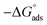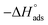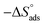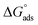DOI:
10.1039/D1RA01405F
(Paper)
RSC Adv., 2021,
11, 13497-13512
Retracted Article: Experimental and surface morphological studies of corrosion inhibition on carbon steel in HCl solution using some new hydrazide derivatives
Received
21st February 2021
, Accepted 23rd March 2021
First published on 12th April 2021
Abstract
The corrosion inhibition of C-steel in 1 M HCl was assessed using three newly synthesized hydrazide derivatives (H1, H2 and H3) using weight loss (WL), potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS) techniques. Also, the adsorption of these compounds was confirmed using several techniques such as atomic force microscopy (AFM), Fourier-transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS). High inhibition efficiencies were obtained resulting from the constitution of the protective layer on the C-steel surface, which increased with increasing concentration and temperature and reached 91.7 to 96.5% as obtained from the chemical method at 20 × 10−6 M at 45 °C. The polarization curves refer to these derivatives belonging to mixed-type inhibitors. The adsorption of (H1, H2 and H3)on the CS surface follows the Temkin adsorption isotherm. Inhibition influence of hydrazide derivatives at the molecular level was greatly proven using quantum chemical calculations and Monte Carlo simulation methods. Furthermore, the molecular simulation results evidenced the adsorption of these derivatives on the carbon steel surface.
1 Introduction
Corrosion is the natural destruction of a material due to reaction with its surroundings. It is a natural, slow, continuous, and spontaneous process. It cannot be avoided, but it can be mitigated by several techniques such as reducing the aggressiveness of media, using inhibitors, cathodic, anodic protection and applying preventive coatings.1,2 Several processes place C-steel adjacent to corrosive media, such as chemical plants, petroleum refineries and power plants. During the rust removal from the C-steel surface process, as an example, C-steel material needs to be immersed in an acidic solution such as hydrochloric acid (HCl), which cleans the surface from rust and other scales, this process is known as acid pickling. However, after rust removal, HCl continues to attack the original metal and dissolves it, hence a corrosion inhibitor is needed to stop or mitigate the acid influence on the C-steel surface.3 The inhibitor molecules cover the C-steel surface and prevent acid from further metal dissolution. The adsorption of inhibitors occurs by two mechanisms; first is physisorption, which results from electrostatic interactions between opposite charges on the metal surface and inhibitor molecules, and the second type is chemisorption, which demands charge sharing or charge transfer from the inhibitor molecules to the C-steel surface to form a coordinated bond.4–6 Indeed, there is a huge necessity to prospect new corrosion inhibitors with low cost and high corrosion inhibition efficiency to cover several industries and applications. Heterocyclic corrosion inhibitors have elucidated their effectiveness in the previous years in protecting metal surfaces from oxidation by covering the surface through a consistent protective film, which prevents or decreases the rate of metal dissolution.7,8 Heterocyclic compounds, which contain electron-donating atoms such as N, O and S atoms or donating group such as (CH3, NH2) and (OH) are considered excellent corrosion inhibitors because they can fill the unoccupied orbitals of metal as iron with the required electrons, hence they can return the metal to its steady-state and inhibit its oxidation.9 Low electronegativity and high polarization tendency of those compounds allow them to cover the metal surface efficiently, such that electrons can transfer easily to the unoccupied orbitals of the metal surface.10 It was agreed that the nitrogen-containing compounds are effective inhibitors for metals in HCl medium.11 The inhibition efficiency of any compounds bearing various heteroatoms follows the reverse order of their electronegativities.12 Hydrazide derivatives are a kind of organic compound having a nitrogen–nitrogen covalent bond with one of the substituents being an acyl group. The hydrazide derivatives have various applications in medicine and engineering. Their effectiveness as anticancer, antibacterial, anti-inflammatory, analgesic and antioxidant factors has been helucidated.13 Furthermore, they are also used as effective inhibitors for the mitigation of corrosion in various metals such as C-steel. Many scientists such as Fouda et al. and Pradeep et al. have studied various hydrazide derivatives and proved their effective corrosion inhibition efficacy, some of the investigated compounds followed Temkin isotherm and the others followed Langmuir isotherm.14–16
The objective of this research is to examine the effectiveness of three newly synthesized hydrazide derivatives as C-steel corrosion inhibitors by introducing various electron-donating atoms such as N and O atoms or donating groups such as (CH3) and (OH) in these compounds. The investigated compounds are similar in the structure in the hydrazide nucleus but differ in the substituents that affect the compound's inhibition abilities. The corrosion inhibition of the investigated compounds was further deduced using quantum chemical calculations and Monte Carol simulation techniques.
2 Materials and methods
2.1 Preparation of inhibitors
The investigated compounds were synthesized as described before17 and are listed in Table 1. All the structures of the prepared compounds were elucidated by elemental analysis, IR, 1H-NMR 13C-NMR, and mass spectral data.
Table 1 Nomenclature, structures, molecular formulas, and molecular weights of the investigated compounds
| Code |
Name |
Structure |
Mol. Formula |
M.Wt. |
| H1 |
8-Hydroxy-N′-((2-methoxynaphthalen-1-yl)methylene)-2,3-dimethyl-8H-pyrimido[1,2-b][1,2,4]triazine-7-hydrazide |
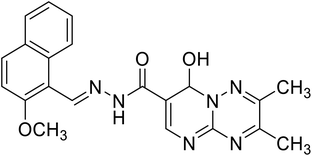 |
C21H20N6O3 |
404.42 |
| H2 |
N′-((2-Methoxynaphthalen-1-yl)methylene)-8-oxo-4a,5-dihydro-8H-pyrimido[1,2-b][1,2,4]triazine-7-hydrazide |
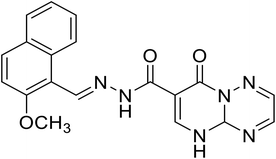 |
C19H16N6O3 |
376.36 |
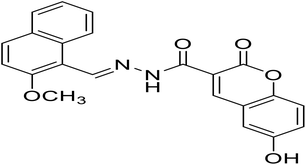
| H3 |
6-Hydroxy-N′-((2-methoxynaphthalen-1-yl)methylene)-2-oxo-2H-chromene-3-hydrazide |
 |
C22H16N2O5 |
388.37 |
Typically, 0.0001 gm of each inhibitor was dissolved in 3 ml of dimethylformamide (DMF) then the solution was added 99.5% absolute ethyl alcohol to make up 100 ml of the stock solution. The applied corrosive medium was 1 M HCl. Sodium carbonate was used to titrate HCl using methyl orange as an indicator.
2.2 Carbon steel (C-steel) sheets
All the experiments were performed using C-steel sheets with dimensions of 1 × 1 × 0.2 cm approximately. C-steel was mainly composed of iron with traces of some different elements (weight %: 0.06% Si, 0.001% Ti, 0.022% P, 0.08% C 0.010% S, 0.030% Al, 0.3% Mn).18 The working electrode for electrochemical experiments was made of carbon steel piece (area of 0.5 cm2) attached to a bar of copper and covered with glass. The auxiliary anode was a platinum sheet (1 cm2), the reference electrode was the saturated calomel electrode (SCE).
2.3 Methods used for corrosion calculations
2.3.1 Weight loss (WL) method. WL measurements were performed using carbon steel sheets measuring 1 × 1 × 0.2 cm. These sheets were first abraded carefully with (400, 600, 800 and 1200 grit size) coarseness emery sheet, washed with bidistilled water and dried before being weighed and dipped in 100 ml glass beakers of 1 M HCl + gradual concentrations of (H1, H2 and H3). Glass beakers were put in a water bath with an automatic thermostat to achieve experiments with different temperatures; all experiments were performed in air atmosphere. Sheets were removed from beakers every 30 minutes for 3 hours, washed with distilled water, dehydrated, and weighed exactly. WL was estimated for each period, the degree of surface coverage (θ) and % IE of H1, H2 and H3 inhibition was determined from eqn (1):| | |
% IE = θ × 100 = [(W° − W)/W°] × 100
| (1) |
where W and W° are the values of the WLs in the presence and absence of the inhibitors, respectively.
2.3.2 Electrochemical measurements.
2.3.2.1 Potentiodynamic polarization (PDP) method. The PDP experiments were performed in a cell of three electrodes, the working electrode (WE) of a C-steel sheet was attached to a copper bar and covered with glass, saturated calomel electrode (SCE) was used as a reference electrode, and a platinum plate was used as a counter electrode (CE). 100 ml beakers containing 1 M HCl, without and with the addition of several concentrations of inhibitors were investigated in each run. The work was carried out using a potentiostat/galvanostat/ZRA (Gamry reference 3000), the experiment was adjusted by a computer provided with electrochemical software. The WE was adjusted for at least 30 min to achieve the equilibrium before measurements. The polarization diagrams were performed by sweeping the electrode potential in the interval from −500 to +500 mV versus open circuit potential under 1 mV s−1 sweep rate and under air atmosphere. To calculate the corrosion current densities, the cathodic branch of the Tafel curve was extrapolated to the corrosion potential, Ecorr. The experiments were completed with gradual concentrations of inhibitors at 25 °C. The level of (θ) and % IE were determined from eqn (2):| | |
% IE = θ × 100 = [(Icorr. − Icorr.(inh.)/Icorr.] × 100
| (2) |
where Icorr.(inh.) and Icorr. are the inhibited and uninhibited corrosion current density, respectively.
2.3.2.2 Electrochemical impedance spectroscopy (EIS) method. The experimental EIS was estimated after OCP measurement using the equivalent circuit. The EIS measurements were carried out between 1 Hz to 100 kHz frequency, the perturbation was conducted with a signal amplitude of 10 mV. Essential parameters deduced from the Nyquist graph (that is produced from the computer program-Gamry) are impedance Rp and the double layer capacitance Cdl,19 Bode diagrams were also drawn. % IE was obtained from eqn (3):| |
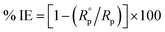 | (3) |
where 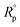 and Rp are the polarization resistances without and with the inhibitor, respectively,where fmax is the angular frequency; Cdl is the double-layer capacitance.
and Rp are the polarization resistances without and with the inhibitor, respectively,where fmax is the angular frequency; Cdl is the double-layer capacitance.
2.3.3 Atomic force microscopy (AFM) analysis. AFM is a device generating a topographic surface map with a unique resolution, so the outer surface roughness can be measured; the surface roughness is caused due to deviations of a surface from its ideal shape due to corrosion or inhibitor adsorption.
2.3.4 Fourier-transform infrared spectroscopy (FTIR) analysis. FT-IR spectra were measured using a PerkinElmer 1600 spectrophotometer on pure solutions of (H1, H2 and H3) and those in which carbon steel sheets were immersed in 1 M HCl + the optimal concentration of each inhibitor for 24 hours.
2.3.5 X-ray photoelectron spectroscopy (XPS) examination. These tests were performed using a highly efficient device that determines the binding energies of different bonds found on the carbon steel surface using (XPS), hence the adsorbed atoms and functional groups on the metal surface could be deduced. The XPS test was performed using K-ALPHA (Thermo Fisher Scientific, USA).
2.3.6 Quantum chemical calculations. The calculations were performed using methods from Material studio Dmol6 DFT software, GGA basis Set RPBE. Parameters (global and local indicators) were calculated as (ELUMO), (EHOMO), (ΔE), (χ), (η), (ΔN), and (μ). The following equations were used in the calculations:20| | |
Pi = (ELUMO − EHOMO)/2
| (8) |
where φ is the work function of the metal surface (φFe = 4.81 eV).
2.3.7 Monte Carlo simulation. The interaction between the three investigated hydrazide derivatives and the carbon steel surface (Fe) was studied using Monte Carlo simulations. To interpret the more adapted metallic surfaces for the simulations process, the Monte Carlo simulation method was used on Materials Studio 2017. The hydrazide derivatives adsorption energy (Eads) on the metal surface was estimated from the following equation.
| Eads = ET − (Esurf + Eads) |
where, ET is the total energy of all systems, Esurf is the metal surface energy, and Eads is the energy of one of the three derivative compounds adsorbed on the CS surface.
3 Results and discussions
3.1 Weight loss (WL) tests
WL tests were conducted for H1, H2 and H3 compounds and corrosion rates of carbon steel were estimated. Fig. 1 illustrates a change of WL in the absence and presence of gradual concentrations of H1 at 25°, for example. The curves for H2 and H3 are not shown. The (θ) and % IE are illustrated in Table 2. As shown from the table, the % IE rises with increasing hydrazide derivative concentrations, which means that more hydrazide derivative molecules were adsorbed on the surface, which increases the surface coverage. For example, the ideal concentration needed to provide % IE of 93.1% was seen at 20 × 10−6 M for the H1 inhibitor.
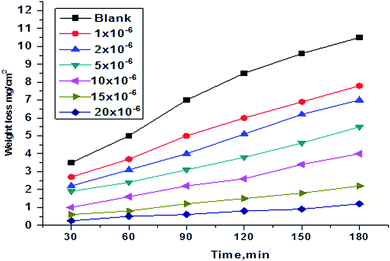 |
| | Fig. 1 WL vs. time curves for the corrosion of C-steel in 1.0 M HCl in the absence and presence of various concentrations of compound (H1) at 25 °C. | |
Table 2 WL tests for H1, H2 and H3 at 25 °C [corrosion rate (C.R.), surface coverage (θ) and percentage of inhibition (% IE)]
| Comp. |
Conc., M |
C.R., mg cm−2 min−1 |
θ |
% IE |
| Blank |
0.082 ± 0.0017 |
| H1 |
1 × 10−6 |
0.062 ± 0.00230 |
0.242 |
24.2 |
| 2 × 10−6 |
0.055 ± 0.00260 |
0.325 |
32.5 |
| 5 × 10−6 |
0.037 ± 0.00288 |
0.554 |
55.4 |
| 10 × 10−6 |
0.030 ± 0.00202 |
0.633 |
63.3 |
| 15 × 10−6 |
0.017 ± 0.00233 |
0.797 |
79.7 |
| 20 × 10−6 |
0.006 ± 0.000281 |
0.931 |
93.1 |
| H2 |
1 × 10−6 |
0.048 ± 0.002309 |
0.415 |
41.5 |
| 2 × 10−6 |
0.039 ± 0.002027 |
0.526 |
52.6 |
| 5 × 10−6 |
0.033 ± 0.002333 |
0.592 |
59.2 |
| 10 × 10−6 |
0.028 ± 0.001763 |
0.653 |
65.3 |
| 15 × 10−6 |
0.020 ± 0.001452 |
0.755 |
75.5 |
| 20 × 10−6 |
0.012 ± 0.002886 |
0.852 |
85.2 |
| H3 |
1 × 10−6 |
0.058 ± 0.002027 |
0.287 |
28.7 |
| 2 × 10−6 |
0.039 ± 0.00233 |
0.522 |
52.2 |
| 5 × 10−6 |
0.034 ± 0.002309 |
0.581 |
58.1 |
| 10 × 10−6 |
0.031 ± 0.001732 |
0.623 |
62.3 |
| 15 × 10−6 |
0.017 ± 0.002309 |
0.787 |
78.7 |
| 20 × 10−6 |
0.004 ± 0.000176 |
0.946 |
94.6 |
As shown in Fig. 1, the curves in inhibitors' presence were found to lie below those recorded in their absence. Also, the curves are approximately straight lines due to the absence of oxide film or any corrosion product on the steel surface.
3.1.1 Adsorption isotherms. H1, H2 and H3 inhibitors have shown protection from oxidation of carbon steel in the corrosive medium by adsorption on its surface because the binding energy between the H2O particles and the outer surface is lower than that between the particles of the inhibitor and the surface of the metal.21| | |
xH2Oads + Orgsol ↔ Orgads + xH2O
| (11) |
where x is the number of single organic particles substituted for H2O particles. The particles on the outer surface of a metal can be physisorbed or chemisorbed. By minimizing the cathodic response, the physisorbed molecules face metal corrosion, while chemisorbed particles impede the anodic response by decreasing the possible reaction of the corroding metal at the sites of adsorption.22 After the examination of all adsorption isotherms, we conclude that the best isotherm that fits our results is Temkin isotherm,23 so:| |
aθ = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KC KC
| (12) |
where C is the concentration (M) of H1, H2 and H3; K is the adsorption equilibrium constant, (a) is a molecular interaction parameter. A graph of θ against log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C gives straight lines as shown in Fig. 2, note, the slope is 2.303/a and the intercept is 2.303/a
C gives straight lines as shown in Fig. 2, note, the slope is 2.303/a and the intercept is 2.303/a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kads. Thermodynamic parameters of the adsorption process were calculated and are tabulated in Table 3. The essential parameters were calculated as
Kads. Thermodynamic parameters of the adsorption process were calculated and are tabulated in Table 3. The essential parameters were calculated as 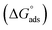 ,
, 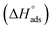 and
and 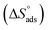 after assessment of Kads at various temperatures (Fig. 2).24 The change in free energy can be calculated from eqn (13):
after assessment of Kads at various temperatures (Fig. 2).24 The change in free energy can be calculated from eqn (13):| |
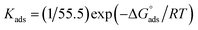 | (13) |
where 55.5 is the amount of H2O in mol L−1, T is the temperature and R is the universal gas constant. Enthalpy of adsorption 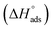 , the entropy of adsorption
, the entropy of adsorption 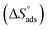 can be determined from eqn (14):
can be determined from eqn (14):| |
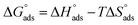 | (14) |
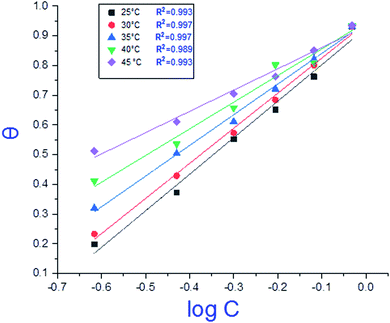 |
| | Fig. 2 Temkin adsorption isotherm of compound (H1) on C-steel surface in 1.0 M HCl at various temperatures. | |
Table 3 Thermodynamic parameters for the adsorption of (H1, H2 and H3) derivatives on the C-steel surface in 1 M HCl at different temperatures
| Comp. |
Temp., °C |
Kads, M−1 |
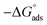
, kJ mol−1 |
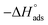
, kJ mol−1 |
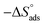
, J mol−1 K−1 |
| H1 |
25 |
5.67 |
14.25 |
48.8 |
169 |
| 30 |
6.29 |
14.75 |
| 35 |
8.21 |
15.68 |
| 40 |
11.33 |
16.77 |
| 45 |
20.03 |
18.55 |
| H2 |
25 |
5.38 |
14.12 |
40.3 |
188 |
| 30 |
5.90 |
14.59 |
| 35 |
7.45 |
15.43 |
| 40 |
9.80 |
16.39 |
| 45 |
15.52 |
17.87 |
| H3 |
25 |
5.67 |
14.25 |
48.8 |
212 |
| 30 |
6.29 |
14.75 |
| 35 |
8.21 |
15.68 |
| 40 |
11.33 |
16.77 |
| 45 |
20.03 |
18.55 |
By plotting 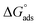 vs. T, (Fig. 3), the obtained curve is a straight line and the slope is equal to
vs. T, (Fig. 3), the obtained curve is a straight line and the slope is equal to 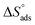 and the intercept is equal to
and the intercept is equal to 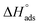 .
.
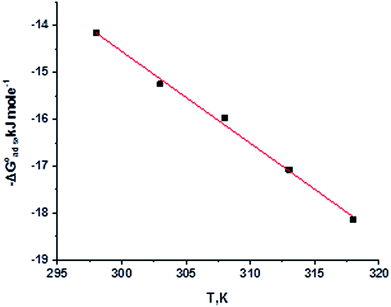 |
| | Fig. 3 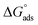 were plotted against T (K) for H1 derivative. were plotted against T (K) for H1 derivative. | |
Table 3 represents the calculated thermodynamic parameters and clarifies that the values of 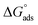 were negative, which indicates that (H1, H2 and H3) the stability of the adsorbed layer on the metal surface and to the extent the spontaneity of the adsorption process. As stated before, from different researches, the type of adsorption was ascribed as physisorption if
were negative, which indicates that (H1, H2 and H3) the stability of the adsorbed layer on the metal surface and to the extent the spontaneity of the adsorption process. As stated before, from different researches, the type of adsorption was ascribed as physisorption if 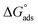 values were −20 kJ mol−1 or lower, the reaction occurred because of the electrostatic attraction between oppositely charged (inhibitor and metal), whereas the values of about −40 kJ mol−1 or more were ascribed as chemisorption because of sharing of the charge or transfer of charge between the inhibitor and C-steel.25–27 The calculated values of
values were −20 kJ mol−1 or lower, the reaction occurred because of the electrostatic attraction between oppositely charged (inhibitor and metal), whereas the values of about −40 kJ mol−1 or more were ascribed as chemisorption because of sharing of the charge or transfer of charge between the inhibitor and C-steel.25–27 The calculated values of 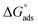 lie between −18.55 kJ mol−1 and −14.25 kJ mol−1, which refers to physical adsorption. The negative value of
lie between −18.55 kJ mol−1 and −14.25 kJ mol−1, which refers to physical adsorption. The negative value of 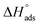 indicates that the adsorption type of inhibitor particle is an exothermic reaction. The exothermic reaction refers to either physisorption or chemisorption while an endothermic reaction is referred to as chemisorption.28 Also, enthalpy values are around 41.9 kJ mol−1, which is related to physisorption and those up to 100 kJ mol−1 or larger are related to chemisorption. The determined
indicates that the adsorption type of inhibitor particle is an exothermic reaction. The exothermic reaction refers to either physisorption or chemisorption while an endothermic reaction is referred to as chemisorption.28 Also, enthalpy values are around 41.9 kJ mol−1, which is related to physisorption and those up to 100 kJ mol−1 or larger are related to chemisorption. The determined 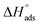 assessments are negative and ranged from 40.3 to 48.8 kJ mol−1 demonstrating that these compounds (H1, H2 and H3) might be physisorbed or chemisorbed. The
assessments are negative and ranged from 40.3 to 48.8 kJ mol−1 demonstrating that these compounds (H1, H2 and H3) might be physisorbed or chemisorbed. The 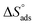 estimations are negative, which is related to the ordering of the adsorbed molecules on the C-steel surface and the absence of intermolecular interaction between the adsorbed molecules at these used concentrations. In addition, Fig. 3. Shows
estimations are negative, which is related to the ordering of the adsorbed molecules on the C-steel surface and the absence of intermolecular interaction between the adsorbed molecules at these used concentrations. In addition, Fig. 3. Shows 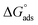 against various T (K) for H1 inhibitor.
against various T (K) for H1 inhibitor.
The activation factors for the dissolution procedure were calculated according to the Arrhenius eqn (15):
| |
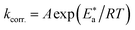 | (15) |
where k
corr. is the corrosion ratio,
A is the Arrhenius constant,
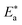
is the activation energy,
R is the universal gas constant, and
T is the absolute temperature. Estimations of
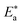
of C-steel corrosion in the presence of measured amounts of (H1, H2 and H3) were obtained from the relation of log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kcorr.
kcorr. against 1000/
T graphs as appeared in
Fig. 4, the transition state relation is obtained from
eqn (16):
| | |
kcorr. = (RT/Nh)exp(ΔS*/R)exp(−ΔH*/RT)
| (16) |
where
N is the Avogadro's factor,
h is referred to as Planck's parameter, Δ
S* is activated entropy and Δ
H* represents activated enthalpy. Plots of log(
kcorr./
T)
versus (1000/
T) give straight lines, as shown in
Fig. 5, with a slope is (Δ
H*/2.303
R) and an intercept is log(
R/
Nh) + Δ
S*/2.303
R. All calculations are listed in
Table 4, the increase in
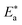
values demonstrated that (H1, H2 and H3) is physisorbed on the C-steel surface.
29 The positive indications of Δ
H* values give the endothermic idea of the C-steel dissolution procedure. The negative indications of Δ
S* demonstrated that in the rate-determining step, the association of unstable coordinated particles is larger than the dissociation.
21,30
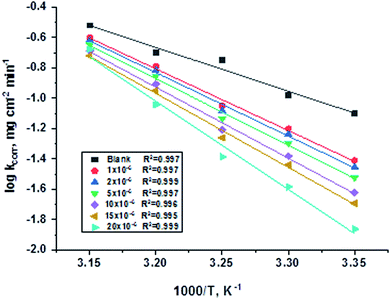 |
| | Fig. 4 Arrhenius diagrams for C-steel corrosion rates (kcorr.) against 1000/T after 120 minutes of immersion in 1.0 M HCl in the absence and presence of gradual concentrations of compound (H1). | |
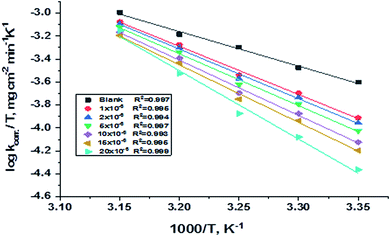 |
| | Fig. 5 Diagrams of (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kcorr./T) vs. 1000/T for corrosion of C-steel in 1.0 M HCl in the absence and presence of gradual concentrations of compound (H1). kcorr./T) vs. 1000/T for corrosion of C-steel in 1.0 M HCl in the absence and presence of gradual concentrations of compound (H1). | |
Table 4 Activation parameters for the dissolution of C-steel in the presence and absence of gradual concentrations of derivatives in 1.0 M HCl
| Conc. |
Activation parameters |
| Comp. |
Conc. (M) |
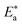
kJ mol−1 |
ΔH* kJ mol−1 |
−ΔS* J mol−1 K−1 |
| Blank |
55.14 |
57.71 |
73.30 |
| H1 |
1 × 10−6 |
77.74 |
80.29 |
3.31 |
| 2 × 10−6 |
80.07 |
82.62 |
3.49 |
| 5 × 10−6 |
83.96 |
86.51 |
15.15 |
| 10 × 10−6 |
89.40 |
91.94 |
31.47 |
| 15 × 10−6 |
93.28 |
95.83 |
43.13 |
| 20 × 10−6 |
111.34 |
113.88 |
99.92 |
| H2 |
1 × 10−6 |
77.74 |
80.29 |
3.31 |
| 2 × 10−6 |
80.86 |
83.41 |
5.74 |
| 5 × 10−6 |
84.73 |
87.28 |
17.48 |
| 10 × 10−6 |
93.67 |
96.21 |
44.42 |
| 15 × 10−6 |
97.17 |
99.71 |
54.79 |
| 20 × 10−6 |
126.94 |
129.46 |
144.61 |
| H3 |
1 × 10−6 |
80.42 |
82.97 |
4.93 |
| 2 × 10−6 |
81.62 |
84.17 |
8.15 |
| 5 × 10−6 |
82.40 |
84.95 |
10.33 |
| 10 × 10−6 |
86.29 |
88.84 |
22.15 |
| 15 × 10−6 |
94.03 |
96.57 |
45.82 |
| 20 × 10−6 |
108.54 |
111.08 |
88.45 |
3.1.2 Effect of temperature. The influence of temperature on the corrosion rates of C-steel in 1 M HCl and in the presence of gradual inhibitor amounts was considered in the temperature from 25 to 45 °C using the WL method. As the temperature rises, the rate of corrosion decreases and the % IE of the added inhibitors slightly increases, which are the characteristics of chemisorption, the results are summarized in Table 5.
Table 5 WL results for C-steel sheets in 1 M HCl solution without and with gradual concentrations of (H1, H2 and H3) at 30–45 °C
| Conc |
Temp. |
30 °C |
35 °C |
40 °C |
45 °C |
| Comp. |
Conc., (M) |
θ |
% IE |
θ |
% IE |
θ |
% IE |
θ |
% IE |
| H1 |
1 × 10−6 |
0.288 |
28.8 |
0.383 |
38.3 |
0.422 |
42.2 |
0.433 |
43.3 |
| 2 × 10−6 |
0.385 |
38.5 |
0.442 |
44.2 |
0.461 |
46.1 |
0.482 |
48.2 |
| 5 × 10−6 |
0.588 |
58.8 |
0.626 |
62.6 |
0.683 |
68.3 |
0.691 |
69.1 |
| 10 × 10−6 |
0.659 |
65.9 |
0.664 |
66.4 |
0.695 |
69.5 |
0.732 |
73.2 |
| 15 × 10−6 |
0.827 |
82.7 |
0.852 |
85.2 |
0.854 |
85.4 |
0.862 |
86.2 |
| 20 × 10−6 |
0.934 |
93.4 |
0.930 |
93.0 |
0.932 |
93.2 |
0.944 |
94.4 |
| H2 |
1 × 10−6 |
0.481 |
48.1 |
0.491 |
49.1 |
0.563 |
56.3 |
0.595 |
59.5 |
| 2 × 10−6 |
0.565 |
56.5 |
0.584 |
58.4 |
0.581 |
58.1 |
0.602 |
60.2 |
| 5 × 10−6 |
0.632 |
63.2 |
0.675 |
67.5 |
0.692 |
69.2 |
0.743 |
74.3 |
| 10 × 10−6 |
0.684 |
68.4 |
0.724 |
72.4 |
0.731 |
73.1 |
0.746 |
74.6 |
| 15 × 10−6 |
0.755 |
75.5 |
0.762 |
76.2 |
0.765 |
76.5 |
0.774 |
77.4 |
| 20 × 10−6 |
0.876 |
87.6 |
0.903 |
90.3 |
0.908 |
90.8 |
0.917 |
91.7 |
| H3 |
1 × 10−6 |
0.344 |
34.4 |
0.381 |
38.1 |
0.427 |
42.7 |
0.432 |
43.2 |
| 2 × 10−6 |
0.552 |
55.2 |
0.573 |
57.3 |
0.571 |
57.1 |
0.582 |
58.2 |
| 5 × 10−6 |
0.601 |
60.1 |
0.622 |
62.2 |
0.644 |
64.4 |
0.651 |
65.1 |
| 10 × 10−6 |
0.643 |
64.3 |
0.645 |
64.5 |
0.663 |
66.3 |
0.685 |
68.5 |
| 15 × 10−6 |
0.802 |
80.2 |
0.824 |
82.4 |
0.825 |
82.5 |
0.844 |
84.4 |
| 20 × 10−6 |
0.955 |
95.5 |
0.953 |
95.3 |
0.952 |
95.2 |
0.965 |
96.5 |
3.2 Potentiodynamic polarization (PDP) method
The polarization curves for carbon steel in corrosive media with increasing amounts of H1, H2 and H3 at 25 °C are illustrated in Fig. 6. Kinetic parameters as corrosion current (Icorr.), corrosion potential (Ecorr.), and Tafel slopes βa and βc were gained from the obtained figures and are shown in Table 6 for carbon steel in 1 M HCl corrosive medium with and without concentrations of H1, H2 and H3. % IE rises with increasing concentrations of compounds. Fig. 6 shows that the Icorr. values decrease upon the addition of additives, which decreases the carbon steel oxidation. The increase of the concentration of the compounds influences the anodic and cathodic directions of the polarization curves. The increase in concentrations of additives moved the Ecorr. values towards the negative values when compared with the blank. Hence, the addition of H1, H2 and H3 decreases carbon steel corrosion and suppresses hydrogen release as demonstrated from the equal cathodic Tafel curves shown in Fig. 6. The parallel lines of the Tafel lines after the addition of H1, H2 and H3 indicate that there is no change in the mechanism of both H2 release and metal consumption processes. In fact, the inhibitor is categorized as cathodic or anodic kind if the moving of corrosion potential in the existence of the inhibitor is ±85 mV from that in the absence of the inhibitor. The presence of H1, H2 and H3 shift Ecorr. to values not exceeding 15 mV, and Tafel slopes of βa and βc at 25 °C did not notably change, indicating that H1, H2 and H3 can be categorized as mixed-type inhibitors.31
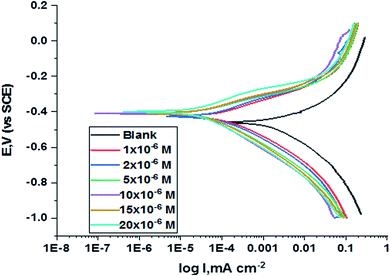 |
| | Fig. 6 PDP curves for the corrosion of C-steel in 1.0 M HCl in the absence and presence of gradual concentrations of compound (H1) at 25 °C. | |
Table 6 The effect of concentration of H1, H2 and H3 on the (Ecorr.), (Icorr.), (βa & βc), (C.R.), (θ) and (% IE) for the corrosion of C-steel 1 M HCl at 25 °C
| Comp. |
Conc. |
Icorr., μA cm−2 |
−Ecorr., mV (vs. SCE) |
βa mV dec−1 |
−βc mV dec−1 |
C.R, mpy |
θ |
% IE |
| Blank |
216 ± 2.6457 |
413 ± 2.3094 |
101.6 ± 2.0275 |
155.7 ± 2.3184 |
98.77 ± 2.333 |
| H1 |
1 × 10−6 |
140 ± 2.3333 |
425 ± 2.6034 |
100.6 ± 1.7320 |
163.2 ± 2.3094 |
64.10 ± 2.603 |
0.35 |
35.19 |
| 2 × 10−6 |
104 ± 2.6039 |
425 ± 1.7320 |
99.4 ± 2.3094 |
160.4 ± 1.7320 |
47.40 ± 1.452 |
0.52 |
51.85 |
| 5 × 10−6 |
53.4 ± 2.30947 |
406 ± 2.4037 |
79.2 ± 1.4529 |
157.4 ± 2.3092 |
24.42 ± 1.732 |
0.75 |
75.28 |
| 10 × 10−6 |
46.2 ± 2.3333 |
409 ± 2.3333 |
76.3 ± 2.0275 |
149.8 ± 2.0270 |
16.49 ± 2.645 |
0.79 |
78.61 |
| 15 × 10−6 |
36.1 ± 2.3094 |
408 ± 2.0275 |
83.5 ± 2.6034 |
157.3 ± 1.7638 |
21.13 ± 2.027 |
0.83 |
83.29 |
| 20 × 10−6 |
24.7 ± 2.9059 |
400 ± 1.1547 |
80.1 ± 1.7638 |
146.9 ± 2.3333 |
11.29 ± 2.309 |
0.89 |
88.56 |
| H2 |
1 × 10−6 |
112 ± 2.3094 |
428 ± 2.0816 |
99.6 ± 2.3094 |
157.1 ± 2.3094 |
51.22 ± 1.154 |
0.48 |
48.15 |
| 2 × 10−6 |
105 ± 2.027 |
424 ± 1.7320 |
89.4 ± 2.0275 |
152.4 ± 2.0245 |
42.02 ± 1.763 |
0.51 |
51.39 |
| 5 × 10−6 |
92 ± 2.6034 |
418 ± 2.3211 |
86.9 ± 2.3221 |
156.8 ± 2.0277 |
47.97 ± 1.754 |
0.57 |
57.41 |
| 10 × 10−6 |
62.6 ± 2.333 |
424 ± 2.6034 |
91.1 ± 2.333 |
156.1 ± 2.4037 |
27.75 ± 1.73 |
0.71 |
71.02 |
| 15 × 10−6 |
60.7 ± 1.7411 |
415 ± 2.3094 |
92 ± 2.6034 |
151.7 ± 2.0275 |
28.62 ± 2.081 |
0.72 |
71.90 |
| 20 × 10−6 |
42.3 ± 1.7320 |
405 ± 2.6171 |
84.3 ± 2.354 |
149.1 ± 1.7322 |
19.32 ± 2.603 |
0.80 |
80.42 |
| H3 |
1 × 10−6 |
152 ± 1.763 |
406 ± 1.763 |
91.8 ± 1.732 |
162.5 ± 1.765 |
69.29 ± 2.309 |
0.30 |
29.63 |
| 2 × 10−6 |
115 ± 2.027 |
405 ± 2.603 |
77.1 ± 1.452 |
144.9 ± 2.027 |
52.42 ± 2.309 |
0.47 |
46.76 |
| 5 × 10−6 |
44.1 ± 2.309 |
406 ± 1.452 |
70.1 ± 2.024 |
146.3 ± 1.763 |
20.16 ± 2.333 |
0.80 |
79.58 |
| 10 × 10−6 |
43.7 ± 2.403 |
409 ± 2.027 |
73.4 ± 2.021 |
145.9 ± 2.021 |
15.39 ± 2.027 |
0.80 |
79.77 |
| 15 × 10−6 |
33.7 ± 2.645 |
408 ± 2.905 |
79.9 ± 2.028 |
155.9 ± 1.201 |
19.95 ± 2.333 |
0.84 |
84.40 |
| 20 × 10−6 |
26.1 ± 2.024 |
400 ± 2.333 |
81.8 ± 2.309 |
151.2 ± 1.732 |
11.95 ± 1.732 |
0.88 |
87.92 |
3.3 (EIS) measurements
The corrosion of C-steel in 1 M HCl medium with and without various concentrations of H1, H2 and H3 were researched by the EIS technique at 25 °C after 30 min of immersion. Fig. 7 shows the circuit model used to carry out the EIS experiment and Fig. 8 shows the Nyquist plot for carbon steel in 1 M HCl medium in the absence and presence of various amounts of H1, H2 and H3. The appearance of impedance charts indicating around semi-round appearance clarifies that the oxidation of C-steel in 1 M HCl is faced by a charge transfer impedance process.32 The capacitive circle diameter rises with an increase in the amount of H1, H2 and H3, and is demonstrative of the level of the inhibitory influence of the corrosion process.33 Fig. 9 shows the Bode plot for C-steel in 1 M HCl at gradual concentrations of (H1) at 25 °C, which shows the same behavior. The circuit shown in Fig. 7 was used to calculate (RP) from eqn (17):| | |
RP = (Rct × RL)/(Rct + RL)
| (17) |
where (L), is the inductance, RL is the inductive resistance.34
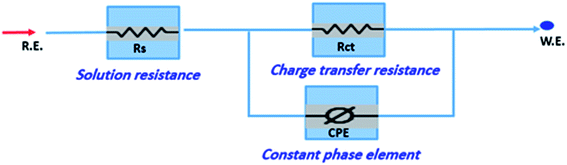 |
| | Fig. 7 Circuit used to investigate EIS data. | |
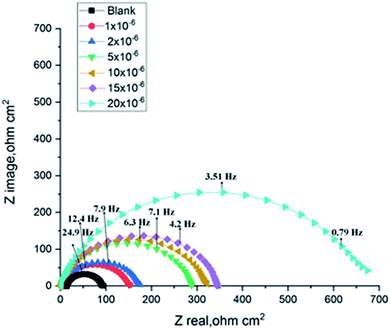 |
| | Fig. 8 Nyquist diagrams for corrosion of C-steel in 1.0 M HCl in the absence and presence of gradual concentrations of compound (H1) at 25 °C. | |
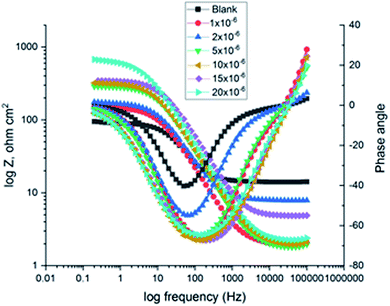 |
| | Fig. 9 The Bode diagrams for C-steel in 1 M HCl at gradual concentrations of (H1) at 25 °C. | |
EIS information from Table 7 illustrates that the RP values were raised (due to an increase in the thickness of the double layer) and Cdl values decreased with the increase in the amounts of H1, H2 and H3. This is because of the continuous substitution of H2O particles by the adsorbed H1, H2 and H3 molecules on the carbon steel surface and decreasing the degree of dissolution reaction. The large RP values are referred to as a small corrosion procedure.35 Cdl is lowered with increasing concentration because of the decrease of the dielectric constant or may be due to the enlargement of the double layer,36 which assures that the adsorption of H1, H2 and H3 mitigates CS corrosion to a large extent.
Table 7 Electrochemical kinetic parameters obtained from the EIS technique for the corrosion of C-steel in 1 M HCl without and with various concentrations of investigated compounds at 25 °C
| C. |
Conc., M |
Rp, Ω cm2 |
Cdl, μF cm−2 |
θ |
% IE |
| Blank |
77.72 |
105.25 ± 1.731 |
| H1 |
1 × 10−6 |
147.2 |
94.76 ± 1.734 |
0.472 |
47.2 |
| 2 × 10−6 |
164.5 |
89.91 ± 1.742 |
0.528 |
52.8 |
| 5 × 10−6 |
282.8 |
55.65 ± 2.333 |
0.725 |
72.5 |
| 10 × 10−6 |
316.9 |
53.40 ± 2.603 |
0.755 |
75.47 |
| 15 × 10−6 |
338.5 |
52.17 ± 2.309 |
0.770 |
77.0 |
| 20 × 10−6 |
654 |
42.98 ± 1.201 |
0.881 |
88.1 |
| H2 |
1 × 10−6 |
143.2 |
88.59 ± 1.452 |
0.457 |
45.7 |
| 2 × 10−6 |
155.7 |
85.05 ± 1.732 |
0.501 |
50.1 |
| 5 × 10−6 |
155.7 |
74.28 ± 2.024 |
0.501 |
50.1 |
| 10 × 10−6 |
155.9 |
69.95 ± 2.021 |
0.502 |
50.2 |
| 15 × 10−6 |
298.8 |
64.75 ± 2.022 |
0.740 |
74.0 |
| 20 × 10−6 |
354.3 |
51.20 ± 1.732 |
0.781 |
78.1 |
| H3 |
1 × 10−6 |
123.1 |
79.40 ± 2.027 |
0.369 |
36.9 |
| 2 × 10−6 |
148.2 |
72.31 ± 2.33 |
0.476 |
47.6 |
| 5 × 10−6 |
286.3 |
56.45 ± 2.34 |
0.729 |
72.9 |
| 10 × 10−6 |
317.1 |
51.22 ± 1.45 |
0.755 |
75.5 |
| 15 × 10−6 |
338.5 |
48.11 ± 2.027 |
0.770 |
77.0 |
| 20 × 10−6 |
653.1 |
43.19 ± 3.179 |
0.881 |
88.1 |
3.3 Atomic force microscopy (AFM) examination
AFM gives microscopic images for carbon steel surface topography perfectly, which assesses the roughness of the examined metal. The 3D AFM morphologies of the outer surface of pure carbon steel and carbon steel in 1 M HCl in the absence and presence of H1, H2 and H3 are shown in Fig. 10. The images of the outer surface of carbon steel in 1 M HCl have a larger roughness (993.8 nm) than that in the free carbon steel sample (17.5 nm), which clarifies that the carbon steel blank sample is severely corroded because of corrosive attacks. The obtained roughness of inhibited carbon steel shown in Table 8 and Fig. 10 were reduced to low values (105.6 nm in H1, 53.9 nm in H2 and 215.6 nm in H3) because of the effectiveness of the adsorbed layer of inhibitors on the outer surface, hence impeding the corrosion of carbon steel.37
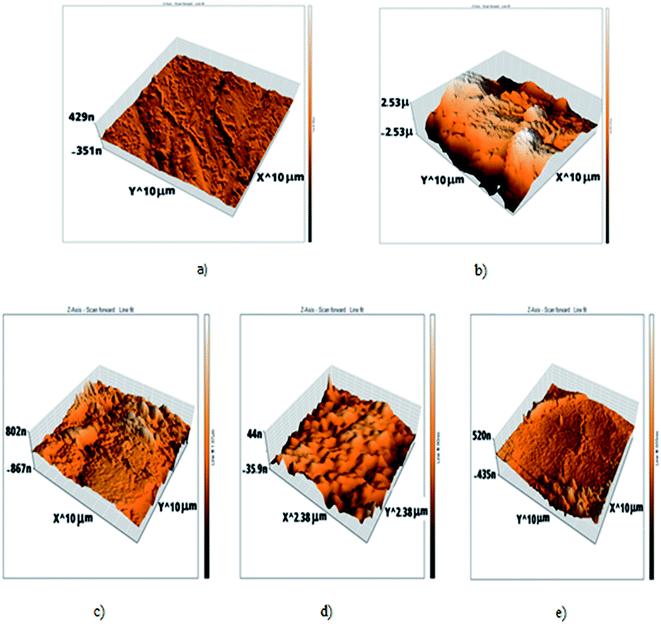 |
| | Fig. 10 AFM 3d photos of: (a) C-steel free surface, (b) C-steel in 1 M HCl only, (c) C-steel in 1 M HCl + 20 × 10−6 M of H, (d) C-steel in 1 M HCl + 20 × 10−6 M of H2, (e) C-steel in 1 M HCl + 20 × 10−6 M of H3. | |
Table 8 Roughness of all samples that appeared through atomic force microscope (AFM) examinations
| Sample |
Roughness (nm) |
| Free |
17.5 |
| Blank |
993.8 |
| H1 |
105.6 |
| H2 |
53.9 |
| H3 |
215.6 |
3.4 FT-IR spectroscopy analysis
FT-IR spectra show functional groups of the solutions and their behavior on the metal surface after adsorption, with high precision38 as shown in Fig. 11–13 concerning H1 inhibitor. The FTIR data could be interpreted as illustrated in Table 9. Fig. 11–13 illustrates FT-IR spectra of pure inhibitor liquids and the layers formed on C-steel samples after dipping in 1.0 M HCl for a day in the presence of 20 × 10−6 M of (H1). When comparing the spectrum of the inhibitor solution with the spectrum of the C-steel surface after immersion, the two spectra have the same properties, which means that the compounds were adsorbed on the C-steel surface.17 The obtained results illustrate the mechanism of interference between H1, H2 and H3 and carbon steel surface. The shifting and missing of the peaks in the spectrum after immersion showed that the interaction between H1, H2 and H3 and carbon steel surface happened through functional groups mentioned in Table 9.
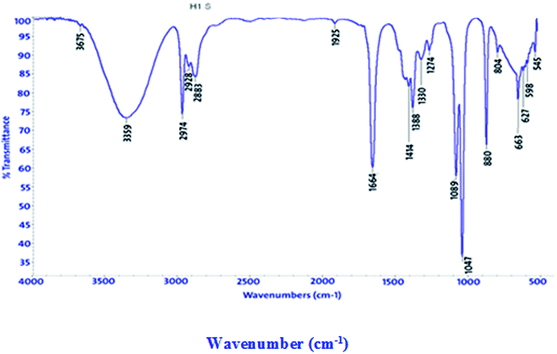 |
| | Fig. 11 IR spectra of 20 × 10−6 M of compound (H1) solution at 25 °C. | |
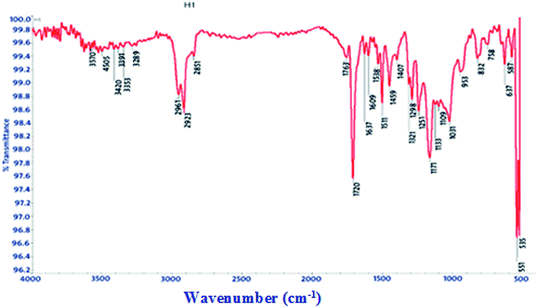 |
| | Fig. 12 IR spectra of carbon steel surface after 3 hours immersion in 20 × 10−6 M of compound (H1) at 25 °C. | |
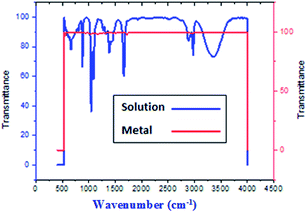 |
| | Fig. 13 Combined IR chart of pure solution and C-steel surface after 3 hours immersion in 20 × 10−6 M of compound (H1) at 25 °C. | |
Table 9 IR spectra of H1, H2 and H3 pure solutions and the spectra of the metal surface after inhibitor adsorption
| Comp. |
Solution beaks & frequencies (cm−1) |
Frequencies refer to |
Shifting and missing of beaks & frequencies (cm−1) after adsorption |
| H1 |
3359 |
OH, N–H stretching |
Missed |
| 2974, 2928 and 2883 |
(CH3) and (C–H) extending |
2961, 2923, 2851 |
| 1664 |
(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) attached to NH O) attached to NH |
1720 |
| 1047 |
(C–O) stretch |
1171 |
| 1388 |
(C–H) |
Missed |
| 880 |
(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) C–H) |
Missed |
| H2 |
3375 |
OH, N–H stretching |
Missed |
| 2974, 2928 and 2882 |
(CH3) and (C–H) extending |
(3036, 2965, 2876) |
| 1665 |
(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) attached to NH O) attached to NH |
1720 |
| 1047 |
(C–O) stretch |
1169 |
| 1388 |
(C–H) |
Missed |
| 881 |
(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) C–H) |
Missed |
| H3 |
3382 |
OH, N–H stretching |
Missed |
| (2974, 2929 and 2883) |
(CH3) and (C–H) extending |
Reduced to one beak at 2968 |
| 1663 |
(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) attached to NH O) attached to NH |
1720 |
| 1047 |
(C–O) stretch |
1169 |
| 1389 |
(C–H) holding |
Missed |
| 881 |
(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) C–H) |
Missed |
3.5 X-ray photoelectron spectroscopy (XPS) examination
It is a perfect system that can predict the adsorbed atoms on the metal surface. XPS examination of H1 mainly prospected for definite atoms such as (C, O, N and Fe), the obtained results are shown in Fig. 14 for carbon steel after immersion in 1 M HCl with 20 × 10−6 M of (H1) at 25 °C. Analysis of the obtained data39–41 for three inhibitors are summarized in Table 10.
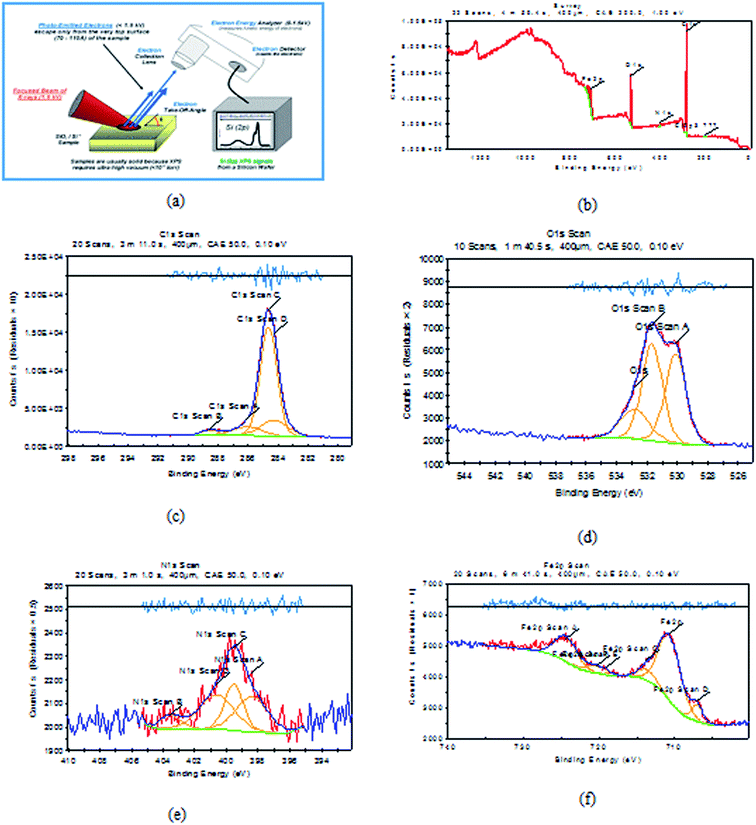 |
| | Fig. 14 XPS graphs of (a) XPS device, (b) general survey, (c) C 1s scan (d) O 1s scan (e) N 1s scan (f) Fe 2p scan of C-steel after immersion in 1 M HCl + 20 × 10−6 M of (H1) inhibitor for 24 h. | |
Table 10 Binding energies of different surveys and their expected bonds
| Comp. |
Scan type |
Binding energies peaks (ev) |
Peak refers to |
| H1 |
C 1s |
284.6 |
C–C |
| 288.5 |
–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
| 286.08 |
C–N |
| O 1s |
530.1 |
O2− (Fe2O3 mainly) |
| 531.66 |
OH− of FeOOH |
| 532.81 |
O2 of adsorbed water |
| N 1s |
398.4 |
N–Fe |
| 403.5 |
Protonated nitrogen atoms of the hydrazine group |
| Fe 2p |
706.9 |
Fe0 |
| 710.6 |
Fe2O3/Fe3O4/FeOOH |
| 713.69 |
FeCl3 |
| 718.96 |
Ferric compounds satellites |
| H2 |
C 1s |
285.02 |
C–C |
| 286.6 |
–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
| 288 |
C–N |
| O 1s |
530.1 |
O2− (Fe2O3 mainly) |
| 531.67 |
OH− of FeOOH |
| 532.79 |
O2 of adsorbed water |
| N 1s |
398.68 |
N–Fe |
| 402.48 |
Protonated nitrogen atoms of hydrazine group |
| Fe 2p |
710.6 |
Fe2O3/Fe3O4/FeOOH |
| 712.8 |
FeCl3 |
| 719.65 |
Ferric compounds satellites |
| H3 |
C 1s |
284.5 |
C–C |
| 289 |
–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
| 286.4 |
C–N |
| O 1s |
529.82 |
O2− (Fe2O3 mainly) |
| 530.24 |
OH− of FeOOH |
| 532.62 |
O2 of adsorbed water |
| N 1s |
398.8 |
N–Fe |
| 403.9 |
Protonated nitrogen atoms of the hydrazine group |
| Fe 2p |
707.01 |
Fe0 |
| 710.89 |
Fe2O3/Fe3O4/FeOOH |
| 713.98 |
FeCl3 |
| 719.3 |
Ferric compounds satellites |
All mentioned groups and bonds are found in the investigated inhibitor, so the experiment elucidated the adsorption of the investigated inhibitors on the metal surface.
3.6 Quantum chemical calculation
Theoretical chemistry is frequently used to interpret the mechanism of corrosion inhibition; the most popular technique is quantum chemical calculations. This technique has been elucidated to be a very excellent system for investigating the mechanism of interactions. Quantum chemical analysis using the density-functional theory (DFT) method was performed on the three investigated compounds to evaluate their efficiency. Indeed, the effectiveness of an inhibitor depends on its molecular structure. Frontier orbital theory is used to predict the adsorption sites of the inhibitor molecules, which interact with Fe atoms. As reported, effective corrosion inhibitors are those, which donate electrons to an empty orbital of the metal and at the same time receive electrons from the metal surface.42 Frontier orbital theory stated that any chemical reaction mainly happened between HOMO (the highest occupied molecular orbital) of the first reactant and LUMO (the lowest unoccupied molecular orbital) of the second reactant, the interaction between these orbitals constitutes the adsorption mechanism. Hence, it is essential to assess the presence of HOMO and LUMO orbitals of the investigated compounds to interpret the inhibition mechanism. Inhibitors with a high energy level of HOMO can give electrons to the unoccupied orbitals of the acceptor. EHOMO refers to the ability of the molecule to give electrons and ELUMO refers to the ability of the molecule to accept an electron. Table 11 shows the three inhibitors' geometrical structures with various HOMO and LUMO sites. The adsorption of inhibitor on the metal surface was performed using two approaches: the first is that the inhibitor molecule gives electrons to unoccupied (d) orbitals of the Fe atom to form a coordinate bond, the second is that the inhibitor molecule received electrons from the Fe atom, which establishes a back-bond between the metal surface and inhibitor. It was previously shown that the lower the values of ΔE higher are the inhibition efficiencies because the energy needed for separating an electron from the highest occupied molecular orbital (HOMO) is low. Table 11 shows that low values of ΔE were obtained for the three inhibitors that increased in the following order: H3 > H1 > H2. The results of quantum chemical calculations were not in accordance with the experimental results in this arrangement, this could be due to the adsorption of inhibitors occurred via a combination of the physical and chemical adsorption process. However, ΔE back-donation was increased in the following order H2 > H1 > H3, (H3 has the lowest ΔE back-donation). The investigated compounds differed in adsorption ability that could be explained according to Gece and Bilgic43 who stated that when the sites of N and O atoms were changed in their structure, the corrosion inhibition efficiency is consequently changed, which explains the difference in the obtained efficiencies between the three inhibitors. In Fe [Ar] 4s2 3d6, the 3d orbitals are not totally occupied with electrons. N atom with electronic configuration [He] 2s2 2p3 and O atom with electronic configuration, [He] 2s2 2p4, have a lone pair of electrons that are highly needed by Fe for completing unfilled 3d orbitals, as such, Fe was able to adsorb inhibitor molecules on its surface.42 As shown in Fig. 15, the electron density concentrated on N atoms. N and O atoms are the active sites, which have the best ability of binding with the Fe surface, Table 12 interprets the colors of orbitals that appeared in Fig. 15. Most reactivity parameters were evaluated to assure the effectiveness of hydrazide derivatives as corrosion inhibitors. These include softness σ(S), electronegativity (v), chemical hardness (η), ΔN, and Δw. All quantum parameters are shown in Table 9. The optimized geometries HOMO and LUMO distributions of H1, H2 and H3 in their non-protonated form are shown in Fig. 15.
Table 11 Quantum calculation parameters: (EHOMO), (ELUMO), (ΔE), (η), (σ), (χ), (ω) ΔN, and Δw
| Code |
H1 |
H2 |
H3 |
| Program |
MS-Dmol6 |
MS-Dmol6 |
MS-Dmol6 |
| Method |
DFT |
DFT |
DFT |
| Basis set |
DNP (4.4) |
DNP (4.4) |
DNP (4.4) |
| Function |
GGA-RPBE |
GGA-RPBE |
GGA-RPBE |
| EHOMO (ev) |
−5.1422 |
−5.2789 |
−5.3527 |
| ELUMO (ev) |
−3.3394 |
−3.4795 |
−3.4827 |
| ΔE = E LUMO − EHOMO |
1.80274 |
1.79942 |
1.86997 |
| η = ΔE/2 |
0.90137 |
0.89971 |
0.93499 |
| σ(S) = 1/η |
1.10942 |
1.11147 |
1.06953 |
| Pi = (EHOMO + ELUMO)/2 |
−4.2408 |
−4.3792 |
−4.4177 |
| X = −Pi |
4.24086 |
4.37922 |
4.41777 |
| ΔNmax |
2.35246 |
2.43369 |
2.36247 |
| ΔN (FET) |
0.32126 |
0.24496 |
0.21510 |
| ω |
9.97642 |
10.6576 |
10.4368 |
| ε |
0.10024 |
0.09383 |
0.09581 |
| ΔE back-donation |
−0.2253 |
−0.2249 |
−0.2337 |
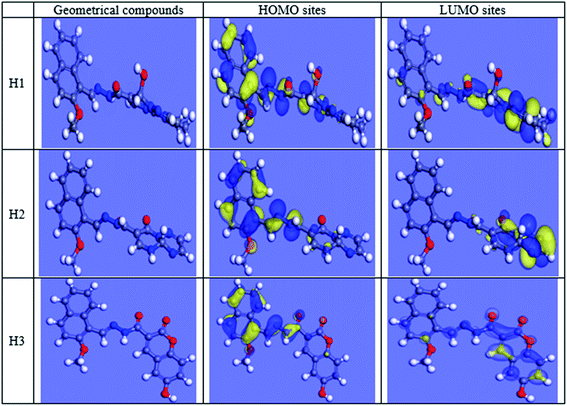 |
| | Fig. 15 HOMO, LUMO centers of inhibitors and carbon steel surface. | |
Table 12 Orbital Colors interpretation
| Orbital type |
Color |
Refer to |
| Molecular orbital |
Red/blue |
Filled orbitals |
| Yellow |
Unfilled orbitals |
| Electrostatic potential |
Red |
Negative sites |
| Blue |
Positive sites |
| Electrophilic (HOMO) |
Blue |
Site most susceptible to attack by a electrophile |
| Nucleophilic (LUMO) |
Blue |
Site most susceptible to attack by a nucleophile |
3.7 Molecular simulation results
The computational Monte Carlo (MC) method was performed to study the adsorption behavior of H1, H2 and H3 on the carbon steel surface in the solution presence of H3O+, Cl− and H2O. Side and top views of the adsorbed (H1, H2 and H3) molecules on the Fe surface are shown in Fig. 16. Computer simulations were performed to understand how the inhibitors react with the C-steel surface and how the geometrical structures of inhibitor molecules are arranged on the Fe surface, the figure shows the protonated form of the inhibitor in a solution. All inhibitor molecules are arranged in a superficial orientation on the Fe surface, which aids in forming ideal coverage of the carbon steel surface. The results in Tables 13 and 14 give the estimated energies.44 The negative values for adsorption energy prove to stiffen binding of the H1, H2 and H3 on the CS surface.45 Effective binding is ascribed to the presence of N and O atoms, which processes the coordination bonds. Adsorption energies of H1, H2 and H3 on C-steel surface can be arranged in the following order: H3 > H1 > H2. This order agrees with the practical inhibition percentage of the three inhibitors. The adsorption energy from the wet medium as (HCl) is higher than the H3O medium, H2O and vacuum media. This elucidates that the presence of H2O with the Cl− species strengthens the adsorption.
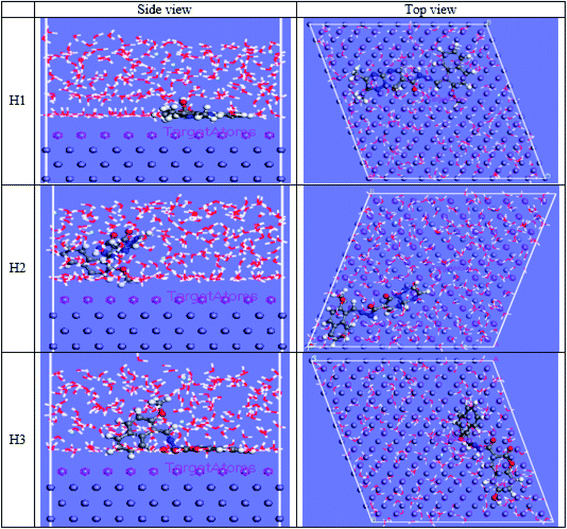 |
| | Fig. 16 Side and top views for MC simulations of the most stable configuration of the investigated compounds on the iron surface in acid solution conditions. | |
Table 13 Simulation results (total energy, adsorption energy, rigid absorption energy, and deformation energies) in vacuum
| Structures |
Fe–H1 |
Fe–H2 |
Fe–H3 |
| Total energy |
−239.003 |
−310.522 |
−205.550 |
| Adsorption energy |
−218.075 |
−207.526 |
−221.927 |
| Rigid adsorption energy |
−220.250 |
−212.893 |
−226.584 |
| Deformation energy |
2.174 |
5.366 |
4.657 |
| Inh: dEad/dNi |
−218.075 |
−207.526 |
−221.927 |
Table 14 Simulation results (total energy, adsorption energy, rigid absorption energy, and deformation energies) in acid solutions
| Structures |
Fe–H1 |
Fe–H2 |
Fe–H3 |
| Total energy |
−4055.159 |
−3929.906 |
−4046.015 |
| Adsorption energy |
−4102.05 |
−4135.034 |
−4135.472 |
| Rigid adsorption energy |
−4139.396 |
−4128.955 |
−4213.68 |
| Deformation energy |
37.34644 |
−6.078894 |
78.20721 |
| INH: dEad/dNi |
−345.7965 |
−323.8458 |
−375.1785 |
| H2O: dEad/dNi |
−7.605077 |
−7.513984 |
−13.41925 |
| H3O+: dEad/dNi |
−156.6965 |
−155.6190 |
−147.6683 |
| Cl−: dEad/dNi |
−138.1235 |
−146.6183 |
−154.2356 |
3.8 Mechanism of corrosion inhibition
Corrosion happens by two essential reactions, the oxidation reaction and the reduction of hydrogen. Most possibly, the organic compounds prevent corrosion by reducing both reactions. The mechanism of inhibition of the examined inhibitors cannot be considered as only chemical or physical adsorption because Tafel polarization results and the change in Ecorr. demonstrate that H1, H2 and H3 appear as mixed-type inhibitors. The inhibitive mechanism can be explained with two interpretations, the first is chemisorption, which happened when some electron-donating groups found in H1, H2 and H3 inhibitors give their electrons to the unoccupied d-orbitals in carbon steel. There are many donating atoms and groups found in H1, H2 and H3 with lone pairs to give, such as O−, OH−, NH−, NR, OCH3, alkyl group (CH3), and conjugated π-electrons. These atoms and functional groups can cover large metallic surface areas on carbon steel and electrons can transfer from them to the empty d-orbitals of Fe.45 The second interpretation is the physical adsorption, which is the attraction between inhibitors that protonated and charged C-steel surface since the C-steel surface has a positive charge, Cl− ions are firstly adsorbed onto the metal surface, then inhibitor molecules form a protective layer by the electrostatic attraction between the negatively charged metal surface and the positively charged inhibitor molecules.45 Hence, the adsorption of H1, H2 and H3 was developed and confirmed from AFM, FT-IR and XPS results. Quantum calculations and Monte Carlo simulations have assured the above-deduced mechanism with some variations. The investigated inhibitors from previous experiments can be arranged in the order of their inhibition efficiencies as H3 > H1 > H2.
4 Conclusions
The results from all experiments demonstrated that the percentage inhibition of C-steel corrosion rises with rising concentrations of the additives H1, H2 and H3, the percentage of inhibition also increases with increasing temperatures, i.e., these derivatives work well at higher temperatures. The adsorption of compounds on the C-steel surface follows Temkin isotherm. Tafel polarization results demonstrate that these derivatives (H1, H2 and H3) appear as mixed-type inhibitors. The % IE examined by different techniques is in acceptable agreement. The FT-IR and XPS examination assured the foundation of a protective layer from H1, H2 and H3 on the C-steel surface. The investigated compounds, which have shown inhibition efficiency greater than 90% are the most suitable inhibitors for any industrial application at higher temperatures as corrosion inhibitors for C-steel. The investigation by Monte Carlo simulation verifies the experimental results.
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- C. Verma, L. O. Olasunkanmi, I. B. Obot, E. E. Ebenso and M. A. Quraishi, RSC Adv., 2016, 6, 53933 RSC.
- Y. Sasikumar, A. S. Adekunle, L. O. Olasunkanmi, I. Bahadur, R. Baskar, M. M. Kabanda, I. B. Obot and E. E. Ebenso, J. Mol. Liq., 2015, 211, 105 CrossRef CAS.
- A. Khadiri, R. Saddik, K. Bekkouche, A. Aouniti, B. Hammouti, N. Benchat, M. Bouachrine and R. Solmaz, J. Taiwan Inst. Chem. Eng., 2016, 58, 552 CrossRef CAS.
- A. A. Khadom, A. S. Yaro, A. S. AlTaie and A. A. H. Kadum, Port. Electrochim. Acta, 2009, 27, 699 CrossRef CAS.
- A. Y. Musa, A. A. H. Kadhum, A. B. Mohamad, M. S. Takriff, A. R. Daud and S. K. Kamarudin, Corros. Sci., 2010, 52, 526 CrossRef CAS.
- A. A. Khadom, A. Y. Musa, A. A. H. Kadhum, A. B. Mohamad and M. S. Takriff, Port. Electrochim. Acta, 2010, 28, 221 CrossRef CAS.
- K. K. Alaneme, Y. S. Daramola, S. J. Olusegun and A. S. Afolabi, Int. J. Electrochem. Sci., 2015, 10, 3553 CAS.
- E. A. Noor and A. H. Al-Moubaraki, Mater. Chem. Phys., 2008, 110, 145 CrossRef CAS.
- F. Bentiss, M. Lebrini and M. Lagrenée, Corros. Sci., 2005, 47, 2915 CrossRef CAS.
- K. Bhrara, H. Kim and G. Singh, Corros. Sci., 2008, 50, 2747–2754 CrossRef CAS.
- M. A. Quraishi, Ind. Eng. Chem. Res., 2014, 53, 2851 CrossRef.
- A. Y. Musa, A. A. H. Kadhum, A. B. Mohamad and M. S. Takriff, Corros. Sci., 2010, 52, 3331 CrossRef CAS.
- M. Saini, P. Kumar, M. Kumar, K. Ramasamy, V. Mani, R. K. Mishra, A. B. A. Majeed and B. Narasimhan, Arabian J. Chem., 2014, 7, 448 CrossRef CAS.
- A. S. Fouda, M. T. Mohamed and M. R. Soltan, J. Electrochem. Sci. Technol., 2013, 4, 61 CrossRef CAS.
- C. B. P. Kumar and K. N. Mohana, J. Taiwan Inst. Chem. Eng., 2014, 45, 1031–1042 CrossRef CAS.
- P. Shetty, S. Afr. J. Chem., 2018, 71, 46 CrossRef CAS.
- H. M. Refat and A. A. Fadda, J. Heterocycl. Chem., 2016, 53, 1129 CrossRef CAS.
- A. S. Fouda, M. A. Ismail, A. A. Al-Khamri and A. S. Abousalem, J. Mol. Liq., 2019, 290, 111178 CrossRef CAS.
- A. S. Fouda, F. I. El-Dossoki, A. El-Hossiany and E. A. Sello, Surf. Eng. Appl. Electrochem., 2020, 56, 491 CrossRef.
- S. A. Umoren, I. B. Obot, A. Madhankumar and Z. M. Gasem, Carbohydr. Polym., 2015, 124, 280 CrossRef CAS PubMed.
- A. S. Fouda, H. E. Megahed, N. Fouad and N. M. Elbahrawi, Journal of Bio- and Tribo-Corrosion, 2016, 2, 16 CrossRef.
- A. S. Fouda, M. A. Abd El-Ghaffar, M. H. Sherif, A. Taher El-Habab and A. El-Hossiany, Protect. Met. Phys. Chem. Surface, 2020, 56, 189 CrossRef CAS.
- A. S. Fouda, G. El-Ewady and A. H. Ali, Green Chem. Lett. Rev., 2017, 10, 88 CrossRef CAS.
- A. S. Fouda, M. A. Ismael, R. M. A. Shahba, L. A. Kamel and A. A. El-Nagggar, Int. J. Electrochem. Sci., 2017, 12, 3361 CrossRef CAS.
- A. S. Fouda, K. Shalabi, G. Y. Elewady and H. F. Merayyed, Int. J. Electrochem. Sci., 2014, 9, 7038 Search PubMed.
- A. S. Fouda, S. A. Abd El-Maksoud, A. El-Hossiany and A. Ibrahim, Int. J. Electrochem. Sci., 2019, 14, 6045 CrossRef CAS.
- Y. Baymou, H. Bidi, M. E. Touhami, M. Allam, M. Rkayae and R. A. Belakhmima, Journal of Bio- and Tribo-Corrosion, 2018, 4, 11 CrossRef.
- A. S. Fouda, M. Abdel Azeem, S. A. Mohamed, A. El-Hossiany and E. El-Desouky, Int. J. Electrochem. Sci., 2019, 14, 3932 CrossRef CAS.
- M. B. Ibrahim, Z. Sulaiman, B. Usman and M. A. Ibrahim, World, 2019, 4, 45 Search PubMed.
- A. S. Fouda and A. A. Nazeer, Journal of Bio- and Tribo-Corrosion, 2018, 4, 7 CrossRef.
- A. S. Fouda, M. Eissa and A. El-Hossiany, Int. J. Electrochem. Sci., 2018, 13, 11096 CrossRef CAS.
- A. S. Fouda, E. El-Gharkawy, H. Ramadan and A. El-Hossiany, Biointerface Res. Appl. Chem., 2012, 11, 9786 Search PubMed.
- M. Abdallah, E. M. Kamar, S. Eid and A. Y. El-Etre, J. Mol. Liq., 2016, 220, 755 CrossRef CAS.
- A. S. Fouda, K. Shalabi and A. El-Hossiany, Journal of Bio- and Tribo-Corrosion, 2016, 2, 18 CrossRef.
- A. Morales, O. Piamba and J. Olaya, Coatings, 2019, 9, 507 CrossRef CAS.
- P. N. Devi, J. Sathiyabama and S. Rajendran, Int. J. Corros. Scale Inhib., 2017, 6, 18 CAS.
- S. Y. Al-Nami and A. E.-A. S. Fouda, Int. J. Electrochem. Sci., 2019, 14, 6902 CrossRef CAS.
- P. Muthukrishnan, B. Jeyaprabha and P. Prakash, Arabian J. Chem., 2017, 10, S2343 CrossRef CAS.
- N. El Hamdani, R. Fdil, M. Tourabi, C. Jama and F. Bentiss, Appl. Surf. Sci., 2015, 357, 1294 CrossRef CAS.
- A. S. Fouda, H. Ibrahim, S. Rashwaan, A. El-Hossiany and R. M. Ahmed, Int. J. Electrochem. Sci., 2018, 13, 6327 CrossRef CAS.
- M. Outirite, M. Lagrenée, M. Lebrini, M. Traisnel, C. Jama, H. Vezin and F. Bentiss, Electrochim. Acta, 2010, 55, 1670 CrossRef CAS.
- N. Anusuya, P. Sounthari, J. Saranya, K. Parameswari and S. Chitra, Orient. J. Chem., 2015, 31, 1741 CrossRef CAS.
- G. Gece and S. Bilgic, Corros. Sci., 2010, 52, 3435 CrossRef CAS.
- M. El Faydy, R. Touir, M. E. Touhami, A. Zarrouk, C. Jama, B. Lakhrissi, L. O. Olasunkanmi, E. E. Ebenso and F. Bentiss, Phys. Chem. Chem. Phys., 2018, 20, 201677 RSC.
- P. Singh, M. Makowska-Janusik, P. Slovensky and M. A. Quraishi, J. Mol. Liq., 2016, 220, 71 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2021 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a,
Samir A. Abd El-Maksoudb,
Elsherbiny H. El-Sayedb,
Hazem A. Elbazbc and
Ashraf S. Abousalem
*a,
Samir A. Abd El-Maksoudb,
Elsherbiny H. El-Sayedb,
Hazem A. Elbazbc and
Ashraf S. Abousalem ad
ad
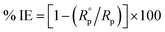
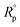 and Rp are the polarization resistances without and with the inhibitor, respectively,
and Rp are the polarization resistances without and with the inhibitor, respectively,
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KC
KC
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C gives straight lines as shown in Fig. 2, note, the slope is 2.303/a and the intercept is 2.303/a
C gives straight lines as shown in Fig. 2, note, the slope is 2.303/a and the intercept is 2.303/a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kads. Thermodynamic parameters of the adsorption process were calculated and are tabulated in Table 3. The essential parameters were calculated as
Kads. Thermodynamic parameters of the adsorption process were calculated and are tabulated in Table 3. The essential parameters were calculated as 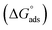 ,
, 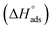 and
and 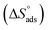 after assessment of Kads at various temperatures (Fig. 2).24 The change in free energy can be calculated from eqn (13):
after assessment of Kads at various temperatures (Fig. 2).24 The change in free energy can be calculated from eqn (13):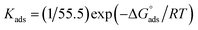
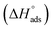 , the entropy of adsorption
, the entropy of adsorption 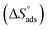 can be determined from eqn (14):
can be determined from eqn (14):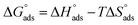

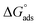 vs. T, (Fig. 3), the obtained curve is a straight line and the slope is equal to
vs. T, (Fig. 3), the obtained curve is a straight line and the slope is equal to 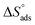 and the intercept is equal to
and the intercept is equal to 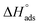 .
.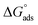 were negative, which indicates that (H1, H2 and H3) the stability of the adsorbed layer on the metal surface and to the extent the spontaneity of the adsorption process. As stated before, from different researches, the type of adsorption was ascribed as physisorption if
were negative, which indicates that (H1, H2 and H3) the stability of the adsorbed layer on the metal surface and to the extent the spontaneity of the adsorption process. As stated before, from different researches, the type of adsorption was ascribed as physisorption if 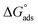 values were −20 kJ mol−1 or lower, the reaction occurred because of the electrostatic attraction between oppositely charged (inhibitor and metal), whereas the values of about −40 kJ mol−1 or more were ascribed as chemisorption because of sharing of the charge or transfer of charge between the inhibitor and C-steel.25–27 The calculated values of
values were −20 kJ mol−1 or lower, the reaction occurred because of the electrostatic attraction between oppositely charged (inhibitor and metal), whereas the values of about −40 kJ mol−1 or more were ascribed as chemisorption because of sharing of the charge or transfer of charge between the inhibitor and C-steel.25–27 The calculated values of 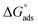 lie between −18.55 kJ mol−1 and −14.25 kJ mol−1, which refers to physical adsorption. The negative value of
lie between −18.55 kJ mol−1 and −14.25 kJ mol−1, which refers to physical adsorption. The negative value of 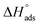 indicates that the adsorption type of inhibitor particle is an exothermic reaction. The exothermic reaction refers to either physisorption or chemisorption while an endothermic reaction is referred to as chemisorption.28 Also, enthalpy values are around 41.9 kJ mol−1, which is related to physisorption and those up to 100 kJ mol−1 or larger are related to chemisorption. The determined
indicates that the adsorption type of inhibitor particle is an exothermic reaction. The exothermic reaction refers to either physisorption or chemisorption while an endothermic reaction is referred to as chemisorption.28 Also, enthalpy values are around 41.9 kJ mol−1, which is related to physisorption and those up to 100 kJ mol−1 or larger are related to chemisorption. The determined 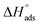 assessments are negative and ranged from 40.3 to 48.8 kJ mol−1 demonstrating that these compounds (H1, H2 and H3) might be physisorbed or chemisorbed. The
assessments are negative and ranged from 40.3 to 48.8 kJ mol−1 demonstrating that these compounds (H1, H2 and H3) might be physisorbed or chemisorbed. The 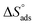 estimations are negative, which is related to the ordering of the adsorbed molecules on the C-steel surface and the absence of intermolecular interaction between the adsorbed molecules at these used concentrations. In addition, Fig. 3. Shows
estimations are negative, which is related to the ordering of the adsorbed molecules on the C-steel surface and the absence of intermolecular interaction between the adsorbed molecules at these used concentrations. In addition, Fig. 3. Shows 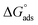 against various T (K) for H1 inhibitor.
against various T (K) for H1 inhibitor.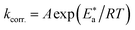
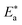 is the activation energy, R is the universal gas constant, and T is the absolute temperature. Estimations of
is the activation energy, R is the universal gas constant, and T is the absolute temperature. Estimations of 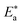 of C-steel corrosion in the presence of measured amounts of (H1, H2 and H3) were obtained from the relation of log
of C-steel corrosion in the presence of measured amounts of (H1, H2 and H3) were obtained from the relation of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kcorr. against 1000/T graphs as appeared in Fig. 4, the transition state relation is obtained from eqn (16):
kcorr. against 1000/T graphs as appeared in Fig. 4, the transition state relation is obtained from eqn (16):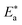 values demonstrated that (H1, H2 and H3) is physisorbed on the C-steel surface.29 The positive indications of ΔH* values give the endothermic idea of the C-steel dissolution procedure. The negative indications of ΔS* demonstrated that in the rate-determining step, the association of unstable coordinated particles is larger than the dissociation.21,30
values demonstrated that (H1, H2 and H3) is physisorbed on the C-steel surface.29 The positive indications of ΔH* values give the endothermic idea of the C-steel dissolution procedure. The negative indications of ΔS* demonstrated that in the rate-determining step, the association of unstable coordinated particles is larger than the dissociation.21,30


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kcorr./T) vs. 1000/T for corrosion of C-steel in 1.0 M HCl in the absence and presence of gradual concentrations of compound (H1).
kcorr./T) vs. 1000/T for corrosion of C-steel in 1.0 M HCl in the absence and presence of gradual concentrations of compound (H1).



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) attached to NH
O) attached to NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H)
C–H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) attached to NH
O) attached to NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H)
C–H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) attached to NH
O) attached to NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H)
C–H)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O
O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O
O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O
O