Open Access Article
Open Access ArticleInsights into antimicrobial agent sulfacetamide transformation during chlorination disinfection process in aquaculture water†
Yaoguang Guo a,
Zhiyuan Liuab,
Xiaoyi Lou*b,
Changling Fangb,
Pu Wanga,
Genying Wuc and
Jie Guan*a
a,
Zhiyuan Liuab,
Xiaoyi Lou*b,
Changling Fangb,
Pu Wanga,
Genying Wuc and
Jie Guan*a
aSchool of Environmental and Materials Engineering, Shanghai Polytechnic University, Shanghai 201209, China. E-mail: guanjie@sspu.edu.cn
bLaboratory of Quality Safety and Processing for Aquatic Product, East China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Shanghai 200090, China. E-mail: huoxingmayi@126.com
cLongquan Branch of Lishui Municipal Ecological Environment Bureau, Longquan 323700, China
First published on 20th April 2021
Abstract
Antibiotic addition and chlorination are two common processes in fishery culture. Antibiotic residues not only pollute aquaculture water, but are also one of the potential precursors of disinfection by-products (DBPs) during chlorination. The degradation kinetics, products identification and reaction mechanism of sulfacetamide (SFA), a new sulfonamides antibiotics, and potential formation of haloacetic acids (HAAs) in chlorination were explored. The results showed that the degradation of SFA followed pseudo first-order kinetic model, and chlorinating agent dose, pH of water, water temperature, NH4+, HCO3− and humic acid (HA) had various effects on the degradation of SFA and the yields of HAAs. The presence of Br− accelerated both the degradation rate of SFA and more formation of Br-DBPs. Through the identification of intermediate products, we proposed the transformation pathway of SFA during the chlorination disinfection process. Namely, in this NaClO disinfection system, the C–S bond between the sulfonyl group and benzene ring, and S–N bond between sulfonyl and acylamino of SFA were broken, and then the primary formed groups were further oxidized to produce intermediates, such as chloroanilines and chlorophenols. And then chlorophenols were subsequently chlorinated to form toxic HAAs. The present study might be of significance for the evaluation of effective degradation of SFA and potential production of halogenate-DBPs (H-DBPs) during the chlorination disinfection process in aquaculture water.
1. Introduction
Aquaculture is a very large and mature industry in the world, and China is the largest producer and exporter of aquatic products as well.1 Due to the occurrence of various aquatic diseases in the development of aquaculture, antibiotics have become one of the indispensable drugs in aquaculture.2 China is the largest antibiotic user in the world in which animal consumption accounts for half of the total.3 Nowadays, the most widely used antibiotics in aquaculture are quinolones, sulfonamides, macrolides, β-lactams, tetracyclines, furans, etc.4 Antibiotics are added to the feed of aquatic animals in order to assure the safety in aquaculture, but they cannot be completely digested, therefore, the residual antibiotics would enter the aquaculture water body. After human consumption of aquatic products contaminated by antibiotics, allergic symptoms and chronic intoxication might be caused.5 For instance, sulfonamides and aureomycin may lead to fulminant liver necrosis, agranulocytosis, aplastic anemia and other diseases.6,7 Ciprofloxacin may result in interstitial nephritis, hepatitis, liver necrosis, and furazolidone may cause hemolytic anemia and multiple neuritis.8 Hence, more and more attention has been paid to the residue, transportation and transformation of antibiotics in aquaculture water, especially in the disinfection process.Chlorination is the most common process in aquaculture water, with the disinfectants of chlorine compounds.9 Residual antibiotics are degraded during the chlorination process; however, studies have found that antibiotics undergo side reactions with chlorine disinfectants to generate halogenated disinfection by-products (H-DBPs) such as trihalomethanes (THMs) and haloacetic acids (HAAs).10,11 These H-DBPs have attracted extensive attention in the field of scientific research owing to the latent health risks.12 In seawater, hypobromous acid (HOBr) can be generated by the chlorine oxidation to be dominant oxidant species, leading to preferential formation of various brominated by-products.13,14 Rong et al.15 reported that norfloxacin (NOR) in seawater was substituted with HOBr to form two brominated DBPs (Br-DBPs) during chlorination, and the toxicity of Br-DBPs was far higher than that of chlorinated DBPs (Cl-DBPs). Marsà et al.16 reported that bromoacetic acid was 89.8 times higher than that of chloroacetic acid in human urothelial cells. THMs exposure resulted in adverse effect for upgrowth and significant tail shortening in zebrafish, and tribromoacetic acid (TBAA) and dichloroacetic acid (DCAA) can significantly increase the deformity rate of zebrafish embryos.17,18 In brief, possible derivative H-DBPs from antibiotics during chlorination disinfection process in aquaculture water might threaten health to aquatic animals, and subsequently intimidate the fishery economy. Therefore, the transformation fate of H-DBPs in aquaculture water during disinfection is significant for effective assessment of fishery health risk.
Sulfonamides (SAs) are broad-spectrum antibiotics used in aquaculture waters, and the most related researches focus on the degradation kinetics during chlorination disinfection process.19 In contrast, the transformation mechanism and the potential generation of H-DBPs in the chlorination process still need to be further studied to explore the threat to fisheries aquaculture. Consequently, in the present study, we selected a new SA antibiotic (sulfacetamide, SFA) as the model substrate (the detailed information was shown in Table S1 in the ESI†). We studied the relationship between reaction kinetics of SFA in chlorination and the parameters, such as available chlorine concentration, pH, temperature, and other common components (i.e. NH4+, HCO3− and HA) in aquaculture water. Based on the above analysis, the potential formation of H-DBPs represented by HAAs and transformation mechanism of SFA in the chlorination process were explored. The present research might provide technical support for the effective removal of SFA and the control of the production of HAAs during the chlorination process in aquaculture water.
2. Materials and methods
2.1 Chemicals and reagents
All chemicals were analytical grade except as noted. Mixed standard of 9 kinds of halogenated acetic acids (i.e. monochloroacetic acid (MCAA), dichloroacetic acid (DCAA), trichloroacetic acid (TCAA), monobromoacetic acid (MBAA), dibromoacetic acid (DBAA), tribromoacetic acid (TBAA), bromochloroacetic acid (BCAA), bromodichloroacetic acid (BDCAA), and chlorodibromoacetic acid (CDBAA)) were obtained from Accustandard (America). Humic acid (HA, BR) were purchased from Yuanye Biological Technology (Shanghai, China). Sodium hypochlorite solution (available chlorine, HOCl/ClO−/Cl2O, ∼10% w/w) and ascorbic acid were purchased from Aladdin Co., Ltd. (Shanghai, China) Need of special note is the pH was maintained at 5 with 10 mM phosphate buffer expect for the effect of pH in the examined experiments. Therefore, [Cl2] was used to represent available chlorine species in the full text. Methyl tert-butyl ether (MTBE) and acetonitrile (HPLC grade) were obtained from Shanghai Anpu Co., Ltd., China. Sulfacetamide (SFA, 99.5%), concentrated sulfuric acid (H2SO4, 98%), sodium bicarbonate (NaHCO3, AR), formic acid (HCOOH, AR), potassium dihydrogen phosphate (KH2PO4, AR), dipotassium hydrogen phosphate (K2HPO4, AR), sodium hydroxide (NaOH, AR), sodium chloride (NaCl, AR), and sodium bromide (NaBr, AR) were all from Sinopharm Chemical Reagent Co., Ltd., China. All solutions were prepared using ultra-pure water (18.2 MΩ cm) produced by Hetai Co., Ltd. of Shanghai, China.2.2 Experimental procedure
For SFA oxidation by [Cl2], the experiments were conducted using a 150 mL conical flask with 100 mL SFA solution of 1 mg L−1. The experiments were started by adding certain volumes of the [Cl2] stock solution. At fixed points in time, 1 mL of samples was rapidly quenched with excessive ascorbic acid to stop the reaction, and then transferred into a high performance liquid chromatography (HPLC) instrument to perform analyses of the data. The pH of all experiments was maintained with 10 mM phosphate buffer. The temperature of all experiments was maintained by DF-101S constant temperature magnetic stirrer. The effects of [Cl2] (50–125 μM), reaction pH (5–9), reaction temperature (10–40 °C), NH4+ (0–5 mg L−1), HCO3− (0–100 mg L−1), HA (0–15 mg L−1) and Br− (0–65 mg L−1) on SFA degradation were examined. In order to investigate the oxidation of SFA and the formation of HAAs in different types of aquaculture water during chlorination disinfection process, we also prepared water samples with specific Cl− and Br− concentrations at pH = 5 as models of marine culture water (containing 6.6 g L−1 of Cl− and 22 mg L−1 of Br−) and seawater (containing 19 g L−1 of Cl− and 65 mg L−1 of Br−). All the experiments were conducted at least duplicate, and the error bars were provided in the figures.To examine the potential formation of HAAs, 40 mL of chlorinated water sample for 24 h was transferred to a 100 mL centrifuge tube, and then 2 mL of concentrated sulfuric acid was added to make the pH of the solution lower than 0.5, and subsequently 9 g of baked sodium chloride was quickly added to dissolve with shaking. The water samples were then extracted twice with 4 mL MTBE, and each extraction was shaken for 5 min and left to stand for 5 min, ultimately the extracts were combined. Afterwards, 3 mL of the extract was transferred into a 50 mL centrifuge tube, and after adding 3 mL of a newly prepared 10% of sulfuric acid methanol solution, mixed well and placed it into 50 °C water bath for 2 h for derivatization. After the derivative water sample was cooled, 7 mL of 250 g L−1 sodium chloride solution was added, quickly followed shaken up. After leaving the water sample quietly for 5 min, a pipette was used to remove the water phase, and 1 mL of saturated sodium bicarbonate solution was slowly added to the organic layer to exhaust the gas. Finally, 1 mL of the upper organic phase was placed in a 1.5 mL brown sample bottle for GC-ECD analysis.
2.3 Analytical methods
A Shimadzu 20A HPLC equipped with a C18 column (4.6 mm × 150 mm, 5 μm) and a UV detector was used to determine the concentration changes of SFA in the experiments. The mobile phase used was 0.1% formic acid and acetonitrile (Va/Vb = 70/30) at a flow rate of 1.0 mL min−1. The detection wavelength was set at 275 nm, the column temperature was 30 °C, and the injection volume was 20 μL (see HPLC chromatogram of SFA in Fig. S1†). The concentration change of HA was monitored by UV-2600 spectrophotometer of Shimadzu company (Japan), and the analytical wavelength was set at 254 nm.The analytical method to determine HAAs refers to ‘water quality-determination of haloacetic acids-gas chromatography’ (HJ 758-2015), a national environmental protection standard of the People's Republic of China. An Agilent 7890A gas chromatography spectrometer (GC) equipped with a DB-5 column (30 m × 0.32 mm×0.25 μm) and an electron capture detector (ECD) was used to determine the concentrations of HAAs in NaClO disinfection process. The injections were made in the splitless mode using an injection temperature of 210 °C and the injection volume was 1 μL. Nitrogen was used as carrier gas and make-up gas, the flow rate of carrier gas was 2.5 mL min−1. The temperature of ECD detector was 260 °C. The GC column was operated in a temperature programmed mode with an initial temperature of 40 °C held for 5 min, ramp first to 65 °C with a 5 °C min−1 rate, then to 75 °C with 1 °C min−1 rate, then to 135 °C with 5 °C min−1 rate, and then to 280 °C with 20 °C min−1 rate and held at that temperature for 5 min. The external standard method was used for quantitative analyses of halogenated methyl acetate derivatives of the nine HAAs mixed standards after methyl esterification pretreatment. The organic intermediates produced during the SFA degradation were identified by gas chromatography-mass spectrometry (GC-MS, Shimadzu GCMS-QP2010 plus) and the details are shown in Text S1.†
3. Results and discussion
3.1 Chlorination kinetics of SFA
The chlorination of SFA is much complex in the actual disinfection of aquaculture water, but the main reaction process can be roughly described as eqn (1):| [Cl2] + SFA → products | (1) |
The rate equation of reaction (1) should be:
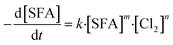 | (2) |
The degradation of SFA at a concentration of 5 μM was examined at initial [Cl2] concentrations of 50, 75, 100, and 125 μM. Fig. S2† and 1 show that the degradation effect of SFA increased with the increase of the concentration of [Cl2], and the degradation of SFA satisfied the pseudo first-order kinetics with R2 > 0.998 (Fig. 1a). In the actual disinfection of aquaculture water, the concentration of disinfectant is usually much more than that of antibiotic.21 In the present study, the initial mole ratio of SFA to [Cl2] was set at lower than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Therefore, the concentration of [Cl2] was far excessive and can be approximated as constant compared to SFA in the whole chlorination process. Therefore, the reaction kinetics between disinfectants [Cl2] and antibiotics SFA are recognized as pseudo first-order model. As such, eqn (2) can be simplified to eqn (3).22
10. Therefore, the concentration of [Cl2] was far excessive and can be approximated as constant compared to SFA in the whole chlorination process. Therefore, the reaction kinetics between disinfectants [Cl2] and antibiotics SFA are recognized as pseudo first-order model. As such, eqn (2) can be simplified to eqn (3).22
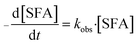 | (3) |
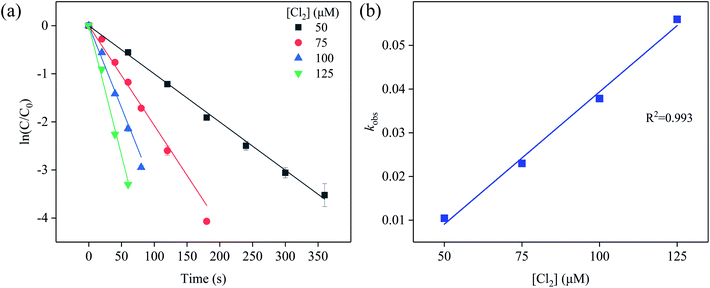 | ||
| Fig. 1 (a) Effect of [Cl2] dose on the degradation of SFA, and (b) the linear relationship between kobs and [Cl2] dose. Experimental conditions: [SFA]0 = 5 μM, pHini = 5, T = 20 °C. | ||
We can also observe that at pH 5 and temperature of 20 °C, as the concentration of chlorine disinfectant increased, the reaction rate constant kobs were 0.01044, 0.02298, 0.03783, and 0.05596 s−1, respectively, and there was a linear relationship between kobs and [Cl2] dose (R2 = 0.993) (Fig. 1b). Hence, it can be noted that the concentration of [Cl2] was an important factor for accelerating reaction kinetics.
The degradation of SFA in NaClO disinfection system at pH 5–9 was presented in Fig. S3† and 2, and the results of the experiment showed that the reaction rate of SFA was the fastest under neutral condition, and slowed down under weak acid or weak base condition. The observed pseudo first-order kinetic constants of SFA degradation first increased from 0.01044 to 0.03003 s−1 with the pH extended from 5 to 7, and then decreased to 0.00273 s−1 when pH further reached to 9. The macroscopic Ka1 and Ka2 constants of SFA (Fig. S4†) are related to amino and amide groups, respectively. The pKa1 and pKa2 of SFA were 1.76 and 5.22 (Table S1†),23 and the pKa of HOCl was 7.54,24 indicating both SFA and some available chlorine species existed in molecular state (i.e. SFA and HOCl) at pH 5, respectively. Although HOCl had strong oxidizability under acidic conditions, the molecular state of SFA had relatively weaker ability to provide electrons for molecular HOCl. When the pH value was in the range from 5.22 to 7.49, SFA was in the form of deprotonated state, and HOCl existed in the molecular state, thus, the deprotonated amide group of SFA can provide electrons more easily to HOCl with higher oxidizability. Furthermore, trace Cl2O, potent chlorinating agent at pH ≲ 8 dependent on the existence of HOCl (eqn (4)), is more potent electrophiles than HOCl/ClO−.25 As such, the electrophilic action to SFA by slight Cl2O can explain the effective removal of SFA and the increased reaction rate under neutral conditions. In alkaline conditions (pH > 7.49), SFA existed in deprotonic state and the available chlorine species mainly existed in the form of ClO−, however, the oxidative capbility and eletrophilicity of ClO− was much weaker than that of HOCl and Cl2O,24,25 which led to the slower degradation of SFA.
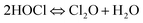 | (4) |
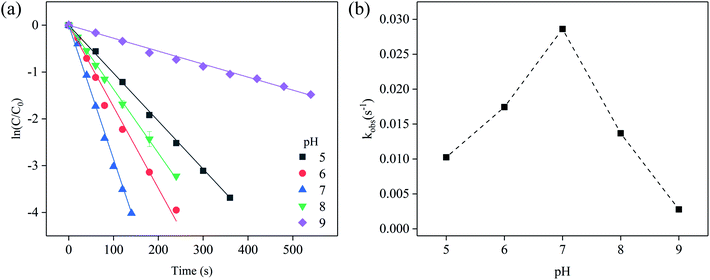 | ||
| Fig. 2 (a) Oxidation of SFA by [Cl2], and (b) the variation of kobs at different pH values. Experimental conditions: [SFA]0 = 5 μM, [Cl2]0 = 50 μM, T = 20 °C. | ||
The temperature had a great influence on the degradation rate of SFA, and the degradation of SFA at high temperature was faster than that at low temperature (Fig. S5†). Fig. 3a shows the pseudo first order kinetic constants of SFA increased from 0.00507 to 0.01994 s−1 with the temperature elevated from 10 to 40 °C. The effect of temperature on chemical reaction rate can also be expressed by Arrhenius equation (eqn (5)).26
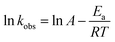 | (5) |
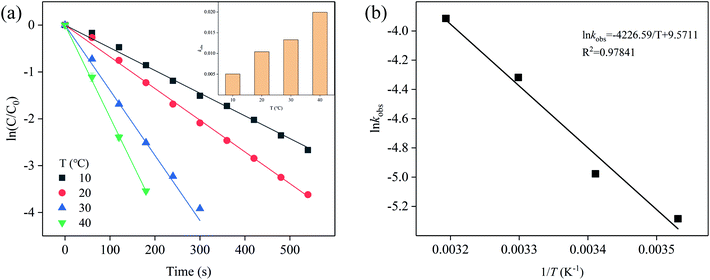 | ||
| Fig. 3 (a) Effect of temperature, and (b) Arrhenius plot. Experimental conditions: [SFA]0 = 5 μM, [Cl2]0 = 50 μM, pHini = 5. | ||
According to Fig. 3b, the calculated observed activation energy was 35.14 kJ mol−1. Zhang et al.15 reported that the observed activation energies of the reactions of NOR and roxithromycin (ROX) with [Cl2] were 105.00 and 79.34 kJ mol−1, respectively. In contrast, the obtained observed activation energy of [Cl2] to oxidize SFA is much lower, indicating the reaction rate is faster under the same experimental conditions, and the reaction between SFA and [Cl2] is easier to occur from the perspective of activation energy as well.
NH4+ and HCO3− are common cation and anion in aquaculture water.27 The presence of NH4+ can inhibit the oxidation of SFA by [Cl2] (Fig. S6a† and 4a). With the concentration of NH4+ increased from 0 to 5 mg L−1, the kobs decreased from 0.01044 to 0.00076 s−1. When the concentration of NH4+ reached 5 mg L−1, only less than 40% of SFA was degraded within 10 min (Fig. S6a†). These phenomena might be attributed that HOCl could react with NH4+ to form NH2Cl, NHCl2 and NCl3 (eqn (6)–(9)),28,29 whose oxidation abilities were weaker than that of HOCl,30 leading to the decreased oxidation of SFA (Fig. S6a†).
| NH4+ + H2O → NH3 + H3O+ | (6) |
| HOCl + NH3 → NH2Cl + H2O | (7) |
| HOCl + NH2Cl → NHCl2 + H2O | (8) |
| HOCl + NHCl2 → NCl3 + H2O | (9) |
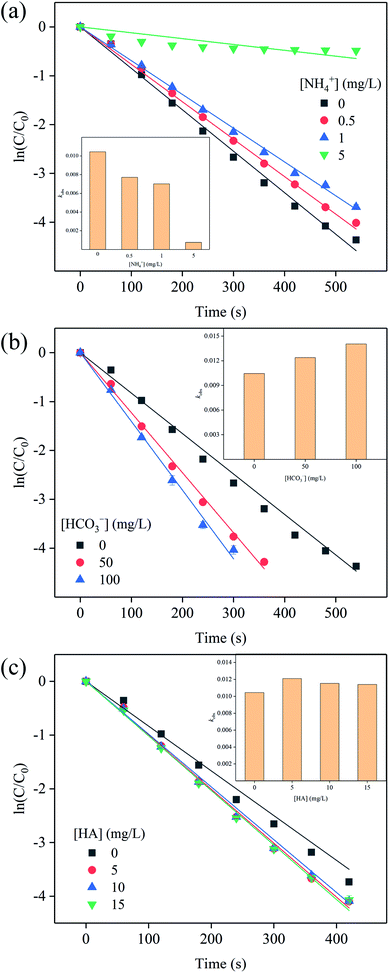 | ||
| Fig. 4 Effects of concentrations of (a) NH4+, (b) HCO3−, and (c) HA on the oxidation of SFA by [Cl2]. Experimental conditions: [SFA]0 = 5 μM, [Cl2]0 = 50 μM, pHini = 5, T = 20 °C. | ||
Fig. S6b† and 4b showed the HCO3− had little effect on SFA degradation. With the concentration of HCO3− increased from 0 to 100 mg L−1, kobs varied much more slightly and negligibly, i.e. only from 0.01044 to 0.01403 s−1. This phenomenon indicated that HCO3− had neglected influence on the transformation of SFA.
Humic acid (HA) is the main component of natural organic matter, which accounts for 50–90%.31,32 HA is commonly found in natural water bodies, and the concentration in water bodies ranges from 0 to 30 mg L−1.33 Therefore, the effects of HA on the degradation of SFA by [Cl2] were also evaluated. Fig. S6c† and 4c showed the negligible effect of altered concentrations of HA on the oxidation of SFA by [Cl2]. When the concentration of HA increased from 0 to 15 mg L−1, the changes of kobs were ignorable (Fig. 4c). At the same time, we measured the concentration changes of HA during the reaction. The results showed that HA hardly degraded in 420 s. [Cl2] with relatively low redox potential (e.g. EHOCl = 740 mV), has limited oxidation capacity to degrade HA in the examined 420 s.34
3.2 Influence of different water bodies
According to the characteristics of water quality, there are not only freshwater aquaculture models, but also mariculture models. The intricacy of chemical characteristics and features of chlorination in seawater greatly differ from those of freshwater, as a result of the oxidation of bromide (∼65 mg L−1) present in seawater, thereby resulting in HOBr as the dominant oxidant species, unlike HOCl in freshwater.35 Fig. S7a† and 5a showed the effects of different water bodies on the degradation of SFA by [Cl2]. Most of SFA was degraded in 100 s in marine culture water and seawater. However, in freshwater, it took about 550 s to be degraded completely. The degradation rate of SFA in various media was decreased in the order of seawater > marine culture water > freshwater, i.e. the kobs of them were in the sequence of 0.02854 s−1 > 0.01762 s−1 > 0.01044 s−1 through the fitting of the first-order kinetic model (eqn (3)). Considering that the reaction rate constant is likely to depend on the change of the concentration of Br−, therefore, the concentration of Cl− was fixed at 6.6 g L−1, and the Br− concentration effect was examined. The results in Fig. S7b† and 5b illustrated that the degradation of SFA increased with the Br− concentration amplified from 0 to 65 mg L−1, and the reaction rate constant increased from 0.04027 to 0.11078 s−1. This phenomenon indicated that the presence of Br− accelerated the reaction rate. The oxidation rate of Br− by HOCl to form HOBr was much higher than that of organic compounds with HOCl, thus generating a large amount of HOBr with the characteristic of higher electrophilicity, and subsequently, the amino and other electron donating groups in SFA react more easily and rapidly with HOBr, leading to promoted degradation of SFA (eqn (10) and (11)).36–38| HOCl + Br− → HOBr + Cl− | (10) |
| HOBr + SFA → products | (11) |
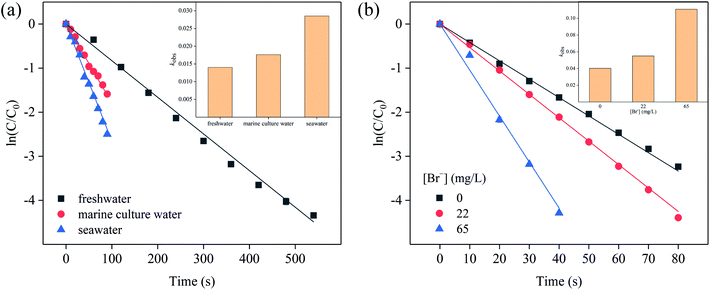 | ||
| Fig. 5 Effects of (a) different water bodies and (b) Br− concentration on SFA degradation. Experimental conditions: [SFA]0 = 5 μM, [Cl2]0 = 50 μM, pHini = 5, T = 20 °C. | ||
3.3 Potential formation of HAAs
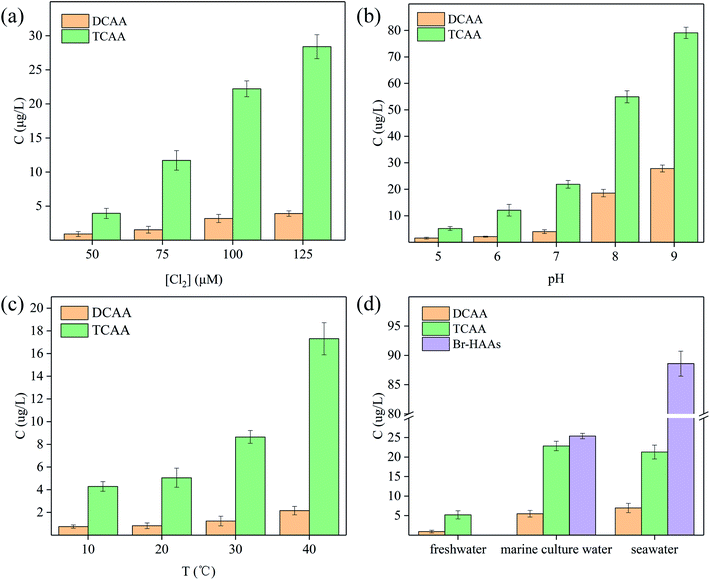
The formation potential of HAAs during the chlorination of SFA in the simulated aquaculture water was examined. It was found that two main kinds of HAAs, i.e. DCAA and TCAA, were produced in the chlorination of SFA in the fresh aquaculture water (Fig. 6), which bore a resemblance to the chlorination in drinking water.39 With the increase of chlorine disinfectant, the generation of DCAA and TCAA gradually increased, and the total concentration of HAAs increased from 5.11 to 31.33 μg L−1 (Fig. 6a), indicating that the dosage of chlorine disinfectants was a key parameter affecting Cl-DBPs production. When the dosage of [Cl2] is insufficient, the organic pollutants and some bacteria cannot be completely eliminated, further influencing the ideal disinfection effect. When the chlorine disinfectants dosage is immoderate, the yields of Cl-DBPs will increase, which in turn poses threatening risk for fishery. Therefore, in the actual disinfection process, the chlorine ratio should be strictly controlled to ensure the disinfection effect and the safety of aquaculture water.21As can be seen from Fig. 6b, the formation of DCAA and TCAA increased with the pH rise, the reason for which might be alkaline pH promoted the reaction equilibrium movement towards the direction for formation of chloroacetic salts, further improving the generation of HAAs. In addition, the temperature also has a positive influence on the yield of HAAs (Fig. 6c). During the chlorination process, the concentrations of DCAA and TCAA gradually increased, and the total concentration of HAAs enlarged from 4.63 to 18.92 μg L−1, as the temperature increased from 10 to 40 °C. The degradation kinetics of SFA indicated the obtained lower observed activation energy encouraged the easily occurred reaction between SFA and [Cl2], possibly supporting the susceptive formation of HAAs.
Fig. 6d showed the effect of different types of aquaculture waters on the generation potential of HAAs. The production of DCAA and TCAA in marine culture water and seawater were much more than that in freshwater. Furthermore, the Br-HAAs, such as BCAA, BDCAA, DBAA, CDBAA and TBAA, can be detected in both marine culture water and seawater (Fig. 6d and S8†), which might attribute to high concentration Br− participating in the generation of higher oxidative HOBr (eqn (9)), further accelerating the formation of Br-HAAs (Fig. S8†).40
3.4 Proposed reaction pathway of SFA during the chlorination process
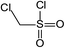
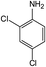
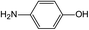
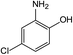
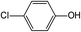
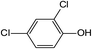
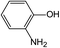
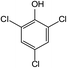
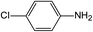
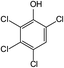
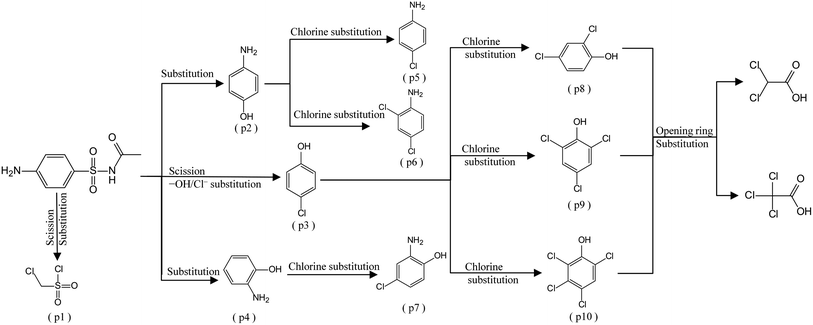
In addition to the maternal SFA and the final two DBPs (DCAA, TCAA), we also detected the intermediate products of SFA in NaClO disinfection system by GC-MS. Based on the mass spectrum data, several main transformation products were detected, and listed in Table 1, Fig. S9–S20.† Based on the reasonable analysis of the experimental results,20,41–44 the degradation route of SFA and potential formation pathway of HAAs were proposed in Fig. 7.The transformation process of SFA in NaClO disinfection system can be divided into three steps: (1) with the oxidation of [Cl2], the C–S bond between sulfonyl group and benzene ring and S–N bond between sulfonyl and acylamino groups of SFA were scissored, and then the intermediate sulfonyl group formed p1(chloromethanesulfonyl chloride), and the rest intermediate aminobenzene were oxidized to produce p2 (4-hydroxyaniline), p3 (4-chlorophenol) and p4 (2-hydroxyaniline).20,43 (2) Due to the chlorination mechanism in NaClO system, the intermediate products p2 and p4 were substituted by chlorine to produce p5 (4-chloroaniline), p6 (2,4-dichloroaniline) and p7 (2-amino-4-chlorophenol). The intermediate product p3 was substituted by chlorine to form polychlorophenols (p8–p10).44 (3) The chlorophenols could be cleaved under the action of [Cl2], and then fully combined with Cl in the subsequent chlorination process to produce HAAs and other products.12,41,42
4. Conclusions
This study demonstrated that the reaction of SFA with [Cl2] followed pseudo first-order kinetic model. The results showed that chlorine disinfectant dosage and temperature had positive effects on the degradation of SFA and Cl-HAAs formation; and neutral pH favored the degradation of SFA, but alkaline pH was more beneficial to generate Cl-HAAs. The common NH4+ cation addition inhibited removal of SFA; in comparison, HCO3− and HA had a negligible effect on the degradation of SFA. The presence of Br− not only accelerated the degradation rate of SFA, but also led to the production of Br-HAAs. In the case of excess [Cl2] ([Cl2]/[SFA] > 10), C–S and S–N bonds around sulfonyl group of SFA were scissored, and then the primary formed groups were further oxidized to produce intermediate products, such as chloroanilines and chlorophenols. Then the chlorophenols were further chlorinated to form HAAs with toxicity.Author contributions
Yaoguang Guo: investigation, visualization, writing original draft, and funding acquisition. Zhiyuan Liu: methodology, investigation, and writing original draft. Xiaoyi Lou: funding acquisition, writing – review & editing, supervision. Changling Fang: software, and formal analysis. Pu Wang: methodology, and investigation. Genying Wu: methodology. Jie Guan: resources, funding acquisition, and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present work received financial support from Shanghai Sailing Program (18YF1429900 and 19YF1459900), Shanghai Natural Science Foundation (20ZR1421100), Natural Science Foundation of China (52070127), the Central-Public interest Scientific Institution Basal Research Fund (2019T13, 2019T14), and postgraduate Program Fund of Shanghai Polytechnic University (EGD19YJ0079). Dr Y. Guo also appreciates the Cultivation Discipline Fund of Shanghai Polytechnic University (XXKPY1601), the Project of Key Undergraduate Courses (Instrumental Analysis) from Shanghai Municipal Education Commission.References
- X. B. Bai, Z. T. Fu, N. Li, S. Stankovski, X. S. Zhang and X. X. Li, J. Clean. Prod., 2021, 288, 125633 CrossRef CAS.
- M. Ramesh, T. Thilagavathi, R. Rathika and R. K. Poopal, Antioxidant status, Aquaculture, 2018, 491, 10–19 CrossRef CAS.
- X. Liu, J. C. Steele and X. Z. Meng, Environ. Pollut., 2017, 223, 161–169 CrossRef CAS PubMed.
- L. Santos and F. Ramos, Trends Food Sci. Technol., 2016, 52, 16–30 CrossRef CAS.
- R. C. Okocha, I. O. Olatoye and O. B. Adedeji, Publ. Health Rev., 2018, 39, 1–22 Search PubMed.
- N. Hamid, M. Junaid and D. S. Pei, J. Hazard. Mater., 2020, 382, 121106 CrossRef CAS PubMed.
- M. Hajji, H. Jebali, A. Mrad, Y. Blel, N. Brahmi, R. Kheder, S. Beji, L. B. Fatma, W. Smaoui, M. Krid, F. B. Hmida, L. Rais and M. K. Zouaghi, Drug Safety – Case Reports, 2018, vol. 5 Search PubMed.
- B. H. Ali, Pharmacological, Vet. Res. Commun., 1999, 23, 343–360 CrossRef CAS PubMed.
- Z. H. Pan, Y. J. Zhu, M. Wei, Y. Y. Zhang and K. K. Yu, J. Environ. Sci., 2021, 102, 170–184 CrossRef PubMed.
- H. Sanawar, Y. H. Xiong, A. Alam, J. P. Croué and P. Y. Honga, Aquaculture, 2017, 480, 94–102 CrossRef CAS.
- H. C. Hong, F. Q. Huang, F. Y. Wang, L. X. Ding, H. J. Lin and Y. Liang, J. Hydrol., 2013, 476, 274–279 CrossRef CAS.
- H. C. Hong, Y. J. Xiong, M. Y. Ruan, F. L. Liao, H. J. Lin and Y. Liang, Sci. Total Environ., 2013, 444, 196–204 CrossRef CAS PubMed.
- R. K. Padhi, S. Subramanian, A. K. Mohanty, S. N. Bramha, M. V. R. Prasad and K. K. Satpathy, Environ. Eng. Res., 2021, 17, 57–62 Search PubMed.
- R. K. Padhi, S. Subramanian, A. K. Mohanty and K. K. Satpathy, Environ. Monit. Assess., 2019, 191, 1–13 CrossRef CAS PubMed.
- Y. Y. Zhang, C. Rong, Y. Q. Song, Y. H. Wang, J. Y. Pei, X. Y. Tang, R. J. Zhang and K. F. Yu, Chemosphere, 2017, 182, 245–254 CrossRef CAS PubMed.
- A. Marsà, C. Cortés, A. Hernández and R. Marcos, Toxicol. Appl. Pharmacol., 2018, 347, 70–78 CrossRef PubMed.
- T. Elisabet, P. Esther, G. L. Javier, J. M. Llobet and G. C. Jesús, J. Water Health, 2015, 13, 54–66 CrossRef PubMed.
- Y. Huang, H. Li, Q. Zhou, A. Li, C. Shuang, Q. Xian, B. Xu and Y. Pan, Water Res., 2018, 146, 298–306 CrossRef CAS PubMed.
- F. L. Dong, C. Li, J. Crittenden, T. Q. Zhang, Q. F. Lin, G. L. He, W. Q. Zhang and J. M. Luo, J. Hazard. Mater., 2019, 366, 88–97 CrossRef CAS PubMed.
- V. d. J. Gaffney, V. V. Cardoso, M. J. Benoliel and C. M. M. Almeida, J. Environ. Manage., 2016, 166, 466–477 CrossRef CAS PubMed.
- S. Q. Zhou, Y. S. Shao, N. Y. Gao, S. M. Zhu, Y. Ma and J. Deng, Ecotoxicol. Environ. Saf., 2014, 107, 30–35 CrossRef CAS PubMed.
- P. Wang, Y. L. He and C. H. Huang, Water Res., 2011, 45, 1838–1846 CrossRef CAS PubMed.
- M. J. B. Mengelers, P. E. Hougee, L. H. M. Janssen and A. S. J. P. A. M. V. Miert, J. Vet. Pharmacol. Therapeut., 1997, 20, 276–283 CrossRef CAS PubMed.
- B. Li and T. Zhang, Water Res., 2012, 46, 3703–3713 CrossRef CAS PubMed.
- M. R. Rose, S. S. Lau, C. Prasse and J. D. Sivey, Environ. Sci. Technol. Lett., 2020, 7, 360–370 CrossRef CAS.
- S. Norzaee, M. Taghavi, B. Djahed and F. K. Mostafapour, J. Environ. Manage., 2018, 21, 316–323 CrossRef PubMed.
- L. J. Binkowski and B. Rzonca, Water, Air, Soil Pollut., 2014, 225, 2217 CrossRef PubMed.
- Y. J. Zhu, M. Wei, Z. H. Pan, L. Y. Li, J. Y. Liang, K. F. Yu and Y. Y. Zhang, Sci. Total Environ., 2020, 705, 135960 CrossRef CAS PubMed.
- F. X. Tian, W. K. Ye, B. Xu, X. J. Hu, S. X. Ma, F. Lai, Y. Q. Gao, H. B. Xing, W. H. Xia and B. Wang, Chem. Eng. J., 2020, 398, 125570 CrossRef CAS PubMed.
- L. Jing and B. Ernest, Environ. Sci. Technol., 2009, 43, 60–65 CrossRef PubMed.
- M. R. Yazdani, N. Duimovich, A. Tiraferri, P. Laurell, M. Borghei, J. B. Zimmerman and R. Vahalac, J. Mol. Liq., 2019, 291, 104353 CrossRef.
- X. X. Cheng, H. Liang, A. Ding, F. S. Qu, S. L. Shao, B. Liu, H. Wang, D. J. Wu and G. B. Li, J. Membr. Sci., 2016, 505, 15–25 CrossRef CAS.
- X. Wang, B. Qiong, H. L. Zhai, M. Y. Xiong and Y. Liu, Food Chem., 2016, 190, 1033–1039 CrossRef CAS PubMed.
- X. F. Wang, B. H. Zhou and X. Shao, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2019, 54, 528–535 CrossRef CAS PubMed.
- R. K. Padhi, S. Subramanian and K. K. Satpathy, Chemosphere, 2019, 218, 540–550 CrossRef CAS PubMed.
- Y. Du, X. T. Lv, Q. Y. Wu, D. Y. Zhang, Y. T. Zhou, L. Peng and H. Y. Hu, J. Environ. Sci., 2017, 58, 51–63 CrossRef CAS PubMed.
- J. Criquet, E. M. Rodriguez, S. Allard, S. Wellauer, E. Salhi, C. A. Joll and U. V. Gunten, Water Res., 2015, 85, 476–486 CrossRef CAS PubMed.
- H. Y. Dong, Z. M. Qiang, X. J. Yuan and A. J. Luo, Sep. Purif. Technol., 2018, 193, 415–420 CrossRef CAS.
- Z. L. Chen, A. Li, S. J. Wang, Y. Han, F. Liu and J. M. Shen, Adv. Mater. Res., 2014, 250–253, 3425–3428 Search PubMed.
- J. Wang, Z. N. Hao, F. Q. Shi, Y. G. Yin, D. Cao, Z. W. Yao and J. F. Liu, Environ. Sci. Technol., 2018, 5, 5662–5670 CrossRef PubMed.
- T. Y. Zhang, B. Xu, S. J. Yao, Y. R. Hu, K. F. Lin, H. Ye and C. Z. Cui, Water Res., 2019, 160, 188–196 CrossRef CAS PubMed.
- V. d. J. Gaffney, V. V. Cardoso, M. J. Benoliel and C. M. M. Almeida, J. Environ. Manage., 2016, 166, 466–477 CrossRef CAS PubMed.
- R. Nassar, A. Rifai, A. Trivella, P. Mazellier, S. Mokh and M. A. Iskandarani, J. Mass Spectrom., 2018, 53, 614–623 CrossRef CAS PubMed.
- W. H. Gan, V. K. Sharma, X. Zhang, L. Yang and X. Yang, J. Hazard. Mater., 2015, 292, 197–204 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01605a |
| This journal is © The Royal Society of Chemistry 2021 |