DOI:
10.1039/D1RA01300A
(Paper)
RSC Adv., 2021,
11, 17914-17923
Retracted Article: Investigation of the biological activity, mechanical properties and wound healing application of a novel scaffold based on lignin–agarose hydrogel and silk fibroin embedded zinc chromite nanoparticles†
Received
17th February 2021
, Accepted 3rd May 2021
First published on 18th May 2021
Abstract
Given the important aspects of wound healing approaches, in this work, an innovative biocompatible nanobiocomposite scaffold was designed and prepared based on cross-linked lignin–agarose hydrogel, extracted silk fibroin solution, and zinc chromite (ZnCr2O4) nanoparticles. Considering the cell viability technique, red blood cell hemolysis in addition to anti-biofilm assays, it was determined that after three days, the toxicity of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite was less than 13%. Moreover, the small hemolytic effect (1.67%) and high level of prevention in forming a P. aeruginosa biofilm with low OD value (0.18) showed signs of considerable hemocompatibility and antibacterial activity. Besides, according to an in vivo assay study, the wounds of mice treated with the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold were almost completely healed in five days. Aside from these biological tests, the structural features were evaluated by FT-IR, EDX, FE-SEM, and TG analyses, as well as swelling ratio, rheological, and compressive mechanical study tests. Additionally, it was concluded that adding silk fibroin and ZnCr2O4 nanoparticles could enhance the mechanical tensile properties of cross-linked lignin–agarose hydrogel, and also an elastic network was characterized for this designed nanobiocomposite.
1 Introduction
Among the unprecedented level of recent progress in recreating medicine as well as tissue engineering approaches, wound healing processes have created new therapeutic prospects in tissue repair and regeneration. In this complex and dynamic process, a series of well-orchestrated biochemical and cellular phenomena occur in order to reform and restore damaged tissues.1,2 The consecutive steps of tissue regeneration include hemostasis, inflammation, and the proliferative phase, in addition to the tissue remodelling phase, which are connected with the dynamic integrity and biochemical activity of soluble mediators, red blood cells (RBCs), and parenchymal cells.1 In terms of this physiological process, different substantial factors such as having a biomimetic structure with excellent similarity to the extracellular matrix and in vitro hemocompatibility must be considered in designing new engineered scaffolds.3 On the other hand, these designed structures must have a useful construction aimed at cellular migration in addition to proliferation, and also a designed porosity aimed at enhancement in cellular ingrowth and vascularization.3 So far, a diverse range of non-natural and natural-based polymers with different applications such as catalysis with high yield efficiency4–6 and adsorption of heavy metal ions7 have been applied. On the other hand, different biomedical approaches such as tissue engineering,8,9 drug delivery systems,10–12 hyperthermia cancer therapy,13,14 and wound healing15,16 have been exclusively developed by using these types of material. Also, the formation of polymer-based nanomaterials as new biosensing probes has led to advanced detection of pathogenic viruses,17,18 toxic mycotoxins,19 neurotoxic proteins,20 and cancer biomarkers.21–23 In this context, lignin is a polyaromatic-containing biopolymer with an amorphous nature, and a complex and mutative chemical structure consisting of phenylpropanoid building units.24 The remarkable advantages such as antioxidant, antibacterial and antifungal properties, inherent biocompatibility, and intrinsic swelling capacity have extended the use of this phenolic polymer in food science and health care.24 Given the lignin structure and presence of rich phenolic and aliphatic groups, this bio-renewable polymer can be considered as an effective candidate for chemical modification and reactions.25 Making lignin into a three-dimensional hydrogel network and its content in the hydrogel structure can significantly influence the mechanical properties.25,26 On the other hand, agarose as a neutral and biocompatible polysaccharide with very good self-gelling properties can generate thermo-reversible hydrogels, which is due to the reversible cross-linking process via hydrogen bonds.27 This feature of agarose hydrogels leads to size and shape control as well as providing self-healing performance.28 Hydrocolloid agarose hydrogel-based scaffolds with porous architecture can promote cellular mobility through the gel matrix; also, the transportation of adequate oxygen and needed nutrients can be well conducted for related cells embedded in the matrix.29 Aside from natural polysaccharides, natural proteins have been exclusively highlighted because of showing highly advantageous biological efficiencies in various biomedical fields.30 Following these considerations, silk fibroin (SF) has been a focus for researchers because of its outstanding features including non-cytotoxicity, low immunogenicity, robust mechanical strength, as well as non-carcinogenic and hemostatic features.31,32 On the other hand, having a biocompatible structure and promoting the adhesion in addition to proliferation of fibroblasts as well as keratinocytes are features of this natural polymer.32–34 Besides, in different research studies, it has been determined that the formation of SF-based composites and combining SF with other materials like metal nanoparticles,35,36 natural polymers,33,37 and graphene derivatives38 can boost its antimicrobial property as a required factor for wound dressing use.
Herein, a new cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold is designed and introduced for the first time. Conducting the cross-linking reaction between lignin and agarose biopolymers by cross-linking agent epichlorohydrin, formation of cross-linked lignin–agarose hydrogel and addition of SF solution and ZnCr2O4 nanoparticles (ZnCr2O4 NPs) led to the synthesis of this nanobiocomposite scaffold (Scheme 1). FT-IR, EDX, FE-SEM, and TG analyses as well as mechanical tensile experiments were applied to characterize the structural properties of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite. Following the structural characterization, the cytotoxicity of this nanobiocomposite scaffold was checked via 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay by means of Hu02 cells in 1, 2 and 3 days. In addition to this, RBC hemolytic and anti-biofilm assays were undertaken to determine its in vitro blood biocompatibility as well as antibacterial activity.
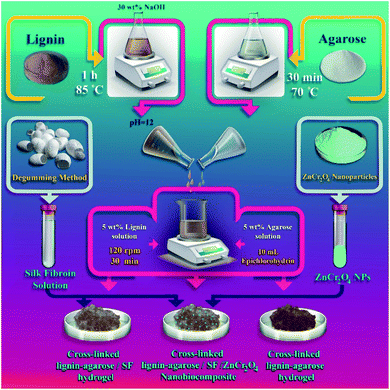 |
| | Scheme 1 Synthesis process of cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold. | |
2 Results and discussion
Following the fabrication process of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite in three main synthesis steps, the structural features of this natural-based scaffold such as the emergence of new chemical bonds and functional groups, structural elements, morphology and structural shapes, as well as the thermal stability and thermogravimetric behaviour were examined and investigated through FT-IR, EDX, FE-SEM, and TG analyses. Furthermore, the tensile properties, including tensile strength, elongation at break, and elastic modulus, of this nanobiocomposite were comparatively characterized.
2.1 Characterization of cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite
2.1.1 FT-IR analysis. According to the three main synthesis steps of cross-linked the lignin–agarose/SF/ZnCr2O4 nanobiocomposite, the formation of new functional groups was evaluated from each step (Fig. S1A–C, ESI†). As observed in the FT-IR spectrum of cross-linked lignin–agarose hydrogel substrate (Fig. S1A, ESI†), a broad band at 3200 cm−1 to 3600 cm−1 can be assigned to the O–H stretching vibration mode and formation of hydrogen bonds between the phenolic and alcoholic hydroxyl groups.39 Two small absorption bands in the region of 2878 cm−1 to 2916 cm−1 and 2850 cm−1 can be assigned to the C–H stretching vibration modes of cross-linked structure of hydrogel and methoxy groups of lignin.39 Bands attributed to the vibration mode of aromatic rings in the lignin structure and symmetric CH2 bending vibration mode of agarose can be observed at 1512 cm−1 and 1419 cm−1.39 The C–O–C stretching vibration mode of aryl ether, C–O–C stretching vibration mode of alkyl ether bonds, and the C–O stretching vibration mode of alkyl substituted ether which are the results of cross-linking reaction are assigned to bands at 1621 cm−1, 1118 cm−1, and 1064 cm−1.39 After the addition of SF solution, new absorption bands were observed (Fig. S1B, ESI†). In general, three different vibrational bands, namely the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration mode of amide I at 1625 cm−1 to 1660 cm−1, the N–H bending vibration mode of amide II at 1520 cm−1 to 1540 cm−1, and the C–N stretching vibration mode of amide III at 1230 cm−1 to 1270 cm−1, can define the presence of SF biopolymeric structure.40 As illustrated in Fig. S1B, ESI,† two observed absorption bands at 1654 cm−1 and 1535 cm−1 which are related to the C
O stretching vibration mode of amide I at 1625 cm−1 to 1660 cm−1, the N–H bending vibration mode of amide II at 1520 cm−1 to 1540 cm−1, and the C–N stretching vibration mode of amide III at 1230 cm−1 to 1270 cm−1, can define the presence of SF biopolymeric structure.40 As illustrated in Fig. S1B, ESI,† two observed absorption bands at 1654 cm−1 and 1535 cm−1 which are related to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration mode of amide I and the N–H bending vibration modes of amide II confirm the random coil conformation of SF.40,41 Also, the broad absorption band around 3300 cm−1 indicates the β-sheet conformation of SF.42 Fig. S1C, ESI,† shows the FT-IR spectrum of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite. Alongside the mentioned absorption bands from two previous synthesis steps, the Zn–O and Cr–O stretching vibration modes of ZnCr2O4 NPs can be observed corresponding to two small absorption bands at 488 cm−1 and 545 cm−1.43 Furthermore, it can be mentioned that the construction of new intramolecular hydrogen bonds is the reason for increased intensity in the band of the O–H stretching vibration mode.
O stretching vibration mode of amide I and the N–H bending vibration modes of amide II confirm the random coil conformation of SF.40,41 Also, the broad absorption band around 3300 cm−1 indicates the β-sheet conformation of SF.42 Fig. S1C, ESI,† shows the FT-IR spectrum of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite. Alongside the mentioned absorption bands from two previous synthesis steps, the Zn–O and Cr–O stretching vibration modes of ZnCr2O4 NPs can be observed corresponding to two small absorption bands at 488 cm−1 and 545 cm−1.43 Furthermore, it can be mentioned that the construction of new intramolecular hydrogen bonds is the reason for increased intensity in the band of the O–H stretching vibration mode.
2.1.2 EDX analysis. Considering the qualitative EDX technique for detecting the structural elements of materials, the elemental composition of the synthesized cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite is identified (Fig. 1A and B). As observed in the EDX spectrum (Fig. 1A), the carbon and oxygen peaks can be attributed to the presence of lignin and agarose biopolymers, as well as the epichlorohydrin cross-linking agent. The chlorine peaks can be associated with the epichlorohydrin structure. Besides, observing zinc and chromium peaks confirms the existence of ZnCr2O4 NPs. Moreover, the distribution pattern of detected structural elements is well detected via the elemental mapping images (Fig. 1B).
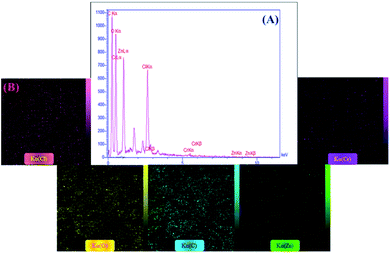 |
| | Fig. 1 (A) EDX spectrum and (B) elemental mapping images of cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite. | |
2.1.3 FE-SEM imaging. Using FE-SEM imaging of the freeze-dried form of cross-linked lignin–agarose hydrogel and cross-linked lignin–agarose/SF hydrogel (Fig. 2A–C), the developed interconnected porosity was observed for this three-dimensional structure. This porous architecture could provide an extended surface area for cell attachment, and also it could simplify cellular growth.44 In addition, it should be mentioned that the addition of SF biopolymers does not show any specific structural alteration in the three-dimensional architecture of the cross-linked lignin–agarose hydrogel. Following that, the synthesized ZnCr2O4 NPs with spherical morphology and uniform distribution were characterized in the structure of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite (Fig. 2D).
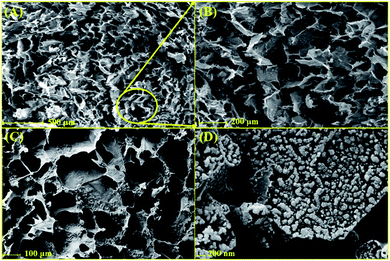 |
| | Fig. 2 FE-SEM images of (A and B) cross-linked lignin–agarose hydrogel, (C) cross-linked lignin–agarose/SF hydrogel, and (D) cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite (freeze-dried forms). | |
2.1.4 Thermogravimetric analysis. As depicted in Fig. S2, ESI,† the synthesized cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite undergoes two different degradation steps. The first step of weight loss (90 °C to 160 °C) is related to the evaporation of smaller molecules or evaporation of adsorbed water. The vast majority of the weight loss occurs at higher temperature (>250 °C). Taking into account previously reported research, this weight loss can be ascribed to the breaking of the amino acid side chains of SF45 or breaking of the methyl–aryl ether bonds, and also the breakdown of lignin aromatic rings.46,47 At a temperature of 570 °C, the hydrogel loses about 40% of its weight; after that, the sample does not decompose due to the presence of dense aromatic structures and the hydrogel weight remains constant.
2.1.5 Swelling ratio study. The swelling ratio (SR) of film samples was evaluated by immersing a pre-weighed film (W1) sample (2.5 cm × 2.5 cm) in 20 mL ultra-pure water (UPW) for 1 h and measuring the weight of the film (W2) after gently removing the surface water using a blotting paper. The SR of the samples was calculated using the following eqn (1):| |
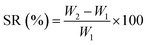 | (1) |
The SR of the neat cross-linked lignin–agarose hydrogel film was 897 ± 211% and was significantly (p < 0.05) decreased to 815 ± 14% for the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite. The reduced SR of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite film may be due to ZnCr2O4 NPs with low interfacial area and low swelling property.
2.1.6 Rheological study. The samples were swollen in UPW at room temperature for 24 h before rheological measurements were conducted using an RMS/MCR 302 rheometer (Anton-Paar Co., USA) equipped with a 20 mm parallel plate. Measurements of the storage modulus (G′) and loss modulus (G′′) were conducted at shear stress range from 0.01 to 1000 Pa at a controlled frequency of 0.1 Hz. The measurements were stopped when both G′ and G′′ began to decrease notably. For this test, three specimens were measured, and the values were averaged. The rheological properties of the cross-linked lignin–agarose hydrogel and cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold were evaluated to establish the elastic properties of these materials, such as gel strength. Dynamical mechanical analysis of the swollen samples was performed by changing the oscillatory stress from 0.01 to 1000 Pa at a constant frequency (1 Hz). The analysis provided information about the storage modulus (G′), the loss modulus (G′′), and the phase angle (Table 1).
Table 1 Viscoelastic parameters of the cross-linked lignin–agarose hydrogel and cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold
| Sample |
G′ (Pa) |
G′′ (Pa) |
Phase angle (°) |
Max. oscillation stress (Pa) |
| Cross-linked lignin–agarose hydrogel |
2365.5 (±101.7) |
953.8 (±24.2) |
20.9 (±3.7) |
3.6 (±0.0) to 5.8 (±0.0) |
| Cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite |
4880.2 (±201.3) |
1715.3 (±54.9) |
24.6 (±4.5) |
7.2 (±0.0) to 9.4 (±0.0) |
Phase angles of 0° and 90° indicate a perfectly elastic material and viscous material, respectively. Also, a larger value of G′ when compared to G′′ indicates that the analysed material has pronounced elastic properties. For the cross-linked lignin–agarose hydrogel and cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold, it was found that the storage modulus is higher than the loss modulus (G′/G′′ > 1), indicating the formation of an elastic network.
2.1.7 Compressive mechanical tests. One of the disadvantages of hydrogels in wound healing approaches is their poor mechanical strength. In many studies, the combination of these cross-linked structures with SF and nanomaterials has been suggested to solve this problem and increase their stability.48,49 In this study, the thickness of sample scaffolds, including cross-linked lignin–agarose hydrogel, cross-linked lignin–agarose/SF hydrogel, and cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite, was measured using a digital micrometer (Digital Outside Micrometer, Model 312-001-01, ACCUD, Austria) with a resolution of 0.001 mm. For each sample, five random measurements were performed and the mean of these values was considered as the thickness. The tensile strength (MPa) was calculated using the following eqn (2):| |
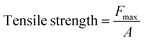 | (2) |
where Fmax is the maximum force (N) and A is the initial cross-sectional area of the sample (m2). Besides, the elongation at break was also calculated using the following eqn (3):| |
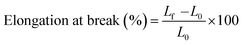 | (3) |
where Lf is the sample elongation at the moment of failure and L0 is the initial grip length of the sample. Finally, the elastic modulus (GPa) was determined from the slope of a linear section of the stress–strain curve.50 The results of the tensile tests were compared with the tensile properties of a number of other nanocomposites films, as listed in Table 2. As can be seen, the mechanical properties of our synthesized cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold are dramatically improved compared to other similar scaffolds. The thickness of the hydrogel film increases after mixing SF and ZnCr2O4 NPs. The increase in the thickness of the composite films is mainly due to the increased solid content of the SF and ZnCr2O4 NPs.49 Fig. 3 also shows comparative graphs of the tensile strength, elongation at break, and elastic modulus of the samples. The mechanical properties of the cross-linked lignin–agarose hydrogel are greatly influenced by the addition of SF biopolymer and ZnCr2O4 NPs. The tensile strength of this cross-linked hydrogel film is 52.7 ± 2.5 MPa, which is increased to 174.1 ± 3.7 MPa after combining with SF. This indicates that SF has packed between the hydrogel fibers and its molecular chains, thereby strengthening and tightening the structure.51 In general, fibroin has been reported to have very great mechanical strength.52 Besides, the tensile strength of the cross-linked lignin–agarose/SF hydrogel was increased after adding ZnCr2O4 NPs to 176.2 ± 1.4 MPa. This increase in its tensile strength might be due to high compatibility between the nanofiller and cross-linked lignin–agarose/SF hydrogel.49 The effect of SF and ZnCr2O4 NPs on the elastic modulus of the film is similar to that on the tensile strength, so that the elastic modulus of the cross-linked lignin–agarose hydrogel increases from 1.87 ± 0.11 GPa to 28.43 ± 0.19 GPa after the addition of SF. The addition of ZnCr2O4 NPs to the cross-linked lignin–agarose/SF hydrogel also increases the Young's modulus to 29.51 ± 0.05 GPa. On the other hand, the elongation at break of the cross-linked lignin–agarose hydrogel was decreased from 25.4 ± 3.7% to 10.6 ± 1.9% after the addition of SF, indicating that blending with SF renders the films less flexible due to the hardness characteristic of SF.48 But the addition of ZnCr2O4 NPs to the cross-linked lignin–agarose/SF hydrogel increased the elongation at break to 12.3 ± 3.5%. In fact, the NPs could act as a reinforcing filler of the blend films53 and composites.54 The results showed that the strength and stiffness of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite film increased without affecting its flexibility.
Table 2 Comparing the tensile properties of synthesized scaffolds with those reported in previous studies
| Sample (film) |
Thickness (μm) |
Tensile strength (MPa) |
Elongation at break (%) |
Elastic modulus (GPa) |
Ref. |
| Agar |
45.1 ± 2.3 |
40.3 ± 4.0 |
19.4 ± 4.2 |
1.39 ± 0.23 |
49 |
| Agar/lignin |
55.9 ± 2.6 |
44.1 ± 3.6 |
16.1 ± 4.3 |
1.48 ± 0.13 |
| Agar/lignin/AgNP0.5 |
54.9 ± 7.4 |
45.5 ± 4.6 |
16.2 ± 5.1 |
1.49 ± 0.15 |
| Agar/lignin/AgNP1.0 |
54.3 ± 2.9 |
49.7 ± 3.3 |
16.7 ± 4.7 |
1.60 ± 0.08 |
| Agar/lignin/AgNP1.5 |
57.2 ± 1.9 |
47.0 ± 3.5 |
16.2 ± 3.4 |
1.52 ± 0.23 |
| Agar/lignin/AgNP2.0 |
53.0 ± 1.4 |
45.6 ± 2.2 |
16.7 ± 2.9 |
1.50 ± 0.13 |
| Agar |
51.8 ± 0.9 |
34.9 ± 0.8 |
12.0 ± 0.8 |
1.17 ± 0.01 |
50 |
| Agar/CuS0.25 |
58.8 ± 1.5 |
40.6 ± 1.1 |
18.9 ± 2.1 |
1.29 ± 0.53 |
| Agar/CuS0.50 |
59.2 ± 1.2 |
47.6 ± 1.1 |
17.4 ± 1.4 |
1.49 ± 0.09 |
| Agar/CuS1.0 |
60.9 ± 1.4 |
43.0 ± 0.4 |
15.6 ± 0.5 |
1.42 ± 0.02 |
| Agar/CuS2.0 |
60.2 ± 1.3 |
41.1 ± 1.4 |
16.1 ± 1.5 |
1.35 ± 0.04 |
| Agar |
60.5 ± 1.0 |
45.5 ± 2.7 |
26.7 ± 2.7 |
1.07 ± 0.06 |
55 |
| Agar/lignin1% |
60.3 ± 1.3 |
48.2 ± 3.2 |
27.6 ± 2.8 |
1.15 ± 0.09 |
| Agar/lignin3% |
61.1 ± 1.0 |
52.1 ± 2.8 |
27.0 ± 2.0 |
1.35 ± 0.09 |
| Agar/lignin5% |
57.1 ± 1.9 |
50.7 ± 3.1 |
22.4 ± 2.7 |
1.46 ± 0.09 |
| Agar/lignin10% |
57.8 ± 2.4 |
51.8 ± 3.3 |
22.1 ± 2.6 |
1.47 ± 0.09 |
| SF hydrogel (NaAc-HAc buffer) |
— |
— |
— |
0.09 ± 10.0 |
56 |
| SF hydrogel (PBS buffer) |
— |
— |
— |
0.08 ± 8.5 |
| Alkaline lignin/SF (NaAc-HAc buffer) |
— |
— |
— |
0.18 ± 32.9 |
| Alkaline lignin/SF (PBS buffer) |
— |
— |
— |
0.17 ± 10.7 |
| Cross-linked lignin–agarose hydrogel |
58.6 ± 3.4 |
52.7 ± 2.5 |
25.4 ± 3.7 |
1.87 ± 0.11 |
This study |
| Cross-linked lignin–agarose/SF hydrogel |
65.3 ± 1.8 |
174.1 ± 3.7 |
10.6 ± 1.9 |
28.43 ± 0.19 |
| Cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite |
66.2 ± 2.6 |
176.2 ± 1.4 |
12.3 ± 3.5 |
29.51 ± 0.05 |
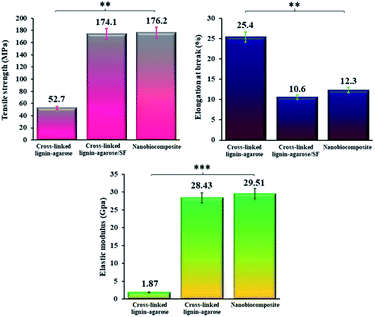 |
| | Fig. 3 Comparing the tensile strength (MPa) (** = significant, P ≤ 0.05), elongation at break (%) (** = significant, P ≤ 0.05), and elastic modulus (GPa) (*** = very significant, P ≤ 0.001) of cross-linked lignin–agarose hydrogel, cross-linked lignin–agarose/SF hydrogel, and cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite. | |
2.2 Bio-application of cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite
2.2.1 In vitro cytotoxicity assay results. The current techniques of wound dressing and skin tissue engineering involve the application of biocompatible scaffolds with a non-toxic nature.57 Therefore, MTT assay was applied to determine the biocompatibility in addition to the cell viability of the synthesized scaffolds. The results showed that the viability of Hu02 cells treated with cross-linked lignin–agarose/SF hydrogel and cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffolds after 1, 2 and 3 days is 93.5%, 92.64%, 89.37%, 91.9%, 88.23% and 87.5% respectively, while for the control group (untreated cells), this value was 100%, 98.5% and 94.34%, respectively. According to the statistical analysis, there is no noteworthy alteration between cell viability of the control group and scaffolds (P ≥ 0.05). Likewise, cisplatin (1 μg mL−1) as positive control kills 86%, 89% and 89.68% of cells after 1, 2 and 3 days, respectively. Results are the average of three independent experiments (Fig. 4A and B). As can be seen in Fig. 4A, the toxicity of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold on the third day is less than 13%; therefore, it is a non-toxic and biocompatible scaffold. The effect of cross-linked lignin–agarose/SF hydrogel and cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite on cell morphology and shape was imaged with reverse microscopy, the results of which can be seen in Fig. 4D and E. These Images indicate that Hu02 cells retain their fibroblast shape after treatment with these synthesized scaffold samples. Also, untreated cells and cisplatin-treated cells were used as negative control and positive control, respectively (Fig. 4C–F). Studies have shown that chromium spinels such as zinc chromate NPs58 and also heavy metal NPs based on zinc,59 chromium,60 and their oxides61,62 exhibit toxicity to human cells. Accordingly, the cell viability has decreased after the addition of ZnCr2O4 NPs to the cross-linked lignin–agarose/SF hydrogel. Additionally, the results of MTT assay in our study show that the toxicity of these NPs in the synthesized scaffold has been moderated. As a result, the viability of cells in the presence of the nanobiocomposite scaffolds is not reduced compared with untreated cells (control group).
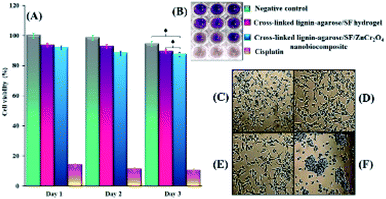 |
| | Fig. 4 (A) Cell viability histogram of untreated Hu02 cells (control group) and cells after treatment with cross-linked lignin–agarose/SF hydrogel, cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite and cisplatin (positive control) after one, two, and three days (* = insignificant, P ≥ 0.05) (B) 96-well plate image. Inverted microscopic images of (C) untreated Hu02 cells (negative control), (D) cross-linked lignin–agarose/SF hydrogel, (E) cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite, and (F) cisplatin treatment. | |
2.2.2 In vivo assay results. Twenty adult male Balb/c mice (20–25 g) were obtained from Pasteur Institute of Iran. They were divided into two groups (test and control) of ten. The mice were subjected to normal and standard conditions: 12 h of darkness/12 h of light. All the experimental techniques were confirmed by Semnan University of Sciences, Ethics Research Committee. Also, the research study was operated based on the principles outlined in the Declaration of Helsinki. In this respect, first of all, mice were anesthetized with ketamine (5 mg kg−1, i.p.) and their back hair was shaved using an electrical clipper. Then, an area of about 1 cm2 was burned on the shaved skin of the mice for 9 second by a hot steel plate (to ∼230 °C) that was attached to a steel rod with a heat-resistant tape.63,64 After the burn, the animals were kept and fed separately. Next, burn wounds of the test group after surgically debriding were covered by 1 cm2 of cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold, and 1 cm2 of sterile gauze was used as negative control in the control group. The length and width of wounds were measured with a calliper every five days until the 20th day. The wound healing process was monitored by evaluating the wound area. Wound area and wound healing percentage in control group and test group were calculated and compared according to the following eqn (4) and (5):65| |
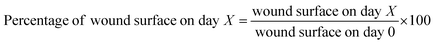 | (4) |
| |
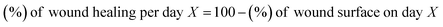 | (5) |
Percentage of wound surface of each mouse in the experimental and control groups on different days of treatment was obtained and the mean of each was calculated. A mouse was selected from each of the treatment and control groups and was monitored in terms of wound area on certain days and the wounds were imaged (Fig. 5B). Next, the percentage of wound healing on each day was calculated (Fig. 5A). The results showed that the wound healing process was faster in mice treated with the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold than in mice in the control group. In the mice of the experimental group, on the third, sixth, ninth and twelfth days, 20.24%, 42.65%, 63.72% and 81.68% of the wound was healed compared to day zero, while for control mice this value was 9.43%, 27.12%, 51.79% and 70.73% respectively. Also, the wounds of mice treated with cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold were almost completely healed on the fifth day, while on the same day the wound healing rate was 91% in the control group. It can also be seen in the images that the infection caused by the wound in the control group is well eliminated, which can be due to the presence of ZnCr2O4 NPs in the composite scaffold that show anti-infective properties.
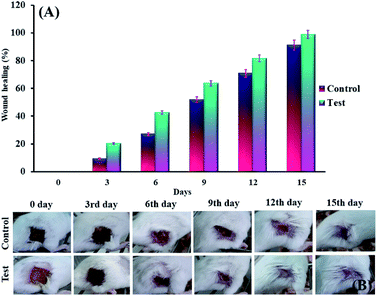 |
| | Fig. 5 (A) The percentage of wound healing on each day. (B) Images of wounds on different days of treatment in test and control groups. | |
2.2.3 In vitro hemolytic assay results. Hemolysis potency is one of the important factors for wound healing scaffolds. Blood compatibility is a crucial feature in deciding whether to use materials that come into direct contact with blood.66 The hemolytic activity of the scaffolds was measured using hemolytic assay on human RBCs. According to the ISO standard (document 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 993-5 1992), when a hemolysis index of a substance is less than 5% it is considered harmless. The results of this test show that there is no significant difference between the hemolytic activity of cross-linked lignin–agarose hydrogel, cross-linked lignin–agarose/SF hydrogel, cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite, and negative control (P ≥ 0.05), the values being 3.93%, 4.91% and 1.67% respectively. This is in contrast to Triton X-100 (as a positive control), which lyses almost all RBCs. The reported results are the mean of 3 separate tests. Fig. 6A and B shows a histogram of the hemolysis results, as well as an image of a 96-well plate, for positive control, cross-linked lignin–agarose/SF hydrogel, cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite, and negative control. Numerous works have confirmed that agar-based66 and lignin-based67 scaffolds are compatible with blood. Also, SF-based scaffolds do not show great hemolysis potential.68 On the other hand, various studies have shown that zinc, chromium and their oxide NPs (ZnO, Cr2O3 etc.) have different hemolytic activity according to their morphology, size and concentration.69–73 In this study, ZnCr2O4 NPs with appropriate concentration (0.5% w/w) were used and, as a result, the hemolytic activity of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite remains almost unchanged. Overall, it can be said that this nanobiocomposite scaffold is fully hemocompatible and is very suitable for use in skin tissue engineering and wound healing.
993-5 1992), when a hemolysis index of a substance is less than 5% it is considered harmless. The results of this test show that there is no significant difference between the hemolytic activity of cross-linked lignin–agarose hydrogel, cross-linked lignin–agarose/SF hydrogel, cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite, and negative control (P ≥ 0.05), the values being 3.93%, 4.91% and 1.67% respectively. This is in contrast to Triton X-100 (as a positive control), which lyses almost all RBCs. The reported results are the mean of 3 separate tests. Fig. 6A and B shows a histogram of the hemolysis results, as well as an image of a 96-well plate, for positive control, cross-linked lignin–agarose/SF hydrogel, cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite, and negative control. Numerous works have confirmed that agar-based66 and lignin-based67 scaffolds are compatible with blood. Also, SF-based scaffolds do not show great hemolysis potential.68 On the other hand, various studies have shown that zinc, chromium and their oxide NPs (ZnO, Cr2O3 etc.) have different hemolytic activity according to their morphology, size and concentration.69–73 In this study, ZnCr2O4 NPs with appropriate concentration (0.5% w/w) were used and, as a result, the hemolytic activity of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite remains almost unchanged. Overall, it can be said that this nanobiocomposite scaffold is fully hemocompatible and is very suitable for use in skin tissue engineering and wound healing.
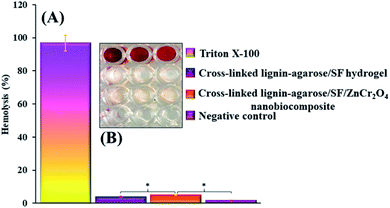 |
| | Fig. 6 (A) Hemolysis histogram of positive control, cross-linked lignin–agarose/SF hydrogel, cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite, and negative control (* = insignificant, P ≥ 0.05). (B) 96-well plate image. These results indicate that the synthesized scaffold has no specific hemolytic activity. | |
2.2.4 Inhibition of anti-biofilm activity results. The absorbance of resulting solutions was determined at 570 nm in the 96-microwell plate, when the biofilms were washed from the pieces. Fig. 7A displays the values for polystyrene, cross-linked lignin–agarose/SF hydrogel, and cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite pieces to be 0.9, 0.57, and 0.18, respectively. Furthermore, the anti-biofilm activity of the cross-linked lignin–agarose/SF/ZnCr2O4 fragment was significantly (P ≤ 0.05) and very significantly (P ≤ 0.001) higher than that of the cross-linked lignin–agarose/SF hydrogel and polystyrene pieces, respectively, based on the statistical analysis. Actually, the reduction in OD of the NB culture medium covering the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold measured at 570 nm indicates that our scaffold can well prevent the formation of P. aeruginosa biofilm on its surface. This is visible in the 96-microwell plate image in Fig. 7B. Furthermore, a perfect tissue adhesive for wound treatment should be antimicrobial and impervious to antibiotic resistance and a promoter of tissue regeneration and wound healing.74 Lignin and lignin-based hydrogels have been reported to have antioxidant and antimicrobial activities.75,76 Agar is also one of the polysaccharides often used in the production of antimicrobial films and composites.50 SF is one of the most desirable wound dressing materials because of its exceptional features including good biocompatibility, biodegradability, morphologic flexibility, and appropriate mechanical features.77 In addition, SF does not have antimicrobial features that frequently cause wound infection. So, enhancing the antimicrobial features of SF scaffolds is considerable in the use of wound dressings.33 Many studies have shown the integration of antimicrobial features into hydrogel-based dressings via mixtures of dissimilar kinds of antimicrobial agents, together with metal NPs, cationic polymers, and antimicrobial peptides.74 Also, Salehi et al. have studied and proven the antimicrobial properties of ZnCr2O4 NPs.78 Accordingly, in our study, the combination of these NPs with cross-linked lignin–agarose/SF hydrogel significantly increased the antimicrobial properties of the hydrogel-based substrate. This property increases the potential of using this scaffold in tissue engineering and wound healing.
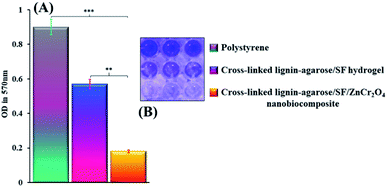 |
| | Fig. 7 (A) Anti-biofilm histogram of polystyrene, cross-linked lignin–agarose/SF hydrogel and cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite pieces (** = significant, P ≤ 0.05; *** = very significant, P ≤ 0.001). (B) 96-well plate image. It is clear that the synthesized scaffold was able to inhibit P. aeruginosa biofilm formation. | |
3 Conclusions
In this current study, the design and synthesis of a cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold depending on cross-linked lignin–agarose hydrogel, adding SF solution and ZnCr2O4 NPs was presented together with particular and eye-catching biological performance and amended mechanical tensile, swelling, and rheological properties. Also, the cell viability of this nanobiocomposite scaffold was less than 13% after three days. Also, this nanobiocomposite could lyse only 1.67% of RBCs. A strong prevention of the formation of a P. aeruginosa biofilm, as evident from a low OD value (0.18), was observed for this nanostructure scaffold. Besides, given the in vivo assay study, in comparison to the control group with 91% healing rate, the wounds of mice treated with the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold were almost completely healed in five days. Compared to cross-linked lignin–agarose hydrogel and cross-linked lignin–agarose/SF, the mechanical aspects of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite scaffold were notable. Taking into account these in vitro and in vivo biological experiments, enhanced mechanical properties, and it having an elastic network, this nanobiocomposite should be evaluated in more detail for biomedical fields such as tissue engineering.
Ethical statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Semnan University of Medical Sciences and approved by the Animal Ethics Committee of this university and the study was conducted in accordance with the principles outlined in the Declaration of Helsinki.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
All authors gratefully acknowledge the partial support from the Research Council of the Iran University of Science and Technology. Furthermore, A. E. S. is grateful for the National Research grants from MINECO, Spain, “Juan de la Cierva” [FJCI-2018-037717]. Also, we thank the Ethics Research Committee and Biotechnology Research Center from Semnan University of Medical Sciences.
References
- A. C. d. O. Gonzalez, T. F. Costa, Z. d. A. Andrade and A. R. A. P. Medrado, An. Bras. Dermatol., 2016, 91, 614–620 CrossRef PubMed.
- M. Hajialyani, D. Tewari, E. Sobarzo-Sánchez, S. M. Nabavi, M. H. Farzaei and M. Abdollahi, Int. J. Nanomed., 2018, 13, 5023 CrossRef CAS PubMed.
- M. Batool, M. F. Nazar, A. Awan, M. B. Tahir, A. Rahdar, A. E. Shalan, S. Lanceros-Méndez and M. N. Zafar, Nano-Struct. Nano-Objects, 2021, 27, 100762 CrossRef.
- S. Asgharnasl, R. Eivazzadeh-Keihan, F. Radinekiyan and A. Maleki, Int. J. Biol. Macromol., 2020, 144, 29–46 CrossRef CAS PubMed.
- M. S. Esmaeili, Z. Varzi, R. Eivazzadeh-Keihan, A. Maleki and H. J. M. C. Ghafuri, Mol. Catal., 2020, 492, 111037 CrossRef CAS.
- A. Maleki, M. Panahzadeh and R. Eivazzadeh-keihan, Green Chem. Lett. Rev., 2019, 12, 395–406 CrossRef CAS.
- R. Eivazzadeh-Keihan, F. Radinekiyan, S. Asgharnasl, A. Maleki and H. Bahreinizad, J. Mater. Res. Technol., 2020, 9, 12244–12259 CrossRef CAS.
- R. Eivazzadeh-Keihan, A. Maleki, M. De La Guardia, M. S. Bani, K. K. Chenab, P. Pashazadeh-Panahi, B. Baradaran, A. Mokhtarzadeh and M. Hamblin, J. Adv. Res., 2019, 18, 185–201 CrossRef CAS PubMed.
- P. Zarrintaj, S. Manouchehri, Z. Ahmadi, M. R. Saeb, A. M. Urbanska, D. L. Kaplan and M. Mozafari, Carbohydr. Polym., 2018, 187, 66–84 CrossRef CAS PubMed.
- T. G. Barclay, C. M. Day, N. Petrovsky and S. Garg, Carbohydr. Polym., 2019, 221, 94–112 CrossRef CAS PubMed.
- H. Liu, C. Wang, C. Li, Y. Qin, Z. Wang, F. Yang, Z. Li and J. Wang, RSC Adv., 2018, 8, 7533–7549 RSC.
- G. D. Mogoşanu, A. M. Grumezescu, L. E. Bejenaru and C. Bejenaru, Nanobiomaterials in Drug Delivery, 2016, 9, 229–284 CrossRef.
- M. S. Bani, S. Hatamie, M. Haghpanahi, H. Bahreinizad, M. H. S. Alavijeh, R. Eivazzadeh-Keihan and Z. H. Wei, Sipin, 2019, 9, 1940003 CrossRef CAS.
- R. Eivazzadeh-Keihan, F. Radinekiyan, A. Maleki, M. S. Bani and M. Azizi, J. Mater. Sci., 2020, 55, 319–336 CrossRef CAS.
- R. Eivazzadeh-Keihan, F. Khalili, H. A. M. Aliabadi, A. Maleki, H. Madanchi, E. Z. Ziabari and M. S. Bani, Int. J. Biol. Macromol., 2020, 162, 1959–1971 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, F. Radinekiyan, H. Madanchi, H. A. M. Aliabadi and A. Maleki, Carbohydr. Polym., 2020, 248, 116802 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, P. Pashazadeh-Panahi, T. Mahmoudi, K. K. Chenab, B. Baradaran, M. Hashemzaei, F. Radinekiyan, A. Mokhtarzadeh and A. Maleki, Microchim. Acta, 2019, 186, 329 CrossRef PubMed.
- A. Mokhtarzadeh, R. Eivazzadeh-Keihan, P. Pashazadeh, M. Hejazi, N. Gharaatifar, M. Hasanzadeh, B. Baradaran and M. de la Guardia, TrAC, Trends Anal. Chem., 2017, 97, 445–457 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, P. Pashazadeh, M. Hejazi, M. de la Guardia and A. Mokhtarzadeh, TrAC, Trends Anal. Chem., 2017, 87, 112–128 CrossRef CAS.
- R. Eivazzadeh-Keihan, P. Pashazadeh-Panahi, B. Baradaran, M. de la Guardia, M. Hejazi, H. Sohrabi, A. Mokhtarzadeh and A. Maleki, TrAC, Trends Anal. Chem., 2018, 103, 184–197 CrossRef CAS.
- K. K. Chenab, R. Eivazzadeh-Keihan, A. Maleki, P. Pashazadeh-Panahi, M. R. Hamblin and A. Mokhtarzadeh, Nanomedicine, 2019, 17, 342–358 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, P. Pashazadeh-Panahi, B. Baradaran, A. Maleki, M. Hejazi, A. Mokhtarzadeh and M. de la Guardia, TrAC, Trends Anal. Chem., 2018, 100, 103–115 CrossRef CAS.
- A. Mohammadinejad, R. K. Oskuee, R. Eivazzadeh-Keihan, M. Rezayi, B. Baradaran, A. Maleki, M. Hashemzaei, A. Mokhtarzadeh and M. de la Guardia, TrAC, Trends Anal. Chem., 2020, 130, 115961 CrossRef CAS.
- L. Musilová, A. Mráček, A. Kovalcik, P. Smolka, A. Minařík, P. Humpolíček, R. Vícha and P. Ponížil, Carbohydr. Polym., 2018, 181, 394–403 CrossRef PubMed.
- Y. Meng, J. Lu, Y. Cheng, Q. Li and H. Wang, Int. J. Biol. Macromol., 2019, 135, 1006–1019 CrossRef CAS PubMed.
- L. Berglund, F. Forsberg, M. Jonoobi and K. Oksman, RSC Adv., 2018, 8, 38219–38228 RSC.
- S. Shin, M. Ikram, F. Subhan, H. Y. Kang, Y. Lim, R. Lee, S. Jin, Y. H. Jeong, J. Y. Kwak and Y. J. Na, RSC Adv., 2016, 6, 46952–46965 RSC.
- Y. Ko, J. Kim, H. Y. Jeong, G. Kwon, D. Kim, M. Ku, J. Yang, Y. Yamauchi, H. Y. Kim and C. Lee, Carbohydr. Polym., 2019, 203, 26–34 CrossRef CAS PubMed.
- F. Topuz, A. Nadernezhad, O. S. Caliskan, Y. Z. Menceloglu and B. Koc, Carbohydr. Polym., 2018, 201, 105–112 CrossRef CAS PubMed.
- S. Gomes, I. B. Leonor, J. F. Mano, R. L. Reis and D. L. Kaplan, Prog. Polym. Sci., 2012, 37, 1–17 CrossRef CAS PubMed.
- H. J. Chun, K. Park, C. H. Kim and G. Khang, Novel Biomaterials for Regenerative Medicine, Springer, 1st edn, 2018 Search PubMed.
- H. W. Ju, O. J. Lee, J. M. Lee, B. M. Moon, H. J. Park, Y. R. Park, M. C. Lee, S. H. Kim, J. R. Chao, C. S. Ki and C. H. Park, Int. J. Biol. Macromol., 2016, 85, 29–39 CrossRef CAS PubMed.
- M. Ghanbari, F. Davar and A. E. Shalan, Ceram. Int., 2021, 47, 9409–9417 CrossRef CAS.
- A. Keirouz, M. Zakharova, J. Kwon, C. Robert, V. Koutsos, A. Callanan, X. Chen, G. Fortunato and N. Radacsi, Mater. Sci. Eng., C, 2020, 110939 CrossRef CAS PubMed.
- S. Calamak, E. A. Aksoy, N. Ertas, C. Erdogdu, M. Sagıroglu and K. Ulubayram, Eur. Polym. J., 2015, 67, 99–112 CrossRef CAS.
- S. De Simone, A. Gallo, F. Paladini, A. Sannino and M. Pollini, J. Mater. Sci.: Mater. Med., 2014, 25, 2205–2214 CrossRef CAS PubMed.
- B. Cui, C. Zhang, B. Gan, W. Liu, J. Liang, Z. Fan, Y. Wen, Y. Yang, X. Peng and Y. Zhou, Mater. Sci. Eng., C, 2020, 109, 110611 CrossRef CAS PubMed.
- S. D. Wang, Q. Ma, K. Wang and H. W. Chen, ACS Omega, 2018, 3, 406–413 CrossRef CAS PubMed.
- S. Sathawong, W. Sridach and K. a. Techato, J. Polym. Environ., 2018, 26, 3307–3315 CrossRef CAS.
- S. W. Ha, A. E. Tonelli and S. M. Hudson, Biomacromolecules, 2005, 6, 1722–1731 CrossRef CAS PubMed.
- X. Zhang and Z. Pan, Polymers, 2019, 11, 1622 CrossRef CAS PubMed.
- M. Narimani, A. Teimouri and Z. J. P. B. Shahbazarab, Polym. Bull., 2019, 76, 725–745 CrossRef CAS.
- H. M. Fahmy, A. M. Mosleh, A. Abd Elghany, E. Shams-Eldin, E. S. Abu Serea, S. A. Ali and A. E. Shalan, RSC. Adv., 2019, 9(35), 20118–20136 RSC.
- B. J. Lawrence and S. V. Madihally, Cell Adhes. Migrat., 2008, 2, 9–16 CrossRef PubMed.
- G. M. Nogueira, R. F. Weska, W. C. Vieira Jr, B. Polakiewicz, A. C. Rodas, O. Z. Higa and M. M. Beppu, J. Appl. Polym. Sci., 2009, 114, 617–623 CrossRef CAS.
- Q. Feng, F. Chen and H. Wu, BioResources, 2011, 6, 4942–4952 CAS.
- S. Sathawong, W. Sridach and K. a. Techato, J. Environ. Chem. Eng., 2018, 6, 5879–5888 CrossRef CAS.
- N. Golafshan, R. Rezahasani, M. T. Esfahani, M. Kharaziha and S. N. Khorasani, Carbohydr. Polym., 2017, 176, 392–401 CrossRef CAS PubMed.
- L. Liu, C. He, M. Xiao, Z. An and S. Lv, Journal of Applied Catalysis A: General, 2020, 597, 117541 CrossRef CAS.
- S. Roy, J. W. Rhim and L. J. F. H. Jaiswal, Food Hydrocolloids, 2019, 93, 156–166 CrossRef CAS.
- C. Narita, Y. Okahisa and K. Yamada, J. Cleaner Prod., 2019, 234, 200–207 CrossRef CAS.
- X. N. Qi, Z. L. Mou, J. Zhang and Z. Q. Zhang, J. Biomed. Mater. Res., Part A, 2013, 102, 366–372 CrossRef PubMed.
- N. Niamsa, Y. Srisuwan, Y. Baimark, P. Phinyocheep and S. Kittipoom, Carbohydr. Polym., 2009, 78, 60–65 CrossRef CAS.
- L. Wang and C. Li, Carbohydr. Polym., 2007, 68, 740–745 CrossRef CAS.
- I. Spiridon, C.-A. Teaca and R. Bodirlau, J. Mater. Sci., 2011, 46, 3241–3251 CrossRef CAS.
- R. Núñez-Flores, B. Giménez, F. Fernández-Martín, M. López-Caballero, M. Montero and M. C. Gómez-Guillén, Food Hydrocolloids, 2013, 30, 163–172 CrossRef.
- A. Rahmani Del Bakhshayesh, N. Annabi, R. Khalilov, A. Akbarzadeh, M. Samiei, E. Alizadeh, M. Alizadeh-Ghodsi, S. Davaran and A. Montaseri, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 691–705 CrossRef CAS PubMed.
- A. L. Holmes, S. S. Wise, S. C. Pelsue, A.-M. Aboueissa, W. Lingle, J. Salisbury, J. Gallagher and J. P. Wise Sr, Chem. Res. Toxicol., 2010, 23, 386–395 Search PubMed.
- M. Tyszka-Czochara, P. Paśko, W. Reczyński, M. Szlósarczyk, B. Bystrowska and W. Opoka, Biol. Trace Elem. Res., 2014, 160, 123–131 CrossRef CAS PubMed.
- H. Sun, J. Brocato and M. Costa, Curr. Environ. Health Rep., 2015, 2, 295–303 CrossRef CAS PubMed.
- H. Papavlassopoulos, Y. K. Mishra, S. Kaps, I. Paulowicz, R. Abdelaziz, M. Elbahri, E. Maser, R. Adelung and C. Röhl, PLoS One, 2014, 9, e84983 CrossRef PubMed.
- D. A. Saud Alarifi and S. Alkahtani, Int. J. Nanomed., 2016, 11, 1253 CrossRef PubMed.
- H. Calum, C. Moser, P. Ø. Jensen, L. Christophersen, D. Maling, M. Van Gennip, T. Bjarnsholt, H. Hougen, M. Givskov, G. K. Jacobsen and N. HØiby, Clin. Exp. Immunol., 2009, 156, 102–110 CrossRef CAS PubMed.
- J. J. Hew, R. J. Parungao, H. Shi, K. H. Y. Tsai, S. Kim, D. Ma, J. Malcolm, Z. Li, P. K. Maitz and Y. Wang, Burns, 2020, 46, 663–674 CrossRef PubMed.
- S. Murthy, M. K. Gautam, S. Goel, V. Purohit, H. Sharma and R. K. Goel, BioMed Res. Int., 2013, 2013, 972028 CAS.
- A. Zuluaga-Vélez, D. F. Cómbita-Merchán, R. Buitrago-Sierra, J. F. Santa, E. Aguilar-Fernández and J. Sepúlveda-Arias, PLoS One, 2019, 14, e0213303 CrossRef PubMed.
- Y. Zhang, M. Jiang, Y. Zhang, Q. Cao, X. Wang, Y. Han, G. Sun, Y. Li and J. Zhou, Mater. Sci. Eng., C, 2019, 104, 110002 CrossRef CAS PubMed.
- M. Andiappan, S. Sundaramoorthy, N. Panda, G. Meiyazhaban, S. B. Winfred, G. Venkataraman and P. Krishna, Prog. Biomater., 2013, 2, 6 CrossRef PubMed.
- H. E. Ahmed Mohamed, S. Afridi, A. T. Khalil, T. Zohra, M. Ali, M. M. Alam, A. Ikram, Z. K. Shinwari and M. Maaza, Nanomedicine, 2020, 15, 1653–1669 CrossRef CAS PubMed.
- E. P. Babu, A. Subastri, A. Suyavaran, K. Premkumar, V. Sujatha, B. Aristatile, G. M. Alshammari, V. Dharuman and C. Thirunavukkarasu, Sci. Rep., 2017, 7, 1–12 CrossRef PubMed.
- D. Hassan, A. T. Khalil, A. R. Solangi, A. El-Mallul, Z. K. Shinwari and M. Maaza, Appl. Organomet. Chem., 2019, 33, e5041 CrossRef.
- M. Rajapriya, S. A. Sharmili, R. Baskar, R. Balaji, N. S. Alharbi, S. Kadaikunnan, J. M. Khaled, K. F. Alanzi and B. Vaseeharan, J. Cluster Sci., 2019, 1–11 Search PubMed.
- R. R. Ray, Interdiscip. Toxicol., 2016, 9, 55–65 CrossRef CAS PubMed.
- N. Annabi, D. Rana, E. S. Sani, R. Portillo-Lara, J. L. Gifford, M. M. Fares, S. M. Mithieux and A. Weiss, Biomaterials, 2017, 139, 229–243 CrossRef CAS PubMed.
- A. Alzagameem, S. E. Klein, M. Bergs, X. T. Do, I. Korte, S. Dohlen, C. Hüwe, J. Kreyenschmidt, B. Kamm and M. Larkins, Polymers, 2019, 11, 670 CrossRef CAS PubMed.
- E. Larrañeta, M. Imízcoz, J. X. Toh, N. J. Irwin, A. Ripolin, A. Perminova, J. Domínguez-Robles, A. Rodríguez and R. Donnelly, ACS Sustainable Chem. Eng., 2018, 6, 9037–9046 CrossRef PubMed.
- S. Çalamak, C. Erdoğdu, M. Özalp and K. Ulubayram, Mater. Sci. Eng., C, 2014, 43, 11–20 CrossRef PubMed.
- M. Salehi, A. Eshaghi and H. Tajizadegan, ACS Sustainable Chem. Eng., 2019, 778, 148–155 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section, FT-IR spectra and TG curve of cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite. See DOI: 10.1039/d1ra01300a |
| ‡ Currently on leave from CMRDI. |
|
| This journal is © The Royal Society of Chemistry 2021 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article bc,
Fateme Radinekiyana,
Mohammad Sobhania,
Farzane khalilia,
Ali Maleki
bc,
Fateme Radinekiyana,
Mohammad Sobhania,
Farzane khalilia,
Ali Maleki *a,
Hamid Madanchi*de,
Mohammad Mahdavi
*a,
Hamid Madanchi*de,
Mohammad Mahdavi f and
Ahmed Esmail Shalan‡
f and
Ahmed Esmail Shalan‡
 gh
gh
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration mode of amide I at 1625 cm−1 to 1660 cm−1, the N–H bending vibration mode of amide II at 1520 cm−1 to 1540 cm−1, and the C–N stretching vibration mode of amide III at 1230 cm−1 to 1270 cm−1, can define the presence of SF biopolymeric structure.40 As illustrated in Fig. S1B, ESI,† two observed absorption bands at 1654 cm−1 and 1535 cm−1 which are related to the C
O stretching vibration mode of amide I at 1625 cm−1 to 1660 cm−1, the N–H bending vibration mode of amide II at 1520 cm−1 to 1540 cm−1, and the C–N stretching vibration mode of amide III at 1230 cm−1 to 1270 cm−1, can define the presence of SF biopolymeric structure.40 As illustrated in Fig. S1B, ESI,† two observed absorption bands at 1654 cm−1 and 1535 cm−1 which are related to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration mode of amide I and the N–H bending vibration modes of amide II confirm the random coil conformation of SF.40,41 Also, the broad absorption band around 3300 cm−1 indicates the β-sheet conformation of SF.42 Fig. S1C, ESI,† shows the FT-IR spectrum of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite. Alongside the mentioned absorption bands from two previous synthesis steps, the Zn–O and Cr–O stretching vibration modes of ZnCr2O4 NPs can be observed corresponding to two small absorption bands at 488 cm−1 and 545 cm−1.43 Furthermore, it can be mentioned that the construction of new intramolecular hydrogen bonds is the reason for increased intensity in the band of the O–H stretching vibration mode.
O stretching vibration mode of amide I and the N–H bending vibration modes of amide II confirm the random coil conformation of SF.40,41 Also, the broad absorption band around 3300 cm−1 indicates the β-sheet conformation of SF.42 Fig. S1C, ESI,† shows the FT-IR spectrum of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite. Alongside the mentioned absorption bands from two previous synthesis steps, the Zn–O and Cr–O stretching vibration modes of ZnCr2O4 NPs can be observed corresponding to two small absorption bands at 488 cm−1 and 545 cm−1.43 Furthermore, it can be mentioned that the construction of new intramolecular hydrogen bonds is the reason for increased intensity in the band of the O–H stretching vibration mode.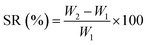
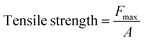
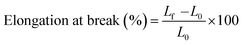
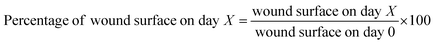
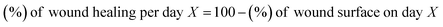
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 993-5 1992), when a hemolysis index of a substance is less than 5% it is considered harmless. The results of this test show that there is no significant difference between the hemolytic activity of cross-linked lignin–agarose hydrogel, cross-linked lignin–agarose/SF hydrogel, cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite, and negative control (P ≥ 0.05), the values being 3.93%, 4.91% and 1.67% respectively. This is in contrast to Triton X-100 (as a positive control), which lyses almost all RBCs. The reported results are the mean of 3 separate tests. Fig. 6A and B shows a histogram of the hemolysis results, as well as an image of a 96-well plate, for positive control, cross-linked lignin–agarose/SF hydrogel, cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite, and negative control. Numerous works have confirmed that agar-based66 and lignin-based67 scaffolds are compatible with blood. Also, SF-based scaffolds do not show great hemolysis potential.68 On the other hand, various studies have shown that zinc, chromium and their oxide NPs (ZnO, Cr2O3 etc.) have different hemolytic activity according to their morphology, size and concentration.69–73 In this study, ZnCr2O4 NPs with appropriate concentration (0.5% w/w) were used and, as a result, the hemolytic activity of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite remains almost unchanged. Overall, it can be said that this nanobiocomposite scaffold is fully hemocompatible and is very suitable for use in skin tissue engineering and wound healing.
993-5 1992), when a hemolysis index of a substance is less than 5% it is considered harmless. The results of this test show that there is no significant difference between the hemolytic activity of cross-linked lignin–agarose hydrogel, cross-linked lignin–agarose/SF hydrogel, cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite, and negative control (P ≥ 0.05), the values being 3.93%, 4.91% and 1.67% respectively. This is in contrast to Triton X-100 (as a positive control), which lyses almost all RBCs. The reported results are the mean of 3 separate tests. Fig. 6A and B shows a histogram of the hemolysis results, as well as an image of a 96-well plate, for positive control, cross-linked lignin–agarose/SF hydrogel, cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite, and negative control. Numerous works have confirmed that agar-based66 and lignin-based67 scaffolds are compatible with blood. Also, SF-based scaffolds do not show great hemolysis potential.68 On the other hand, various studies have shown that zinc, chromium and their oxide NPs (ZnO, Cr2O3 etc.) have different hemolytic activity according to their morphology, size and concentration.69–73 In this study, ZnCr2O4 NPs with appropriate concentration (0.5% w/w) were used and, as a result, the hemolytic activity of the cross-linked lignin–agarose/SF/ZnCr2O4 nanobiocomposite remains almost unchanged. Overall, it can be said that this nanobiocomposite scaffold is fully hemocompatible and is very suitable for use in skin tissue engineering and wound healing.








