Open Access Article
Open Access ArticleA depth-suitable and water-stable trap for CO2 capture†
Zhaofu Zhang *a,
Shuaishuai Liuab,
Jun Maa and
Tianbin Wua
*a,
Shuaishuai Liuab,
Jun Maa and
Tianbin Wua
aBeijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid and Interface and Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: zhangzf@iccas.ac.cn
bSchool of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing 100049, China
First published on 28th April 2021
Abstract
In terms of CO2 capture and storage (CCS), it is highly desired to substitute of high efficiency process for the applied one which is far from the ideal one. Physical processes cannot capture CO2 effectively, meanwhile CO2 desorption is energy-intensive in chemical processes. Herein, a depth-suitable and water-stable trap for CO2 capture was discovered. Carboxylates can react with polybasic acid roots by forming united hydrogen bonds. Carboxylate ionic liquid (IL) aqueous solutions can absorb one equimolar CO2 chemically under ambient pressure, and its CO2 desorption is easy, similar to that in physical absorption/desorption processes. When used as aqueous solutions, carboxylate ILs can replace alkanolamines directly in the applied CCS process, and the efficiency is enhanced significantly due to the low regenerating temperature. CO2 (or polybasic acids) can be used as a polarity switch for ILs and surfactants. A new method for producing carboxylate ILs is also proposed.
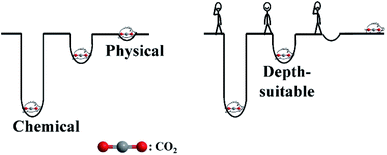
Being the main greenhouse gas, CO2 capture and storage (CCS) is indispensable for achieving carbon neutrality, and it has been a hot topic for many years.1 Besides CO2 generated from the energy demand of human being's daily activities, energy requirement in CCS also produces more CO2, which comes from fuel consumption, and this will lead to an increase in electricity prices.2,3 So, the enhancement of energy efficiency in CCS is highly desired. Alkanolamines are the main absorbents applied for CCS in industry, and their regeneration processes are energy intensive, as shown in Scheme 1 (chemical trap). There are more inherent shortages, such as amine degradation and volatilize, and hence, the CCS process applied in this study is far from the ideal one.
Having many unique features, such as extremely low vapor pressure, ionic liquids (ILs) have aroused the interest of many scientists,4–6 including their ability to absorb CO2 physically.7 Physical absorption is conducted under high pressure and anhydrous conditions. There are several shortcomings as follows: the sorption capacity is limited at low pressure, water is invariably present in almost all ILs,8 CO2 and water are the two main exhaust products in fossil fuel burning. It is difficult to get satisfactory results in physical absorption, as shown in Scheme 1. Functional ILs have also been tested for absorbing CO2 (ref. 9 and 10) and SO2 (ref. 11 and 12) chemically, and some authors claimed that the products obtained are carbamic acids or amidates while absorbing CO2. Recently, Dupont et al. considered that carbonates/bicarbonates are generated because water is inevitable in most cases,8 and this is similar to the alkanolamine aqueous solution in the applied CCS process. The regeneration of those ILs is energy intensive, and the stronger the functional ILs used for absorbing CO2, the more energy is needed in the regeneration process. The high viscosity of ILs is also unfavourable for capturing CO2 effectively. Finding a depth-suitable trap (as shown in Scheme 1) with low viscosity and water-stability for CO2 capture is highly desirable.
Various chemical reactions are going on at this moment in nature and our bodies, and many of them have been discovered and explained clearly.13 Most of them form new chemical bonds, including covalent bonds, ionic bonds, and metal bonds.14 In comparison, hydrogen bonding is a weak interaction that has been known for a century15 and has been redefined recently.16 Although weak, hydrogen bonds are vital for water keeping its state as we know generally17 and for life passing on its genetic code.18,19
Herein, a depth-suitable and water-stable trap for CO2 capture was discovered. Carboxylate roots can react with polybasic acid roots by forming united hydrogen bonds, and this lower energy of the products make them more stable even in water. Carboxylate IL aqueous solutions can replace alkanolamine aqueous solutions directly in the applied CCS process, and the efficiency is enhanced significantly, which comes from the low regenerating temperature. CO2 (or polybasic acids) can be used as a polarity switch for ILs and surfactants. A new way for producing carboxylate ILs is also proposed.
Finding the trap
Strong acids reacting with weak acid salts to produce weak acids are classic reactions that are widely applied in industry. Most carboxylic acids, such as formic acid and acetic acid, are stronger than carbonic acid, and can react with carbonates to form carboxylates, CO2, and water. On the contrary, some carboxylates can absorb CO2 under certain conditions.20–26 So, there must be an intermediate in this reaction, which is relatively stable under certain conditions. Bicarbonate is a reasonable explanation, and Yasaka et al. considered that bicarbonate is stabilized by a hydrogen bond with a carboxylic acid.25 However, those systems are viscous and not stable in water, and the CO2 sorption shrinks down sharply with an increase in water concentration.In this study, 1 mole of sodium 2,2-dimethylbutyrate with 0.5 mol water could absorb 0.5 mol CO2 under ambient conditions in one bottle. In another bottle, 2,2-dimethylbutyric acid and sodium carbonate (molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
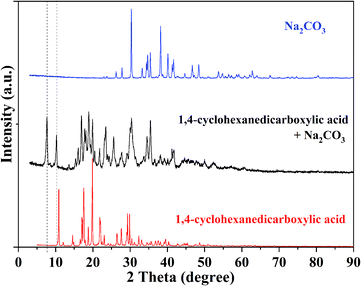
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were added and stirred, and there was no CO2 bubbling out as the bottle was covered with a cap. Proportions of the 2,2-dimethylbutyrate root, sodium ion, and carbonic acid (CO2 plus H2O) in the two bottles are the same, so it is reasonable to consider that the structure of the products from the left and right sides of eqn (1) (in Scheme 2) are the same, and the product has a lower energy level than the reactants of two sides of the reaction under certain conditions. It is an equilibrium reaction, and the equilibrium will shift to the right side by moving CO2 out or adding water. The stability of the intermediate varies with carboxylate anions. As the caps of the bottles are opened, the CO2 release rate from formic acid and sodium carbonate is faster than that from acetic acid and sodium carbonate. X-ray diffraction (XRD) and infrared radiation (IR) analysis results of the product of sodium carbonate reacting with 1,4-cyclohexanedicarboxylic acid are shown in Fig. 1 and S1a,† respectively. The results show that the product is different from both the reactants. The new peaks at 7.7° and 10.3° in XRD indicate that an ordered structure is formed between the two reactants.
1) were added and stirred, and there was no CO2 bubbling out as the bottle was covered with a cap. Proportions of the 2,2-dimethylbutyrate root, sodium ion, and carbonic acid (CO2 plus H2O) in the two bottles are the same, so it is reasonable to consider that the structure of the products from the left and right sides of eqn (1) (in Scheme 2) are the same, and the product has a lower energy level than the reactants of two sides of the reaction under certain conditions. It is an equilibrium reaction, and the equilibrium will shift to the right side by moving CO2 out or adding water. The stability of the intermediate varies with carboxylate anions. As the caps of the bottles are opened, the CO2 release rate from formic acid and sodium carbonate is faster than that from acetic acid and sodium carbonate. X-ray diffraction (XRD) and infrared radiation (IR) analysis results of the product of sodium carbonate reacting with 1,4-cyclohexanedicarboxylic acid are shown in Fig. 1 and S1a,† respectively. The results show that the product is different from both the reactants. The new peaks at 7.7° and 10.3° in XRD indicate that an ordered structure is formed between the two reactants.
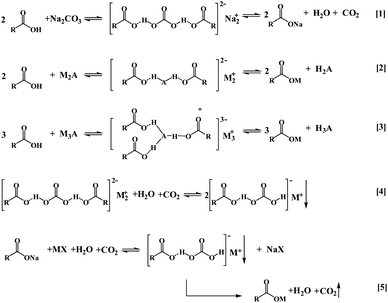 | ||
| Scheme 2 Reactions between carboxylates and polybasic acid roots. M represents inorganic or organic cation. | ||
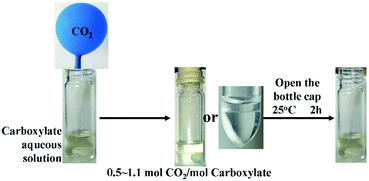
The stability of the intermediates also varies with different cations, including inorganic and organic cations. Tributylhexylphosphonium 2-ethylhexanoate (1) is a hydrophilic IL and miscible with water. When 1 is selected as the carboxylate in eqn (1), the product after the absorption of CO2 is stable in water, and the sorption remains constant with water being added. When the aqueous solution (50 wt%) is exposed to the CO2 atmosphere, a new liquid phase appears during the absorption of CO2, as shown in Fig. 2, and the sorption is 0.49 mol CO2 per mol IL under ambient temperature. This suggests that the sorption of CO2 changes the polarity of the reaction system and induces product separation from water.
With the above understanding, we proposed that there are two hydrogen bonds in the intermediates of eqn (1), and the two united hydrogen bonds tie two carboxylate roots and one carbonate root together, as shown in the spatial conformation obtained from DFT calculation (see Fig. 3 and S2a, b†). The united hydrogen bonds lower the energy of the products and enhance their stabilities even in an aqueous solution. The united hydrogen bonds also change the polarity of the product and cause its separation from water.
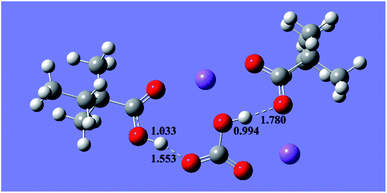 | ||
| Fig. 3 Spatial conformation of the product from 2,2-dimethylbutyric acid + Na2CO3 obtained from DFT calculation. | ||
Reaction of carboxylates with other polybasic acid roots
This reaction is also tested with sulfur dioxide (SO2), hydrogen sulfide (H2S), and phosphoric acid, and they can all react with an aqueous solution of 1 and form two liquid phases. The top phase of aqueous solution 1 absorbing SO2 was tested with 1H NMR spectroscopy (Fig. S3†), and the results show that tributylhexylphosphonium ions and 2-ethylhexanoate ions in the top phase are equimolar. This implies that the top organic phase separating from the aqueous solution is not 2-ethylhexanoic acid, which may come from 2-ethylhexanoate ions reacting with sulfite acid. Some products of 1,4-cyclohexanedicarboxylic acid and 1,3,5-benzenetricarboxylic acid reacting with sodium sulfite and dipotassium hydrogen phosphate were tested with XRD (Fig. S4a–c†), IR spectroscopy (Fig. S1b and c†), and scanning electron microscopy (SEM, Fig. S5a–d†). XRD and IR analysis of the products indicate that the products are different from the raw reactants, and new ordered structures are generated. The SEM images show the shapes of those products, and there is one kind of substance in each photo, while not mixture of two reactants. This represents the reactants reacting with each other and forming new products. The products from 1,3,5-benzenetricarboxylic acid + K2HPO4 (Fig. S5a†) and 1,3,5-benzenetricarboxylic acid + Na2SO3 (Fig. S5d†) are sheets, and this implies that the united hydrogen bond products from 1,3,5-benzenetricarboxylic acid are ready to form a sheet. The shapes of products from 1,4-cyclohexanedicarboxylic acid + K2HPO4 (Fig. S5c†) and 1,4-cyclohexanedicarboxylic acid + Na2SO3 (Fig. S5b†) are different. It is seen from the four images that the shape varies with the type of carboxylate acids and salts. The spatial conformations of sulfite acid and H2S reacting with 1 from DFT calculation (Fig. S2c and d†) show that there are two hydrogen bonds in both of their products.The product of tripotassium phosphate reacting with 1,4-cyclohexanedicarboxylic acid was tested with XRD (Fig. S4d†) and SEM (Fig. S5e†). The product of tripotassium phosphate reacting with 1,3,5-benzenetricarboxylic acid was tested using IR spectroscopy (Fig. S1d†). The results demonstrate that the product is different from that of dipotassium hydrogen phosphate plus carboxylate acid. This means that carboxylate roots can react with phosphate root in two ways. One is two carboxylate ions combining with one hydrogen phosphate ion, and forming two hydrogen bonds (Fig. S2e†). The other is three carboxylate ions combining with one phosphate ion, and forming three hydrogen bonds (Fig. S2f†).
In order to show the reaction clearly, the general equations are given as eqn (2) and (3). The united hydrogen bonds enhance the stability of the products as in DNA,19 and some of them are stable in water. The united hydrogen bonds tie two or three carboxylate ions together, and this induces different polarities of the products with reactants.
Influence of different anions and cations
The influence of different anions and cations on the system of carboxylates absorbing CO2 in water was studied and listed in Table 1. When the salts are strongly hydrophilic, such as sodium carboxylates, they are soluble in water, and there is no change in the phase behaviour of their aqueous solutions with the addition of CO2. When the carboxylate ILs have suitable hydrophilicity, they are miscible with water, and new phases appear in their aqueous solutions with the addition of CO2. Some of them form new liquid phases, and some others form solid phases, as shown in the table. CO2 sorption in the systems forming two phases was measured and marked in the table. For tributylhexylphosphonium aqueous solutions, there are four anions that can form a new liquid phase with the addition of CO2. For 1 and tributylhexylphosphonium 2-propylpentanoate, CO2 sorption is about 0.5 mole per mol IL, and this implies that these two ILs can form stable, united hydrogen bonds in water. For tributylhexylphosphonium 2,2-dimethylbutyrate and tributylhexylphosphonium n-octanoate, the sorptions are 0.11 and 0.18 mol CO2 per mol IL, respectively. This indicates that the united hydrogen bonds in their products are not stable, resulting from the strong hydrophilicity of anions.a For sodium carboxylate aqueous solutions, the concentrations are 10 wt%; for carboxylate ILs, weight ratios of IL: water are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Blue represents the water phase, and red represents the organic phase. The organic phase below water represents the solid depositing out. Numbers present CO2 sorption (on carboxylates) under ambient pressure and temperature. 1. Blue represents the water phase, and red represents the organic phase. The organic phase below water represents the solid depositing out. Numbers present CO2 sorption (on carboxylates) under ambient pressure and temperature. |
|---|
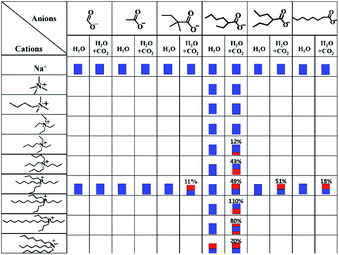 |
When the anion of ILs are selected as 2-ethylhexylate, several IL aqueous solutions form solids with the addition of CO2, and there are great differences in CO2 sorption. For tributyl-n-octylphosphonium-2-ethylhexylate (2), the sorption of CO2 can get to 1.1 mole per mol 2. During CO2 absorption by the aqueous solution of 2, a state of liquid–liquid–solid was found, as shown in Fig. 2. The three-phase state was also found in the CO2 release of 2 + H2O + CO2 system. A liquid–liquid state was found in this system, absorbing CO2 at 80 °C. All these findings suggest that the united hydrogen bond product shown in eqn (1) is an intermediate, and it reacts with CO2 and water further and generates the solid as shown in eqn (4). The solid is the crystallization of the product with some more carbonic acid. The solid has lower energy than the liquid united hydrogen bond product shown in eqn (1) and can tie more CO2. The energy change may come from crystallization, which forms new hydrogen bonds.25 So, this reaction step is a part of the united hydrogen bond reaction. For tributyl-n-dodecylphosphonium-2-ethylhexylate (3) aqueous solution, after it reacts with CO2 for 12 h, beside the solid, there is also a little organic liquid phase in the system (liquid–liquid–solid state), and this shows that the solid of 3 + CO2 + H2O is not as stable as the solid of 2 + CO2 + H2O. For aqueous solutions of triethyl-n-butylammonium-2-ethylhexylate and tetrabutylammonium-2-ethylhexylate, there is no liquid–liquid–solid state observed during the absorption of CO2. It means that the united hydrogen bond products formed from the reaction between these two ILs and carbonic acid do not have enough hydrophobicity to separate from water, but further react with carbonic acid and form solids. Yet, they are more hydrophilic than 2, so only a part of them is deposited.
When ILs are strongly hydrophobic, such as methyltrioctylammonium 2-ethylhexanoate (4), it is immiscible with water at room temperature. Two liquid phases appeared as 4 is mixed with the same weight of water, and there is no change in the phase behaviour of its water mixture with or without CO2. The CO2 sorption is 0.2 mole per mol of 4. The sorption of CO2 in 4 with equimolar water is 0.52 mole per mol IL, as determined in this work. The difference may come from the ability of 4 combining more water than equimolar, and the combined water influences the stability of the united hydrogen bonds, so its CO2 sorption declines with the addition of water.
Utilization of the united hydrogen bond reaction
As mentioned above, aqueous solutions of carboxylate IL can be used for CO2 absorption with relatively low viscosity. For IL 1 and 2, their aqueous solution (50 wt%) can absorb 0.49 and 1.1 mol CO2 per mol IL, respectively, under CO2 atmosphere. High temperature is unfavorable for CO2 sorption. When the temperature rises to 80 °C, the sorption of atmospheric CO2 with an aqueous solution of 1 and 2 can obtain 0.31 and 0.30 mole per mol IL, respectively. For real flue gas, CO2 may be diluted by nitrogen gas, and its partial pressure will be lower than 1 bar. In this work, we test the CO2 absorption of simulated flux gas with CO2 partial pressure 0.1 bar, and the sorption for aqueous solutions of 1 and 2 (50 wt%) are 0.20 and 0.25 mol CO2 per mol IL, respectively.The regeneration of IL solution only requires opening the bottle cap at room temperature for 2 h, as shown in Fig. 2. The CO2 absorbed in the IL aqueous solutions is tied only by two united hydrogen bonds, and the trap of united hydrogen bonds is depth-suitable for CO2 capture, as shown in Scheme 1. On the one hand, it has enough power to capture CO2 effectively under relatively high temperature and low pressure in an aqueous solution. On the other hand, its CO2-desorption process is easy, similar to that in physical-absorption processes. The comparison of the CO2 capture efficiency between carboxylate ionic liquid in this work and monoethanolamine (MEA) applied in industry are shown in Table 2. It can be seen that the CO2 capture efficiency is significantly enhanced. When used as an aqueous solution, carboxylates can replace alkanolamines directly in the applied CCS process.
| Carboxylates | MEA23 | |
|---|---|---|
| Content in water | 50 w% | 30 w% |
| CO2 absorption | 1 bar: ∼1.1 mol L−1, 0.1 bar: ∼0.25 mol L−1 | 3.5 mol L−1 |
| Regenerating temperature | Room temperature | 120 °C |
| Chemical structure to be broken in regeneration | Two hydrogen bonds | [HOCH2CH2NH3]+HCO3− to HOCH2CH2NH2 + CO2 + H2O |
| Need of extra energy for water evaporation | No | Yes |
| Volatilize, decompose | No | Yes |
The united hydrogen bond reaction can also be used to absorb SO2 and H2S, as aforementioned. Many previous works on absorbing CO2 and SO2 with carboxylates need to take this united hydrogen bond reaction mechanism into consideration.
Jessop et al. have reported a kind of CO2 switchable ILs and surfactants.27,28 In their works, CO2 reacts with organic bases generating carbonates and dicarbonates, and the organic solvents transform from nonpolar to polar. In this work, the united hydrogen bond reaction could change the polarity of carboxylate systems, and they can be used as switchable solvents and surfactants. A series of carboxylate ILs were found that can be separated from water and form a new liquid phase with the addition of CO2 or other polybasic acids (SO2 + H2O, H3PO4, etc.). CO2 can be released at room temperature in a short time, and it will be miscible with water again. Selecting long-chain tetraalkylammonium carboxylates and tetraalkylphosphonium carboxylates as surfactants and switching their polarities by CO2 may realize the separation of surfactants from water or other solvents. For tributylhexadecylphosphonium ion, it can be separated out with 2-ethylhexanoate root from aqueous solution by dissolving tributylhexadecylphosphonium bromide and sodium 2-ethylhexanoate, even at a concentration of 1 wt%, in several minutes with CO2 bubbling (Fig. S6†). The separation process is revisable. The mixtures are miscible again and become clear in three minutes as they are exposed to air. This process can separate tetraalkylphosphonium (tetraalkylammonium or other ions) carboxylates from water (solid or a new liquid phase) and leave the corresponding ions in water as shown in eqn (5), and so it provides a new way for producing carboxylate ILs.
Conclusions
In summary, we discovered a depth-suitable and water-stable trap for CO2 capture that can replace the one currently in use, and the efficiency is significantly enhanced. Carboxylates can react with polybasic acid roots by forming united hydrogen bonds, and the united hydrogen bonds enhance the stability of the products even in aqueous solution. Some united hydrogen bond carbonates can interact with CO2 and water further and form water-stable solids. It can be used to capture acidic gases (CO2, SO2, and H2S) chemically. The CO2 sorption can exceed one equimolar for carboxylates under ambient pressure. Having suitable heats of absorption, carboxylates can absorb CO2 chemically, and its CO2-desorption process is easy, almost like that in physical absorption/desorption processes. CO2 (or other polybasic acids) can be used as a polarity switch for carboxylate ionic liquids and surfactants. This reaction also provides a new method for producing carboxylate ILs.Author contributions
Z. Z. proposed the idea, performed most experiments and wrote the manuscript. S. L. and T. W. performed the characterization. J. M. conducted DFT calculations.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the National Natural Science Foundation of China (21673255, 22072156).References
- N. M. Dowell, P. S. Fennell, N. Shah and G. C. Maitland, Nat. Clim. Change, 2017, 7, 243–249 CrossRef.
- R. S. Haszeldine, Science, 2009, 325, 1647–1652 CrossRef CAS PubMed.
- M. E. Boot-Handford, J. C. Abanades, E. J. Anthony, M. J. Blunt, S. Brandani, N. Mac Dowell and J. R. Fernández, et al., Energy Environ. Sci., 2014, 7, 130–189 RSC.
- J. S. Wilkes and M. J. Zaworotko, J. Chem. Soc., Chem. Commun., 1992, 965–967 RSC.
- Y. L. Wang, Chem. Rev., 2020, 120, 5798–5877 CrossRef CAS PubMed.
- Z. F. Zhang, Y. Xie, W. J. Li, S. Q. Hu, J. L. Song, T. Jiang and B. X. Han, Angew. Chem., Int. Ed., 2008, 47, 1127–1129 CrossRef CAS PubMed.
- L. A. Blanchard, D. Hancu, E. J. Beckman and J. F. Brennecke, Nature, 1999, 399, 28–29 CrossRef.
- M. Zanatta, N. M. Simon and J. Dupont, ChemSusChem, 2020, 13, 3101–3109 CrossRef CAS PubMed.
- E. D. Bates, R. D. Mayton, I. Ntai and J. H. Davis Jr., J. Am. Chem. Soc., 2002, 124, 926–927 CrossRef CAS PubMed.
- D. Hospital-Benito, J. Lemus, C. Moya, R. Santiago and J. Palomar, Chem. Eng. J., 2020, 390, 124509 CrossRef CAS.
- W. Z. Wu, B. X. Han, H. X. Gao, Z. M. Liu, T. Jiang and J. Huang, Angew. Chem., Int. Ed., 2004, 43, 2415–2417 CrossRef CAS PubMed.
- C. M. Wang, G. Cui, X. Luo, Y. Xu, H. Li and S. Dai, J. Am. Chem. Soc., 2017, 133, 11916–11919 CrossRef PubMed.
- J. J. Li, Name Reactions, Springer-Verlag Berlin Heidelberg, 4th edn, 2009 Search PubMed.
- L. Pauling, The nature of the chemical bond, Cornell University Press, Ithaca, 3rd edn, 1960 Search PubMed.
- W. M. Latimer and W. H. Rodebush, J. Am. Chem. Soc., 1920, 42, 1419–1433 CrossRef CAS.
- E. Arunan, G. R. Desiraju, R. A. Klein, J. Sadlej and S. Scheiner, et al., Pure Appl. Chem., 2011, 83, 1619–1636 CAS.
- F. N. Keutsch, J. D. Cruzan and R. J. Saykally, Chem. Rev., 2003, 103, 2533–2577 CrossRef CAS PubMed.
- J. D. Watson and F. H. C. Crick, Nature, 1953, 171, 737–738 CrossRef CAS PubMed.
- S. E. Harding, G. Channell and M. K. Phillip-Jones, Biochem. Soc. Trans., 2018, 46, 1171–1182 CrossRef CAS PubMed.
- R. Quinn, Synth. React. Inorg. Met.-Org. Chem., 2001, 31, 359–369 CrossRef CAS.
- R. Quinn, J. B. Appleby and G. P. Pez, J. Am. Chem. Soc., 1995, 117, 329–335 CrossRef CAS.
- G. Wang, W. Hou, F. Xiao, J. Geng, Y. Wu and Z. Zhang, J. Chem. Eng. Data, 2011, 56, 1125–1133 CrossRef CAS.
- K. Anderson, M. P. Atkins, J. Estager, Y. Kuah, S. Ng, A. A. Oliferenko, N. V. Plechkova, A. V. Puga, K. R. Seddon and D. F. Wassell, Green Chem., 2015, 17, 4340–4354 RSC.
- Y. Yasaka and Y. Kimura, J. Chem. Eng. Data, 2016, 61, 837–845 CrossRef CAS.
- Y. Yasaka, Y. Saito and Y. Kimura, ChemPhysChem, 2018, 19, 1674–1682 CrossRef CAS PubMed.
- D. J. Yeadon, J. Jacquemin, N. V. Plechkova, M. Maréchal and K. R. Seddon, ChemPhysChem, 2020, 21, 1369–1374 CrossRef CAS PubMed.
- P. G. Jessop, D. J. Heldebrant, L. Xiaowang, C. A. Eckert and C. L. Liotta, Nature, 2005, 436, 1102 CrossRef CAS PubMed.
- Y. X. Liu, P. G. Jessop, M. Cunningham, C. A. Eckert and C. L. Liotta, Science, 2006, 313, 958–960 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01268a |
| This journal is © The Royal Society of Chemistry 2021 |