Open Access Article
Open Access ArticlePolysaccharide from rubescens: extraction, optimization, characterization and antioxidant activities
Shuang Chenga,
Fei Hea,
Longyang Fua and
Yadong Zhang *ab
*ab
aSchool of Chemical Engineering, Zhengzhou University, Zhengzhou 450001, China
bJiyuan Research Institute, Zhengzhou University, Jiyuan 459000, China
First published on 25th May 2021
Abstract
In this work, rubescens polysaccharide was extracted and extraction conditions were optimized with the response surface method (RSM). The extracted polysaccharide was structurally elucidated and its antioxidant activity was investigated. The best extraction conditions were as follows: the solvent-solid ratio was 33.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the extraction temperature was 87.70 °C and the extraction time was 2.59 h. Under optimum conditions, the predicted yield of the polysaccharide was 11.92%. The preliminary structural characteristics were analyzed by HPLC, X-ray, and FT-IR. Results showed that the polysaccharide composition was mannose
1, the extraction temperature was 87.70 °C and the extraction time was 2.59 h. Under optimum conditions, the predicted yield of the polysaccharide was 11.92%. The preliminary structural characteristics were analyzed by HPLC, X-ray, and FT-IR. Results showed that the polysaccharide composition was mannose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose
glucose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) galactose
galactose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xylose
xylose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) arabinose = 2.27
arabinose = 2.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.65
4.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.57
2.57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.85. In addition, the rubescens polysaccharide possessed typical characteristic absorption peaks of polysaccharides. During the antioxidant experiments, the rubescens polysaccharide showed great reducing capacity and strong antioxidant activities on DPPH, ABTS, and hydroxyl radicals. This proved that rubescens polysaccharide has the potential to be a resource for the development of natural antioxidants. Rubescens has become a research object highly valued by the international oncology community and by western institutions for the research and development of new drugs due to its excellent anti-tumor activity. The research on the extraction of polysaccharide from rubescens provides a technical reference for the further utilization of rubescens resources. Hot-water extraction has the advantages of simple operation, easy access to materials and mild extraction conditions, and it can better preserve the original biological activity of the polysaccharide. The research on the antioxidant activity of rubescens polysaccharide provides theoretical support for its use as a natural antioxidant.
1.85. In addition, the rubescens polysaccharide possessed typical characteristic absorption peaks of polysaccharides. During the antioxidant experiments, the rubescens polysaccharide showed great reducing capacity and strong antioxidant activities on DPPH, ABTS, and hydroxyl radicals. This proved that rubescens polysaccharide has the potential to be a resource for the development of natural antioxidants. Rubescens has become a research object highly valued by the international oncology community and by western institutions for the research and development of new drugs due to its excellent anti-tumor activity. The research on the extraction of polysaccharide from rubescens provides a technical reference for the further utilization of rubescens resources. Hot-water extraction has the advantages of simple operation, easy access to materials and mild extraction conditions, and it can better preserve the original biological activity of the polysaccharide. The research on the antioxidant activity of rubescens polysaccharide provides theoretical support for its use as a natural antioxidant.
1. Introduction
Rubescens, also known as bingling grass, is a famous traditional medicine. Modern research shows that rubescens has efficacy in clearing heat and detoxicating, reducing inflammation, promoting blood circulation and dissipating swelling, and anti-tumor activity.1,2 Jiyuan has rich resources and unique population of rubescens. As early as 2006, Jiyuan rubescens was identified as a geographical indication protected product. Rubescens contains oridonin, ponicidin, flavonoids, and polysaccharides. Modern pharmacological and toxicological studies have proved that rubescens polysaccharide (RP) has many pharmacological activities, such as immune regulation and anti-tumor activity.3,4 However, the current research on rubescens is mostly focused on its fat-soluble components, such as diterpenoids,5–7 and there are few studies on RP.Currently, there are many methods of extracting polysaccharides, such as heating, enzyme or ultrasonic-assisted methods. However, as is known, solvents are not environmentally friendly and ultrasonic or enzyme-assisted extraction is uneconomical, which limits the usage of these techniques. Hot-water extraction has the advantages of simple operation, easy access to materials and mild extraction conditions, and it can better preserve the original biological activity of polysaccharides.8,9 Therefore, the purpose of this work was to apply response surface methodology (RSM) to optimize the extraction conditions of RP. RSM has been used to optimize extraction processes of many natural active ingredients in a wide range of fields, such as pharmaceutical and food industries.10–12 Box–Behnken design (BBD) is one type of RSM; it is widely used because it is effective, and easy to arrange and explain experiments.13 Therefore, we chose the BBD method.
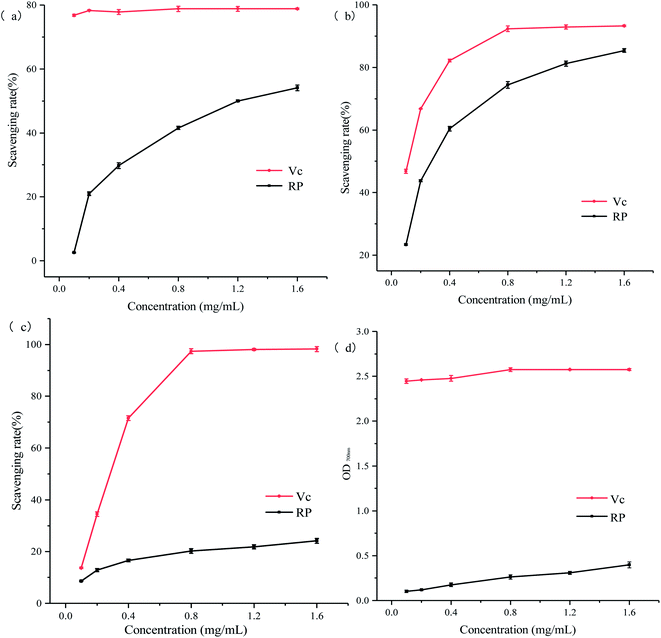
Plant polysaccharides have certain biological and antioxidant activities,14,15 and they have always been an active area of research. It is expected that polysaccharides can be applied to food and medicine to promote human health. Because polysaccharides have good antioxidant properties, they can be developed as natural antioxidants to prevent the occurrence of chronic diseases.16,17 Herein, we studied the antioxidant activity of RP from four aspects: reducing ability, DPPH radical scavenging ability, hydroxyl radical scavenging ability, and ABTS radical scavenging ability. It is hoped that the use of RP will provide some theoretical basis for the development of medicines and functional foods in the future.
2. Materials and methods
The rubescens sample was purchased from Henan Jiyuan Research Institute, crushed and screened with a 20 mesh screen to obtain fractions. All other chemicals used in the experiment were of analytical grade.2.1 Extraction of crude polysaccharides
Rubescens (100 g) was extracted twice with petroleum ether at 80 °C for 2 h each time in order to remove some colored materials and lipids. After being dried at 60 °C for 12 h, the defatted sample (3 g) was extracted under the conditions of liquid–solid ratio (10–50), extraction time (1–5 h) and extraction temperature (60–100 °C). The filtrate was measured in a 250 mL volumetric flask. D-Glucose was used as the standard, and the content of polysaccharide was determined by the phenol-sulfuric acid method.182.2 Experimental design and statistical analysis
Based on single factor research, three factors, namely X1 (liquid–solid ratio, mL g−1), X2 (extraction temperature, °C) and X3 (extraction time, h) were studied to determine their influence on the polysaccharide yield. According to the BBD method, three levels were designed, namely X1 (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 30
1, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 40
1, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), X2 (70 °C, 80 °C, 90 °C) and X3 (1 h, 2 h, 3 h). The design of each factor and level is shown in Table 1; the whole design consisted of the group of 17 experiments in Table 2.
1), X2 (70 °C, 80 °C, 90 °C) and X3 (1 h, 2 h, 3 h). The design of each factor and level is shown in Table 1; the whole design consisted of the group of 17 experiments in Table 2.
| Factor | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Liquid/solid ratio (mL g−1) | 20 | 30 | 40 |
| Extraction temperature (°C) | 70 | 80 | 90 |
| Extraction time (h) | 1 | 2 | 3 |
| Run order | X1 (liquid–solid ratio, mL g−1) | X2 (extraction temperature, °C) | X3 (extraction time, h) | Y (polysaccharide yield, %) | |
|---|---|---|---|---|---|
| Observed | Predicted | ||||
| 1 | 0 | −1 | −1 | 5.95 | 5.86 |
| 2 | 1 | 0 | −1 | 6.39 | 6.66 |
| 3 | 0 | 1 | −1 | 6.82 | 6.77 |
| 4 | −1 | −1 | 0 | 7.41 | 7.63 |
| 5 | 0 | 0 | 0 | 11.06 | 10.92 |
| 6 | 0 | 0 | 0 | 10.92 | 10.92 |
| 7 | 1 | −1 | 0 | 7.85 | 7.67 |
| 8 | 1 | 0 | 1 | 9.89 | 10.02 |
| 9 | 0 | 0 | 0 | 10.92 | 10.92 |
| 10 | −1 | 0 | −1 | 5.66 | 5.53 |
| 11 | −1 | 1 | 0 | 8.58 | 8.76 |
| 12 | 0 | 1 | 1 | 11.36 | 10.45 |
| 13 | 1 | 1 | 0 | 10.92 | 10.70 |
| 14 | 0 | 0 | 0 | 11.06 | 10.92 |
| 15 | −1 | 0 | 1 | 9.46 | 9.19 |
| 16 | 0 | 0 | 0 | 10.62 | 10.92 |
| 17 | 0 | −1 | 1 | 8.14 | 8.193 |
A second-order polynomial model was proposed for the response (Yi), and the regression coefficients were as follows:
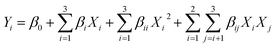 | (1) |
2.3 Physical and chemical properties
The chemical properties were determined by α-naphthol reaction, phenol-sulfuric acid reaction, FeCl3 reaction, Fehling's test, iodination reaction, carbazole reaction and ninhydrin test.2.4 Preliminary analysis of polysaccharides
The HPLC system was equipped with a C18 column (250 mm × 4.6 mm) and a DAD detector (250 nm). The mobile phase was 0.1 mol L−1 phosphate buffer (pH 6.7)–acetonitrile (82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18) at a flow rate of 1.0 mL min−1. The column temperature was 35 °C, and 10 μL of the resulting solution was injected each time.
18) at a flow rate of 1.0 mL min−1. The column temperature was 35 °C, and 10 μL of the resulting solution was injected each time.
2.5 Antioxidant activities in vitro
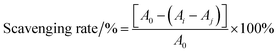 | (2) |
2.6 Statistical analyses
Each experiment was repeated three times, and the average was taken as the observed response value. All the data are shown as the mean ± standard deviation (SD); analysis of variance (ANOVA) and P values were obtained by employing statistical software (SPSS 13.0.) P values below 0.05 were regarded as statistically significant.3. Results and discussion
3.1 Optimization of polysaccharide extraction
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
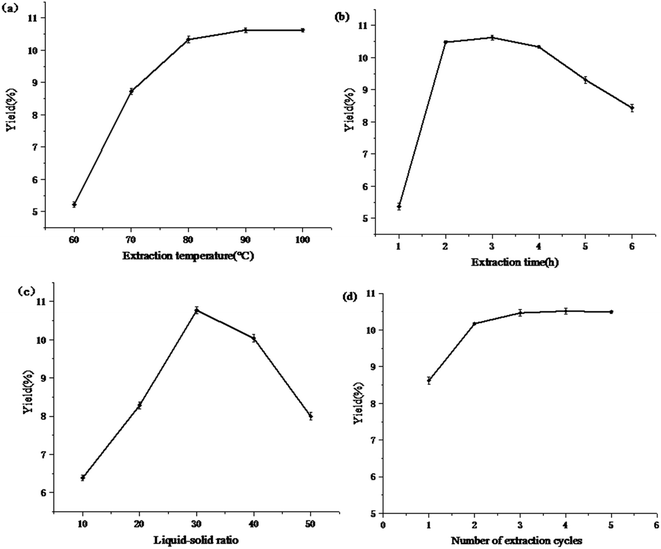
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40. As shown in Fig. 1(a), the extraction rate of polysaccharides rose sharply, but when the temperature exceeded 80 °C, the curve gradually became flat. The reason was that the increase in temperature was beneficial to the mass transfer rate of polysaccharide molecules and increased the solubility of the polysaccharides at the same time.25,26 When the temperature reached 80 °C, the polysaccharides in the solution almost reached a saturated state. Therefore, 80 °C was considered as the optimal extraction temperature.
40. As shown in Fig. 1(a), the extraction rate of polysaccharides rose sharply, but when the temperature exceeded 80 °C, the curve gradually became flat. The reason was that the increase in temperature was beneficial to the mass transfer rate of polysaccharide molecules and increased the solubility of the polysaccharides at the same time.25,26 When the temperature reached 80 °C, the polysaccharides in the solution almost reached a saturated state. Therefore, 80 °C was considered as the optimal extraction temperature.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and a number of extraction cycles of 2. As the extraction time increases, the extraction rate of polysaccharides gradually increases, as shown in Fig. 1(b). This may be because the polysaccharides were fully leached in the early stage of extraction, but the long-term extraction process may cause some hydrolysis and oxidation of polysaccharides and some polysaccharides may adhere to the macromolecular proteins to form precipitates.26,27 From an economical point of view, the optimized extraction time was determined to be 2 h.
40 and a number of extraction cycles of 2. As the extraction time increases, the extraction rate of polysaccharides gradually increases, as shown in Fig. 1(b). This may be because the polysaccharides were fully leached in the early stage of extraction, but the long-term extraction process may cause some hydrolysis and oxidation of polysaccharides and some polysaccharides may adhere to the macromolecular proteins to form precipitates.26,27 From an economical point of view, the optimized extraction time was determined to be 2 h.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 was favorable for polysaccharide extraction.
30 was favorable for polysaccharide extraction.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. As shown in Fig. 1(d), the polysaccharide yield reached 10.47% as the number of extraction periods increased from 1 to 3. However, there was no significant change in the yield of polysaccharides when the number of extractions continued to increase. The greater the number of extraction times, the greater the energy consumption. The number of extraction cycles was 3.
30. As shown in Fig. 1(d), the polysaccharide yield reached 10.47% as the number of extraction periods increased from 1 to 3. However, there was no significant change in the yield of polysaccharides when the number of extractions continued to increase. The greater the number of extraction times, the greater the energy consumption. The number of extraction cycles was 3.According to the single-parameter study, extraction temperatures of 70–90 °C, extraction times of 1–3 h, and liquid–solid ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20–1
20–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 were adopted for the RSM experiments.
40 were adopted for the RSM experiments.
| Y = 10.92 + 0.49X1 + 1.04X2 + 1.75X3 + 0.48X1X2 − 0.075X1X3 + 0.59X2X3 − 1.22X12 − 1.00X22 − 1.84X32 | (3) |
| Sources | Sum of squares | Degrees of freedom | Mean square | F Value | P Value |
|---|---|---|---|---|---|
| a R2 = 0.9924; Radj2 = 0.9825; Rpred2 = 0.9026; C.V.% = 2.97. | |||||
| Model | 65.03 | 9 | 7.23 | 101.04 | <0.0001 |
| X1 | 1.94 | 1 | 1.94 | 27.13 | 0.0012 |
| X2 | 8.67 | 1 | 8.67 | 121.29 | <0.0001 |
| X3 | 24.61 | 1 | 24.61 | 344.06 | <0.0001 |
| X1X2 | 0.90 | 1 | 0.90 | 12.62 | 0.0093 |
| X1X3 | 0.022 | 1 | 0.022 | 0.31 | 0.5923 |
| X2X3 | 1.38 | 1 | 1.38 | 19.31 | 0.0032 |
| X12 | 6.28 | 1 | 6.28 | 87.88 | <0.0001 |
| X22 | 4.25 | 1 | 4.25 | 59.38 | 0.0001 |
| X32 | 14.32 | 1 | 14.32 | 200.26 | <0.0001 |
| Residual | 0.50 | 7 | 0.072 | ||
| Lack of fit | 0.37 | 3 | 0.12 | 3.84 | 0.1134 |
| Pure error | 0.13 | 4 | 0.032 | ||
| Cor. total | 65.53 | 16 | |||
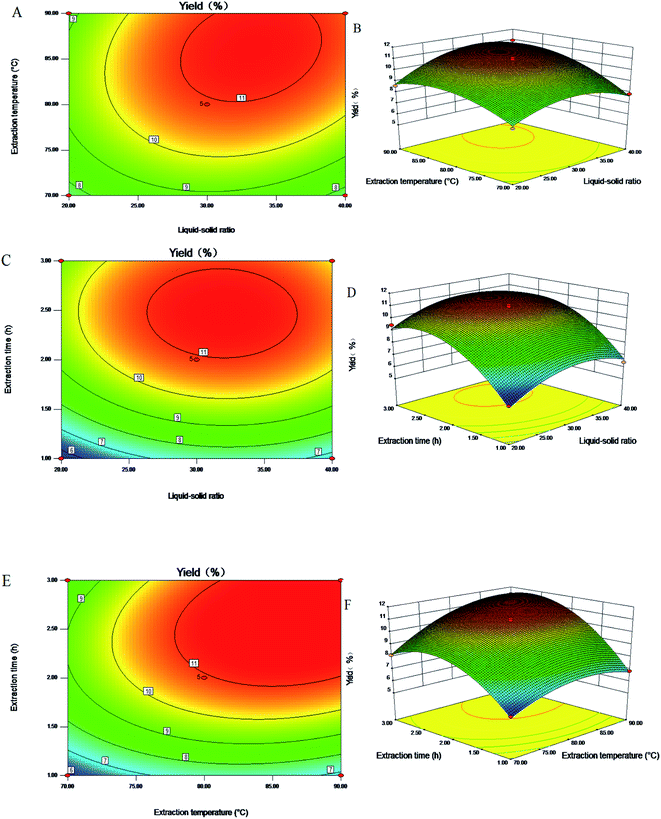
The values of R2 represent the fit ability of the model. For the quadratic regression model, the value of R2 and the predicted R2 were 0.9924 and 0.9062, respectively, which indicates that the experimental value has high accuracy and good reliability. In addition, the adjusted coefficient of determination (Radj2 = 0.9825) showed that the model was of great significance, and the coefficient of variation (C.V.% = 2.97) clearly indicated a very high degree of the accuracy and reliability of the polynomial model.29 Compared with the pure error, the lack of fit term of the equation was 0.11, with a p-value less than 0.05. The lack of fit was not significant, indicating that the model was stable and could be applied to predict the extraction rate within the experimental range adequately. As shown in Table 3, the linear coefficients (X1, X2 and X3), quadratic coefficients (X12, X22 and X32) and cross product coefficients (X1X2, X2X3) of the model were of great significance, and their p-values were less than 0.05. At the same time, other coefficients (X1X3) had no great effect on the extraction rate of RP. A conclusion could be obtained: the order of factors that had great effects on the response value of the extraction ratio of RP by observing the quadratic and linear coefficients was extraction time > extraction temperature > liquid–solid ratio.
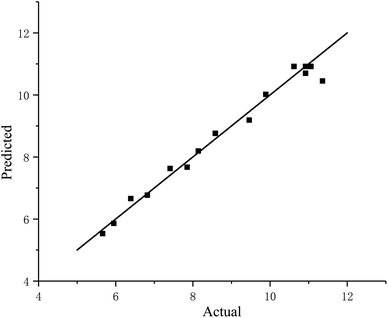
As shown in Fig. 2, the observed value was compared to the predicted value calculated from the regression model, and the predicted value well matched the experimental value. This result suggests that the model was able to identify the operating conditions for the extraction of RP.
There are few studies on the extraction of rubescens; however, there are more similar studies using other materials. From previous studies, the effects of the linear terms and interaction terms were diverse between different species and different materials.30 Song et al.31 studied the extraction of defatted peanut cake polysaccharide and drew a conclusion that the best extraction conditions were extraction temperature 48.7 °C, extraction time 1.52 h, and ethanol concentration 61.9% (v/v), respectively. Luo et al.32 worked on dioscorea nipponica makino polysaccharide and found that the optimum conditions were liquid–solid ratio 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, extraction time 134 min, and extraction temperature 95 °C; they also found that the extraction temperature has a significant effect on the yield of polysaccharides. Dried materials compared with fresh materials take more energy to extract the polysaccharide, and a higher extraction temperature and a longer extraction time are needed.33 Different types of polysaccharides have different physical and chemical properties and structural characteristics; therefore, different types of polysaccharides have different solubilities in water and different extraction conditions.
1, extraction time 134 min, and extraction temperature 95 °C; they also found that the extraction temperature has a significant effect on the yield of polysaccharides. Dried materials compared with fresh materials take more energy to extract the polysaccharide, and a higher extraction temperature and a longer extraction time are needed.33 Different types of polysaccharides have different physical and chemical properties and structural characteristics; therefore, different types of polysaccharides have different solubilities in water and different extraction conditions.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.33) was incorporated into the regression equation, a yield of 11.91% of the polysaccharides could be obtained.
33.33) was incorporated into the regression equation, a yield of 11.91% of the polysaccharides could be obtained.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33, and extraction time 2.06 h. Taking into account the feasibility of the actual operation, the extraction conditions of the polysaccharides were modified to extraction temperature 85 °C, material–liquid ratio 1
33, and extraction time 2.06 h. Taking into account the feasibility of the actual operation, the extraction conditions of the polysaccharides were modified to extraction temperature 85 °C, material–liquid ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33, and extraction time 2 h. Under these conditions, three parallel experiments were performed, and the average value was taken to obtain an RP extraction rate of 11.16% with an error of 0.11%. This value was close to the theoretical prediction value, indicating that the use of this model to optimize the process of extracting polysaccharides has certain practical significance.
33, and extraction time 2 h. Under these conditions, three parallel experiments were performed, and the average value was taken to obtain an RP extraction rate of 11.16% with an error of 0.11%. This value was close to the theoretical prediction value, indicating that the use of this model to optimize the process of extracting polysaccharides has certain practical significance.3.2 Physical and chemical properties analysis
The RP content was 74.3 ± 1.2% measured by the phenol-sulfuric acid method after deproteinization and decolorization. Additionally, it was free of amino acids, starch, and proteins.3.3 Preliminary structural analysis
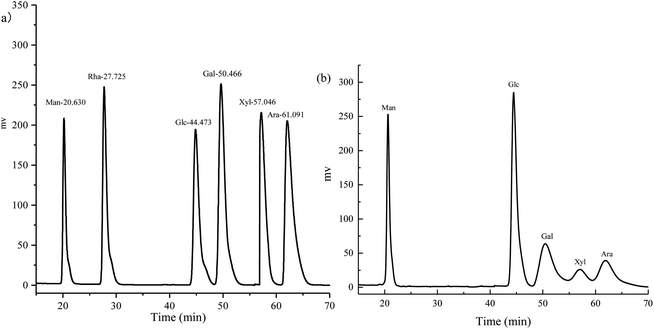
The monosaccharide composition of the RP is illustrated in Fig. 4. The sugar compositional analysis of RP was mannose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose
glucose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) galactose
galactose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xylose
xylose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) arabinose = 2.27
arabinose = 2.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.65
4.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.57
2.57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.85. The molar ratio is illustrated in Table 4.
1.85. The molar ratio is illustrated in Table 4.
| Compound | Molar ratio (%) |
|---|---|
| Mannose | 18.4 |
| Glucose | 37.7 |
| Galactose | 20.8 |
| Xylose | 8.1 |
| Arabinose | 15.0 |
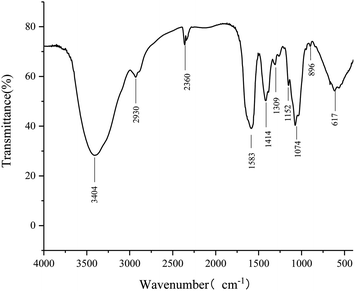
The FT-IR spectrum is shown in Fig. 5. Three strong absorption bands at 1152, 1074, and 1039 cm−1 in the range of 1200–1000 cm−1 indicated that the monosaccharide in RP had a pyranose ring.31 The characteristic strong peak at 3404 cm−1 was due to O–H stretching vibrations, and the vibration at 2930 cm−1 exhibited C–H stretching vibrations. The absorption peak at 1583 cm−1 was due to associated water.35 The absorption peak at 1414 cm−1 was dominated by C–O stretching vibrations, and the peak at 1070 cm−1 indicated that RP contains the ether bond (C–O–C) and the hydroxyl of the pyranose ring. The absorption at 896 cm−1 revealed that the polysaccharide contained a β-glycosidic linkage.36,37
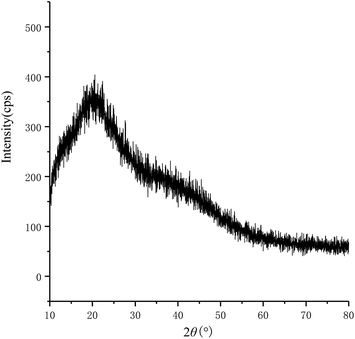
As shown in Fig. 6, when 2θ was close to 20°, the characteristic diffraction curve of the polysaccharides was shown. This indicated that the polysaccharides existed in both crystalline and amorphous states and were semi-crystalline polymers.
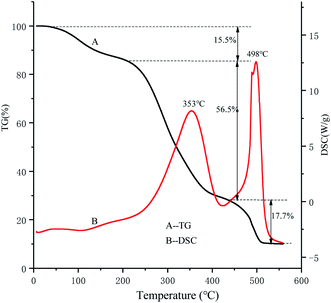
As shown in Fig. 7, the TG curve was divided into three different stages. The first stage weight loss range was 25–205 °C, mainly due to the loss of water, and the mass loss rate was about 15.5%. The second rapid weight loss stage occurred at 205–433 °C, and the weight loss was 56.5%, which may be related to the thermal decomposition of the polysaccharides. When the third weight loss was in the range of 433–528 °C, the quality of the carbon was gradually reduced due to the thermal decomposition of carbon.
3.4 Antioxidant activity assays
4. Conclusions
RSM was applied to optimize the extraction parameters in this study. The experimental value of the polysaccharide yield varied from 5.66% to 11.36%. The variable with the largest effect was extraction time. The optimal conditions for the extraction of polysaccharide were as follows: extraction time 2 h, material–liquid ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 and extraction temperature 85 °C. The preliminary characterization of RP was mainly mannose
33 and extraction temperature 85 °C. The preliminary characterization of RP was mainly mannose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose
glucose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) galactose
galactose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xylose
xylose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) arabinose = 2.27
arabinose = 2.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.65
4.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.57
2.57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.85 by HPLC. Regarding antioxidant activity, the antioxidant capacity of RP was generally lower than that of ascorbic acid. However, RP performed well in the scavenging ability of ABTS free radicals. RP had a certain scavenging ability for DPPH and hydroxyl radicals. This result proved that RP can be used as a potential natural antioxidant for development in the future. In addition, further studies on the exact chemical structure of RP and the mechanism of its antioxidant activity are ongoing.
1.85 by HPLC. Regarding antioxidant activity, the antioxidant capacity of RP was generally lower than that of ascorbic acid. However, RP performed well in the scavenging ability of ABTS free radicals. RP had a certain scavenging ability for DPPH and hydroxyl radicals. This result proved that RP can be used as a potential natural antioxidant for development in the future. In addition, further studies on the exact chemical structure of RP and the mechanism of its antioxidant activity are ongoing.
Conflicts of interest
There are no conflicts to declare.References
- N. Bai, K. He, Z. Zhou, M.-L. Tsai and L. Zhang, Ent-Kaurane Diterpenoids from Rabdosia rubescens and Their Cytotoxic Effects on Human Cancer Cell Lines, Planta Med., 2010, 76(2), 140–145 CrossRef CAS PubMed.
- N. Bai, H. Kan, Z. Zhou, C. S. Lai, Z. Li, Z. Quan, X. Shao, M. H. Pan and C. T. Ho, Flavonoids from Rabdosia rubescens exert anti-inflammatory and growth inhibitory effect against human leukemia HL-60 cells, Food Chem., 2010, 122(3), 831–835 CrossRef CAS.
- Y. Ding, C. Y. Ding, N. Ye, Z. Q. Liu, E. A. Wold, H. Y. Chen, C. Wild, Q. Shen and J. Zhou, Discovery and development of natural product oridonin-inspired anticancer agents, Eur. J. Med. Chem., 2016, 47, 102–117 CrossRef PubMed.
- S. X. Huang, Y. Zhou, J. X. Pu, R. T. Li and H. D. Sun, Cytotoxic ent-kauranoid derivatives from Isodon rubescens, Tetrahedron, 2006, 62(20), 4941–4947 CrossRef CAS.
- H. D. Sun, Q. Z. Zhou and T. Fujita, Rubescensin D, a diterpenoid from Rabdosia rubescens, Phytochemistry, 1992, 31, 1418–1419 CrossRef CAS.
- Q. B. Han, J. X. Zhang, A. H. Zhao, H. D. Sun, Y. Lu, Y. S. Wu and Q. T. Zheng, Two novel tricyclic diterpenoids from Isodon rubescens var. taihangensis, Tetrahedron, 2004, 60(10), 2373–2377 CrossRef CAS.
- J. Yang, W. G. Wang, H. Y. Wu, M. Liu, H. Y. Jiang, X. Du, Y. Li, J. X. Pu and H. D. Sun, Ent-Kaurene diterpenoids from Isodon phyllostachys, Tetrahedron Lett., 2016, 58(4), 349–351 CrossRef.
- P. Gong, S. Y. Wang, M. Liu, F. X. Chen and X. F. Chen, Extraction methods, chemical characterizations and biological activities of mushroom polysaccharides: a mini-review, Carbohydr. Res., 2020, 494, 108037 CrossRef CAS PubMed.
- S. Lei, Bioactivities, isolation and purification methods of polysaccharides from natural products: a review, Int. J. Biol. Macromol., 2016, 37–48 Search PubMed.
- S. S. J. Chelladurai, K. Murugan, A. P. Ray, M. Upadhyaya, V. Narasimharaj and S. Gnanasekaran, Optimization of process parameters using response surface methodology: a review, Mater. Today: Proc., 2020, 1301–1304 Search PubMed.
- Y. X. Sun, T. B. Li, J. W. Yan and J. C. Liu, Technology optimization for polysaccharides (POP) extraction from the fruiting bodies of Pleurotus ostreatus by Box–Behnken statistical design, Carbohydr. Polym., 2010, 80(1), 242–247 CrossRef CAS.
- H. Zhao, J. Wang and Z. Lu, Optimization of process parameters of the Pholiota squarrosa extracellular polysaccharide by Box–Behnken statistical design, Carbohydr. Polym., 2009, 77(3), 677–680 CrossRef CAS.
- J. P. Maran, S. Manikandan, K. Thirugnanasambandham, C. V. Nivetha and R. Dinesh, Box-Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide, Carbohydr. Polym., 2013, 92(1), 604–611 CrossRef PubMed.
- J. Fan, H. Feng, Y. Yu, M. Sun, Y. Liu, T. Li, X. Sun, S. Liu and M. Sun, Antioxidant activities of the polysaccharides of Chuanminshen violaceum, Carbohydr. Polym., 2017, 157, 629–636 CrossRef CAS PubMed.
- S. Y. Tian, C. C. Hao, G. K. Xu, J. J. Yang and R. G. Sun, Optimization conditions for extracting polysaccharide from Angelica sinensis and its? antioxidant activities, J. Food Drug Anal., 2017, 766–775 CrossRef CAS PubMed.
- Y. Zhang, D. Ling, X. Kong and L. Chen, Characterization and in vitro antioxidant activities of polysaccharides from Pleurotus ostreatus[J]., Int. J. Biol. Macromol, 2012, 51(3), 259–265 CrossRef CAS PubMed.
- J. Fang, Z. Wang, P. Wang and M. Wang, Extraction, structure and bioactivities of the polysaccharides from Ginkgo biloba: a review, Int. J. Biol. Macromol, 2020, 162, 1897–1805 CrossRef CAS PubMed.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Colorimetric Method for Determination of Sugars and Related Substances, Anal. Chem., 1956, 28(3), 350–356 CrossRef CAS.
- J. J. Zhang, Q. B. Zhang, J. Wang, X. L. Shi and Z. S. Zhang, Analysis of the monosaccharide composition of fucoidan by precolumn derivation HPLC, Chin. J. Oceanol. Limnol., 2009, 27(3), 578–582 CrossRef CAS.
- L. Nenad, S. Vojin, N. Stevo, P. Milenko and U. Dragan, A study of HAp/PLLA composite as a substitute for bone powder, using FT-IR spectroscopy, Biomaterials, 2001, 22(6), 571–575 CrossRef.
- S. Kazuko, F. Kuniko, Y. Keiko and N. Takashi, Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion, J. Agric. Food Chem., 1992, 40(6), 945–948 CrossRef.
- C. J. Yue, J. Chen, R. R. Hou, J. Liu and X. P. Li, Effects of Selenylation Modification on Antioxidative Activities of Schisandra chinensis Polysaccharide, PLoS One, 2015, e0134363 CrossRef PubMed.
- J. Tang, J. Nie, D. P. Li, W. J. Zhu, S. P. Zhang, F. Ma, Q. Sun, J. Song, Y. L. Zheng and P. Chen, Characterization and antioxidant activities of degraded polysaccharides from Poria cocos sclerotium, Carbohydr. Polym., 2014, 105, 121–126 CrossRef CAS PubMed.
- Y. M. Pan, K. Wang, S. Q. Huang, H. S. Wang and X. M. Mu, Antioxidant activity of microwave-assisted extract of longan (Dimocarpus Longan Lour.) peel, Food Chem., 2008, 106(3), 1264–1270 CrossRef CAS.
- E. V. Vinogradov, L. Brade and H. Brade, Structural and serological characterisation of the O-antigenic polysaccharide of the lipopolysaccharide from Acinetobacter baumannii strain 24, Carbohydr. Res., 2003, 2751–2756 CrossRef CAS PubMed.
- M. E. M. Braga, S. R. M. Moreschi and M. A. A. Meireles, Effects of supercritical fluid extraction on Curcuma longa L. and Zingiber officinale R. starches, Carbohydr. Polym., 2006, 63(3), 340–346 CrossRef CAS.
- E. V. Vinogradov, L. Brade, H. Brade and O. Holst, Structural and serological characterisation of the O-antigenic polysaccharide of the lipopolysaccharide from Acinetobacter baumannii strain 24, Carbohydr. Res., 2003, 338(23), 2751–2756 CrossRef CAS PubMed.
- M. B. Hu, W. Peng, Y. J. Liu, N. Wu, C. B. Zhao, D. S. Xie, D. Yan, X. F. Zhang, X. B. Tao and C. J. Wu, Optimum Extraction of Polysaccharide from Areca catechu Using Response Surface Methodology and its Antioxidant Activity, J. Food Process. Preserv, 2017, 41(1), e12798 CrossRef.
- W. Li, S. W. Cui and Y. Kakuda, Extraction, fractionation, structural and physical characterization of wheat β-D-glucans, Carbohydr. Polym., 2006, 63(3), 408–416 CrossRef CAS.
- X. Guo, X. Zou and M. Sun, Optimization of extraction process by response surface methodology and preliminary characterization of polysaccharides from Phellinus igniarius, Carbohydr. Polym., 2010, 80(2), 344–349 CrossRef CAS.
- Y. Song, B. J. Du, T. Zhou, B. Han, F. Yu, R. Yang, X. S. Hu, Y. Y. Ni and Q. H. Li, Optimization of extraction process by response surface methodology and preliminary structural analysis of polysaccharides from defatted peanut (Arachis hypogaea) cakes, Carbohydr. Res., 2011, 346(2), 305–310 CrossRef CAS PubMed.
- D. H. Luo, Optimization of total polysaccharide extraction from Dioscorea nipponica Makino using response surface methodology and uniform design, Carbohydr. Polym., 2012, 90(1), 284–288 CrossRef CAS PubMed.
- Y. X. Sun, Y. J. Li, M. Q. Li, H. B. Tong, X. D. Yang and J. C. Liu, Optimization of extraction technology of the Anemone raddeana polysaccharides (ARP) by orthogonal test design and evaluation of its anti-tumor activity, Carbohydr. Polym., 2009, 75(4), 575–579 CrossRef CAS.
- R. Muralidhar, R. Chirumamila, R. Marchant and S. N. N. Poonam, A response surface approach for the comparison of lipase production by Candida cylindracea using two different carbon sources, Biochem. Eng. J., 2001, 9(1), 17–23 CrossRef CAS.
- W. Cao, X. Q. Li, L. Liu, M. Wang, H. T. Fan, C. Li, Z. Lv, X. Wang and Q. Mei, Structural analysis of water-soluble glucans from the root of Angelica sinensis (Oliv.) Diels, Carbohydr. Res., 2006, 341(11), 1870–1877 CrossRef CAS PubMed.
- W. Cao, X. Q. Li, L. Liu, M. C. Wang, H. T. Fan, C. Li, Z. G. Lv, X. J. Wang and Q. B. Mei, Structural analysis of water-soluble glucans from the root of Angelica sinensis (Oliv.) Diels, Carbohydr. Res., 2006, 341(11), 1870–1877 CrossRef CAS PubMed.
- M. J. Wu, D. Z. Jiang and T. M. Liu, Structural Analysis of Water-soluble Polysaccharide PIP1 Extracted from the Cultured Mycelium of Phellinus igniarius, Chem. Res. Chin. Univ., 2006, 22(6), 708–711 CrossRef CAS.
- M. Öztürk, F. Aydoğmuş-Öztürk, M. E. Duru and G. Topçu, Antioxidant activity of stem and root extracts of Rhubarb (Rheum ribes): An edible medicinal plant, Food Chem., 2007, 103(2), 623–630 CrossRef.
- J. H. Xie, M. Y. Shen, M. Y. Xie, S. P. Nie, Y. Chen, C. Li, D. F. Huang and Y. X. Wang, Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of Cyclocarya paliurus (Batal.) Iljinskaja polysaccharides, Carbohydr. Polym., 2012, 89(1), 177–184 CrossRef CAS PubMed.
- R. Z. Chen, S. Z. Li, C. M. Liu, S. M. Yang and X. L. Li, Ultrasound complex enzymes assisted extraction and biochemical activities of polysaccharides from Epimedium leaves, Process Biochem., 2012, 47(12), 2040–2050 CrossRef CAS.
- K. Durgo, M. Koncar, D. Komes, A. Belscak-Cvitanovic, J. Franekic, I. Jakopovich, N. Jakopovich and B. Jakopovich, Cytotoxicity of blended versus single medicinal mushroom extracts on human cancer cell lines: contribution of polyphenol and polysaccharide content, Int. J. Med. Mushrooms, 2013, 15(5), 435–448 CrossRef CAS PubMed.
- X. H. Zhang, L. Liu and C. W. Lin, Isolation, structural characterization and antioxidant activity of a neutral polysaccharide from Sisal waste, Food Hydrocolloids, 2014, 39, 10–18 CrossRef CAS.
- E. Tsiapali, S. Whaley, J. Kalbfleisch, H. E. Ensley, L. W. Browder and D. L. Williams, Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity, Free Radicals Biol. Med., 2001, 30(4), 393–402 CrossRef CAS PubMed.
- S. Q. Li and N. P. Shah, Characterization, antioxidative and bifidogenic effects of polysaccharides from Pleurotus eryngii after heat treatments, Food Chem., 2016, 197, 240–249 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |