Open Access Article
Open Access ArticleThe phytochemistry, pharmacology and traditional medicinal use of Glechomae Herba – a systematic review†
Liu Yang ,
Jiaxu Zhang,
Senwang Zheng,
Ajiao Hou,
Song Wang,
Huan Yu,
Xuejiao Wang,
Yingjie Xu,
Haixue Kuang* and
Hai Jiang*
,
Jiaxu Zhang,
Senwang Zheng,
Ajiao Hou,
Song Wang,
Huan Yu,
Xuejiao Wang,
Yingjie Xu,
Haixue Kuang* and
Hai Jiang*
Key Laboratory of Chinese Materia Medica, Ministry of Education, Heilongjiang University of Chinese Medicine, Harbin 150040, China. E-mail: hxkuang56@163.com; jianghai_777@126.com
First published on 27th May 2021
Abstract
Glechomae Herba is a Chinese herb, which has been used in China for thousands of years, mainly for the treatment of nephrolithiasis. This paper summarizes the modern research progress on Glechomae Herba from the aspects of botany, traditional medicinal use, phytochemistry, pharmacology, pharmacokinetics, analytical methods and quality control. In addition, it also points out the deficiencies of current research on this herb and provides possible directions for its development. So far, more than 190 chemical components have been isolated and identified from Glechomae Herba, including organic acids and their esters, volatile oils, flavonoids and their glycosides, terpenes and other chemical components. Its extracts and compounds have a wide range of pharmacological effects, including anti-stone, anti-inflammatory, bacteriostatic, cholagogic and diuretic, effect on ileum smooth muscle, anti-tumor effect on tumor and hypoglycemic effects. However, future studies should focus on drug metabolism, clarify its pharmacodynamic mechanism, and establish a reasonable quality control standards for Glechomae Herba.
1. Introduction
Lamiaceae is a family of dicotyledons, with more than 3500 species in more than 200 genera. It is widely distributed in the world, mainly in the Mediterranean and Central Asia.1 Glechomae Herba (GH) is the dried whole grass of Lamiaceae plant Glechoma longituba (Nakai) Kupr. (GLK), harvested in spring and autumn, removed impurities washed, cut into sections, dried and preserved. It is cool in property and bitter and pungent in flavour. It affects the kidney, liver and bladder meridians, exerting the functions of moisture-removing tonglin, clearing heat and detoxification, dispersing blood stasis and detumescence. Clinically, GH is mainly used for urination, bladder calculi, kidney stones, ureteral calculi, nephritis edema, jaundice with heat and humidity, cholecystitis, cholelithiasis, and burns.2 GH is often used to treat diarrhea in Hubei Province, and the therapeutic effect is good. The general clinical dosage of GH is shown as follows. The recommended dosage for the dry product is 15–30 g and 30–60 g for fresh product.In recent years, with the deepening of the study on hepatolithiasis of the hepatobiliary system, GH has attracted more and more attention due to its significant curative effects and little toxic and side effects. It is generally considered non-toxic or weakly toxic, with only one case of GH-induced drug dermatitis reported in 1988.3,4 At present, and phytochemical studies have shown that there are organic acids and their esters, volatile oils, flavonoids and their glycosides, terpenoids, lignin, anthraquinone and other components in GH.5–8 Pharmacology studies have verified that GH has the effects of anti-calculus, anti-inflammatory, anti-bacterial, cholagogic diuretic treatment of diarrhea and anti-tumor.9–11 Since 2008, more and more patents have been reported on the potential development value of GH. In the medical field, GH's traditional Chinese medicine preparation has the effects of clearing away heat and detoxifying, treating urinary and gallstone. In addition, polysaccharides in GH can be used to prepare anti-complement drugs.
A comprehensive review of GH is necessary to advance its research. Thus, available research articles from 1975 to 2021 were collected and analyzed. We summarized its achievements in botany, traditional medicinal use, phytochemistry, pharmacology, pharmacokinetics and quality control, with the hope that this will help in the search for effective strategies for the prevention and treatment of diseases by using GH, as well as in the design of GH clinical trials. At the same time, it is also hoped that this article will be conducive to the search for bioactive compounds in future studies, and the development of new drugs containing these compounds, so as to realize the greater therapeutic potential of GH.
2. Botany
GH is a perennial herb. The stems of GH recorded in Jiangsu New Medical College were thin, square, covered with fine pilose hair, with the lower part prostrate and the upper part erect. Leaves opposite, kidney-shaped to round-cordate, 1.5–3 cm in length and 1.5–5.5 cm in diameter, margin rounded serrate, hairy or subglabrous on both sides, glandular below; petiole length is 1–2 times of leaf blade. Cymes axillary, 2–6 flowers per cyme; bracts punctate; calyx campanulate, with the length of 7–10 mm, calyx teeth narrowly triangular-lanceolate, apical awn-shaped, outer with hair and glandular points; corolla 2-lipped, pale blue to purple, 1.7–2.2 cm in length, lower lip with dark markings, middle lobe kidneylike; stamens 4, chamber spread. Nutlets oblong, brown. The flowering period is from March to April, and the fruiting period is from April to June. The image of GH is shown in Fig. 1.GH likes wet, grows in the field, the edge of the forest, roadside, forest grass, river stream or village side wet grass. It is not strict with the soil requirements, but loose, fertile, well-drained sandy loam is better. Moreover, GH is suitable for growth in a warm and humid climate.12 GH is distributed all over China, except in northwest China and Inner Mongolia. GH in folk is also known as GLK, Lysimachiae Herba (LH) (Shanghai, Jiangsu), Glechoma hederacea, Habenaria aitchisonii Rchb. f, Sanicula lamelligera Hance, Glechoma biondiana (Sichuan). Due to various reasons in history and geography, confusion of homonyms or homonyms often occurs in the process of treating diseases. In the Supplements to Compendium of Materia Medica (Zhao, 1765), GLK was named “LH” by Zhao Xuemin. Hence, the saying “GH” and “LH” are the same kinds of traditional Chinese medicine. LH is a dried whole plant of the family primula Lysimachia christinae Hance (LCH), often entwined into clumps, glabrous or sparsely pilose, stems twisted, surface brown or dark brown, longitudinally striated, lower stem nodes sometimes have fibrous roots, section solid. It can be used for the treatment of heat shower, acerbity of urine, jaundice, urinary red skin, carbuncles, skullcap, venomous snake bite, hepatolithiasis, urinary calculi, etc.13 Both have a significant curative effect on calculi, but GH is more effective in the treatment of kidney stones.14
3. Traditional uses
GH is widely used as an important traditional Chinese medicine to treat urogenital diseases for a long period. In the Chinese Pharmacopoeia (version 2020), it has been used to treat conditions such as heat strangury, urethral calculus, damp-heat jaundice, sore carbuncle sore and traumatic injury, and its recommended dosage is 15–30 g. According to traditional Chinese medicine (TCM) records, the first record of GH was kept as notes in the Newly Revised Materia Medica (Tang, 659), which refers to the Medicine Chart written by Yi Xu in the Tang dynasty, as the heteronym of Centella asiatica (L.) Urban.15 Later, Bencao Tujing (Song, 1061) records: “GH, sweet, flat, non-toxic. The folk says that the leaves of GH are similar to mint and are often taken orally by women who have pain in the lower abdomen, and it turns out to be treatable for this condition.” Based on the description in the book above and the efficacy of the drug, it can be concluded that this drug is consistent with GLK. This kind of plant was described in detail and pictures were noted in the book “An Illustrated Book of Plants”. The 1977 edition of Chinese Pharmacopoeia officially collected the dry ground part of GLK, a plant of the Labiatae family, and named it GH.In the folk, there are quite a few ways to use GH. “Zhejiang Folk Herbal Medicine (1960)” records GH, Juncus effusus, Plantaginis herba 15 grams each, water decoction can benefit urination, treatment of bladder stones; “Chinese Herbs In Common Use In Shanghai (1970)” records the fresh GH mashed external application, can treat boils, mumps, skin bruises and swelling; “Annals Of Medicinal Plants Of Zhejiang” records GH, Taraxaci Herba 30 grams each, 15 grams of Cyperus rotundus, decocted for a day, 1 dose, can treat cholecystitis and cholelithiasis. Other common compatibility and pharmacological effects of GH are shown in Table 1. With the development of the pharmaceutical industry, GH is now used in the treatment of calculi. Paishi Granules, a prescription with GH as the Monarch drug, has the functions of clearing heat, benefiting water and draining stone through pouring. In clinical practice, it is widely used in the treatment of kidney calculi, ureteral calculi.16 In addition to nephrolithiasis, GH is used to treat falls and mumps.17,18 Examples of common formulations containing GH are listed in Table 2. Among them, Danle Capsules, Niaoganning Granule and Paishi Granules are included in the Pharmacopoeia. In addition, in these 3 formulations, GH is the Monarch drug. Shexiang Dieda Fengshi adhesive, as a prescription containing GH, is also included in the Pharmacopoeia.
| No. | Composition | Method of use | Traditional and clinical uses | Ref. |
|---|---|---|---|---|
| 1 | Glechomae Herba 100 g, Nodus Nelumbinis Rhizomatis 100 g | Take orally after decocting | Benefit urinate, treat bladder stone | Jilin Chinese Herbal Medicine |
| 2 | Glechomae Herba 120 g | After the medicine is fried with water, add honey and take it twice a day | For kidney and ureteral calculi | Jilin Chinese Herbal Medicine |
| 3 | Glechomae Herba 30 g, Polygoni Avicularis Herba 30 g, flower of Shepherdspurse 15 g | Take orally after decocting | Treat nephritis edema | Chinese Herbs In Common Use In Shanghai |
| 4 | Glechomae Herba 60 g, Bidens bipinnata 75 g | Take orally after decocting | Treatment of jaundice | Annals Of Medicinal Plants Of Zhejiang |
| 5 | Glechomae Herba (fresh) 30 g, Asarum forbesii Maxim (fresh) 3 g | Mash the herbs. Drink it with water or wine, mash and apply the rest to the affected area | Treatment of bruising injuries | Jiangxi Herbal Medicine |
| 6 | Glechomae Herba (fresh), Portulacae Herba (fresh) | After frying two Chinese medicines of the same quality with water, fumigate the affected area with the obtained water | Governance carbuncle swollen | Chinese Herbs In Common Use In Shanghai |
| 7 | Glechomae Herba (fresh), Plantaginis Herba (fresh) | Mash the two drugs of the same quality, squeeze the juice, then add the same amount of white wine and wipe the affected area | Cure sore furuncle, erysipelas | Jilin Chinese Herbal Medicine |
| 8 | Glechomae Herba (fresh) 15 g | The herbs are stewed with an appropriate amount of animal livers and taken orally | Treatment of infantile malnutrition | Chinese Herbs In Common Use In Shanghai |
| 9 | Glechomae Herba (fresh) 25–40 g, crystal sugar 25 g | Add boiling water, simmer for 1 hour, take twice a day | Cure a cold and cough | Fujian Folk Herbal Medicine |
| 10 | Glechomae Herba 35–40 g, Imperatae Rhizoma 20 g, Plantaginis Herba 20 g, Dichondra repens Forst 25 g | Take it orally after frying in water | Treatment of jaundice and tympanitis | Zhejiang Folk Herbal Medicine |
| No. | Preparation name | Traditional and clinical uses |
|---|---|---|
| 1 | Danle capsules | Used for abdominal pain and gallbladder distension caused by liver stagnation and qi stagnation. Chronic cholecystitis, cholelithiasis to see the above symptoms |
| 2 | Huoluo tincture | Comfort the sinews, dispel the cold, promote blood circulation and relieve pain. For pain of muscle and bones, acute sprain, muscle pain and rheumatism, pain of liver area |
| 3 | Ginqiandantong oral liquid | Qingli damp heat, dredge the liver and gallbladder, pain row stone. For cholelithiasis damp-heat stasis in Shao Yang bile-bladder pain |
| 4 | Niaoganning granule | Clear heat detoxify, drench diuresis. Used for the treatment of dampness-heat syndrome of bladder: frequency of urination, urgency of urination, painful urethra acerbity, yellowish urine color, excessive wet urination, etc. Acute, chronic urinary tract infection is seen with above symptoms |
| 5 | Lithoexpulsium paste | Li Shui, tong drench, row stone. For kidney stones, ureteral stones, bladder stones and other urinary calculi |
| 6 | Paishi granules | Clear heat and benefit the water, dripping stone. This product is used for the stone shower caused by the heat and damp of the lower coke. Qin urinary calculi with the above syndrome |
| 7 | Qingrelidan granule | Clearing heat and dampness, anti-inflammatory and cholagogic. For cholecystitis, gallstones with cholecystitis |
| 8 | Shexiang Dieda Fengshi adhesive plaster | Dispel wind to wet, remove blood stasis and relieve pain. For rheumatic pain, injury, swelling and pain |
| 9 | Shuangxiangpaishi granules | Benefit water, tong drench, discharge stone, detoxify. Used for stone shower and removal of urinary stones |
| 10 | Tianqidiedafengshi ointment | Promote blood circulation and remove blood stasis, relax the tendons and collaterals, relieve swelling and pain, and remove wind and dampness. For soft tissue contusion, rheumatic lumbago |
4. Phytochemistry
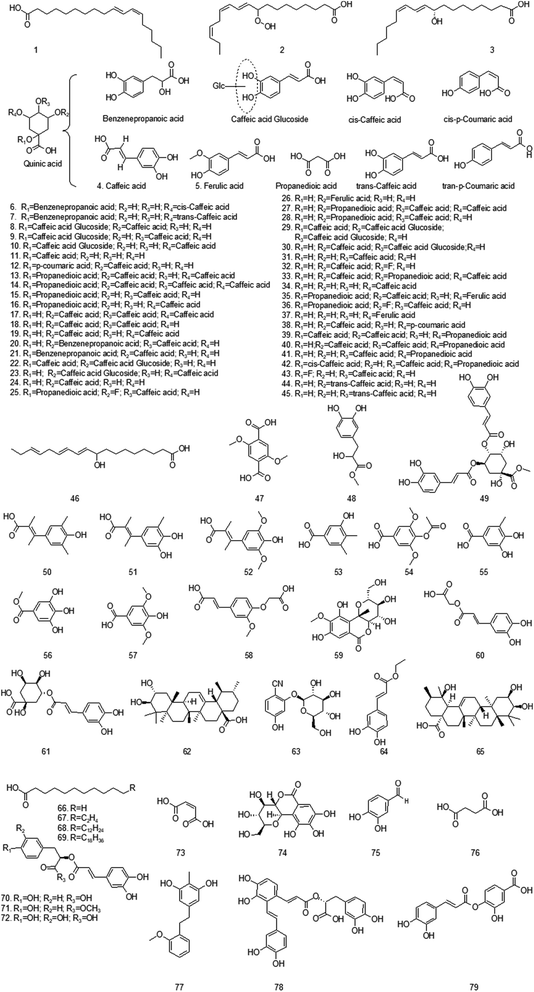
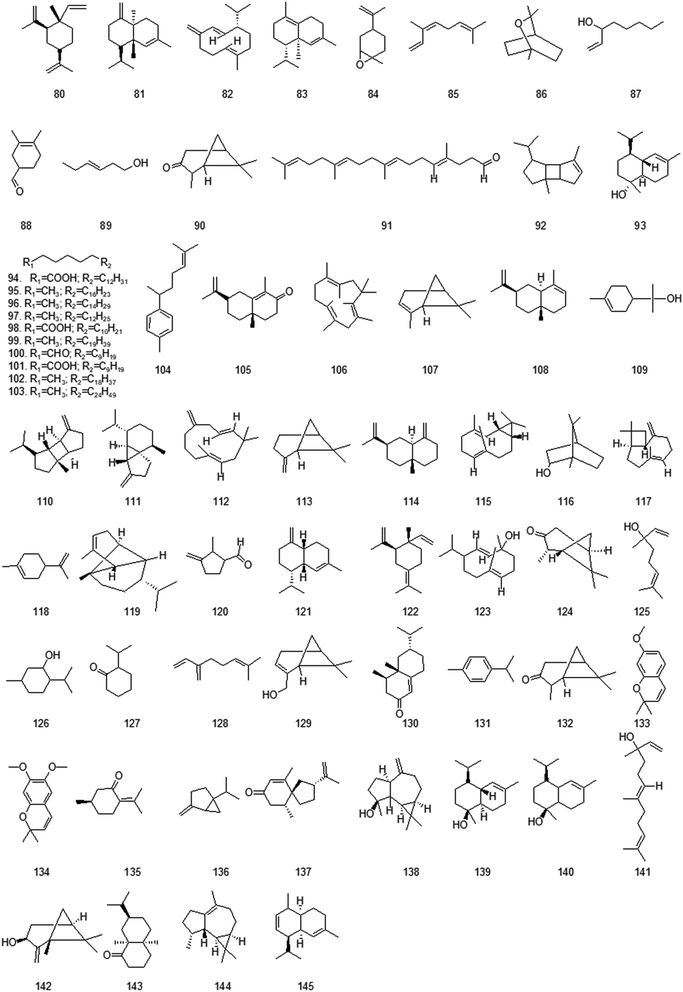
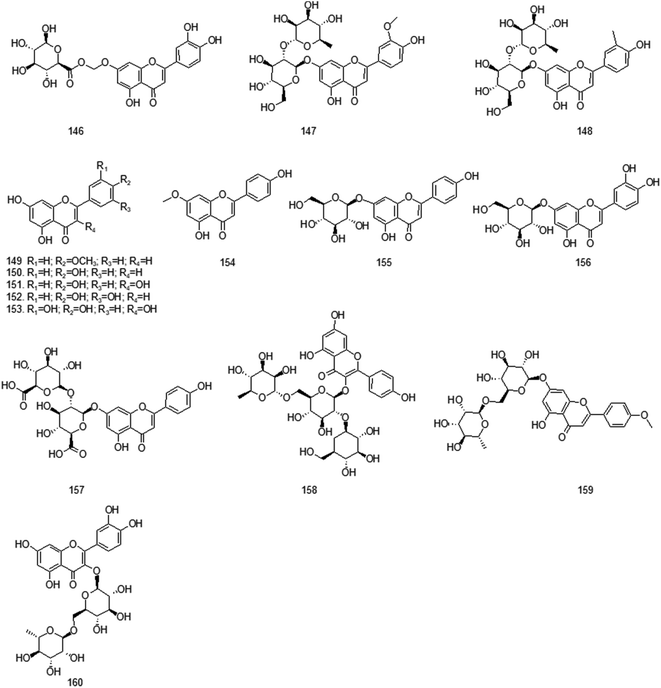
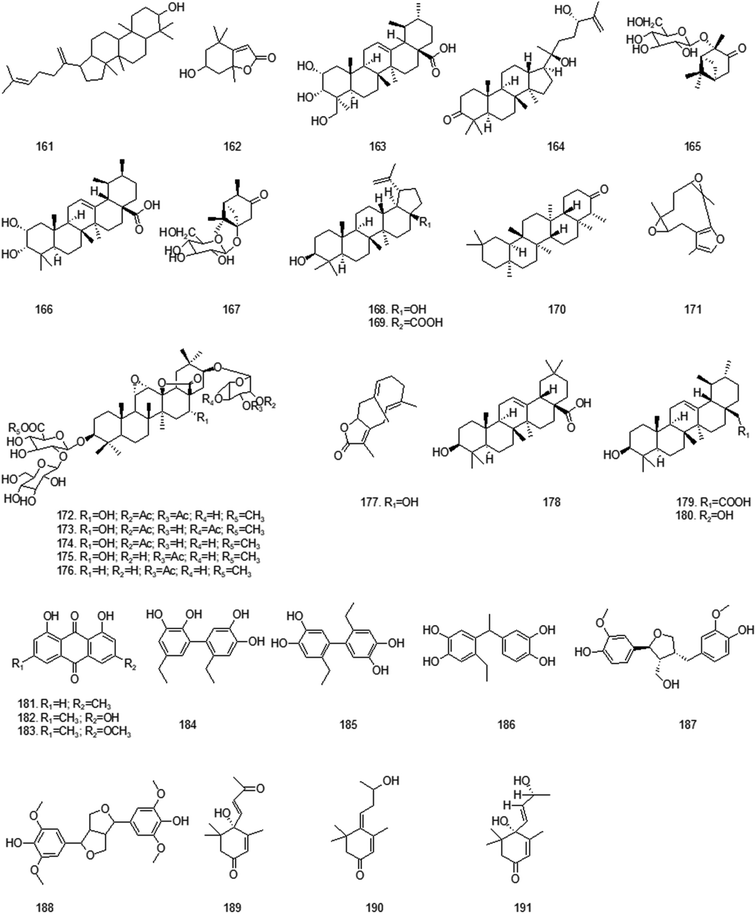
At present, many chemical compounds, including flavonoids and their glycosides, terpenoids, organic acids and their esters, volatile oils, alcohols and others, have been isolated and identified from GH. Among these, organic acids and flavonoids are believed to be the principal non-volatile ingredients with important biological properties. Until now, the compounds isolated are listed in Table 3.| No. | Compound | Ref. | No. | Compound | Ref. |
|---|---|---|---|---|---|
| Organic acids and their esters | |||||
| 1 | (10E,12Z)-Octadeca-10,12-dienoic acid | 24 | 2 | (10E,12Z,15Z)-9-Hydroperoxyoctadeca-10,12,15-trienoic acid | 24 |
| 3 | (9S,10E,12Z)-9-Hydroxyoctadeca-10,12-dienoic acid | 24 | 4 | Caffeic acid | 25 |
| 5 | Ferulic acid | 9 | 6 | 1-Benzenepropanoic acid-cis-5-caffeoylquinic acid | 25 |
| 7 | 1-Benzenepropanoic acid-trans-5-caffeoylquinic acid | 25 | 8 | 1-Caffeic acid glucoside-3-caffeoylquinic acid | 25 |
| 9 | 1-Caffeic acid glucoside-4-caffeoylquinic acid | 25 | 10 | 1-Caffeic acid glucoside-5-caffeoylquinic acid | 25 |
| 11 | 1-Caffeoylquinic acid | 25 | 12 | 1-p-Coumaric acid-3-caffeoylquinic acid | 25 |
| 13 | 1-Propanedioic acid-3,5-di-caffeoylquinic acid | 25 | 14 | 1-Propanedioic acid-4,5-di-caffeoylquinic acid | 25 |
| 15 | 1-Propanedioic acid-4-caffeoylquinic acid | 25 | 16 | 1-Propanedioic acid-5-caffeoylquinic acid | 25 |
| 17 | 3,4,5-Tri-caffeoylquinic acid | 25 | 18 | 3,4-Di-caffeoylquinic acid | 25 |
| 19 | 3,5-Di-caffeoylquinic acid | 25 | 20 | 3-Benzenepropanoic acid-4-caffeoylquinic acid | 25 |
| 21 | 3-Benzenepropanoic acid-1-caffeoylquinic acid | 25 | 22 | 3-Caffeic acid glucoside-1-caffeoylquinic acid | 25 |
| 23 | 3-Caffeic acid glucoside-5-caffeoylquinic acid | 25 | 24 | 3-Caffeoylquinic acid | 25 |
| 25 | 3-Ferulic acid-1-propanedioic acid-4-caffeoylquinic acid | 25 | 26 | 3-Feruloylquinic acid | 25 |
| 27 | 3-Propanedioic acid-4,5-di-caffeoylquinic acid | 25 | 28 | 3-Propanedioic acid-4-caffeoylquinic acid | 25 |
| 29 | 4-Caffeic acid glucoside-1-caffeoylquinic acid | 25 | 30 | 4-Caffeic acid glucoside-3-caffeoylquinic acid | 25 |
| 31 | 4-Caffeoylquinic acid | 25 | 32 | 4-Propanedioic acid-3-caffeoylquinic acid | 25 |
| 33 | 4-Propanedioic acid-4,5-di-caffeoylquinic acid | 25 | 34 | 5-Caffeoylquinic acid | 25 |
| 35 | 5-Ferulic acid-1-propanedioic acid-3-caffeoylquinic acid | 25 | 36 | 5-Ferulic acid-1-propanedioic acid-4-caffeoylquinic acid | 25 |
| 37 | 5-Feruloylquinic acid | 25 | 38 | 5-p-Coumaric acid-3-caffeoylquinic acid | 25 |
| 39 | 5-Propanedioic acid-1,3-di-caffeoylquinic acid | 25 | 40 | 5-Propanedioic acid-3,4-di-caffeoylquinic acid | 25 |
| 41 | 5-Propanedioic acid-4-caffeoylquinic acid | 25 | 42 | cis-1-Caffeic acid-5-feruloylquinic acid | 25 |
| 43 | Ferulic acid-5-caffeoylquinic acid | 25 | 44 | trans-3-Caffeoylquinic acid | 25 |
| 45 | trans-4-Caffeoylquinic acid | 25 | 46 | (9S,10E,12Z,15Z)-9-Hydroxy-10,12,15-octadecatrienoic acid | 24 |
| 47 | 2,5-Dimethoxyterephthalic acid | 8 | 48 | 3,4-Dihydroxyphenyllactic acid methyl ester | 26 |
| 49 | 3,4-Di-O-caffeoylquinic acid methyl ester | 27 | 50 | 4-Hydroxycinnamic acid | 26 |
| 51 | Dihydrocaffeic acid | 28 | 52 | Sinapic acid | 9 |
| 53 | 3-Hydroxybenzoic acid | 21 | 54 | 4-Acetyloxy-3,5-dimethoxybenzoic acid | 8 |
| 55 | Protocatechuic acid | 26 | 56 | Methyl gallate | 26 |
| 57 | Syringate | 8 | 58 | 3-[4-(Carboxymethoxy)-3-methoxyphenyl] acrylic acid | 8 |
| 59 | Bergenin | 26 | 60 | Caffeoylglycolic acid | 27 |
| 61 | Chlorogenic acid | 5 | 62 | Corosolic acid | 5 |
| 63 | Ehretioside B | 26 | 64 | Ethyl 3-(4-hydroxy-3-methoxyphenyl)acrylate | 26 |
| 65 | Euscaphic acid | 22 | 66 | Lauric acid | 20 |
| 67 | Myristic acid | 5 | 68 | Tetracosanoic acid | 20 |
| 69 | Triacontanoic acid | 20 | 70 | Maleic acid | 20 |
| 71 | Methyl rosmarinate | 21 | 72 | Rosmarinic acid | 21 |
| 73 | Isorinic acid | 27 | 74 | Norbergenin | 26 |
| 75 | Protocatechualdehyde | 21 | 76 | Succinic acid | 20 |
| 77 | Stilbostemin D | 26 | 78 | Salvianolic acid A | 27 |
| 79 | Trilepisiumic acid | 26 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Volatile oils | |||||
| 80 | (−)-β-Elemene | 30 | 81 | (−)-γ-Cadinene | 31 |
| 82 | (−)-Germacrene D | 32 | 83 | (+)-δ-Cadinene | 32 |
| 84 | (±)-Limonene | 30 | 85 | (Z)-β-Ocimene | 32 |
| 86 | 1,8-Cineole | 6 | 87 | 1-Octen-3-ol | 32 |
| 88 | 3,4-Dimethyl-3-cyclohexenylmethanal | 32 | 89 | 3-Hexen-1-ol | 32 |
| 90 | 3-Pinanone | 32 | 91 | 4,9,13,17-Tetramethyl-4,8,12,16-octadecatetraenal | 31 |
| 92 | α-Bourbonene | 32 | 93 | α-Cadinol | 32 |
| 94 | 9,12-Octadecadienoic acid | 31 | 95 | Docosane | 31 |
| 96 | Eicosane | 32 | 97 | Octadecane | 32 |
| 98 | Palmitic acid | 31 | 99 | Pentacosane | 31 |
| 100 | Pentadecanal | 31 | 101 | Pentadecanoic acid | 31 |
| 102 | Tetracosane | 32 | 103 | Triacontane | 31 |
| 104 | α-Curcumene | 31 | 105 | α-Cyperone | 32 |
| 106 | α-Humulene | 32 | 107 | α-Pinene | 32 |
| 108 | α-Selinene | 31 | 109 | α-Terpineol | 32 |
| 110 | β-Bourbonene | 32 | 111 | β-Cubebene | 30 |
| 112 | β-Humulene | 32 | 113 | β-Pinene | 6 |
| 114 | β-Selinene | 31 | 115 | Bicyclogermacrene | 32 |
| 116 | Borneol | 32 | 117 | Caryophyllene | 30 |
| 118 | Cinene | 6 | 119 | Copaene | 31 |
| 120 | Cyclopentanecarboxaldehyde, 2-methyl-3-methylene | 32 | 121 | γ-Amorphene | 32 |
| 122 | γ-Elemene | 30 | 123 | Germacrene D-4-ol | 32 |
| 124 | Isopinocamphone | 30 | 125 | Linalool | 6 |
| 126 | Menthol | 6 | 127 | Menthone | 6 |
| 128 | Myrcene | 32 | 129 | Myrtenol | 32 |
| 130 | Nootkatone | 32 | 131 | p-Cymene | 6 |
| 132 | Pinocamphone | 6 | 133 | Precocene 1 | 30 |
| 134 | Precocene 2 | 30 | 135 | Pulegone | 6 |
| 136 | Sabinene | 32 | 137 | Solavetivone | 32 |
| 138 | Spathulenol | 30 | 139 | T-Cadinol | 32 |
| 140 | T-Muurolol | 32 | 141 | trans-Nerolidol | 32 |
| 142 | trans-Pinocarveol | 32 | 143 | Valeranone | 32 |
| 144 | Viridiflorene | 32 | 145 | Zizanene | 32 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Flavonoids and their glycosides | |||||
| 146 | [2-(3,4-Dihydroxyphenyl)-5-hydroxy-4-oxochromen-7-yl]oxymethyl (2S,3S,4S,5R,6R)-3,4,5,6-tetrahydroxyoxane-2-carboxylate | 33 | 147 | 7-O-[α-L-Rhamnosyl-(1->2)-β-D-glucosyl]chrysoeriol | 34 |
| 148 | Rhoifolin | 33 | 149 | Acacetin | 34 |
| 150 | Apigenin | 33 | 151 | Kaempferol | 36 |
| 152 | Luteolin | 33 | 153 | Quercetin | 5 |
| 154 | Genkwanin | 35 | 155 | Apigenin 7-O-β-D-glucoside | 33 |
| 156 | Luteolin 7-O-β-D-glucoside | 33 | 157 | Clerodendrin | 34 |
| 158 | Kaempferol 3-(6′′′-rhamnosyl-2′′′-glucosyl-glucoside) | 33 | 159 | Linarin | 34 |
| 160 | Rutin | 33 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Terpenoids | |||||
| 161 | (3S,5R,8R,9R,10R,14R)-4,4,8,10,14-Pentamethyl-17-(5-methyl-1-methylene-hex-4-enyl)-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | 7 | 162 | (6S,7aR)-6-hydroxy-4,4,7α-trimethyl-6,7-dihydro-5H-1-benzofuran-2-one | 8 |
| 163 | 2,3,24-Trihydroxy-12-ursen-28-oic acid | 7 | 164 | 20S,24S-Dihydroxydammer-25-en-3-one | 7 |
| 165 | 2α-Pinan-3-one-2-O-β-glucopyranoside | 33 | 166 | 2α,3α-Dihydroxy-19β-methyl-29-noroleana-12-ene-28-oic acid | 35 |
| 167 | 5α-Pinan-3-one-5-O-β-glucopyranoside | 33 | 168 | Betulin | 7 |
| 169 | Betulinic acid | 7 | 170 | Friedelin | 34 |
| 171 | Glechomafuran | 24 | 172 | Glechomanoside A | 38 |
| 173 | Glechomanoside B | 38 | 174 | Glechomanoside C | 38 |
| 175 | Glechomanoside D | 38 | 176 | Glechomanoside E | 38 |
| 177 | Glechomanolide | 24 | 178 | Oleanolic acid | 35 |
| 179 | Ursolic acid | 35 | 180 | Uvaol | 7 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Anthraquinones | |||||
| 181 | Chrysophanic acid | 34 | 182 | Emodin | 8 |
| 183 | Physcion | 34 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Lignans | |||||
| 184 | Glechomols A | 6 | 185 | Glechomols B | 6 |
| 186 | Glechomols C | 6 | 187 | Lariciresinol | 6 |
| 188 | Syringaresinol | 6 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Alcohols | |||||
| 189 | (+)-Dehydrovomifoliol | 39 | 190 | 6R,9R-3-oxo-α-Ionol | 39 |
| 191 | Vomifoliol | 39 | |||
4.1 Organic acids and their esters
Organic acids are organic compounds that are acidic. The most common organic acid is the carboxylic acid, which is derived from the carboxylic group (–COOH), sulfonic acid (–SO3H), sulfinic acid (–RSOOH), thiocarboxylic acid (–RCOSH) are also organic acids. Organic acids can react with alcohols to form esters.19 In 2006, Lin et al. first isolated chlorogenic acid, corosolic acid, from whole ground grass of GH.5 Yun et al. also isolated tetracosanoic acid, lauric acid, maleic acid and other substances in the same year.20 Later, Huang isolated rosmarinic acid in 2008.21 Since then, various organic acids and their ester compounds in GH have been isolated. In 2019, Luo et al. used ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight tandem mass analysis (UHPLC-QTOF-MS/MS) to identify 40 chlorogenic acids.22 In the same year, Li et al. also used this method to identify the chemical constituents such as caffeoylquinic acid, feruloylquinic acid, p-coumaroylquinic acid and di-caffeoylquinic acid in GH.23 It was concluded that chlorogenic acids had anti-complement activity. The corresponding chemical structure is shown in Fig. 2.4.2 Volatile oil
Volatile oils, also known as Essential oils, are a kind of volatile liquid that can be distilled with water vapor. Most of them have aroma. It is a mixture of several types of compounds, among which aliphatic compounds, aromatic compounds, sulfur and nitrogen compounds, terpenes and their oxygen-containing derivatives are more abundant than other compounds.6 The chemical composition of the volatile oil from GH is very complex, which is in the order of ketones, terpenes, alcohols, alkanes and steroids according to the relative content.29,30 Volatile alcohols mostly send out exciting and harmonious odors, and also have anti-infection and antiviral effects. Most terpenoids have antibacterial, analgesic and anti-inflammatory effects.18 Among them, caryophyllene is effective in the treatment of digestive tract ulcers and skin inflammation, as well as the treatment of chronic respiratory diseases.30 Cadinene has strong analgesic and antipyretic, anti-inflammatory and bactericidal, cough relieving and expectorant functions.31 These are consistent with the functions of GH in detoxification and heat-clearing, swelling and blood stasis elimination, and drenching and dampness removal. Structures of volatile oils from GH are shown in Fig. 3.4.3 Flavonoids and their glycosides
Flavonoids generally refer to a series of compounds formed by the interlinkage of two benzene rings (A- and B-rings) with phenolic hydroxyl groups through the central tri-carbon atom, whose primary parent nucleus is 2-phenylchromone. In the structure of flavonoids, phenolic hydroxyl, methoxy, methyl, isopentenyl and other functional groups are often connected. In addition, it is often combined with sugars to form glycosides. Most of the known flavonoids in GH were isolated by Yang in 2006. Among them, quercetin has a strong biological activity and can play a pharmacological role of antioxidant, anti-tumor, anti-inflammatory, antibacterial and cardiovascular agents by reducing oxidative stress, interfering with the renin-angiotensin-aldosterone system and down-regulating the downstream signaling pathway mediated by reactive oxygen species. Apigenin in GH is also considered to have a variety of pharmacological activities, and it has significant effects in anti-tumor, neuroprotection, myocardial injury protection, reproductive endocrine regulation, anti-oxidation, hypoglycemic, anti-inflammatory, prevention and treatment of cardiovascular diseases and other aspects. Luteolin is also one of the main active components of GH, and its application in anti-inflammatory, antioxidant and anti-tumor fields has been a hot topic in recent studies. The specific structure of flavonoids and their glycoside compounds is shown in Fig. 4.4.4 Other compounds
Terpenoids are the main chemical constituents of GLK. According to literature reports, terpenes, especially triterpenes, are the material basis of good cytotoxic activity, anti-tumor activity, immunomodulatory functions, promoting blood circulation and removing blood stasis, etc. Glechomanoside A–E are expected to have an antithrombotic effect. Anthraquinone compounds have the pharmacological effects of hemostasis, antimicrobial, detoxification, diarrhea and diuresis. In recent years, studying chemical components of GLK is a research hotspot mainly on the efficacy of such compounds in the treatment of acquired immunodeficiency syndrome. In addition, lignin and alcohol can also be isolated from GH. The structures of compounds from this relatively common GH are shown in Fig. 5.5. Pharmacology
GH is considered to have a variety of pharmacological activities, and its crude extracts have strong biological activities in vivo and in vitro. It is generally believed that GH extract has antiurolithic activity, anti-inflammatory activity, antibacterial activity, diuretic and cholagogic effects, effects on ileum smooth muscle, anti-tumor effect and hypoglycemic effects. The pharmacological activity of the plant will be described next. The main pharmacological activities of GH extracts or certain ingredients are shown in Table 4.| Extracts/compounds dose | Animal/cell line | Study design | Control | Effect | Ref. |
|---|---|---|---|---|---|
| Antiurolithic activity | |||||
| WGH (0.05 g mL−1 15, 30, 45 d) | In vitro | Saline, ursodeoxycholic acid | All extracts had an obvious dissolution effect on cholesterol calculus in vitro on days 15, 30 and 45, and the liposoluble extract was stronger than the water-soluble extract in dissolving calculus | 40 | |
| AGH (0.05 g mL−1 15, 30, 45 d) | |||||
| EGH (0.05 g mL−1 15, 30, 45 d) | |||||
| WGH (3.3 g kg−1 d−1 28 d) | Male British short-haired guinea pigs | In vivo | Saline, Peginterferon alfa-2b | The extract has a significant therapeutic effect on experimental cholesterol calculus in guinea pigs | 40 |
| AGH (3.3 g kg−1 d−1 2 8 d) | |||||
| EGH (3.3 g kg−1 d−1 28 d) | |||||
| WGH (1000, 500, 250 mg kg−1 d−1 28 d) | SPF rats | In vivo | Paishi Granules | WGH can increase the concentration of urinary crystallization inhibitors and reduce the concentration of lithogenic substances, which is conducive to reducing the deposition of calcium oxalate and effectively inhibiting the formation of stones. It can also make the urine become acidic and dissolve and disperse stones, and promote the excretion and metabolism of Ca2+ in renal tissues and blood. At the same time, improve the metabolism and function of renal tissue cells, accelerate urine excretion | 9 |
| WGH (0.5, 1.0, 2.0, 4.0 mg mL−1 24 h) | The human kidney epithelial cell line HK-2 | In vitro | Cells exposed to 66 g cm−2 of CaOx for 24 hours, potassium citrate | After pretreatment with WGH at the concentration of 2.0 mg mL−1, MDA significantly decreased, and SOD and CAT levels significantly increased compared with the control group | 45 |
| WGH (220, 440, 880 mg kg−1 d−1 28 d) | SD rats | In vivo | None | GH has a potential oxalic acid reduction capacity and has a protective effect on the epithelial cells of the kidney. Glechoma longituba could regulate the urolithiasis-related protein OPN and KIM-1 expression in rat experiments | 45 |
| WGH (1.0, 2.0, 4.0 mg mL−1) | Human kidney HK-2 cells | In vitro | Glechoma longituba has antioxidant effects that attenuate the cell oxidative damage and apoptosis induced by CaOx via the Nrf2/HO-1 signalling pathway, in a dose-dependent manner | 46 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Anti-inflammatory activity | |||||
| WGH (1 g mL−1 d−1) | KM mice | In vivo | Saline, aspirin | The extract of GH has a strong anti-inflammatory effect | 10 |
| EGH (1 g mL−1 d−1) | |||||
| Methyl isoferuloyl-7-(3,4-dihydroxyphenyl) lactate | HepG2 cells | In vitro | None | The compounds potent anti-inflammatory activity by the inhibition of NF-κB activation, iNOS and COX-2 expression | 48 |
| Benzyl-40-hydroxy-benzoyl-30-O-b-D-glucopyranoside | |||||
| Methyl rosmarinate | |||||
| Ethyl rosmarinate | |||||
| 30-O-Methyl-rosmarinic acid | |||||
| Rosmarinic acid | |||||
| WGH (8, 4, 2 g kg−1 9 d) | Wistar rats | In vivo | Aspirin | The dosage of WGH (8 g kg−1) significantly reduced the toe swelling rate caused by carrageenan in rats 1, 2, 3 and 5 h after inflammation. The dosage of WGH (4 g kg−1) could significantly reduce the toe swelling rate caused by caraway gum in rats 2 h after inflammation | 49 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Antibacterial activity | |||||
| WGH (1 g mL−1 d−1) | Escherichia coli, Proteus, Staphylococcus aureus, Pseudomonas aeruginosa | In vitro | None | GH has a strong bacteriostatic effect. The EC50 of petroleum ether extract to these 4 fungi were 0.837 (3 mg mL−1), 3.517 (8 mg mL−1), 1.496 (0 mg mL−1) and 2.351 (7 mg mL−1), respectively, and the EC50 of chloroform extract were 0.619 (3 mg mL−1), 4.458 (6 mg mL−1), 1.689 (8 mg mL−1) and 1.556 (3 mg mL−1), respectively | 11 |
| EGH (1 g mL−1 d−1) | |||||
| EGH (5, 2.5, 1.25, 0.625, 0.312 g L−1) | Cytospora sp., Rhizoctonia solani, Fusarium oxysporum f.s p. cucumerinum, Botrytis cinerea Pers. | In vitro | None | 51 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Cholagogic and diuretic effect | |||||
| WGH (20 g kg−1) | SD rats | In vivo | Distilled water, furosemide tablets | GH has a strong cholagogic and diuretic effect | 54 |
| EGH (20 g kg−1) | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Effect on ileum smooth muscle | |||||
| WGH (0.1, 0.2,0.3 mg mL−1) | Isolated ileum smooth muscle of guinea pig | In vitro | Promethazine, atropine sulfate injection, epinephrine hydrochloride injection | WGH significantly antagonized the inhibitory effect of atropine and epinephrine on isolated ileum smooth muscle in guinea pigs, but not the inhibitory effect of promethazine hydrochloride. EGH significantly antagonized the excitation effect of histamine, acetylcholine and BaCl2 on ileum smooth muscle | 53 |
| EGH (0.05, 0.1, 0.2, 0.3 mg mL−1) | |||||
| EGH (1 g mL−1) | KM mice | In vivo | Neostigmine | EGH had an obvious antagonistic effect on diarrhea in mice, inhibited the advanced rate of charcoal powder in the small intestine of mice, and also resisted the hypermotility of the small intestine caused by neostigmine | 55 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Anti-tumor activity | |||||
| Euscaphic acid G | NCI-H460, HepG2, T24, SKOV3, MGC-803, HL-7702 cells | In vitro | None | Euscaphic acid G showed potential anticancer effects against lung cancer cells via inducing cell cycle arrest and apoptosis, at least partly, through NF-κB signalling pathways | 20 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Hypoglycemic activity | |||||
| EGH (0.1, 0.2, 0.4 g kg−1 3 d) | KM mice | In vivo | Phenformin hydrochloride | GH can reduce hyperglycemia caused by streptozotocin in mice, improve SOD activity in mice, reduce MDA content, inhibit the damage of oxygen free radicals to cells, and protect cells. | 63 |
5.1 Antiurolithic activity
Calculosis involves the formation of a solid mass in the lumen of a catheter or in the lumen of sexual organs (such as the kidney, ureter, gallbladder or bladder) in a human or animal body. There are many kinds of calculosis, including extrahepatic and intrahepatic bile duct calculi, cholecystolithiasis and common bile duct calculi. There are lithiasis in the urinary system, ureteral calculi, bladder calculi, gastrointestinal calculi and so on. The constitution in some people is more special, and they may have lung stones, muscle stones and other diseases. GH is commonly used for kidney stones and gallstones and is more effective in the treatment of kidney stones than LCH.14 In 2005, Xue discovered that water extract of GH (WGH) could cure urinary stones in domestic animals.40,41 Ge et al. carried out in vivo and in vitro experiments on the antiurolithic activity of GH in 2017.43 In vitro experiments were conducted to observe the effect of GH extract on lowering the weight of cholesterol calculi in humans. However, in vitro experiments were somewhat simple, considering the anti-calculi effect of GH from the perspective of weight only, and more detailed in vitro experiments were lacking. In vivo experiments showed that the extract of GH had the effect of lowering the level of blood lipid and inhibiting the formation of cholesterol stones.41 In 2012, Xiao He summed up that GH with LCH, Desmodium styracifolium (Osh.) Merr. and other drugs can be commonly used as medicines for the treatment of urolithiasis.10 Yang et al. observed in 2014 that GH extract obtained after water extraction and alcohol precipitation had a preventive and therapeutic effect on kidney stone model rats and discussed its possible mechanism of action.Experimental studies preliminarily confirmed that GH extract does have the advantage of overall regulation in kidney stones. They consider that the supernatant of GH after water extraction and alcohol precipitation mainly contains a variety of organic acids and flavonoids, which can form a soluble salt or complex with Ca2+, increase the concentration of urinary crystallization inhibitors and reduce the concentration of lithogenic substances, which is conducive to reducing calcium oxalate deposition and effectively inhibiting the formation of stones. In addition, it can make urine acidic and dissolve stones, promote the excretion and metabolism of Ca2+ in renal tissue and blood, and reduce the content of Ca2+ and oxalic acid in renal tissue. At the same time, it can improve the metabolism and function of renal tissue cells, accelerate urine excretion, promote the discharge of microcalculi from the body, reduce the formation of calculi in renal tissue, reduce the damage to the kidney, so as to protect renal tissue and prevent and treat renal calculi in rats.42 However, the experiments of Yang et al. still lack verification at the cellular level, and the pharmacological mechanism of GH antiurolithiasis is still in the conjecture stage.
In 2016, Qiang et al. conducted a more in-depth study on antiurolithic activity of GH. Currently, urolithiasis composed of calcium oxalate (CaOx) alone or in combination with calcium phosphate accounts for more than 80% of calculi in clinical practice.47 Studies on the pathogenesis of CaOx stone formation suggest that oxalate-induced oxidative stress (OS) may be the initial trigger of a vicious cycle of urolith formation.44 Under this premise, in vivo and in vitro experiments of Qiang et al. demonstrated that the extract of GH could significantly reduce CaOx-induced expression of osteopontin, kidney injury molecule-1 expression, and OS compared with the positive control group. Moreover, in vivo experiments proved that the extract of GH could significantly reduce the deposition of CaOx in diseased rats and slow down the pathological changes of urolithic rats. Compared with the model group, oxalate, creatinine and urea levels were significantly lower while citrate levels increased in the administered group.45 However, the research did not address which specific chemical substances in the extract had antiurolithic activity, and further studies in this direction should be considered for future experiments. Subsequently, Wang et al. concluded that WGH could attenuate CaOx-induced apoptosis and oxidative stress through the nuclear factor-k-gene binding (Nrf2/HO-1) signaling pathway.48 However, these two research works did not address which specific chemical substances in the extract had antiurolithic activity, and further studies in this direction should be considered through future experiments.
5.2 Anti-inflammatory activity
Inflammation is a defense response of living tissue with a vascular system to damage factors. It can also be considered as a defense response of the body to stimulation, manifested as redness, swelling, heat, pain and dysfunction. This is a normal physiological response to an injury or infection. However, conditions associated with inflammation, such as allergies, fibrosis and arthritis, last a long time and can cause major problems, such as tissue damage, if left untreated.47,48 WGH and ethanol extract from GH (EGH) had a strong inhibitory effect on inflammatory models such as xylene-induced auricle swelling and acetic acid-induced increased capillary permeability in the abdominal cavity of mice. WGH could significantly inhibit the relative content of 5-hydroxytryptamine (5-HT) and histamine in inflammatory tissues, but could not inhibit the relative content of prostaglandin E2 in inflammatory tissues, while the effect of EGH on 5-HT and histamine was weak.10 In 2011, Kim et al. isolated rosmarinic acid and its analogues and phenolic compounds from the EGH, and demonstrated that three rosmarinic acid analogues can inhibit NF-κB production and the induction of cyclooxygenase-2 (COX-2) and inducible nitric-oxide synthase (iNOS) mRNA in HepG2 cells, thus producing anti-inflammatory effects.51 However, the results obtained at the cellular level are not enough, and animal experiments need to be supplemented for further verification. The research on WGH has made new progress in 2014. He et al. found that the WGH (8.4 g kg−1) significantly inhibited the rate of toenail swelling and the weight of rat cotton granulation tissue in rats induced by carrageenan.52 However, the specific molecular mechanism of the experiment is still unclear and further studies are needed.5.3 Antibacterial activity
GH also has a strong antibacterial activity. In 1999, in Chinese Materia Medica, it was recorded that through the bacteriostatic test, the WGH and EGH were extremely sensitive to Staphylococcus aureus and moderately sensitive to Shigella sonnei. Yong et al. also selected common intestinal pathogens to investigate the bacteriostatic effect of GH. It was found that WGH and volatile oil had good in vitro bacteriostasis effects on Escherichia coli, Proteus species and P. aeruginosa. Besides, the essential oil of GH is the main part of its bacteriostatic effect.11 On this basis, Zhang et al. found that GLK, the whole grass of GH, its alcohol extract, water extract and ultrasonic alcohol extract had a better inhibitory effect on Pseudomonas aeruginosa and Staphylococcus aureus than Escherichia coli and had the strongest inhibitory effect on Pseudomonas aeruginosa.53 It may be due to the fact that volatile alcohols in volatile oil have anti-putrefying, anti-filtering virus and other characteristics, while terpenes generally have refreshing, antibacterial, anti-inflammatory and analgesic effects, suggesting that the effect of GH on diarrhea may be related to the bacteriostatic effect of the volatile oil and water extract.The petroleum ether extract and chloroform extract of GH also have strong inhibitory activity against Cytospora sp., Rhizoctonia solani, Fusarium oxysporum f.s p. cucumerinum, and Botrytis cinerea Pers.50 All four are plant pathogenic fungi. The inhibition effect of chloroform extract on Rhizoctonia solani was the strongest, and EC50 was 0.619 (3 mg mL−1). The EC50 of petroleum ether extract in these 4 fungi were 0.837 (3 mg mL−1), 3.517 (8 mg mL−1), 1.496 (0 mg mL−1) and 2.351 (7 mg mL−1) and EC50 in chloroform extract were 0.619 (3 mg mL−1), 4.458 (6 mg mL−1), 1.689 (8 mg mL−1) and 1.556 (3 mg mL−1).51 In addition, 80% EGH also has a certain bacteriostatic effect on Bacillus subtilis and Pneumococcus.52 However, the active mechanism of these extracts and the specific mechanisms that produce this antibacterial effect remains to be further studied.
5.4 Cholagogic and diuretic effect
WGH and EGH can promote bile secretion of liver cells, bile duct bile increase, internal pressure increase and relaxing the biliary sphincter to facilitate bile excretion. Large doses and high concentrations of GH decoction, juice, and various extracts can enhance the contraction of isolated rabbit intestines. At the same time, it enhances the contraction of smooth muscle in the uterus and intestine and has the effect of choline.53 By screening and comparison of the choledogenic activity and diuretic activity of GH, Hu et al. found that extracts of GH and LCH could effectively promote bile excretion in experimental animals, reduce the concentration of total bilirubin and direct bilirubin in the bile, increase the concentration of bile acid and reduce the formation of gallstones. The effect of GH on urine output was greater than that of LCH, but the cholagogic effect was weaker than that of LCH.54 However, the active mechanisms of these extracts and the specific mechanisms that produce these cholagogic and diuretic effects require further investigation.5.5 Effect on ileum smooth muscle
Yong et al. found that the WGH could significantly stimulate the spontaneous activity of guinea pig ileum and strengthen the contractile force. It had an antagonistic effect on ileum contraction reduction induced by atropine and epinephrine, but had no antagonistic effect on ileum contraction reduction induced by promethazine hydrochloride. EGH could significantly inhibit the spontaneous activity of guinea pig ileum, weaken the contractile force, and have an antagonistic effect on the strengthening of ileum contractile caused by acetylcholine, histamine and BaCl2.53 They then looked at the effects of GH extracts on small intestinal propulsion in mice, on drug-induced diarrhea in mouse models, and on the contractions of isolated ileum smooth muscle in guinea pigs. It was found that the EGH had an obvious antagonistic effect on diarrhea in mice, and inhibited the rate of carbon particles in the small intestine of mice, as well as the hyperkinetic activity of the small intestine caused by neostigmine.55 WGH excites the smooth muscle of the guinea pig ileum, which may be mediated by the choline and adrenaline receptors in the gastrointestinal tract, but not by the histamine receptors. EGH can inhibit the ileum movement in guinea pigs, which may be mediated by choline receptors and histamine receptors in the gastrointestinal tract, or directly on ileum smooth muscle cells. Both in vitro and in vivo experiments have proved that the extracts of GH have an effect on ileum smooth muscle, but the specific mechanism of the effect is still unclear, so it is necessary to study the extracts at the cellular level.5.6 Anti-tumor activity
Cancer is a major cause of disease and death worldwide, and the development of effective anti-tumor therapies is a difficult problem in medical research. WGH can partially block the process of Epstein -Barr virus (EBV) activated by the combined action of the cancer-promoting compound crotonis oleum and n-butyl ether.56 Relevant studies have also demonstrated that ursinic acid in GH has significant cytotoxicity to some tumor cell lines and can induce apoptosis in a variety of cancer cells, including acute myelogenous leukemia, breast cancer, prostate cancer, melanoma, and liver cancer.57,58 Rosmarinic acid is also one of the important compounds of GH. It was also shown that rosmarinic acid inhibited the growth of human uterine carcinoma, human gastric adenocarcinoma, and murine melanoma cells in vitro and induced apoptosis in Jurkat cells.59 In 2019, Xi-Lin et al. found that euscaphic acid G isolated from GH exerted strong antiproliferative activity against NCI-H460 cells in a concentration- and time-dependent manner. By acting on NF-κB signaling pathways, euscaphic acid G showed a potential anticancer effect on lung cancer cells by inducing cell cycle arrest and apoptosis.23 However, Xi-Lin et al. only conducted cell experiments, lacking experimental studies at the animal and clinical levels. Other substances in GH, such as quercetin, oleanolic acid, and apigenin, have also been shown to have anti-cancer properties.60–625.7 Hypoglycemic activity
Diabetes mellitus is a metabolic disease characterized by hyperglycemia. Hyperglycemia is caused by defective insulin secretion or impaired biological action, or both. Chronic hyperglycemia in diabetes leads to chronic damage and dysfunction of various tissues, especially the eyes, kidneys, heart, blood vessels and nerves. Yuan studied normal mice and diabetic mice induced by streptozotocin. The blood glucose of mice was measured by a blood glucose meter. The activity of serum superoxide dismutase (SOD) and the content of malondialdehyde (MDA) were determined by colorimetry. At the same time, Mallory dyeing and other methods were used. The results showed GH had no effect on the blood glucose of normal mice, but it could significantly reduce the blood glucose level of diabetic mice, increase the activity of serum SOD and reduce the content of serum MDA. Its hypoglycemic mechanism is to increase the number of cells in the islet.63 However, this study is conducted only at the animal experiment level, and should also be evaluated in relevant cell experiments and clinical trials. Besides, the mechanism and protein targets of GH extract on islet cells in vivo should be discussed accordingly, so as to provide help rational clinical use of drugs and the development of new drugs and new dosage forms.5.8 Other activities
Ge et al. found that GH extract had an obvious dissolving effect on human cholesterol, and could effectively reduce the concentration of total cholesterol, triglyceride, low-density lipoprotein cholesterol, cholesterol and egg quality in the bile of guinea pigs and increase the content of bile acid and lecithin in bile.43 In addition to its lipid-lowering effect, GH also has antioxidant effects. Deok Jo et al. found that linoleic acid accounted for 45% of the fatty acid content, and the antioxidant property of aqueous solution containing 10% of the fatty acid in Lycium chinense was similar to that of 50 ppm of tocopherol, and it had the function of nitrite decomposition at pH 1.2–6.0, so it had the antioxidant property.64 Moreover, the effect of GH on the cardiovascular system cannot be ignored. The total alcohol extract and total ethyl acetate extract of GH had a relaxing effect on the vascular loop of the intact endothelium and the endotheliated superior mesenteric artery. At the same time, GH also has antithrombotic, anti-complement and free radical scavenging activities.37,65–676. Pharmacokinetics
Pharmacokinetic studies on the extracts or monomers of GH are rarely reported. In 2010, Ni adopted rapid and automatic ultra-performance liquid chromatography (UPLC) coupled to a quadrupole time-of-flight (QTOF) method, represented by protocatechuic aldehyde, chlorogenic acid, caffeic acid, ferulic acid, rosmarinic acid, luteolin-7-O-glucoside, luteolin, apigenin, acacetin, to study the chemical changes of ethanol extract from GH after absorption and metabolism in rats. The results showed that the metabolites of phenolic acids and flavonoids were mainly present in the plasma and urine of rats, including hydroxylation, methylation, thiourea and sulfation conjugates.68,69 However, this experiment only studied the chemical transformation of the ethanol extract of GH and did not carry out a detailed study on the pharmacokinetic properties such as bioavailability, plasma concentration of the drug at different times, time to peak and other common pharmacokinetic parameters.In 2019, Yun et al. used UHPLC-QTOF-MS/MS method to study mucous, bile, plasma and feces of rats induced by oral ethanol extracts of GH. Ninety-one components, including 13 prototypical compounds and 78 metabolites, were clearly or reasonably identified in rat biological samples.70,71 Metabolites were excreted mainly through urine (82 metabolites) and bile (19 metabolites), and significantly fewer metabolites were excreted through feces (4 metabolites). The main metabolic pathways of phenolic acids and flavonoids are glucosylation, methylation and sulfation.72 However, this study also lacks the investigation of the common pharmacokinetic parameters of GH.
7. Analysis and quality control
GH has a fragrant, slightly bitter taste. GH resources in China are affected by different factors such as provenance, growing environment, harvesting time, processing method and storage conditions, and the quality of GH in different producing areas is uneven. All these factors lead to uncontrolled quality and clinical efficacy. In the Chinese Pharmacopoeia (version 2020), luteolin is used as the standard to identify the authenticity of GH by thin-layer chromatography. The quality of crude drugs was controlled by determining the content of alcohol-soluble extracts. There is no provision for the determination of specific chemical components. At present, the analysis methods of chemical components in GH are mainly focused on organic acid compounds and flavonoids. Both compounds are often detected using high-performance liquid chromatography (HPLC).71–78 In addition, other methods have also been used to study the quality control of GH, including HPLC-ESI-QTOF/MS, UV/Visible spectrophotometry, LC-MS/MS, HPLC-UV and UHPLC-QTOF-MS/MS.79–83 For the volatile components, gas chromatography-mass spectrometry is generally used for the determination.31 Phenolic acid compounds are often used as the standard for quality testing.It is a characteristic of traditional Chinese medicine that multi-component and multi-target exert a curative effect. It is not enough to evaluate GH from the content determination of a few compounds. At present, there is no literature to prove which compound is the most important pharmacological active component of GH. With further study of the compound, the quality control of GH will eventually be solved. The method for establishing the spectral effect correlation can be used to screen the active compounds, or network pharmacology can be used to predict disease-related compounds, and finally in vitro and in vivo studies can be used to verify the results. These two methods may contribute significantly to the establishment of GH quality control.
8. Conclusion and discussion
GH is a kind of TCM, which is widely used in ancient China. Many ancient books have recorded its medicinal value. This paper mainly summarizes the research results of GH in botany, traditional application, phytochemistry, pharmacology, pharmacokinetics and quality control in recent years, and puts forward some corresponding deficiencies. It provides new thoughts for future researchers to further develop plant-derived drugs and compounds.With the further development and wide application of modern medical technology, China has developed and clinically applied a variety of proprietary Chinese medicine of GH. Paishi Granules and Danle Capsules are typical examples. However, the current studies on GH are still in the preliminary stage. There are few studies on the active target of GH, and few reports on the tissue distribution of GH after it enters the human body. The chemical components, especially the active components absorbed into the body and the processes most likely to change, also need to be studied. Therefore, in future studies, the combination of blood pharmacodynamics, pharmacokinetics and pharmacokinetics can be used to further study the substance basis of GH in vivo and the mechanism of disease treatment. Carrying out the analysis of effective components of GH in the body, and making clear the changes in the effective components in the blood after its action on the body. Only the chemical components absorbed into the body can be the material basis for the efficacy of traditional Chinese medicine. Considering that improper operation in the treatment of blood samples is easy to produce errors, if the sampling and testing can be completed in one step, the reliability of the experimental results will be greatly improved. It is also very necessary for the establishment of GH quality standards.
The plant resources of the GH are abundant, and it is widely used as a medicinal plant in folk areas. The theory of traditional Chinese medicine is not only traditional Chinese culture but also the treasure of world culture. As an excellent traditional medicine for the treatment of urinary system diseases, GH deserves more comprehensive and in-depth research. With the development of science and technology, it will make a greater contribution to the prevention and treatment of diseases.
Abbreviations
| GH | Glechomae herba |
| GLK | Glechoma longituba (Nakai) Kupr |
| LH | Lysimachiae herba |
| LCH | Lysimachia christinae Hance |
| TCM | Traditional Chinese medicine |
| UHPLC-QTOF-MS/MS | Ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight tandem mass analysis |
| WGH | Water extract of GH |
| CaOx | Calcium oxalate |
| OS | Oxidative stress |
| NF-kB | Nuclear factor-k-gene binding |
| EGH | Ethanol extract from GH |
| COX-2 | Cyclooxygenase-2 |
| iNOS | Inducible nitric-oxide synthase |
| 5-HT | 5-Hydroxytryptamine |
| EBV | Epstein-Barr virus |
| SOD | Serum superoxide dismutase |
| MDA | Malondialdehyde |
| AGH | Ethyl acetate extract from GH |
| UPLC | Ultra-performance liquid chromatography |
| QTOF | Quadrupole time-of-flight |
| HPLC | High-performance liquid chromatography |
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the Innovative Talents Funding of The Heilongjiang University of Chinese Medicine Doctoral Innovation Foundation [No. 2014bs05]; the Graduate Innovative Research Project Foundation of Heilongjiang University of Chinese Medicine [No. 2019yjscx013]; the Application Technology Research and Development Projects of Harbin Technology Bureau [No. 2014RFQXJ149]; the Natural Science Foundation of Heilongjiang Province [No. H2017052]; Heilongjiang University of Chinese Medicine [No. 2018RCD25]; the National Natural Science Foundation Matching Project [No. 2018PT02]; the National Natural Science Foundation Matching Project [No. 2017PT01]; Heilongjiang Postdoctoral Scientific Research Developmental Fund [No. LBH Q16210 and LBH-Q17161]; the National Natural Science Foundation of China [No. 81703684, 81803690, 81973604]; the Postdoctoral Initial Fund of Heilongjiang Province, the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province [No. UNPYSCT 2017219 and UNPYSCT 2017215].References
- P. D. Cantino and R. W. Sanders, Syst. Bot., 1986, 11, 163 CrossRef.
- Y. Yongyang, World J. Tradit. Chin. Med., 2015, 10, 1503–1505 Search PubMed.
- State Administration of Traditional Chinese Medicine, Chinese Materia Medica, Shanghai Scientific and Technical Publishers, Shanghai, 1999, pp. 494–498 Search PubMed.
- Y. Tao and M. Y. Shi, Chin. J. Hosp. Pharm., 2011, 23, 21–23 Search PubMed.
- Y. Lin, China J. Chin. Mater. Med., 1988, 13, 9 Search PubMed.
- N. Y. Yang, J. A. Duan, P. Li and S. H. Qian, Chin. J. Nat. Med., 2006, 41, 431–434 CAS.
- Y. Zhang and Z. Wang, Chem. Nat. Compd., 2007, 43, 625–628 CrossRef CAS.
- Q. J. Zhang, X. S. Yang, H. Y. Zhu, Y. Yao, X. J. Hao and B. A. Song, Chin. Tradit. Herb. Drugs, 2006, 037, 1780–1781 CAS.
- R. G. Shu, H. Cai, X. M. Wang, H. Y. Deng and P. F. Tu, Chin. Tradit. Herb. Drugs, 2017, 48, 4215–4218 Search PubMed.
- X. He, Urolithiasis, 2012, 539–541 Search PubMed.
- Y. Tao, Y. X. Xiao, M. Y. Shi, X. L. Nie and C. X. Liu, Herb. Med., 2007, 8, 840–843 Search PubMed.
- Y. Tao and M. Y. Shi, Chin. J. Hosp. Pharm., 2011, 023, 21–23 Search PubMed.
- M. Warda, E. Stamirowskakrzaczek and M. Kulik, J. Water Land Dev., 2013, 19, 77–82 CAS.
- X. Yang, B. C. Wang, X. Zhang, W. Q. Liu, J. Z. Qian, W. Li, J. Deng, G. K. Singh and H. Su, J. Ethnopharmacol., 2011, 137, 57–63 CrossRef PubMed.
- F. G. Ruan and Y. Zhou, Strait Pharm. J., 2012, 024, 43–45 Search PubMed.
- S. W. Tang, Jing Shi Zheng Lei Beiji Bencao, Huaxia Publishing House, Beijing, 1993, p. 265 Search PubMed.
- J. B. Li, Jiangxi J. Tradit. Chin. Med., 2004, 035, 21 Search PubMed.
- X. S. Zeng, Chin. J. Tradit. Med. Sci. Technol., 2004, 011, 125 Search PubMed.
- Y. J. Chen, Chin. Clin. Dr., 1975, 19 Search PubMed.
- S. Rui, Z. Yun, X. D. Cong and B. C. Cai, Chin. Arch. Tradit. Chin. Med., 2010, 28, 2511–2514 Search PubMed.
- H. S. Huang, L. Jiang, J. Liu, Y. B. Zhang, G. C. Wang and Y. L. Li, Chin. Med. Mater., 2017, 040, 844–847 Search PubMed.
- Y. Luo, Q. Wen, S. Yang, Y. Feng and T. Tan, Biomed. Chromatogr., 2020, 34, e4762 CAS.
- J. Li, Q. H. Shao, J. Kai, E. Mahmoud, X. Fang and C. Yu, Cancer Res., 2013, 73, 3290 Search PubMed.
- Q. Wen, Y. Feng, J. Zhang, Y. Luo and T. Tan, J. Sep. Sci., 2019, 42, 1312–1322 CrossRef PubMed.
- Z. B. Yu, X. Wu and Y. H. Ye, Nat. Prod. Res. Dev., 2008, 02, 73–75 Search PubMed.
- H. Y. Deng, M. M. Yuan, Y. Deng, L. G. Zhou, S. Zeng and G. P. Zhou, Chin. Med. Mater., 2018, 41, 1103–1107 Search PubMed.
- Q. F. Zhu, Y. Y. Wang and H. B. Qu, Chin. Tradit. Herb. Drugs, 2013, 44, 387–390 CAS.
- Q. J. Zhang, X. S. Yang, H. Y. Zhu, Y. Yao, X. J. Hao and B. A. Song, Nat. Prod. Res. Dev., 2006, 06, 55–56 Search PubMed.
- K. Hosni, A. Kerkenni, W. Medfei, N. B. Brahim and H. Sebei, Org. Chem. Int., 2010, 2010, 2010 CrossRef.
- J. F. Ma, Plant Cell Physiol., 2000, 41, 383–390 CrossRef CAS PubMed.
- Y. H. Fan, G. Zhou, L. Zhang, X. Y. Chen and L. I. Cui-Ping, Chin. J. Exp. Tradit. Med. Formulae, 2010, 13, 49–52 Search PubMed.
- Z. Y. Zhou, G. Y. Lin, J. L. Li and C. X. Wang, Chin. J. Mod. Appl. Pharm., 2011, 028, 737–739 CAS.
- X. Ouyang, F. Qin, R. Huang, D. Liang, C. Wang, H. Wang and Z. Liao, Phytomedicine, 2019, 63, 153037 CrossRef CAS PubMed.
- J. Fang, Chin. Med. Pharm., 2012, 028, 737–739 Search PubMed.
- T. Tan, J. Zhang, X. Xu, W. P. Huang and Y. Luo, Biomed. Chromatogr., 2018, 32, e4239 CrossRef PubMed.
- N. Y. Yang, J. A. Duan, P. Li, S. H. Qian and W. C. Hu, Chin. J. Nat. Med., 2014, 39, 695–698 Search PubMed.
- Y. N. Yun, D. J. Ao, L. I. Ping and Q. S. Hui, J. China Pharm. Univ., 2005, 36, 210–212 Search PubMed.
- Q. J. Zhang, Studies on the chemical constituents of Glechoma longituba (Nakai) Kupr, Guizhou University, 2006 Search PubMed.
- Y. Zhu, J. Zou and W. Zhao, J. Asian Nat. Prod. Res., 2008, 10, 199–204 CrossRef CAS PubMed.
- X. Ouyang, W. Mao, C. Wang, Y. Pan, D. Liang and H. Wang, Fitoterapia, 2019, 138, 104345 CrossRef CAS PubMed.
- Q. F. Zhu, Y. Y. Wang, W. Jiang and H. B. Qu, J. Asian Nat. Prod. Res., 2013, 15, 258–264 CrossRef CAS PubMed.
- Z. C. Xue, Henan J. Anim. Husb. Vet. Med., 2005, 026, 47 Search PubMed.
- S. X. Ge, D. Y. Peng, J. Q. Liu and L. Ye, Chin. Herb. Med., 2007, 30, 842–845 Search PubMed.
- Y. Nianyun, L. Pei and G. Jianming, Chin. J. Mod. Appl. Pharm., 2014, 31, 918–920 Search PubMed.
- M. S. J. Schepers, E. S. Van Ballegooijen, C. H. Bangma and C. F. Verkoelen, Kidney Int., 2005, 68, 1660–1669 CrossRef CAS PubMed.
- S. R. Khan, Urol. Res., 2006, 34, 86–91 CrossRef PubMed.
- Q. Liang, X. Li, W. Zhou, Y. Su and Z. Yue, Oxid. Med. Cell. Longevity, 2016, 2016, 1–11 CrossRef PubMed.
- S. Wang, P. Liang, Y. Han, B. Zhou and J. Chen, Trop. J. Pharm. Res., 2018, 17, 225 CrossRef CAS.
- R. d. Lima, J. C. Brondani, R. C. Dornelles, C. L. Lhamas and M. P. Manfron, Braz. J. Pharm. Sci., 2020, 56, e17419 CrossRef.
- M. M. Brandenburg, F. G. Rocha, P. L. Pawloski, B. d. S. Soley and M. F. Otuki, J. Ethnopharmacol., 2020, 259, 112840 CrossRef CAS PubMed.
- J. P. Kim, S. B. Song, I. S. Lee and Y. H. Bioorg, Med. Chem. Lett., 2011, 21, 3483–3487 CrossRef CAS PubMed.
- P. He, J. J. Zou and S. J. Fei, Yunnan J. Tradit. Chin. Med. Mater. Med., 2014, 035, 63–64 Search PubMed.
- Y. Zhang, J. J. Wen, L. I. Xiao-Feng, H. Zhang, W. X. Han, B. J. Wang, Z. J. Chen, Y. Zhu, X. M. University and X. M. University, Res. Pract. Chin. Med., 2018, 32, 31–33 Search PubMed.
- Y. Qu, H. Jiang, Y. Zhang, Z. Lan, W. He and D. Ma, Pestic. Sci. Adm., 2012, 4, 58–61 Search PubMed.
- F. M. Tian, Z. H. Huang, H. Wang, Q. Chen, P. Q. Zhuo and W. D. Chen, Journal of Gansu Normal Colleges, 2016, 3, 42–45 Search PubMed.
- Y. Tao, Y. X. Xiao, J. P. Yi and M. Y. Shi, Chin. Herb. Med., 2003, 026, 746–747 Search PubMed.
- W. C. Hu, Y. Guo, and X. J. Yu, in Seminar on inheritance innovation and development of traditional Chinese Medicine, 2007 Search PubMed.
- T. Yong, Y. X. Xiao and M. Y. Shi, Chin. J. Hosp. Pharm., 2004, 24, 65–67 Search PubMed.
- D. Jo, J. Lee, J. Noh, O. K. Kim and J. Kwon, Food Sci. Nutr., 2001, 6, 142–146 CAS.
- Y. L Hsu, P. L. Kuo and C. C. Lin, Life Sci., 2004, 75, 2303–2316 CrossRef PubMed.
- C. M. Ma, S. Q. Cai, J. R. Cui, R. Q. Wang, P. F. Tu, M. Hattori and M. Daneshtalab, Eur. J. Med. Chem., 2005, 40, 582–589 CrossRef CAS PubMed.
- M. Yoshida, M. Fuchigami, T. Nagao, H. Okabe, K. Matsunaga, J. Takata, Y. Karube, R. Tsuchihashi, J. Kinjo and K. Mihashi, Biol. Pharm. Bull., 2005, 28, 173–175 CrossRef CAS PubMed.
- C. S. Neethu, V. B. Kavitha, K. Krishnakumar, J. Anish and B. Dineshkumar, J. Drug Discovery Ther., 2015, 9–17 CAS.
- R. Zhou, Z. Zhang, L. Zhao, C. Jia, S. Xu, Q. Mai, M. Lu, M. Huang, L. Wang and X. Wang, J. Orthop. Res., 2011, 29, 846–852 CrossRef CAS PubMed.
- C. L. Yuan, P. Q. Wang and W. Y. Guo, Chin. Med. Pharmaco. Clinic, 2008, 024, 57–58 Search PubMed.
- J. Li, Q. Wen, Y. Feng, J. Zhang, Y. Luo and T. Tan, J. Sep. Sci., 2019, 1312–1322 CrossRef CAS PubMed.
- Y. Kumarasamy, P. J. Cox, M. Jaspars, L. Nahar and S. D. Sarker, Fitoterapia, 2002, 73, 721–723 CrossRef CAS PubMed.
- C. H. Tan and J. L. Li, Cancer Res. Clin., 1994, 006, 73–76 Search PubMed.
- Y. Zhang, H. H Liu and H. H. Guo, Res. Pract. Chin. Med., 2019, 033, 14–18 Search PubMed.
- S. Ni, D. Qian, J. A. Duan, J. Guo, E. X. Shang, Y. Shu and C. Xue, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2010, 878, 2741–2750 CrossRef CAS PubMed.
- Y. Luo, J. Zhang, Y. Feng, Q. Wen and T. Tan, J. Pharmaceut. Biomed. Anal., 2019, 164, 615–629 CrossRef CAS PubMed.
- Q. Wang, J. A. Duan, D. W. Qian, M. Kong and N. Y. Yang, J. Nanjing Univ. Tradit. Chin. Med., 2006, 22, 44–46 CAS.
- L. Y. Zhu, D. W. Qian, J. A. Duan and S. H. Qian, Lishizhen Med. Mater. Med. Res., 2009, 1, 121–123 Search PubMed.
- X. L. Yang and W. U. Yun-Fei, Herb. Med., 2009, 4, 26–29 Search PubMed.
- C. B. Liu, D. Y. Peng and Z. Q. Liu, Anhui Med. Pharm. J., 2010, 02, 52–53 Search PubMed.
- Y. L. Liao, A. N. Wei and H. U. Guang-Xu, Herb. Med., 2011, 30, 1075–1080 CrossRef CAS PubMed.
- T. C. Huang, J. Nie and L. Q. Zhang, Chin. J. Exp. Tradit. Med. Formulae, 2013, 19, 132–135 CAS.
- X. T. Deng, China Pract. Med., 2014, 29, 255–256 Search PubMed.
- Y. Deng, Y. S. Chen and Q. Wang, J. Pharm. Anal., 2018, 038, 643–647 Search PubMed.
- S. M. Ni, D. W. Qian, J. A. Duan, N. Y. Yang and J. M. Guo, Nat. Prod. Commun., 2011, 6, 17–20 CrossRef CAS PubMed.
- Y. Bo, H. Yu, X. Cai and H. Xi, Guangdong Chem. Ind., 2014, 17, 171–172 Search PubMed.
- H. Cai, B. Cai, Q. Shan and G. Cao, Pharmacogn. Mag., 2013, 9, 216 CrossRef PubMed.
- L. Yang, A. J. Hou, M. L. Yan, X. D. Xing, X. Y. Guo, H. Jiang, B. Y. Yang, H. X. Kuang, K. Chan and Q. H. Wang, World J. Tradit. Chin. Med., 2019, 5, 50–60 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01366a |
| This journal is © The Royal Society of Chemistry 2021 |