 CrossRef CAS.
- N. Phasukarratchai, Fuel, 2019, 243, 125–132 CrossRef CAS.
- I. Pires de Oliveira, A. R. L. Caires, K. Baskar, S. Ponnusamy, P. Lakshmanan and V. Veerappan, Fuel, 2020, 263, 116753 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |
