Open Access Article
Open Access ArticleEnvironmental fate and impacts of microplastics in aquatic ecosystems: a review
Sen Dua,
Rongwen Zhua,
Yujie Caia,
Ning Xua,
Pow-Seng Yapb,
Yunhai Zhanga,
Yide He *a and
Yongjun Zhang*a
*a and
Yongjun Zhang*a
aSchool of Environmental Science and Engineering, Nanjing Tech University, Jiangsu 211816, P. R. China. E-mail: heyd@njtech.edu.cn; y.zhang@njtech.edu.cn; Tel: +86-25-58139656
bDepartment of Civil Engineering, Xi'an Jiaotong-Liverpool University, Suzhou, 215123, China
First published on 27th April 2021
Abstract
Wide usage of plastic products leads to the global occurrence of microplastics (MPs) in the aquatic environment. Due to the small size, they can be bio-ingested, which may cause certain health effects. The present review starts with summarizing the main sources of various types of MPs and their occurrences in the aquatic environment, as well as their transportation and degradation pathways. The analysis of migration of MPs in water environments shows that the ultimate fate of most MPs in water environments is cracked into small fragments and sinking into the bottom of the ocean. The advantages and disadvantages of existing methods for detection and analysis of MPs are summarized. In addition, based on recent researches, the present review discusses MPs as carriers of organic pollutants and microorganisms, and explores the specific effects of MPs on aquatic organisms in the case of single and combined pollutants. Finally, by analysing the causes and influencing factors of their trophic transfer, the impact of MPs on high-level trophic organisms is explored.
1. Introduction
Plastic products are widely used in many fields such as packaging materials (39.5% of total plastic output), building materials (20.1%), automotive parts (8.6%), electrical appliances (5.7%) and agricultural materials (3.4%), and the rest including household appliances, sports equipments, and other products.1 Since the 1950s, plastics have been produced on a large scale.2 In 2013, the global output of plastics had already reached 300 million tons and it is estimated that plastics production will increase to 33 billion tons by 2050.3 There are various materials of plastics, such as polyethylene terephthalate (PET), polystyrene (PS), polyvinyl chloride (PVC), polypropylene (PP), polyethylene (PE), poly amide (PA), polyethylene terephthalate (PET), low density polyethylene (LDPE), polymethyl methacrylate (PMMA) and others.4The ineffectively disposed plastic garbage in daily life has been entering the aquatic environment, and migrating with the hydrodynamic process over a long distance, which results in worldwide pollution.2 In the marine environment, plastic accounts for about 60–80% of marine garbage.5 To reduce the plastic pollution, a large number of studies have been carried out to reuse plastics. For example, researchers have explored the use of waste PET plastic bottles to make an innovative nonwoven packing material for wastewater treatment plants, and found that PET spunbonded nonwovens can be used as cost-effective, reliable and efficient packing materials for wastewater treatment.6,7
Microplastics (MPs) are generally defined as plastic particles with effective diameter less than 5 mm, and there is generally no specific lower size limitation.8,9 They are distributed all over the world, from the mainland to the ocean, from cities to remote areas. Their sources can be divided into two main categories. One is specially manufactured in the micron size range, which can be called primary plastic particles, such as industrial abrasives (acrylic acid or polyester beads), plastic beads added into toothpaste and cosmetics, etc.10,11 The other is plastic particles or fragments which are split or decomposed from large plastics in the environment, which can be called secondary plastic particles.12
Because of their small size, MPs are difficult to be identified in water. The detection and analysis of MPs is always done by visual inspection followed by Fourier-transform infrared spectroscopy (FTIR), Raman spectroscopy, and pyrolysis gas chromatography mass spectrometry (Pyro GC-MS), and so on.13,14 The large specific surface area leads to the absorption of organic matters, which can act as a medium for pollutants to enter the aquatic food webs. Moreover, when plastic additives carried in MPs are released into the aquatic environment, harm to aquatic organisms could be caused.15,16
The present review is characterized by a more comprehensive introduction to the pollution, migration and biological effects of MPs in aquatic ecosystem. It emphasizes the influence of MPs on organisms in aquatic environment, which is analysed from the perspectives of MPs as carriers and transferring through trophic level respectively, and the role of chemistry is also discussed. The pollution situation and transportation of MPs in aquatic systems around the world are summarized. At the same time, degradation and fate of MPs in the aquatic environment are clarified. The existing detection and analysis methods of MPs, and the challenges in this field are also discussed. Suggestions are proposed for future research works in the end of the present review.
2. Occurrence and fate of MPs in the aquatic environment
2.1. MPs in the aquatic environment
Another important terrestrial source is farmland. The fertilizer,25 plastic film or plastic greenhouse for heat preservation of crops,26 and agricultural irrigation leads to the existence of a large number of MPs in agricultural soil.27 They can migrate into the surrounding rivers and surface water through the rain and irrigation runoff of farmland. Firstly, synthetic fibers or deposited MPs in sewage sludge have been identified by many studies that MPs have been used as fertilizer or repair material.28 So large amount of plastics enter into the terrestrial environment. After that, they enter the freshwater environment through rainfall or infiltration of the land. In European Union (EU), 4 to 5 million tons of sewage sludge are used annually for cultivating land.29 Secondly, the extensively used mulch film in agriculture (especially in facility agriculture) is also an origin of plastic pollution in terrestrial environment.30 However, the recovery rate of agricultural film is low, and a large amount of agricultural film remains in the environment after use.31 Finally, the wastewater containing MPs was directly used for agriculture irrigation, resulting in the accumulation of MPs in the soil, and then migrating into the freshwater bodies. The type of MPs is one factor influencing the migration in soils, since microbeads and microfibers show different interaction with soil, which may affect the transportation of MPs in soil.32
Atmospheric deposition is also confirmed as a source of MPs in freshwater environment. For example, Dris et al. investigated atmospheric dust in Paris urban areas, and found that it contained man-made fibers.33 Studies have shown that MPs in atmospheric environment can be transferred to water environment through transportation and sedimentation.34 At the same time, sewage treatment plant is also one of the sources that can not be ignored, even it can remove part of MPs.35 Generally speaking, the removal rate of MPs from wastewater in sewage treatment plants can reach 72%, most of which go to sewage sludge.35 Many of the sludge from the sewage treatment plants are used to make crop fertilizer or directly applied, which make them as one major source of MPs into the ecological environment.35
Researches on the occurrence, accumulation, environmental effects, and methods of detecting plastics in freshwater have been carried out in recent decades, which showed that more attention has been paid to the existence of MPs in freshwater.36 Table 1 shows the MPs particles detected in different freshwater environments around the world.37 The detected MPs show different shapes, sizes and colours. At the same time, they have various chemical compositions and may carry other pollutants.38
| Region | Concentration | Shape | Type | Reference |
|---|---|---|---|---|
| North shore channel (North America) | 1.94–17.93 items per m3 | Fragments | PS | 158 |
| Pellets | ||||
| Fibers | ||||
| Garda Lake (Italy) | 1108 ± 983 items per m2 | Debris | PS | 159 |
| PE | ||||
| PP | ||||
| PA | ||||
| PVC | ||||
| Danube River (Europe) | 316.8 ± 4664.6 items per m3 | Pellets | — | 22 |
| Flakes | ||||
| Spherules | ||||
| Fragments | ||||
| Antuã River (Portugal) | 58–1265 items per m3 | Fibers | PE | 160 |
| Fragments | PP | |||
| Hovsgol Lake (Mongolian) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 264 items per km2 264 items per km2 |
Fragments | — | 161 |
| Films | ||||
| Pellets | ||||
| St. Lawrence River (North America) | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 832 ± 13 832 ± 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 677 items per m2 677 items per m2 |
Micro-beads | PE | 162 |
| Jiao Jiang (China) | 960 items per m3 | — | — | 117 |
| San Gabriel River (California) | 0–153 items per m3 | Fragments | PS | 163 |
| Ou Jiang (China) | 680 items per m3 | — | — | 117 |
| Nakdong River (South Korea) | 83–5242 items per m3 | Fibers | PP | 164 |
| Min Jiang (China) | 1300 items per m3 | — | — | 117 |
| Raritan River (North America) | 7.7–24 items/m3 | Fragments | — | 165 |
| Films | ||||
| Ulansuhai Lake (China) | 1760–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 n per m3 120 n per m3 |
Fibers | PE | 166 |
| PS | ||||
| PBT | ||||
| Winnipeg Lake (Canada) | 193![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420–115567 items per km2 420–115567 items per km2 |
Fibers | — | 165 |
| Taihu Lake (China) | 3.4–25.8 items per L | Fibers | — | 167 |
| Saigon River (Vietnam) | 10–223 items per m3 | Fragments | — | 168 |
| Films | ||||
| Tibet Plateau Lake (China) | 563 ± 1219 items per m2 | — | PS | 169 |
| PP | ||||
| PE | ||||
| PET | ||||
| PVC | ||||
| Seine River (France) | 0.28–47 items per m3 | Fibers | PP | 170 |
| Particles | PE | |||
| PS | ||||
| Three Gorges reservoir (China) | 1597–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 611 n per m3 611 n per m3 |
Fibers | PS | 171 |
| Particles | PP | |||
| PE |
| Location | Range of MPs concentration | MPs type | Reference |
|---|---|---|---|
| Arabian Bay | 4.4 × 104 to 1.5 × 106 items per km2 | LDPE | 172 |
| PP | |||
| PET | |||
| South Pacific Ocean | 0–4.0 × 105 items per km2 | — | 173 |
| East Asian seas around Japan | 1.7 × 106 items per km2 | — | 174 |
| Western North Atlantic Ocean | 0–5.8 × 105 items per km2 | PS | 175 |
| Mediterranean | 0–9.0 × 105 items per km2 | PE | 176 |
| Northwestern Pacific | 6.4 × 102 to 4.2 × 104 items per km2 | PE | 177 |
| PP | |||
| PA | |||
| PVC | |||
| PS | |||
| Mid-West Pacific Ocean | 0.6–9.5 × 104 items per km2 | PP | 178 |
| PE | |||
| PET | |||
| PMMA | |||
| Chesapeake Bay | 0.007–1.25 × 103 items per km2 | PE | 176 |
| PP | |||
| East China seacoasts | 0.167 items per m3 | — | 179 |
| North Western Mediterranean Sea | 0.12 items per m2 | PS | 176 |
| Northeast Pacific Ocean | 1710 n per m3 | — | 179 |
| Southern coast of Korea | 23 n per L | PA | 180 |
| PS | |||
| Bohai Sea of China | 0.22–0.53 items per m3 | PE | 181 |
| PET | |||
| Yellow Sea of China | 0.05–0.174 items per m3 | PE | 182 |
| PET | |||
| East Sea of China | 0.011–2.20 items per m3 | PE | 183 |
| PP | |||
| Northeast Atlantic Ocean | 2.46 items per m3 | — | 184 |
For water column, previous studies have shown that the abundance of MPs in water column is lower than that in surface water.42,43 The MPs suspended in the water column are settling slowly every day, the number of MPs detected in the vertical distribution of deep seabed is four times of that in the surface layer.44 So, the MPs in the water column tend to sink to the bottom. The water column distribution of MPs in the ocean is mainly horizontal or vertical. The main reasons for the horizontal migration of MPs are tidal ocean current and wind drive.45 The results show that the areas with serious accumulation of MPs in the ocean basically coincide with the ocean current, so the ocean current plays a significant role in the horizontal transportation of MPs in the ocean.46 For the vertical level, the main factor affecting the distribution in the water column is the density of MPs.47 Generally, MPs made from materials with density greater than that of water are more likely to sink into the water column.47
For sediments, the amount of MPs in the sediment varies with geographical location. In terms of vertical distribution, the investigation results showed that the number concentration of plastic particles in coastal sediments ranges from 0.21 to 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 items per m2, which is higher than those in deep-sea sediments.48 In terms of the horizontal distribution, the concentrations of MPs are lower in remote areas. In the Yellow Sea and Xiangshan Bay of China, the concentration of MPs in sediments was 37.1 items per kg and 1739 items per kg, respectively.49,50 In the Balearic Islands of Spain, the sediment contains around 106 items per km2 of MPs.51 In the Northwest Pacific Ocean, sediments are rich in MPs of 1.0 × 104 items per km2.52 In the remote Atlantic, the average concentration of MPs in the sediments was 1.15 items per m3.53
000 items per m2, which is higher than those in deep-sea sediments.48 In terms of the horizontal distribution, the concentrations of MPs are lower in remote areas. In the Yellow Sea and Xiangshan Bay of China, the concentration of MPs in sediments was 37.1 items per kg and 1739 items per kg, respectively.49,50 In the Balearic Islands of Spain, the sediment contains around 106 items per km2 of MPs.51 In the Northwest Pacific Ocean, sediments are rich in MPs of 1.0 × 104 items per km2.52 In the remote Atlantic, the average concentration of MPs in the sediments was 1.15 items per m3.53
For organisms, such as zooplankton,54 mussels,55 fish,56 etc., it is easy to intake MPs, which pose a risk to their life and bring MPs to somewhere else. The intake of MPs and distribution in organisms will be elucidated in the following Section 2.2.
The influence of ocean currents on the distribution of MPs includes the movement of ocean currents, the drive of wind and other factors. Some factors also affect the migration of MPs when affecting the distribution of MPs, which will be explained in detail in the migration of MPs in the following Section 2.2.
The sources of MPs in marine environment include land-based input, shipping, marine aquaculture and fishing.57 Firstly, as land-based waste, in many countries plastic garbages are dumped directly into the marine environment. Oztekin et al. investigated the marine garbage of Sarikum lagoon coast, and found that the garbages on the beach mainly came from 25 neighbouring countries, the most common type of garbages was plastic, accounting for 95.61%.58 Secondly, the dumping from coastal cruise ships and merchant ships is another source for marine plastic pollution. In 2005, the United Nations Environment Program (UNEP) estimated that the amount of plastic wastes dumped into the sea by ships alone was as high as 5 million tons across the globe.59 Thirdly, due to the development of aquaculture, fishing boats use more affordable plastic fishing nets to catch fish. When the old fishing products are replaced, the outdated fishing gears have not been properly disposed and then intentionally or unintentionally scattered in the ocean. According to U.S. official statistics, about 30 million pounds of plastic fishing gear pollution come from all over the world each year.60
2.2. Migration of MPs in aquatic systems
Most of the MPs in freshwater will eventually gather in the ocean, and further migration will take place.61 The high concentration of MPs in the sea area is close to the industrialized cities.62 For example, many MPs are found on the beach of Cartagena, an industrialized city near the Caribbean Sea.62 The Mediterranean sea is surrounded by many industrialized and densely populated countries.63 It is found that the concentration of MPs pollution in Iskenderun Bay and Mersin Bay, located on the north east coast of Levantine, Turkey, could reach up to 906 items per m2.63 Moreover, the increase of population density leads to the concentration increase of MPs from inland to estuary, and finally into the ocean.64 The U.S. Environmental Protection Agency have shown that the link between marine pollution and rivers is clear.65 Rivers act as a transport route, transporting MPs to the ocean. Sadri et al. surveyed the flow of MPs in Tamar River in England, found that the amount of MPs entering the estuary was consistent with the amount of MPs flowing out of the estuary.66 Thus, it can be speculated that estuaries that receive MPs input from highly industrialized or densely populated catchment areas may contribute more to the ocean.67 The migration of MPs in aquatic system can be divided into horizontal movement and vertical movement, which is closely related to the flow velocity, depth, flow change, bottom topography of water,67,68 wind speed, and particle density.69For the horizontal transportation, as the main transportation mode of MPs, the ocean current movement contributes greatly to the migration, distribution and concentration of MPs in the open ocean. The Spanish Marine Science and Technology Research Team found that the five major plastics pollution areas in the world's marine areas roughly coincide with the five major circulation flow on the surface of the sea area.70 In the subtropical circulation zone of the North Pacific, the number concentration of plastic particles from 0 mg L−1 increased to 0.116 mg L−1 and the mass concentration of plastics from 0 particles/m3 increased to 0.086 items per m3.70 Research shows that the influence of ocean current on concentration, the total amount of plastic particles in the North Pacific subtropical eddy enrichment area increased by two orders of magnitude from 1980 to 2000.71 Similarly, other researchers have found that the accumulative concentration of MPs in subtropical circulation is very high.72 In addition, due to the low density, MPs floating with the water flow and finally arriving at the sea.73 After the MPs are transported to different water spaces, the spatial distribution of MPs changes significantly, whose concentration changes in order of magnitude in different regions.74
For the vertical transportation, density or buoyancy and the adsorption of MPs results in “dirt” on the surface, which increase the weight of MPs and leads them to sink.75 During the decline process, these “dirty” MPs return to the surface.76 Similarly, when biofilm formed on MPs, the hydrophobicity will decrease and the particle density will change, thus affecting its distribution in water.77 For the vertical transportation, the driving force of wind also plays an important role. Driven by the wind, the flow could be turbulent, which could transfer the MPs from the surface to the bottom.78 The sinking speed of MPs in water is related to the particle density, particle size, fluid density and their shape. And the MPs will be aged or biologically polluted after a long time in the environment, which leads to vary of their mass. All these factors will change the settlement behavior of MPs.79 Wind will not only drive the MPs to sink, but also drive them to float up from the bottom. For example, when the turbulent flows generate storms, the MPs at the lower water layer will rise to middle water layer.80 Also, the intake by aquatic organisms is also an important reason for affecting the vertical transportation of MPs.80,81 The aquatic organisms can absorb or retain MPs in vivo with different time periods, then transport MPs to a large extent, some of which will enter the deep sea.82
2.3. Degradation and fate of MPs in aquatic environment
For MPs, there are many types of degradation, including biological degradation, physical degradation, and chemical degradation.83 The microorganisms get involved in biological degradation, mainly including bacteria, mold (fungi) and algae.83 Microorganisms degrade MPs through hydrolysis and enzyme catalysis.83 Physical degradation includes photo-degradation, thermal degradation and mechanical degradation, while chemical degradation mainly includes hydrolysis degradation and thermal oxidation degradation (Fig. 1).83There are many factors affecting the degradation rate of MPs. Firstly, it is the type of the MPs.83 Different types of MPs have different crystallinity, biodegradability, surface properties, oxidation resistance and residual monomers, which lead to different degradation rates.84 Secondly, the interaction between MPs (especially for the non-biodegradable MPs) and microorganisms results in the biofilm formation on the surface of MPs, which increases the difficulty of degradation.61 When the degradation rate is reduced, MPs are more vulnerable to microbial adhesion and pollution. The biofilm on the surface of polluted microorganism, a protective layer is formed, which further reduces the degradation rate.74 Thirdly, the complex environmental conditions can affect the degradation rate of MPs. Their degradation rate in pure water environment is higher than those in simulated sea water environment.74 Easily exposed to sunlight, the MPs can be degraded by ultraviolet radiation even at the bottom, so that MPs are more easily degraded in shallow lakes with smaller area85 Similarly, in the marine system, the breakage or degradation rate of MPs near the coastline or beach will be several orders of magnitude higher than those floating in water column and sinking in marine sediments.101 In the freshwater system, some rivers and lakes with large area will produce strong waves, which will lead to the breakage of MPs.48 However, compared with the ocean, there is a lack of frequent turbulence, ocean current or wave and other ocean current movements.85 Thus, the aging, fragmentation or mineralization of MPs will not occur easily in freshwater.85
After entering the marine environment, the degradation of MPs includes size variation and mineralization. This means that the polymer chain of the MPs is further destroyed, and the molecular weight of the polymer is reduced.86 Due to the needs of microorganisms' own growth, microbial colonies will convert organic matters from MPs into carbon dioxide and incorporated into biomass.87 However, research shows that it takes hundreds of years for MPs to be fully mineralized.86 Hence, MPs are considered to be relatively persistent in the aquatic environment, and the degradation of MPs are more likely to occur at the surface water, beach or coastline.74
After entering the aquatic environment, it is generally believed that the fate of MPs occurs in the ocean.61 The fate of MPs in the marine system is affected by many environmental factors, such as the ocean current, food abundance of marine organisms and others.88 In the ocean, it is found that 70–80% of MPs finally enter the sea bottom or exist in the seabed sediments.61 The remaining stays in the coastal areas or the surface water respectively.89 Now, the amount of MPs found in the ocean is only 1% of the amount that was dumped into the ocean theoretically. The remaining 99% of the MPs are not found because they are broken down into pieces that are too small to be found, or they are not detected on the bottom of the ocean, rather than disappearing.
2.4. Detection and analysis methods of MPs in the aquatic environment
The MPs fragments suspended in aquatic environment are generally collected by nets or trawls, then followed by sample preparation, usually including sample digestion and density separation.90 At present, the quantitative and qualitative detection technologies for MPs include visual method, spectroscopic method, chromatographic method, and so on.14 Visual inspection with the help of stereoscope or microscope for direct observation is commonly used.28,91 However, this method is highly subjective, time-consuming, and easy to lead to some types and colors of MPs recognition errors due to experience and fatigue.92 FTIR is well-established, fast, quite-reliable and non-destructive method, which can be used to analyze the molecules with polar functional groups such as carbonyl groups.93,94 However, it is always limited to certain diffraction range, the size of MPs down to 20 μm can analyzed by coupling with microscopy.95 Raman spectroscopy can offer structural information of MPs falling in the range of 1 to 20 μm.96 However, the interferences of fluorescence from biological, organic and inorganic impurities can hamper the identification.36 FTIR and Raman spectroscopy are complementary vibrational techniques, which can offer complementary information on MPs.Thermoanalytical methods such as Pyro GC-MS and thermogravimetric analysis-mass spectrometry (TGA-MS) have been applied to MPs analysis, which are destructive methods. MPs at first are thermally degraded, and the products are subsequently sent to MS for analysis. And then the obtained data are compared with reference to get information.36 They can offer sensitive and reliable information about the MPs and the organic plastic additives in one run, without the background interference from solvents,97 which are considered as the best methods for the analysis of oil-based MPs.98 However, they are suitable for the MPs with diameter larger than 500 μm, which can be manually handled.36,99 At the same time, they are largely dependent on the reference database.36,99 Other methods include X-ray fluorescence (XRF) spectrometer, Scanning electron microscope (SEM) and liquid chromatography mass spectrometry (LC-MS), whose advantages and limitations are shown in Table 3.100
| Methods | Advantages | Limitations | References | |
|---|---|---|---|---|
| Visual method | Microscopic counting | Low cost, short detection time, can detect large sample size (>1 μm), detection shape is various (fibres, synthetic particles, fragments, textile fibres) | The nature of the sample cannot be determined and must be used in combination with other identification methods | 14, 91 and 185 |
| Spectroscopic methods | FTIR | Non-destructive testing technology, can quickly obtain thousands of spectra in a region, short analysis time, detection shape is various (fibres, fragments) | Expensive, needs to be operated and processed by special personnel. The detection process and data analysis will be affected by the environment. The detection size range is concentrated in 20 to 500 μm | 14 and 186 |
| Raman Spectroscopy | 1–20 μm small particles and opaque particles can be analyzed, automatic data acquisition and processing | Organic and inorganic impurities need to be removed before sample analysis, time-consuming | 187 and 188 | |
| Scanning Electron Spectroscopy | High resolution image can be obtained for sample analysis, various detection shape (synthetic particle, fibres) | Samples need to be coated in high vacuum for detection. Only large size can be detected (1–5 mm) | 14 | |
| Thermo analytical methods | Pyro GC-MS | The samples can be analyzed with organic plastic additives without solvent pretreatment, detect synthetic particles and fibers with the size larger than 500 μm | Only the selected MPs in the database can be analyzed with thermo analytical methods, and certain requirements for the particle weight to be evaluated | 14 and 189 |
| TGA-MS | ||||
| Thermogravimetric analysis is combined with the solid-phase extraction (TGA-SPE) | TGA-SPE and TDS-GC/MS allow the direct MPs assessment from the field environmental samples with ease in sample preparation | |||
| Thermal desorption gas chromatography mass spectrometer (TDS-GC/MS) | ||||
| Other methods | Tagging method | The method is simple, easy to operate, can quickly screen out the required MPs and identify fluorescent particles | The evaluation of the abundance of MPs is not accurate, and it is on the high side. Only large size MPs samples (1–5 mm) can be detected | 190 |
| Liquid chromatography | Recover high content of MPs. | The sample size of evaluation analysis is small, and only specific MPs can be analyzed | 191 and 192 | |
| SEM dispersive X-ray spectrometer | Provide high-resolution data of surface state and qualitative information about the chemical composition | It is time-consuming and expensive, chemical characterization may be subject to a selection bias | 193 | |
However, there are still a lot of challenges in this field. For example, smaller size plastics, just like nanoplastics, are not easily to be collected and quantified, which leads to underestimation of their pollution. And the non-standardized analysis protocols lead to different explanation of MPs abundance.
3. Impacts of MPs in aquatic system
3.1. MPs as carriers in aquatic system
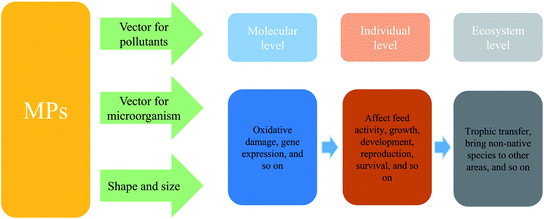
MPs have large specific surface area and good adsorption, which has been proved to be an important carrier for chemicals and microorganisms, which can pose great risk to aquatic biota, even to the ecosystem (Fig. 2).MPs are made of monomers as raw material through addition polymerization or polycondensation reaction. The common monomers of plastics include acrylic and meth acrylic acid monomers, vinyl monomers, functional monomers (crosslinking monomers) and water-soluble monomers (surface active monomers). The common plastic additives are phthalates, aliphatic binary esters, and phosphate epoxy compounds. Among them, phthalate is the most common one.103 Some other by-products are added according to different uses, such as dyes, pigments, etc.
The heavy metals commonly adsorbed by MPs are lead, zinc, copper, chromium, cadmium.104 MPs are very easy to wear under natural conditions, and adsorb sediment, charged minerals and organisms, which leads to the adsorption of metal cations on the surface with charge.105 Studies have shown that the pH value of water environment and the retention time of MPs in the environment are important factors affecting the adsorption capacity of MPs to metal ions.106 MPs can also adsorb some hydrophobic persistent organic pollutants, such as polychlorinated biphenyls, polybrominated diphenyl ethers, organochlorine pesticides, polycyclic aromatic hydrocarbons, petroleum hydrocarbons, bisphenol A.107 For organic chemicals, Wang et al. showed that organic chemicals could be easily adsorbed on MPs due to their hydrophobicity.108 Polychlorinated biphenyls (PCBs) and 1-chloro-2-[2,2-dichloro-1-(4-chlorophenyl)ethenyl]benzene (DDE) were detected on polypropylene MPs collected in the sea near Japan,109 whose highest concentrations are 117 ng g−1 and 3.1 ng g−1 respectively,110 mainly coming from the water. In addition to the properties of the adsorbed chemicals, characteristics of MPs, the weathering/aging effect of plastics themselves, the pH and the ionic strength of water bodies can also affect the adsorption capacity.111–114
In the marine environment, the adsorption of plastic particles starts shortly after invading into water, and a biofilm with stable adsorption can be produced in 7 days.117 About 22% of the MPs particles have small algae and foraminifera on their surface in the French Gulf waters.118 The investigation in the North Atlantic by Zettler et al. showed that the microbial communities adsorbed on plastic particles in coastal waters are heterotrophic, autotrophic or symbiotic.119 The factors that influence this biological attachment are extremely complex, including seasonal changes, geographical location, water temperature, nutrient status of seawater, sediment type, and water flow velocity.120
3.2. Effects of MPs on aquatic biota
The influence of MPs on organisms can be divided into physical and chemical aspects, which is shown in Table 4. On the physical level, MPs debris can have a direct mechanical impact on aquatic organisms through entanglement and swallowing.112 After swallowing, the debris will produce a false sense of satiety, thus affecting appetite, and even cause internal blockage or damage to the digestive system.112 MPs aggregate in the digestive tract of organisms, and smaller particles even can enter and stay in the circulatory system.121 The size affects the accumulation of MPs in the organism, then affecting the growth and reproduction of living organisms. Jeong et al. studied the aggregation effect and adverse effects of different sizes of MPs in rotifers.122 The results showed that smaller size of MPs particles were more easily absorbed and accumulated by organisms, which reduced the growth rate, fecundity and longevity of organisms.122 Other researchers have found that MPs can penetrate intestinal epithelium into biological tissue, thus causing greater harm to organisms.123,124 MPs are not only vary in size but also in shape, such as spheres, fragments or fibers (shown in Table 4). The effects of MPs with different shapes are different in organisms. It is found that, among these shapes, fiber morphology was more toxic to adult T. monodon.125 Studies have also confirmed that residual fibers are more likely to be entangled in the intestine, which ultimately leaded to the death of organisms, since fibers cannot be completely excreted, resulting in long retention in the body.126 Jahan et al. found that fibrous MPs tend to accumulate in oysters, more than spherical or other shapes of MPs.127 The easier retention and less excretion may result in damage to the gastrointestinal function of organisms or death due to blockage of the intestinal tract.128| Cohort | Species | Contamination | MPs | Impact and mechanism | ||
|---|---|---|---|---|---|---|
| Size | Shape | Type | ||||
| Algae | Skeletonema costatum | Cu | 1 μm | Spherical powder | PVC | Copper inhibited the growth of the organism, but the adsorption of Cu2+ and the aggregation of copper nanoparticles and MPs reduced the toxicity of copper nanoparticles due to the presence of MPs194 |
| Chlorella pyrenoidosa | Triphenyltin chloride (TPTCI) | 0.55 μm | Beads | PS | PS exposure will lead to the destruction of algal cell structure, leading to the accelerated uptake of TPTCl by green algae, thus increasing the toxicity of TPTCl195 | |
| 5 μm | ||||||
| Chlorella pyrenoidosa | Dibutyl phthalate (DBP) | 0.1 μm | Beads | PE | The presence of MPs results in the decrease of bioavailability of DBP in microalgae, and the co-action of heteropolymerization and copolymerization leads to the antagonistic effect of DBP in MPs. This effect negatively affected the volume, morphological complexity and chlorophyll fluorescence intensity of microalgae cells196 | |
| 0.55 μm | ||||||
| 5 μm | ||||||
| Phaeodactylum tricornutum | Phenanthrene (Phe) | 150 μm | Powder | PE | The accumulation of lipid in algal fluid was induced by chronic toxic exposure, but the combined exposure of MPs and phenanthrene ether of different sizes had no effect on the growth of algal fluid, and even reduced the toxicity level of single exposure to some extent197 | |
| 250 μm | PVC | |||||
| Chlorella spp., Scenedesmus spp. | — | — | — | PS | The MPs particles block light and air, blocking photosynthesis in algae. At the same time, the presence of MPs leads to the increase of reactive oxygen species (ROS) in algae198 | |
| Scenedesmus obliquus | — | <1 μm | Beads | Nano-PS | That chlorophyll content decreased and some effects on growth and photosynthesis appeared199 | |
| Dunaliella tertiolecta | — | — | Beads | PS | The photosynthetic efficiency of Dunaliella tertiolecta, Thalassiosira pseudonana, and Chlorella vulgaris decreased significantly under the action of a certain range of PS particles137 | |
| Thalassiosira pseudonana | ||||||
| Chlorella vulgaris | ||||||
| Chlamydomonas reinhardtii | — | — | Beads | PP | It can be concluded that the toxicity of MPs to algae generally increases with the decrease of the particle size200 | |
| HDPE | ||||||
| Crustaceans | Hyalella azteca | — | — | — | PE | The mortality rate of Hyalella azteca increased with the increasing of the exposure dosage, showing a significant dose-effect relationship138 |
| PP | ||||||
| Acartia tonsa | Polycyclic aromatic hydrocarbons (PAHs) | 10–200 μm | Micro-beads | PS | Co-exposure to MPs and PAHs improved the bioavailability of free dissolved PAHs, and with the dissolution of MPs to PAHs adsorption, the lethal and bioaccumulation of organisms decreased201 | |
| Calanus finmarchicus | PE | |||||
| Alrchestes compressae | PBDE-28, -47, -99, -100, -153, -154, -183 | 11–700 μm | — | PE | Polybrominated diphenyl ethers (PBDE) can be absorbed into the tissues of organisms, but the presence of PS MPs reduces the uptake of PBDEs by organisms. At the same time, the biological uptake of the homologous substances of different PBDEs was different, and the uptake of PBDE-154 and -153 was higher than that of PBDE-28 and -47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 202 202 |
|
| Tigriopus japonicus | — | — | — | PE | The fatality rate of Tigriopus japonicus also increased significantly with the increase of dosage203 | |
| PP | ||||||
| Daphnia magna | Polychlorinated biphenyls-18 (PCB) | 100 nm | — | PS | As the concentration of PCB-18 decreased, co-existence of PCB-18 with low concentration of PS reduced the biological toxicity, but with the increase of PS concentration, the toxicity of PS was far greater than that of PCB-18, causing death of Daphnia magna204 | |
| Daphnia magna | — | 10 μm, 1 mm | Beads | PS | Daphnia magna can quickly ingest MPs of 0.01 and 1 mm, and accumulate them in fat droplets, which shows that MPs can destroy the filtration process of crustaceans.205 The possible cause of death observed is only blockage in the intestine, not chemicals released from plastic fibers139 | |
| Langoustine (Nephrops norvegicus) | — | — | Fibres | PS | Crustaceans ingest MPs through passive ingestion of sediments or in-taking of prey contaminated with MPs.206 Generally, female lobsters retain more MPs particles than male ones206 | |
| Clyde Sea Area | ||||||
| Eriocheir sinensis | — | — | Fragment | — | For crabs, male crabs consume more MPs than female ones207 | |
| Carcinus maenas | — | 8 μm | Beads | PS | MPs intaken by crabs could significantly reduce oxygen consumption in hemolymph, decrease Na+ concentration, increase Ca2+ concentration and hemocyanin content208 | |
| Bivalves and zooplankton | Blue Mussel Mytilus edulis L. Granulocytoma | — | 0–80 μm | — | HDPE | The intake of MPs can lead to a significant decrease in lysosomal membrane stability and a significant increase in the number of L. Granulocytomas209 |
| Centropages typicus, Calanus helgolandicus | — | 1.7–30.6 μm | Beads | PS | The feeding performance of Centropages typicus was significantly reduced after 7.3 μm MPs was ingested.120,210 The intake, fecundity and survival rate of Calanus helgolandicus showed a downward trend after taking PS particles120,210 | |
| Mytilus edudis | PCB-18 20, 28, 29, 31, 44, 52, 101, 105, 118, 138, 143, 149, 153, 155, 170, 180, 194, 204, and 209 | 400–1300 μm | Beads | PS | A positive relation was observed between PS concentration in the sediment and both uptake of plastic particles and weight loss. A low PS dose of 0.074% increased bioaccumulation of PCBs by a factor of 1−3 mm PS had statistically significant effects on the organisms' fitness and bioaccumulation211 | |
| Mytilus eduli (L.) | — | 2 μm and 6 μm, 30 μm | Beads | PS | The in-taken MPs in hemolymph and circulatory system of marine mussels can exert physiological pressure on organisms.212 For example, when MPs enter into the hemolymph system, the mortality rate of blood cells would increase, leading to the formation of shell and wound healing.213,214 Also, the number and size of oocytes and sperm activity rate was significantly reduced, when oysters were exposed to MPs.215 MPs still cause a negative impact on the population and survival of bivalves216 | |
| Mytilus spp. | 3 μm and 9.6 μm | |||||
| Arenicola marina | PBDE-47 | 230 μm | — | PVC | Intake of different chemicals from PVC has different effects on organisms. Intake of nonylphenol reduces the ability of biological cells to remove pathogenic bacteria, while intake of triclosan can lead to death in severe cases. Exposure to PVC alone causes organisms to be more susceptible to pathogenic stress216 | |
| Nonylphenol | ||||||
| Phenanthrene | ||||||
| Triclosan | ||||||
| Mytilus galloprovincialis | Pyrene | 100 μm | — | PS | Bioingestion of MPs may adversely affect the digestive tract, respiratory system and motor appendages217 | |
| PE | ||||||
| Mysid shrimps | — | — | Beads | PS | Zooplankton have the potential to absorb small plastics.218 The uptake of MPs by zooplankton largely depends on the concentration, size of particles and their feeding mechanism219 | |
| Copepods | ||||||
| Cladocerans | ||||||
| Rotifers polychaete larvae | ||||||
| Vertebrate | Danio rerio | — | <1 μm | — | PE | The result showed that acute exposure may affect the aryl hydrocarbon receptor (AHR) pathway, interrupting the development of eggs and producing neurotoxic effects220 |
| Clarias gariepinus | Phenanthrene | <60 μm | — | LDPE | Exposure to both MPs and phenanthrene inhibits acetylcholinesterase activity and reduces the amount of energy produced by the aerobic pathway. The presence of MPs regulates the bioavailability or biotransformation of phenanthrene221 | |
| Danio rerio | — | <1 μm | — | Nano-PS | Results showed that some nano-plastics can penetrate the intestinal of developing Danio rerio in polluted aquatic ecosystems, accumulate in tissues, and affect their physiology and behaviour222 | |
| Danio rerio | Tetrabromobisphenol A (TBBPA) | 100 μm | Beads | PE | Co-exposure induced significant antioxidative stress than either PE or TBBPA alone when exposed to MP223 | |
| Stellifer brasiliensis | — | 1–5 mm | Nylon fragments | — | The amount of fish that ingested plastic accounted for 6.9% to 9.2% of the total amount of the fish depending on the different size of MPs.224 Also, the Gerreidae consumed much more blue nylon fragments of 1–5 mm than other sizes225 | |
| Stellifer stellifer | ||||||
| Pomatoschistus microps | Cefalexin | 1–5 μm | Beads | PE | The presence of MPs can affect the toxicity of cefalexin, and the biological toxicity of both MPs and cefalexin either alone or in combination with MPs increases with the increase of temperature to P. microps juveniles226 | |
| Danio rerio | — | 5 nm, 70 nm | Beads | PS | MPs with different particle sizes can accumulate in gills, liver and intestines of Danio rerio.227 | |
| Pomatoschistus microps | — | — | — | — | MPs produced a cumulative effect of reducing hepatic glycogen content and fat vacuole degeneration in vivo.221 | |
| Pomatoschistus microps | Chromium | 1–5 μm | Beads | PE | Exposure to low concentrations of Cr(VI) and MPs will not have toxic effects on organisms, but long-term exposure to natural habitats before biological development will affect the sensitivity and response to Cr(VI)228 | |
| Dicentrarchus labrax | — | — | — | PE | The mortality rate of Dicentrarchus labrax increased by about 14% with the increase of exposure dose.229 And for endocrine aspects, such as CYP 1α, vitellogenin I and estrogen receptor alpha expression in females and males, as well as the expression of chorionic protein H in males, there was no significant difference with control ones221 | |
| Danio rerio | Bisphenol A | 1–3 mm | Fragments | PE | Normal life stresses can decrease endocrine disrupting chemicals (EDC) concentrations, but solar irradiation (solar) can increase EDC concentrations in leachates.230 | |
| Bisphenol S | ||||||
| Octylphenol | ||||||
| Nonylphenol | ||||||
| Danio rerio | — | — | — | HDPE | The gene expression profiles of the phase 1 detoxification-related gene (cyp 1α) in the intestine and oogenesis-related gene (vtg 1) in the liver showed significant upregulation.230 | |
| Danio rerio | — | 4–12 μm | Beads | PE | Aging of MPs in low organic-load waters mitigated the toxicity of MPs for organism, while MPs aged in high-organic load waters had the same adverse effect as MPs231 | |
| Danio rerio | — | — | — | PA, PP, PE PVC, PS | Intestinal cell division and GST increased, although there was no significant change in mortality and intestinal villus rupture232 | |
| Pomatoschistus microps | — | 1-5μm | Beads | PE | A large amount of MPs could be ingested, and the mortality rate was significantly increased after the ingestion229 | |
| Dicentrarchus labrax | — | <1 mm | Beads, debris | PE | The intake of MPs also resulted in intestinal obstruction,233 severe glycogen depletion, abnormal proliferation of testicular germ cells and other adverse effects such as liver stress234 | |
| Clarias gariepinus | Mercury | 1-5μm | Micro-spheres | — | MPs influence the bioaccumulation of mercury. MPs, mercury and their mixtures cause neurotoxicity, oxidative stress and damage, and changes in the activities of energy related enzymes in juveniles of this species235 | |
| Dicentrarchus labrax | ||||||
| Gammarus roeseli | — | 50–500 μm | Fragment | LDPE | The transcription levels of forkhead box L2 (foxl2) and tryptophan hydroxylase 2 (tph2) in vivo were significantly reduced236 | |
| Dicentrarchus labrax (Linnaeus, 1758) | Phenanthrene | — | — | PA | The exposure of gammarids in presence of either particle type with phenanthrene resulted after 24 and 48 h in reduced size237 | |
| Dicentrarchus labrax | — | — | Pellets | PVC | Long-term exposure to MPs can lead to significant changes in intestinal structure and function. This may lead to serious damage to the fish development in the early stage, thus adversely affecting the reproductive success, population size and survival of fish238 | |
MPs will transport the carried chemicals to organisms.112 The chemicals absorbed by MPs, such as polychlorinated biphenyls, have carcinogenic, teratogenic and mutagenic effects on organisms.112 Studies have found that MPs with pollutants could promote the accumulation of these pollutants in aquatic organisms.129 Researches have shown that the chemical pollutants adsorbed on the MPs can be bioavailable, thus affecting molecular and cellular pathways, which are summarized in Table 4.
In order to meet the needs of production and usage, many chemical additives, such as plasticizers and dyes, are added into the manufacturing process. Generally, different plastics need different chemical polymers with different plastic additives. Common plastics additives include antioxidant, flame retardant and plasticizer.130 These additives not only can be transferred to aquatic organisms through the intake of MPs by organism, but also can be released during plastics degradation to the aquatic environment. Plastic additives such as bisphenol A, octyl phenol, nonylphenol, brominated flame retardant, boric acid, tri(2-chloroethyl)phosphoric acid, etc.,131 were found in nature waters. The specific effects of these additives and adsorbed chemical pollutants on organisms are shown in Table 4. Although MPs with pollutants could promote the accumulation of these pollutants in aquatic organisms, the toxic effects of the coexistence of MPs with pollutants on organisms are uncertain. Some studies have found that, although MPs can carry into the organism and promote the accumulation of these pollutants in aquatic organisms, the existence of MPs will not increase the impact of these pollutants on the organism.132,133 For example, the coexistence of PS, PA, PE and some chemicals, such as bisphenol, peptides, dimethoate and deltamethrin does not change the 50% effective concentration of these chemicals to organisms.134 For glyphosate and 17α-ethinylestradiol, the presence of MPs may reduce their toxicity.134 For phenanthrene, the presence of MPs may increase it toxicity.134 According to the different endpoint analyzed or treatment methods, the presence of MPs may also increase the toxicity of coexisting chemicals.134 Moreover, the different structure of the same MPs will also lead to different toxic effects on organisms. Studies have shown that the combined toxicity of Ni and PS MPs with –COOH functional group is higher than that of Ni and PS MPs without –COOH functional group.135
For organisms, the existence of MPs in water can exert effects on algae, crustaceans, bivalves, zooplankton and vertebrates in freshwater and marine organisms. For algae, the existence of MPs has a physical impact on the flow of light and air, and has an impact on the chlorophyll of algae.136 All of these factors lead to a significant decrease in the photosynthetic efficiency of algae.137 For crustaceans, the MPs have an impact on the normal life style. The mortality rate will increase with the increasing of the exposure dosage, showing a significant dose-effect relationship, due to the toxicity of MPs.138 At the same time, the presence of MPs can lead to the death of crustaceans, which can lead to intestinal obstruction by destroying crustaceans' filtration systems.139 Many large crustaceans are omnivores, which do not recognize and discard food-related plastic filaments. They can easily ingest large amounts of MPs. Studies have shown that free-swimming crustaceans consume more MPs than sessile ones.140 The retention of in-taken MPs is related to gender, size and molting stage.141 Moreover, the intake of MPs may lead to development retardation of crustacean, decreased fecundity and delayed molting.142 For bivalves, they are self-sufficient compared with other marine invertebrates, because their preferential feeding mechanism allows them to reject non-food particles.143 The bivalves also have other selection mechanisms, such as pre-feeding of lip flaps, gastric post-feeding selection, and so on.143 The inadequate food sources and abundant MPs in biological habitats may be one of the reasons for them to ingest MPs. For vertebrate, there are two main ways for them to ingest MPs. One is to prey on organisms polluted by MPs; the other is to take MPs directly from water column and sediment.
Fish's feeding behavior affects the size and quantity of MPs they consume. Omniphagous consume more MPs than single herbivorous or meat-eating fish because of their wider range of food.144 In comparison with carnivorous fish, herbivorous and omnivorous fish are easier to mistake MPs drifting in water column for food.145 For other large animals or mammals in the ocean, such as baleen whales, which filtered food out of large amounts of water, and tend to ingest MPs. Filter-feeding marine macro-fauna consumed a large amount of MPs mainly because of their filter feeding behavior. Most of the filter feeding animals contact with MPs by means of active or passive ingestion due to its in-distinguishability of feeding mechanisms.146,147 In addition, the marine mammals without filter feeding systems, they still consume MPs.148 When high concentrations of MPs in their habitats and feeding grounds, their intake of MPs is almost inevitable.149
3.3. Trophic transfer
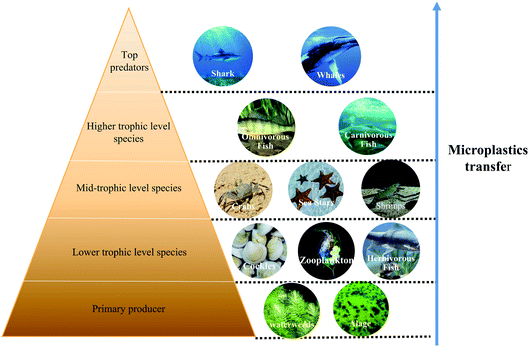
The trophic transfer evidence of MPs is found by quantitative analysis of aquatic organisms collected in the field, their predators and animals raised in laboratory environmental studies.16 It is known that there are nearly 700 species of aquatic organisms affected by MPs, and MPs can be observed in different nutrition levels.150 The accumulation of MPs in low trophic organisms will have a linkage effect on food web.151 MPs will migrate through the food web along with the predation of aquatic organisms. Lusher et al. found that MPs in high nutrient bio-excrement and tissues of different coastal habitats, which can be inferred that MPs could be accompanied by nutrient transfer when they are ingested directly.152 The results showed that mussels Mytilus edulus exposed to PS MPs were fed to crab Carcinus maenas, MPs could be detected in Carcinus maenas.153 Similarly, studies have fed several different zooplankton with PS MPs, and let mysid shrimps ingest these zooplankton, and finally they were transferred to shrimp.154 In addition to crustaceans and plankton, trophic transfer of MPs can also be found in vertebrates. MPs within the size range of many plankton are usually ingested by aquatic invertebrates, which are prone to be transferred to vertebrates at the higher end of the food chain.155 Setala et al. found that the nutrient migration took place after three hours of contacting between macroplankton and medium-sized plankton-ingesting polystyrene MPs.154 Potential pathways of MPs for nutritional level migration in water environment are shown in Fig. 3. Algae, which are located at the bottom of the food chain, are often transferred to higher trophic level organisms (such as plankton) after being ingested, which will have a certain impact on the whole ecosystem. However, some researchers believe that MPs can be rapidly purified in organisms, the possibility of having a great impact on high nutrient level organisms is very small.16The residence time and the accumulation of MPs in biota is an important factor affecting trophic transfer. The longer the residence time, the easier the MPs transfer. For aquatic organisms, the concentration of MPs in the ingested environment may not be high.156 But due to their retention and accumulation, the concentration of MPs in the body will be higher than that in environment.156 The size and shape of MPs are also important factors.40 In addition, individual differences of aquatic organism may affect residence time. The longer the residence time in biota, the easier the transferring of MPs along the trophic levels.40 Moreover, the residence time of MPs varied in different organisms and different organs of the same organism (digestive tract, hepatopancreas, ovaries, gills), but generally shorter than those in blood.153 Farrell and Nelson found that 24 hours after ingestion, the most abundant MPs were detected in vivo.153 Different ways of ingesting MPs also lead to different residence time in organisms. For example, when crabs were exposed to polystyrene MPs, the residence time of MPs in gill obtained by air circulation was longer than that by direct ingestion in vivo.157
The research on the trophic transfer of MPs should not only focus on the MPs alone, but also on the carried pollutants. However, there are few studies on this topic can be found, and the effect of adsorption - desorption kinetics between MPs and pollutants in organisms (especially on advanced predators) is still unknown.150 Due to the lack of data on the transfer of pollutants carried by MPs between organisms of high nutrient level and those of low nutrient level, the ecological risk assessment of MPs still need to be further studied.150
4. Conclusions and recommendations for future work
4.1. Conclusions
In freshwater environment, MPs mainly come from terrestrial and atmosphere environment, through sewage discharge, rainwater scouring and atmospheric sedimentation. For the marine environment, land dumping and freshwater systems contribute most of the MPs. These MPs are distributed in the surface water, water column, sediment and aquatic organisms in the aquatic systems. The most of the MPs in freshwater will flow into the ocean. Due to the degradation, the vast majority of MPs are cracked to the extent that we cannot detect the size or end up in the sediment. The migration of MPs can be divided into horizontal and vertical directions, which is closely related to the flow velocity, water depth, water flow variation, underwater topography, wind speed and MPs particles density.The quantitative and qualitative analysis of MPs usually started with visual inspection with help of stereoscope or microscope, followed by spectroscopic methods, including FTIR, Raman spectroscopy, or thermos-analysis methods. Their advantages and disadvantages are summarized.
MPs as carriers of pollutants in water environment have an impact on biological communities. The factors affecting the ability of MPs to adsorb pollutants include hydrophobicity of adsorbed chemicals (Kow), characteristics of MPs, weathering/aging of plastics, pH value and ionic strengthen of water. The coexistence of MPs with pollutants can promote the accumulation of these pollutants in aquatic organisms, which does not necessarily mean that the toxicity of these pollutant will be increased. Moreover, the hydrophobic surface of MPs can be easily to be colonized by bacteria or algae to form biofilm, which helps the migration of these organisms.
The trophic transfer of MPs along the food chain is mainly related to their residence time, accumulation, size and shape. The longer the residence time in biota, the easier the transferring of MPs along the trophic levels, and this will have a certain impact on the entire ecosystem. Overall, this review may help to enhance the understanding of the distribution, fate, and ecological impacts of MPs in aquatic system.
4.2. Recommendations of future work
In order to facilitate the comparison of MPs, the units to quantify MPs should be consistent or at least easy to convert. Meanwhile, the detection and analysis methods of MPs should be standardized, which is necessary for researchers to make valid comparison. To track and characterize the smaller size MPs, the detection and analysis methods need to be developed. For the ecotoxicological evaluation, large scale experiments need to be conducted to assess the effects of MPs combined with the pollutants they carry on aquatic biota, from molecular level to ecosystem level. Moreover, the negative effects of MPs with the carried pollutants on human health through food web are also encouraged to be explored.Author contributions
All authors have made substantial contributions regarding the design and implement of the study. Yide He, Yunhai Zhang and Yongjun Zhang provided the funding. Yujie Cai, Rongwen Zhu, Ning Xu and Pow-Seng Yap provided the key intellectual input to the study and Sen Du wrote the manuscript. Yide He revised the manuscript. All authors approve the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (19KJA460006), National Natural Science Foundation of China (41807121, 51808284), and Major Science and Technology Program for Water Pollution Control and Treatment (2018ZX07208-004).References
- M. A. Kreiger, M. L. Mulder, A. G. Glover and J. M. Pearce, Life cycle analysis of distributed recycling of post-consumer high density polyethylene for 3-D printing filament, J. Cleaner Prod., 2014, 70, 90–96 CrossRef CAS.
- R. Geyer, J. R. Jambeck and K. L. Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3(7), 1700782 CrossRef PubMed.
- K. L. Law, Plastics in the Marine Environment, Annual Review of Marine Science, 2017, 9, 205–229 CrossRef PubMed.
- S. K. Ghosh, S. Pal and S. Ray, Study of microbes having potentiality for biodegradation of plastics, Environ. Sci. Pollut. Res., 2013, 20(7), 4339–4355 CrossRef CAS PubMed.
- U. Aytan, F. B. E. Sahin and F. Karacan, Beach Litter on Saraykoy Beach (SE Black Sea): Density, Composition, Possible Sources and Associated Organisms, Turkish Journal of Fisheries and Aquatic Sciences, 2020, 20(2), 137–145 CrossRef.
- M. A. El-Khateeb, W. M. Emam, W. A. Darweesh and E. S. Abd El-Sayed, Integration of UASB and downflow hanging non-woven fabric (DHNW) reactors for the treatment of sewage, Desalin. Water Treat., 2019, 164, 48–55 CrossRef CAS.
- M. A. El-Khateeb, M. A. Saad, H. I. Abdel-Shafy, F. A. Samhan and M. F. Shaaban, The feasibility of using non-woven fabric as packing material for wastewater treatment, Desalin. Water Treat., 2018, 111, 94–100 CrossRef CAS.
- D. Eerkes-Medrano and R. Thompson, Occurrence, Fate, and Effect of Microplastics in Freshwater Systems, in Microplastic Contamination in Aquatic Environments, 2018, pp. 95–132 Search PubMed.
- K. Zhang, H. H. Shi, J. P. Peng, Y. H. Wang, X. Xiong, C. X. Wu and P. K. S. Lam, Microplastic pollution in China's inland water systems: A review of findings, methods, characteristics, effects, and management, Sci. Total Environ., 2018, 630, 1641–1653 CrossRef CAS PubMed.
- R. Gouveia, J. Antunes, P. Sobral and L. Amaral, Microplastics from Wastewater Treatment Plants—Preliminary Data, in Proceedings of the International Conference on Microplastic Pollution in the Mediterranean Sea, 2018, pp. 53–57 Search PubMed.
- S. Magni, A. Binelli, L. Pittura, C. G. Avio, C. Della Torre, C. C. Parenti, S. Gorbi and F. Regoli, The fate of microplastics in an Italian Wastewater Treatment Plant, Sci. Total Environ., 2019, 652, 602–610 CrossRef PubMed.
- A. K. Urbanek, W. Rymowicz and A. M. Mironczuk, Degradation of plastics and plastic-degrading bacteria in cold marine habitats, Appl. Microbiol. Biotechnol., 2018, 102(18), 7669–7678 CrossRef CAS PubMed.
- Y. Pico and D. Barcelo, Analysis and Prevention of Microplastics Pollution in Water: Current Perspectives and Future Directions, ACS Omega, 2019, 4(4), 6709–6719 CrossRef CAS PubMed.
- J. Li, H. Liu and J. Paul Chen, Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection, Water Res., 2018, 137, 362–374 CrossRef CAS PubMed.
- Y. Chae and Y. J. An, Effects of micro- and nanoplastics on aquatic ecosystems: Current research trends and perspectives, Mar. Pollut. Bull., 2017, 124(2), 624–632 CrossRef CAS PubMed.
- M. F. M. Santana, F. T. Moreira and A. Turra, Trophic transference of microplastics under a low exposure scenario: Insights on the likelihood of particle cascading along marine food-webs, Mar. Pollut. Bull., 2017, 121(1–2), 154–159 CrossRef CAS PubMed.
- J. K. H. Wong, K. K. Lee, K. H. D. Tang and P. S. Yap, Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions, Sci. Total Environ., 2020, 719, 137512 CrossRef CAS PubMed.
- L. C. M. Lebreton, J. van der Zwet, J.-W. Damsteeg, B. Slat, A. Andrady and J. Reisser, River plastic emissions to the world's oceans, Nat. Commun., 2017, 8, 15611 CrossRef CAS PubMed.
- C. Schmidt, T. Krauth and S. Wagner, Export of Plastic Debris by Rivers into the Sea, Environ. Sci. Technol., 2017, 51(21), 12246–12253 CrossRef CAS PubMed.
- W. Y. Luo, L. Su, N. J. Craig, F. N. Du, C. X. Wu and H. H. Shi, Comparison of microplastic pollution in different water bodies from urban creeks to coastal waters, Environ. Pollut., 2019, 246, 174–182 CrossRef CAS PubMed.
- A. Lechner, H. Keckeis, F. Lumesberger-Loisl, B. Zens, R. Krusch, M. Tritthart, M. Glas and E. Schludermann, The Danube so colourful: A potpourri of plastic litter outnumbers fish larvae in Europe's second largest river, Environ. Pollut., 2014, 188, 177–181 CrossRef CAS PubMed.
- P. Kay, R. Hiscoe, I. Moberley, L. Bajic and N. McKenna, Wastewater treatment plants as a source of microplastics in river catchments, Environ. Sci. Pollut. Res., 2018, 25(20), 20264–20267 CrossRef CAS PubMed.
- R. Hurley, J. Woodward and J. J. Rothwell, Microplastic contamination of river beds significantly reduced by catchment-wide flooding, Nat. Geosci., 2018, 11(4), 251–257 CrossRef CAS.
- S. Wagner, P. Klockner, B. Stier, M. Romer, B. Seiwert, T. Reemtsma and C. Schmidt, Relationship between Discharge and River Plastic Concentrations in a Rural and an Urban Catchment, Environ. Sci. Technol., 2019, 53(17), 10082–10091 CrossRef CAS PubMed.
- N. Yu, B. Huang, M. Li, P. Cheng, L. Li, Z. Huang, W. Gao and Z. Zhou, Single particle characteristics of fine particulate matter in dust, China Environ. Sci., 2017, 37(4), 1262–1268 CAS.
- Z. R. Ma, Y. S. Liu and Q. Q. Zhang, The usage and environmental pollution of agricultural plastic film, Australas. J. Ecotoxicol., 2020, 15 Search PubMed.
- M. Kumar, X. N. Xiong, M. J. He, D. C. W. Tsang, J. Gupta, E. Khan, S. Harrad, D. Y. Hou, Y. S. Ok and N. S. Bolan, Microplastics as pollutants in agricultural soils, Environ. Pollut., 2020, 265, 114980 CrossRef CAS PubMed.
- D. Gao, X. Y. Li and H. T. Liu, Source, occurrence, migration and potential environmental risk of microplastics in sewage sludge and during sludge amendment to soil, Sci. Total Environ., 2020, 742, 140355 CrossRef CAS PubMed.
- A. Willen, C. Junestedt, L. Rodhe, M. Pell and H. Jonsson, Sewage sludge as fertiliser – environmental assessment of storage and land application options, Water Sci. Technol., 2017, 75(5), 1034–1050 CrossRef CAS PubMed.
- H. Y. Sintim, S. Bandopadhyay, M. E. English, A. Bary, J. E. L. Y. Gonzalez, J. M. DeBruyn, S. M. Schaeffer, C. A. Miles and M. Flury, Four years of continuous use of soil-biodegradable plastic mulch: impact on soil and groundwater quality, Geoderma, 2021, 381, 114665 CrossRef CAS.
- F. Corradini, P. Meza, R. Eguiluz, F. Casado, E. Huerta-Lwanga and V. Geissen, Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal, Sci. Total Environ., 2019, 671, 411–420 CrossRef CAS PubMed.
- A. A. D. Machado, C. W. Lau, J. Till, W. Kloas, A. Lehmann, R. Becker and M. C. Rillig, Impacts of Microplastics on the Soil Biophysical Environment, Environ. Sci. Technol., 2018, 52(17), 9656–9665 CrossRef PubMed.
- R. Dris, J. Gasperi, M. Saad, C. Mirande and B. Tassin, Synthetic fibers in atmospheric fallout: A source of microplastics in the environment?, Mar. Pollut. Bull., 2016, 104(1–2), 290–293 CrossRef CAS PubMed.
- K. Liu, X. H. Wang, T. Fang, P. Xu, L. X. Zhu and D. J. Li, Source and potential risk assessment of suspended atmospheric microplastics in Shanghai, Sci. Total Environ., 2019, 675, 462–471 CrossRef CAS PubMed.
- P. U. Iyare, S. K. Ouki and T. Bond, Microplastics removal in wastewater treatment plants: a critical review, Environ. Sci.: Water Res. Technol., 2020, 6(10), 2664–2675 RSC.
- J. Y. Li, H. H. Liu and J. P. Chen, Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection, Water Res., 2018, 137, 362–374 CrossRef CAS PubMed.
- L. M. R. Mendoza and M. Balcer, Microplastics in freshwater environments: A review of quantification assessment, TrAC, Trends Anal. Chem., 2019, 113, 402–408 CrossRef.
- C. Wu, X. L. Pan, H. H. Shi and J. P. Peng, Microplastic Pollution in Freshwater Environment in China and Watershed Management Strategy, China Academic Journal Electronic Publishing House, 2018, vol. 33, ch.10, pp. 1012–1020 Search PubMed.
- Q. P. Li, Z. H. Feng, T. Zhang, C. Z. Ma and H. H. Shi, Microplastics in the commercial seaweed nori, J. Hazard. Mater., 2020, 388, 122060 CrossRef CAS PubMed.
- M. E. Miller, M. Hamann and F. J. Kroon, Bioaccumulation and biomagnification of microplastics in marine organisms: A review and meta-analysis of current data, PLoS One, 2020, 15(10), 240792 Search PubMed.
- A. Mehdinia, R. Dehbandi, A. Hamzehpour and R. Rahnama, Identification of microplastics in the sediments of southern coasts of the Caspian Sea, north of Iran, Environ. Pollut., 2020, 258, 113738 CrossRef CAS PubMed.
- X. Xiong, K. Zhang, X. C. Chen, H. H. Shi, Z. Luo and C. X. Wu, Sources and distribution of microplastics in China's largest inland lake - Qinghai Lake, Environ. Pollut., 2018, 235, 899–906 CrossRef CAS PubMed.
- Z. F. Dai, H. B. Zhang, Q. Zhou, Y. Tian, T. Chen, C. Tu, C. C. Fu and Y. M. Luo, Occurrence of microplastics in the water column and sediment in an inland sea affected by intensive anthropogenic activities, Environ. Pollut., 2018, 242, 1557–1565 CrossRef CAS PubMed.
- P. L. Lenaker, A. K. Baldwin, S. R. Corsi, S. A. Mason, P. C. Reneau and J. W. Scott, Vertical Distribution of Microplastics in the Water Column and Surficial Sediment from the Milwaukee River Basin to Lake Michigan, Environ. Sci. Technol., 2019, 53(21), 12227–12237 CrossRef CAS PubMed.
- Z. W. Zhang, H. Wu, G. Y. Peng, P. Xu and D. J. Li, Coastal ocean dynamics reduce the export of microplastics to the open ocean, Sci. Total Environ., 2020, 713, 136634 CrossRef CAS PubMed.
- D. Barcelo and Y. Pico, Case studies of macro- and microplastics pollution in coastal waters and rivers: Is there a solution with new removal technologies and policy actions?, Case Studies in Chemical and Environmental Engineering, 2020, 2, 100019 CrossRef.
- Y. K. Song, S. H. Hong, S. Eo, M. Jang, G. M. Han, A. Isobe and W. J. Shim, Horizontal and Vertical Distribution of Microplastics in Korean Coastal Waters, Environ. Sci. Technol., 2018, 52(21), 12188–12197 CrossRef CAS PubMed.
- H. K. Imhof, N. P. Ivleva, J. Schmid, R. Niessner and C. Laforsch, Contamination of beach sediments of a subalpine lake with microplastic particles, Curr. Biol., 2013, 23(19), 867–868 CrossRef PubMed.
- L. Zhu, H. Y. Bai, B. J. Chen, X. M. Sun, K. M. Qu and B. Xia, Microplastic pollution in North Yellow Sea, China: Observations on occurrence, distribution and identification, Sci. Total Environ., 2018, 636, 20–29 CrossRef CAS PubMed.
- M. L. Chen, M. Jin, P. R. Tao, Z. Wang, W. P. Xie, X. B. Yu and K. Wang, Assessment of microplastics derived from mariculture in Xiangshan Bay, China, Environ. Pollut., 2018, 242, 1146–1156 CrossRef CAS PubMed.
- L. F. Ruiz-Orejon, R. Sarda and J. Ramis-Pujol, Now, you see me: High concentrations of floating plastic debris in the coastal waters of the Balearic Islands (Spain), Mar. Pollut. Bull., 2018, 133, 636–646 CrossRef CAS PubMed.
- Z. Pan, H. G. Guo, H. Z. Chen, S. M. Wang, X. W. Sun, Q. P. Zou, Y. B. Zhang, H. Lin, S. Z. Cai and J. Huang, Microplastics in the Northwestern Pacific: Abundance, distribution, and characteristics, Sci. Total Environ., 2019, 650, 1913–1922 CrossRef CAS PubMed.
- L. K. Kanhai, R. Officer, O. Lyashevska, R. C. Thompson and I. O'Connor, Microplastic abundance, distribution and composition along a latitudinal gradient in the Atlantic Ocean, Mar. Pollut. Bull., 2017, 115(1–2), 307–314 CrossRef CAS PubMed.
- C. Van Colen, B. Vanhove, A. Diem and T. Moens, Does microplastic ingestion by zooplankton affect predator-prey interactions? An experimental study on larviphagy, Environ. Pollut., 2020, 256, 113479 CrossRef CAS PubMed.
- R. J. E. Vroom, A. A. Koelmans, E. Besseling and C. Halsband, Aging of microplastics promotes their ingestion by marine zooplankton, Environ. Pollut., 2017, 231, 987–996 CrossRef CAS PubMed.
- J. M. Hipfner, M. Galbraith, S. Tucker, K. R. Studholme, A. D. Domalik, S. F. Pearson, T. P. Good, P. S. Ross and P. Hodum, Two forage fishes as potential conduits for the vertical transfer of microfibres in Northeastern Pacific Ocean food webs, Environ. Pollut., 2018, 239, 215–222 CrossRef CAS PubMed.
- M. Jang, W. J. Shim, Y. Cho, G. M. Han, Y. K. Song and S. H. Hong, A close relationship between microplastic contamination and coastal area use pattern, Water Res., 2020, 171, 115400 CrossRef CAS PubMed.
- A. Oztekin, L. Bat and O. Gokkurt Baki, Beach Litter Pollution in Sinop Sarikum Lagoon Coast of the Southern Black Sea, Turkish Journal of Fisheries and Aquatic Sciences, 2020, 20(3), 197–205 CrossRef.
- A. Lohr, H. Savelli, R. Beunen, M. Kalz, A. Ragas and F. Van Belleghem, Solutions for global marine litter pollution, Curr. Opin. Environ. Sustain., 2017, 28, 90–99 CrossRef.
- S. Lynch, OpenLitterMap.com – Open Data on Plastic Pollution with Blockchain Rewards (Littercoin), Open Geospatial Data Software and Standards, 2018, vol. 3, pp. 6–15 Search PubMed.
- A. A. Horton, A. Walton, D. J. Spurgeon, E. Lahive and C. Svendsen, Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities, Sci. Total Environ., 2017, 586, 127–141 CrossRef CAS PubMed.
- I. Acosta-Coley, D. Mendez-Cuadro, E. Rodriguez-Cavallo, J. de la Rosa and J. Olivero-Verbel, Trace elements in microplastics in Cartagena: A hotspot for plastic pollution at the Caribbean, Mar. Pollut. Bull., 2019, 139, 402–411 CrossRef CAS PubMed.
- S. Gündoğdu and C. Cevik, Micro- and mesoplastics in Northeast Levantine coast of Turkey: The preliminary results from surface samples, Mar. Pollut. Bull., 2017, 118(1–2), 341–347 CrossRef PubMed.
- L. Su, S. M. Sharp, V. J. Pettigrove, N. J. Craig, B. X. Nan, F. N. Du and H. H. Shi, Superimposed microplastic pollution in a coastal metropolis, Water Res., 2020, 168, 115140 CrossRef CAS PubMed.
- C. Verster, K. Minnaar and H. Bouwman, Marine and freshwater microplastic research in South Africa, Integr. Environ. Assess. Manage., 2017, 13(3), 533–535 CrossRef PubMed.
- S. S. Sadri and R. C. Thompson, On the quantity and composition of floating plastic debris entering and leaving the Tamar Estuary, Southwest England, Mar. Pollut. Bull., 2014, 81(1), 55–60 CrossRef CAS PubMed.
- T. Kataoka, Y. Nihei, K. Kudou and H. Hinata, Assessment of the sources and inflow processes of microplastics in the river environments of Japan, Environ. Pollut., 2019, 244, 958–965 CrossRef CAS PubMed.
- L. Lin, L. Z. Zuo, J. P. Peng, L. Q. Cai, L. Fok, Y. Yan, H. X. Li and X. R. Xu, Occurrence and distribution of microplastics in an urban river: A case study in the Pearl River along Guangzhou City, China, Sci. Total Environ., 2018, 644, 375–381 CrossRef CAS PubMed.
- E. K. Fischer, L. Paglialonga, E. Czech and M. Tamminga, Microplastic pollution in lakes and lake shoreline sediments - A case study on Lake Bolsena and Lake Chiusi (central Italy), Environ. Pollut., 2016, 213, 648–657 CrossRef CAS PubMed.
- M. C. Goldstein, M. Rosenberg and L. N. Cheng, Increased oceanic microplastic debris enhances oviposition in an endemic pelagic insect, Biol. Lett., 2012, 8(5), 817–820 CrossRef PubMed.
- Q. Q. Chen, J. Reisser, S. Cunsolo, C. Kwadijk, M. Kotterman, M. Proietti, B. Slat, F. F. Ferrari, A. Schwarz, A. Levivier, D. Q. Yin, H. Hollert and A. A. Koelmans, Pollutants in Plastics within the North Pacific Subtropical Gyre, Environ. Sci. Technol., 2018, 52(2), 446–456 CrossRef CAS PubMed.
- B. R. Jiang, A. E. Kauffman, L. Li, W. McFee, B. Cai, J. Weinstein, J. R. Lead, S. Chatterjee, G. I. Scott and S. Xiao, Health impacts of environmental contamination of micro- and nanoplastics: a review, Environ. Health Prev. Med., 2020, 25(1) Search PubMed.
- E. Besseling, J. T. K. Quik, M. Sun and A. A. Koelmans, Fate of nano-and microplastic in freshwater systems: a modeling study, Environ. Pollut., 2017, 220, 540–548 CrossRef CAS PubMed.
- C. Lassen, S. F. Hansen, K. Magnusson, F. Norén, N. I. B. Hartmann, P. R. Jensen, T. G. Nielsen and A. Brinch, Microplastics: Occurrence, effects and sources of releases to the environment in Denmark, Danish Environmental Protection Agency, 2015 Search PubMed.
- L. M. Feng, L. He, S. Q. Jiang, J. J. Chen, C. X. Zhou, Z. J. Qian, P. Z. Hong, S. L. Sun and C. Y. Li, Investigating the composition and distribution of microplastics surface biofilms in coral areas, Chemosphere, 2020, 252, 126565 CrossRef CAS PubMed.
- M. Cole, P. Lindeque, C. Halsband and T. S. Galloway, Microplastics as contaminants in the marine environment: A review, Mar. Pollut. Bull., 2011, 62(12), 2588–2597 CrossRef CAS PubMed.
- C. Tu, T. Chen, Q. Zhou, Y. Liu, J. Wei, J. J. Waniek and Y. M. Luo, Biofilm formation and its influences on the properties of microplastics as affected by exposure time and depth in the seawater, Sci. Total Environ., 2020, 734, 139237 CrossRef CAS PubMed.
- D. O'Connor, S. Z. Pan, Z. T. Shen, Y. A. Song, Y. L. Jin, W. M. Wu and D. Y. Hou, Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles, Environ. Pollut., 2019, 249, 527–534 CrossRef PubMed.
- N. Kowalski, A. M. Reichardt and J. J. Waniek, Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors, Mar. Pollut. Bull., 2016, 109(1), 310–319 CrossRef CAS PubMed.
- M. Eriksen, L. C. M. Lebreton, H. S. Carson, M. Thiel, C. J. Moore, J. C. Borerro, F. Galgani, P. G. Ryan and J. Reisser, Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea, PLoS One, 2014, 9(12), 111913 CrossRef PubMed.
- K. L. Law and R. C. Thompson, Microplastics in the seas, Science, 2014, 345(6193), 144–145 CrossRef CAS PubMed.
- J. Peng, J. Wang and L. Cai, Current understanding of microplastics in the environment: Occurrence, fate, risks, and what we should do, Integr. Environ. Assess. Manage., 2017, 13(3), 476–482 CrossRef PubMed.
- J. H. Yuan, J. Ma, Y. R. Sun, T. Zhou, Y. C. Zhao and F. Yu, Microbial degradation and other environmental aspects of microplastics/plastics, Sci. Total Environ., 2020, 715, 136968 CrossRef CAS PubMed.
- A. L. Andrady, The plastic in microplastics: a review, Mar. Pollut. Bull., 2017, 119(1), 12–22 CrossRef CAS PubMed.
- R. Vaughan, S. D. Turner and N. L. Rose, Microplastics in the sediments of a UK urban lake, Environ. Pollut., 2017, 229, 10–18 CrossRef CAS PubMed.
- V. C. Shruti and G. Kutralam-Muniasamy, Bioplastics: Missing link in the era of Microplastics, Sci. Total Environ., 2019, 697, 134139 CrossRef CAS PubMed.
- J. Jacquin, J. G. Cheng, C. Odobel, C. Pandin, P. Conan, M. Pujo-Pay, V. Barbe, A. L. Meistertzheim and J. F. Ghiglione, Microbial Ecotoxicology of Marine Plastic Debris: A Review on Colonization and Biodegradation by the “Plastisphere”, Front. Microbiol., 2019, 10, 00865 CrossRef PubMed.
- Y. Chae and Y. J. An, Effects of food presence on microplastic ingestion and egestion in Mytilus galloprovincialis, Chemosphere, 2020, 240, 124855 CrossRef CAS PubMed.
- K. Tanaka and H. Takada, Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters, Sci. Rep., 2016, 6, 34351 CrossRef CAS PubMed.
- F. Stock, C. Kochleus, B. Bansch-Baltruschat, N. Brennholt and G. Reifferscheid, Sampling techniques and preparation methods for microplastic analyses in the aquatic environment - a review, TrAC, Trends Anal. Chem., 2019, 113, 84–92 CrossRef CAS.
- V. Hidalgo-Ruz, L. Gutow, R. C. Thompson and M. Thiel, Microplastics in the Marine Environment: a Review of the Methods Used for Identification and Quantification, Environ. Sci. Technol., 2012, 46(6), 3060–3075 CrossRef CAS PubMed.
- J. L. Lavers, S. Oppel and A. L. Bond, Factors influencing the detection of beach plastic debris, Mar. Environ. Res., 2016, 119, 245–251 CrossRef CAS PubMed.
- S. Veerasingam, M. Ranjani, R. Venkatachalapathy, A. Bagaev, V. Mukhanov, D. Litvinyuk, M. Mugilarasan, K. Gurumoorthi, L. Guganathan, V. M. Aboobacker and P. Vethamony, Contributions of Fourier transform infrared spectroscopy in microplastic pollution research: A review, Crit. Rev. Environ. Sci. Technol., 2020, 1807450 Search PubMed.
- I. W. Levin and R. Bhargava, Fourier transform infrared vibrational spectroscopic imaging: Integrating microscopy and molecular recognition, Annu. Rev. Phys. Chem., 2005, 56, 429–474 CrossRef CAS PubMed.
- F. Huth, A. Govyadinov, S. Amarie, W. Nuansing, F. Keilmann and R. Hilenbrand, Nano-FTIR Absorption Spectroscopy of Molecular Fingerprints at 20 nm Spatial Resolution, Nano Lett., 2012, 12(8), 3973–3978 CrossRef CAS PubMed.
- S. P. Zhang, Y. C. Sun, B. B. Liu and R. L. Li, Full size microplastics in crab and fish collected from the mangrove wetland of Beibu Gulf: Evidences from Raman Tweezers (1-20 mu m) and spectroscopy (20-5000 mu m), Sci. Total Environ., 2021, 759, 143504 CrossRef CAS PubMed.
- A. Ceccarini, A. Corti, F. Erba, F. Modugno, J. La Nasa, S. Bianchi and V. Castelvetro, The Hidden Microplastics: New Insights and Figures from the Thorough Separation and Characterization of Microplastics and of Their Degradation Byproducts in Coastal Sediments, Environ. Sci. Technol., 2018, 52(10), 5634–5643 CrossRef CAS PubMed.
- J. Fojt, J. David, R. Prikryl, V. Rezacova and J. Kucerik, A critical review of the overlooked challenge of determining micro-bioplastics in soil, Sci. Total Environ., 2020, 745, 140975 CrossRef CAS PubMed.
- L. Hermabessiere, C. Himber, B. Boricaud, M. Kazour, R. Amara, A. L. Cassone, M. Laurentie, I. Paul-Pont, P. Soudant, A. Dehaut and G. Duflos, Optimization, performance, and application of a pyrolysis-GC/MS method for the identification of microplastics, Anal. Bioanal. Chem., 2018, 410(25), 6663–6676 CrossRef CAS PubMed.
- J. C. Prata, J. P. da Costa, A. C. Duarte and T. Rocha-Santos, Methods for sampling and detection of microplastics in water and sediment: A critical review, TrAC, Trends Anal. Chem., 2019, 110, 150–159 CrossRef CAS.
- W. K. Yuan, Y. F. Zhou, Y. L. Chen, X. N. Liu and J. Wang, Toxicological effects of microplastics and heavy metals on the Daphnia magna, Sci. Total Environ., 2020, 746, 141254 CrossRef CAS PubMed.
- A. Turner and L. A. Holmes, Adsorption of trace metals by microplastic pellets in fresh water, Environ. Chem., 2015, 12(5), 600–610 CrossRef CAS.
- N. Pham, S. Iyer, E. Hackett, B. H. Lock, M. Sandy, L. Zeise, G. Solomon and M. Marty, Using ToxCast to Explore Chemical Activities and Hazard Traits: A Case Study With Ortho-Phthalates, Toxicol. Sci., 2016, 151(2), 286–301 CrossRef CAS PubMed.
- V. Godoy, G. Blazquez, M. Calero, L. Quesada and M. A. Martin-Lara, The potential of microplastics as carriers of metals, Environ. Pollut., 2019, 255, 113363 CrossRef CAS PubMed.
- A. T. Ta and S. Babel, Microplastic contamination on the lower Chao Phraya: Abundance, characteristic and interaction with heavy metals, Chemosphere, 2020, 257, 127234 CrossRef CAS PubMed.
- F. K. Mammo, I. D. Amoah, K. M. Gani, L. Pillay, S. K. Ratha, F. Bux and S. Kumari, Microplastics in the environment: Interactions with microbes and chemical contaminants, Sci. Total Environ., 2020, 743, 140518 CrossRef CAS PubMed.
- T. Wang, L. Wang, Q. Q. Chen, N. Kalogerakis, R. Ji and Y. N. Ma, Interactions between microplastics and organic pollutants: Effects on toxicity, bioaccumulation, degradation, and transport, Sci. Total Environ., 2020, 748, 142427 CrossRef CAS PubMed.
- F. Wang, M. Zhang, W. Sha, Y. D. Wang, H. Z. Hao, Y. Y. Dou and Y. Li, Sorption Behavior and Mechanisms of Organic Contaminants to Nano and Microplastics, Molecules, 2020, 25(8), 1827 CrossRef CAS PubMed.
- H. Takada, Call for pellets! International Pellet Watch Global Monitoring of POPs using beached plastic resin pellets, Mar. Pollut. Bull., 2006, 52(12), 1547–1548 CrossRef CAS PubMed.
- Y. Mato, T. Isobe, H. Takada, H. Kanehiro, C. Ohtake and T. Kaminuma, Plastic resin pellets as a transport medium for toxic chemicals in the marine environment, Environ. Sci. Technol., 2001, 35(2), 318–324 CrossRef CAS PubMed.
- O. S. Alimi, J. F. Budarz, L. M. Hernandez and N. Tufenkji, Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport, Environ. Sci. Technol., 2018, 52(4), 1704–1724 CrossRef CAS PubMed.
- F. Wang, C. S. Wong, D. Chen, X. W. Lu, F. Wang and E. Y. Zeng, Interaction of toxic chemicals with microplastics: A critical review, Water Res., 2018, 139, 208–219 CrossRef CAS PubMed.
- B. Y. Hu, Y. X. Li, L. S. Jiang, X. C. Chen, L. Wang, S. Y. An and F. S. Zhang, Influence of microplastics occurrence on the adsorption of 17 beta-estradiol in soil, J. Hazard. Mater., 2020, 400, 123325 CrossRef CAS PubMed.
- F. F. Liu, G. Z. Liu, Z. L. Zhu, S. C. Wang and F. F. Zhao, Interactions between microplastics and phthalate esters as affected by microplastics characteristics and solution chemistry, Chemosphere, 2019, 214, 688–694 CrossRef CAS PubMed.
- C. R. Nobre, M. F. M. Santana, A. Maluf, F. S. Cortez, A. Cesar, C. D. S. Pereira and A. Turra, Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea), Mar. Pollut. Bull., 2015, 92(1–2), 99–104 CrossRef CAS PubMed.
- J. W. Sun, Characteristics of biofilms and resistance genes o microplastic surfaces in waste water treatment plant, Shandong University, 2020 Search PubMed.
- B. Chen, Review of the Source Distribution and Ecological Effects of Marine Microplastics, Environment Protection Science, 2018, 44(2), 90–97 Search PubMed.
- A. Collignon, J. H. Hecq, F. Galgani, F. Collard and A. Goffart, Annual variation in neustonic microplastic particles and zooplankton in the Bay of Calvi ( Mediterranean-Corsica), Mar. Pollut. Bull., 2014, 293–298 CrossRef CAS PubMed.
- E. R. Zettler, T. J. Mincer and L. A. Amaral-Zettler, Life in the “Plastisphere”: Microbial Communities on Plastic Marine Debris, Environ. Sci. Technol., 2013, 47(13), 7137–7146 CrossRef CAS PubMed.
- M. Cole, P. Lindeque, E. Fileman, C. Halsband, R. Goodhead, J. Moger and T. S. Galloway, Microplastic Ingestion by Zooplankton, Environ. Sci. Technol., 2013, 47(12), 6646–6655 CrossRef CAS PubMed.
- J. Yang, L. Xu, A. X. Lu, W. Luo, J. Y. Li and W. Chen, Research programs on the resources and toxicology of micro (nano) plasticity in environment, Environ. Chem., 2018, 37(3), 383–396 CAS.
- C. B. Jeong, E. J. Won, H. M. Kang, M. C. Lee, D. S. Hwang, U. K. Hwang, B. Zhou, S. Souissi, S. J. Lee and J. S. Lee, Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and p-JNK and p-p38 Activation in the Monogonont Rotifer (Brachionus koreanus), Environ. Sci. Technol., 2016, 50(16), 8849–8857 CrossRef CAS PubMed.
- H. Bouwmeester, P. C. H. Hollman and R. J. B. Peters, Potential Health Impact of Environmentally Released Micro- and Nanoplastics in the Human Food Production Chain: Experiences from Nanotoxicology, Environ. Sci. Technol., 2015, 49(15), 8932–8947 CrossRef CAS PubMed.
- K. Mattsson, L. A. Hansson and T. Cedervall, Nano-plastics in the aquatic environment, Environ. Sci.: Processes Impacts, 2015, 17(10), 1712–1721 RSC.
- A. D. Gray and J. E. Weinstein, Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio), Environ. Toxicol. Chem., 2017, 36(11), 3074–3080 CrossRef CAS PubMed.
- D. G. Austin and E. W. John, Size- and Shape-Dependent Effects of Microplastic Particles on Adult Daggerblade Grass Shrimp (Palaemonetes Pugio), Environ. Toxicol. Chem., 2017, 36(11), 3074–3080 CrossRef PubMed.
- S. Jahan, V. Strezov, H. Weldekidan, R. Kumar, T. Kan, S. A. Sarkodie, J. He, B. Dastjerdi and S. P. Wilson, Interrelationship of microplastic pollution in sediments and oysters in a seaport environment of the eastern coast of Australia, Sci. Total Environ., 2019, 695, 133924 CrossRef CAS PubMed.
- P. Ma, M. W. Wang, H. Liu, Y. F. Chen and J. H. Xia, Research on ecotoxicology of microplastics on freshwater aquatic organisms, Environ. Pollut. Bioavailability, 2019, 31(1), 131–137 CrossRef.
- C. G. Avio, S. Gorbi, M. Milan, M. Benedetti, D. Fattorini, G. d'Errico, M. Pauletto, L. Bargelloni and F. Regoli, Pollutants bioavailability and toxicological risk from microplastics to marine mussels, Environ. Pollut., 2015, 198, 211–222 CrossRef CAS PubMed.
- J. N. Hahladakis, C. A. Velis, R. Weber, E. Iacovidou and P. Purnell, An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling, J. Hazard. Mater., 2018, 344, 179–199 CrossRef CAS PubMed.
- L. Hermabessiere, A. Dehaut, I. Paul-Pont, C. Lacroix, R. Jezequel, P. Soudant and G. Duflos, Occurrence and effects of plastic additives on marine environments and organisms: A review, Chemosphere, 2017, 182, 781–793 CrossRef CAS PubMed.
- R. Almeda, R. Rodriguez-Torres, S. Rist, M. H. S. Winding, P. Stief, B. H. Hansen and T. G. Nielsen, Microplastics do not increase bioaccumulation of petroleum hydrocarbons in Arctic zooplankton but trigger feeding suppression under co-exposure conditions, Sci. Total Environ., 2021, 751, 141264 CrossRef CAS PubMed.
- C. Gambardella, V. Piazza, M. Albentosa, M. J. Bebianno, C. Cardoso, M. Faimali, F. Garaventa, S. Garrido, S. Gonzalez, S. Perez, M. Sendra and R. Beiras, Microplastics do not affect standard ecotoxicological endpoints in marine unicellular organisms, Mar. Pollut. Bull., 2019, 143, 140–143 CrossRef CAS PubMed.
- P. S. Tourinho, V. Koci, S. Loureiro and C. A. M. van Gestel, Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation, Environ. Pollut., 2019, 252, 1246–1256 CrossRef CAS PubMed.
- D. Kim, Y. Chae and Y. J. An, Mixture Toxicity of Nickel and Microplastics with Different Functional Groups on Daphnia magna, Environ. Sci. Technol., 2017, 51(21), 12852–12858 CrossRef CAS.
- Q. J. Wang, X. X. Wangjin, Y. Zhang, N. X. Wang, Y. L. Wang, G. H. Meng and Y. H. Chen, The toxicity of virgin and UV-aged PVC microplastics on the growth of freshwater algae Chlamydomonas reinhardtii, Sci. Total Environ., 2020, 749, 141603 CrossRef CAS PubMed.
- S. B. Sjollema, P. Redondo-Hasselerharm, H. A. Leslie, M. H. S. Kraak and A. D. Vethaak, Do plastic particles affect microalgal photosynthesis and growth?, Aquat. Toxicol., 2016, 170, 259–261 CrossRef CAS PubMed.
- S. Y. Au, T. F. Bruce, W. C. Bridges and S. J. Klaine, Responses of Hyalella azteca to acute and chronic microplastic exposures, Environ. Toxicol. Chem., 2015, 34(11), 2564–2572 CrossRef CAS PubMed.
- A. Jemec, P. Horvat, U. Kunej, M. Bele and A. Krzan, Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna, Environ. Pollut., 2016, 219, 201–209 CrossRef CAS PubMed.
- O. Setala, J. Norkko and M. Lehtiniemi, Feeding type affects microplastic ingestion in a coastal invertebrate community, Mar. Pollut. Bull., 2016, 102(1), 95–101 CrossRef CAS PubMed.
- T. Kogel, O. Bjoroy, B. Toto, A. M. Bienfait and M. Sanden, Micro- and nanoplastic toxicity on aquatic life: Determining factors, Sci. Total Environ., 2020, 709, 136050 CrossRef CAS PubMed.
- C. B. Jeong, H. M. Kang, M. C. Lee, D. H. Kim, J. Han, D. S. Hwang, S. Souissi, S. J. Lee, K. H. Shin, H. G. Park and J. S. Lee, Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana, Sci. Rep., 2017, 7, 41323 CrossRef CAS PubMed.
- R. Goto, A. Kawakita, H. Ishikawa, Y. Hamamura and M. Kato, Molecular phylogeny of the bivalve superfamily Galeommatoidea (Heterodonta, Veneroida) reveals dynamic evolution of symbiotic lifestyle and interphylum host switching, BMC Evol. Biol., 2012, 12, 172 CrossRef PubMed.
- R. Mizraji, C. Ahrendt, D. Perez-Venegas and J. Vargas, Is the feeding type related with the content of microplastics in intertidal fish gut?, Mar. Pollut. Bull., 2017, 116, 498–500 CrossRef CAS PubMed.
- T. D. Garcia, A. L. P. Cardozo, B. A. Quirino, K. Y. Yofukuji, M. J. M. Ganassin, N. C. L. dos Santos and R. Fugi, Ingestion of Microplastic by Fish of Different Feeding Habits in Urbanized and Non-urbanized Streams in Southern Brazil, Water, Air, Soil Pollut., 2020, 231(8), 434 CrossRef CAS.
- L. G. A. Barboza and B. C. G. Gimenez, Microplastics in the marine environment: Current trends and future perspectives, Mar. Pollut. Bull., 2015, 97(1–2), 5–12 CrossRef CAS PubMed.
- A. L. Lusher, C. O'Donnell, R. Officer and I. O'Connor, Microplastic interactions with North Atlantic mesopelagic fish, ICES J. Mar. Sci., 2016, 73(4), 1214–1225 CrossRef.
- E. L. B. Rebolledo, J. A. Van Franeker, O. E. Jansen and S. M. J. M. Brasseur, Plastic ingestion by harbour seals (Phoca vitulina) in The Netherlands, Mar. Pollut. Bull., 2013, 67(1–2), 200–202 CrossRef PubMed.
- M. C. Fossi, L. Marsili, M. Baini, M. Giannetti, D. Coppola, C. Guerranti, I. Caliani, R. Minutoli, G. Lauriano, M. G. Finoia, F. Rubegni, S. Panigada, M. Berube, J. U. Ramirez and C. Panti, Fin whales and microplastics: The Mediterranean Sea and the Sea of Cortez scenarios, Environ. Pollut., 2016, 209, 68–78 CrossRef CAS PubMed.
- M. Carbery, W. O'Connor and T. Palanisami, Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health, Environmental International, 2018, 115, 400–409 CrossRef PubMed.
- E.-V. Armando, M. C. Analicia, C. Jordan, J. G. Micah, A. S. Smit and E. C.-C. Jaclyn, Translocation, trophic transfer, accumulation and depuration of polystyrene microplastics in Daphnia magna and Pimephales promelas, Environ. Pollut., 2020, 259, 113937 CrossRef PubMed.
- A. L. Lusher, N. A. Welden, P. Sobral and M. Cole, Sampling, isolating and identifying microplastics ingested by fish and invertebrates, Anal. Methods, 2017, 9(9), 1346–1360 RSC.
- P. Farrell and K. Nelson, Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.), Environ. Pollut., 2013, 177, 1–3 CrossRef CAS PubMed.
- O. Setala, V. Fleming-Lehtinen and M. Lehtiniemi, Ingestion and transfer of microplastics in the planktonic food web, Environ. Pollut., 2014, 185, 77–83 CrossRef CAS PubMed.
- A. L. Lusher, M. McHugh and R. C. Thompson, Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel, Mar. Pollut. Bull., 2013, 67(1–2), 94–99 CrossRef CAS PubMed.
- S. Y. Au, C. M. Lee, J. E. Weinstein, P. van den Hurk and S. J. Klaine, Trophic transfer of microplastics in aquatic ecosystems: Identifying critical research needs, Integr. Environ. Assess. Manage., 2017, 13(3), 505–509 CrossRef PubMed.
- A. J. R. Watts, C. Lewis, R. M. Goodhead, S. J. Beckett, J. Moger, C. R. Tyler and T. S. Galloway, Uptake and Retention of Microplastics by the Shore Crab Carcinus maenas, Environ. Sci. Technol., 2014, 48(15), 8823–8830 CrossRef CAS PubMed.
- A. McCormick, T. J. Hoellein, S. A. Mason, J. Schluep and J. J. Kelly, Microplastic is an Abundant and Distinct Microbial Habitat in an Urban River, Environ. Sci. Technol., 2014, 48(20), 11863–11871 CrossRef CAS PubMed.
- H. K. Imhof, J. Schmid, R. Niessner, N. P. Ivleva and C. Laforsch, A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments, Limnol. Oceanogr.: Methods, 2012, 10, 524–537 CrossRef CAS.
- M. O. Rodrigues, N. Abrantes, F. J. M. Goncalves, H. Nogueira, J. C. Marques and A. M. M. Goncalves, Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antua River, Portugal), Sci. Total Environ., 2018, 633, 1549–1559 CrossRef CAS PubMed.
- C. M. Free, O. P. Jensen, S. A. Mason, M. Eriksen, N. J. Williamson and B. Boldgiv, High-levels of microplastic pollution in a large, remote, mountain lake, Mar. Pollut. Bull., 2014, 85(1), 156–163 CrossRef CAS PubMed.
- R. A. Castaneda, S. Avlijas, M. A. Simard and A. Ricciardi, Microplastic pollution in St. Lawrence River sediments, Can. J. Fish. Aquat. Sci., 2014, 71(12), 1767–1771 CrossRef CAS.
- C. J. Moore, G. L. Lattin and A. F. Zellers, Quantity and type of plastic debris flowing from two urban rivers to coastal waters and beaches of Southern California, Journal of Integrated Coastal Zone Management, 2011, 11(2), 65–73 Search PubMed.
- S. Eo, S. H. Hong, Y. K. Song, G. M. Han and W. J. Shim, Spatiotemporal distribution and annual load of microplastics in the Nakdong River, South Korea, Water Res., 2019, 160, 228–237 CrossRef CAS PubMed.
- S. Estahbanati and N. L. Fahrenfeld, Influence of wastewater treatment plant discharges on microplastic concentrations in surface water, Chemosphere, 2016, 162, 277–284 CrossRef CAS PubMed.
- Z. C. Wang, Y. M. Qin, W. P. Li, W. H. Yang, Q. Meng and J. L. Yang, Microplastic contamination in freshwater: first observation in Lake Ulansuhai, Yellow River Basin, China, Environ. Chem. Lett., 2019, 17(4), 1821–1830 CrossRef CAS.
- L. Su, Y. G. Xue, L. Y. Li, D. Q. Yang, P. Kolandhasamy, D. J. Li and H. H. Shi, Microplastics in Taihu Lake, China, Environ. Pollut., 2016, 216, 711–719 CrossRef CAS PubMed.
- L. Lahens, E. Strady, T. C. Kieu-Le, R. Dris, K. Boukerma, E. Rinnert, J. Gasperi and B. Tassin, Macroplastic and microplastic contamination assessment of a tropical river (Saigon River, Vietnam) transversed by a developing megacity, Environ. Pollut., 2018, 236, 661–671 CrossRef CAS PubMed.
- K. Zhang, J. Su, X. Xiong, X. Wu, C. X. Wu and J. T. Liu, Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China, Environ. Pollut., 2016, 219, 450–455 CrossRef CAS PubMed.
- A. R. McCormick, T. J. Hoellein, M. G. London, J. Hittie, J. W. Scott and J. J. Kelly, Microplastic in surface waters of urban rivers: concentration, sources, and associated bacterial assemblages, Ecosphere, 2016, 7(11), 1556 CrossRef.
- M. X. Di and J. Wang, Microplastics in surface waters and sediments of the Three Gorges Reservoir, China, Sci. Total Environ., 2018, 616, 1620–1627 CrossRef PubMed.
- O. A. Abayomi, P. Range, M. A. Al-Ghouti, J. P. Obbard, S. H. Almeer and R. Ben-Hamadou, Microplastics in coastal environments of the Arabian Gulf, Mar. Pollut. Bull., 2017, 124(1), 181–188 CrossRef CAS PubMed.
- M. Eriksen, A. Cummins, N. Maximenko, M. Thiel, G. Lattin, S. Wilson, J. Hafner, A. Zellers and S. Rifman, Plastic Pollution in the South Pacific Subtropical Gyre, Plast. Eng., 2013, 68(1–2), 71–76 CrossRef CAS PubMed.
- A. Isobe, K. Uchida, T. Tokai and S. Iwasaki, East Asian seas: A hot spot of pelagic microplastics, Mar. Pollut. Bull., 2015, 101(2), 618–623 CrossRef CAS PubMed.
- K. L. Law, S. Moret-Ferguson, N. A. Maximenko, G. Proskurowski, E. E. Peacock, J. Hafner and C. M. Reddy, Plastic Accumulation in the North Atlantic Subtropical Gyre, Science, 2010, 329(5996), 1185–1188 CrossRef CAS PubMed.
- A. Collignon, J. H. Hecq, F. Galgani, P. Voisin, F. Collard and A. Goffart, Neustonic microplastic and zooplankton in the North Western Mediterranean Sea, Mar. Pollut. Bull., 2012, 64(4), 861–864 CrossRef CAS PubMed.
- S. M. Wang, H. Z. Chen, X. W. Zhou, Y. Q. Tian, C. Lin, W. L. Wang, K. W. Zhou, Y. B. Zhang and H. Lin, Microplastic abundance, distribution and composition in the mid-west Pacific Ocean, Environ. Pollut., 2020, 264, 114125 CrossRef CAS PubMed.
- J. Bikker, J. Lawson, S. Wilson and C. M. Rochman, Microplastics and other anthropogenic particles in the surface waters of the Chesapeake Bay, Mar. Pollut. Bull., 2020, 156, 111257 CrossRef CAS PubMed.
- J. P. W. Desforges, M. Galbraith and P. S. Ross, Ingestion of Microplastics by Zooplankton in the Northeast Pacific Ocean, Arch. Environ. Contam. Toxicol., 2015, 69(3), 320–330 CrossRef CAS PubMed.
- Y. K. Song, S. H. Hong, M. Jang, G. M. Han and W. J. Shim, Occurrence and Distribution of Microplastics in the Sea Surface Microlayer in Jinhae Bay, South Korea, Arch. Environ. Contam. Toxicol., 2015, 69(3), 279–287 CrossRef CAS PubMed.
- J. M. Zhao, W. Ran, J. Teng, Y. L. Liu, H. Liu, X. N. Yin, R. W. Cao and Q. Wang, Microplastic pollution in sediments from the Bohai Sea and the Yellow Sea, China, Sci. Total Environ., 2018, 640, 637–645 CrossRef PubMed.
- T. Liu, Y. F. Zhao, M. L. Zhu, J. H. Liang, S. Zheng and X. X. Sun, Seasonal variation of micro- and meso-plastics in the seawater of Jiaozhou Bay, the Yellow Sea, Mar. Pollut. Bull., 2020, 152, 110922 CrossRef CAS PubMed.
- T. Liu, X. X. Sun and M. L. Zhu, Distribution and composition of microplastics in the surface water of the East China Sea, Oceanol. Limnol. Sin., 2018, 4, 62–69 Search PubMed.
- A. L. Lusher, A. Burke, I. O'Connor and R. Officer, Microplastic pollution in the Northeast Atlantic Ocean: Validated and opportunistic sampling, Mar. Pollut. Bull., 2014, 88(1–2), 325–333 CrossRef CAS PubMed.
- Q. X. Qiu, Z. Tan, J. D. Wang, J. P. Peng, M. M. Li and Z. W. Zhan, Extraction, enumeration and identification methods for monitoring microplastics in the environment, Estuarine, Coastal Shelf Sci., 2016, 176, 102–109 CrossRef CAS.
- J. P. Harrison, J. J. Ojeda and M. E. Romero-Gonzalez, The applicability of reflectance micro-Fourier-transform infrared spectroscopy for the detection of synthetic microplastics in marine sediments, Sci. Total Environ., 2012, 416, 455–463 CrossRef CAS PubMed.
- H. K. Imhof, C. Laforsch, A. C. Wiesheu, J. Schmid, P. M. Anger, R. Niessner and N. P. Ivleva, Pigments and plastic in limnetic ecosystems: A qualitative and quantitative study on microparticles of different size classes, Water Res., 2016, 98, 64–74 CrossRef CAS PubMed.
- S. Y. Zhao, M. Danley, J. E. Ward, D. J. Li and T. J. Mincer, An approach for extraction, characterization and quantitation of microplastic in natural marine snow using Raman microscopy, Anal. Methods, 2017, 9(9), 1470–1478 RSC.
- M. T. Nuelle, J. H. Dekiff, D. Remy and E. Fries, A new analytical approach for monitoring microplastics in marine sediments, Environ. Pollut., 2014, 184, 161–169 CrossRef CAS PubMed.
- W. J. Shim, Y. K. Song, S. H. Hong and M. Jang, Identification and quantification of microplastics using Nile Red staining, Mar. Pollut. Bull., 2016, 113(1–2), 469–476 CrossRef CAS PubMed.
- I. Hintersteiner, M. Himmelsbach and W. W. Buchberger, Characterization and quantitation of polyolefin microplastics in personal-care products using high-temperature gel-permeation chromatography, Anal. Bioanal. Chem., 2015, 407(4), 1253–1259 CrossRef CAS PubMed.
- A. M. Elert, R. Becker, E. Duemichen, P. Eisentraut, J. Falkenhagen, H. Sturm and U. Braun, Comparison of different methods for MP detection: What can we learn from them, and why asking the right question before measurements matters?, Environ. Pollut., 2017, 231, 1256–1264 CrossRef CAS PubMed.
- S. Veerasingam, M. Ranjani, R. Venkatachalapathy, A. Bagaev, V. Mukhanov, D. Litvinyuk, L. Verzhevskaia, L. Guganathan and P. Vethamony, Microplastics in different environmental compartments in India: Analytical methods, distribution, associated contaminants and research needs, TrAC, Trends Anal. Chem., 2020, 133, 116071 CrossRef CAS.
- X. Zhu, W. Zhao, X. Chen, T. Zhao, L. Tan and J. Wang, Growth inhibition of the microalgae Skeletonema costatum under copper nanoparticles with microplastic exposure, Mar. Environ. Res., 2020, 158, 105005 CrossRef CAS PubMed.
- X. Yi, T. Chi, Z. Li, J. Wang, M. Yu, M. Wu and H. Zhou, Combined effect of polystyrene plastics and triphenyltin chloride on the green algae Chlorella pyrenoidosa, Environmental Science Pollution Research Internet, 2019, 26(15), 15011–15018 CrossRef CAS PubMed.
- Z. Li, X. Yi, H. Zhou, T. Chi, W. Li and K. Yang, Combined effect of polystyrene microplastics and dibutyl phthalate on the microalgae Chlorella pyrenoidosa, Environ. Pollut., 2020, 257, 113604 CrossRef CAS PubMed.
- Y. Guo, W. Ma, J. Li, W. Liu, P. Qi, Y. Ye, B. Guo, J. Zhang and C. Qu, Effects of microplastics on growth, phenanthrene stress, and lipid accumulation in a diatom, Phaeodactylum tricornutum, Environ. Pollut., 2020, 257, 113628 CrossRef CAS PubMed.
- P. Bhattacharya, S. J. Lin, J. P. Turner and P. C. Ke, Physical Adsorption of Charged Plastic Nanoparticles Affects Algal Photosynthesis, J. Phys. Chem. C, 2010, 114(39), 16556–16561 CrossRef CAS.
- E. Besseling, A. Wegner, E. M. Foekema, M. J. van den Heuvel-Greve and A. A. Koelmans, Effects of Microplastic on Fitness and PCB Bioaccumulation by the Lugworm Arenicola marina (L.), Environ. Sci. Technol., 2013, 47(1), 593–600 CrossRef CAS PubMed.
- S. Garrido, M. Linares, J. A. Campillo and M. Albentosa, Effect of microplastics on the toxicity of chlorpyrifos to the microalgae Isochrysis galbana, clone t-ISO, Ecotoxicol. Environ. Saf., 2019, 173, 103–109 CrossRef CAS PubMed.
- L. Sorensen, E. Rogers, D. Altin, I. Salaberria and A. M. Booth, Sorption of PAHs to microplastic and their bioavailability and toxicity to marine copepods under co-exposure conditions, Environ. Pollut., 2020, 258, 113844 CrossRef PubMed.
- E. M. Chua, J. Shimeta, D. Nugegoda, P. D. Morrison and B. O. Clarke, Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes compressa, Environ. Sci. Technol., 2014, 48(14), 8127–8134 CrossRef CAS PubMed.
- K. W. Lee, W. J. Shim, O. Y. Kwon and J. H. Kang, Size-Dependent Effects of Micro Polystyrene Particles in the Marine Copepod Tigriopus japonicus, Environ. Sci. Technol., 2013, 47(19), 11278–11283 CrossRef CAS PubMed.
- W. Lin, R. Jiang, Y. Xiong, J. Wu, J. Xu, J. Zheng, F. Zhu and G. Ouyang, Quantification of the combined toxic effect of polychlorinated biphenyls and nano-sized polystyrene on Daphnia magna, J. Hazard. Mater., 2019, 364, 531–536 CrossRef CAS PubMed.
- P. Rosenkranz, Q. Chaudhry, V. Stone and T. F. Fernandes, A Comparison of Nanoparticle and Fine Particle Uptake by Daphnia Magna, Environ. Toxicol. Chem., 2009, 28(10), 2142–2149 CrossRef CAS PubMed.
- N. A. C. Welden and P. R. Cowie, Environment and gut morphology influence microplastic retention in langoustine, Nephrops norvegicus, Environ. Pollut., 2016, 214, 859–865 CrossRef CAS PubMed.
- D. Wojcik-Fudalewska, M. Normant-Saremba and P. Anastacio, Occurrence of plastic debris in the stomach of the invasive crab Eriocheir sinensis, Mar. Pollut. Bull., 2016, 113(1–2), 306–311 CrossRef CAS PubMed.
- A. J. R. Watts, M. A. Urbina, R. Goodhead, J. Moger, C. Lewis and T. S. Galloway, Effect of Microplastic on the Gills of the Shore Crab Carcinus maenas, Environ. Sci. Technol., 2016, 50(10), 5364–5369 CrossRef CAS PubMed.
- N. von Moos, P. Burkhardt-Holm and A. Kohler, Uptake and Effects of Microplastics on Cells and Tissue of the Blue Mussel Mytilus edulis L. after an Experimental Exposure, Environ. Sci. Technol., 2012, 46(20), 11327–11335 CrossRef CAS PubMed.
- M. Cole, P. Lindeque, E. Fileman, C. Halsband and T. S. Galloway, The Impact of Polystyrene Microplastics on Feeding, Function and Fecundity in the Marine Copepod Calanus helgolandicus, Environ. Sci. Technol., 2015, 49(2), 1130–1137 CrossRef CAS PubMed.
- E. Besseling, A. Wegner, E. M. Foekema, M. J. van den Heuvel-Greve and A. A. Koelmans, Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.), J. Environ. Sci. Technol., 2013, 47(1), 593–600 CrossRef CAS PubMed.
- M. A. Browne, A. Dissanayake, T. S. Galloway, D. M. Lowe and R. C. Thompson, Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.), Environ. Sci. Technol., 2008, 42(13), 5026–5031 CrossRef CAS PubMed.
- A. Franchini and E. Ottaviani, Repair of molluscan tissue injury: role of PDGF and TGF-beta, Tissue Cell, 2000, 32(4), 312–321 CrossRef CAS PubMed.
- I. Paul-Pont, C. Lacroix, C. G. Fernandez, H. Hegaret, C. Lambert, N. Le Goic, L. Frere, A. L. Cassone, R. Sussarellu, C. Fabioux, J. Guyomarch, M. Albentosa, A. Huvet and P. Soudant, Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation, Environ. Pollut., 2016, 216, 724–737 CrossRef CAS PubMed.
- R. Sussarellu, M. Suquet, Y. Thomas, C. Lambert, C. Fabioux, M. E. J. Pernet, N. Le Goic, V. Quillien, C. Mingant, Y. Epelboin, C. Corporeau, J. Guyomarch, J. Robbens, I. Paul-Pont, P. Soudant and A. Huvet, Oyster reproduction is affected by exposure to polystyrene microplastics, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(9), 2430–2435 CrossRef CAS PubMed.
- M. A. Browne, S. J. Niven, T. S. Galloway, S. J. Rowland and R. C. Thompson, Microplastic Moves Pollutants and Additives to Worms, Reducing Functions Linked to Health and Biodiversity, Curr. Biol., 2013, 23(23), 2388–2392 CrossRef CAS.
- C. G. Avio, S. Gorbi and F. Regoli, Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea, Mar. Environ. Res., 2015, 111, 18–26 CrossRef CAS PubMed.
- M. W. Hart, Particle captures and the method of suspension feeding by echinoderm larvae, Biol. Bull., 1991, 180(1), 12127 CrossRef.
- M. W. Hart, Particle Captures and the Method of Suspension Feeding by Echinoderm Larvae, Biol. Bull., 1991, 180(1), 12–27 CrossRef CAS PubMed.
- C. W. Mak, K. C. F. Yeung and K. M. Chan, Acute toxic effects of polyethylene microplastic on adult zebrafish, Ecotoxicol. Environ. Saf., 2019, 182, 109442 CrossRef CAS PubMed.
- M. Oliveira, A. Ribeiro, K. Hylland and L. Guilhermino, Single and combined effects of microplastics and pyrene on juveniles (0+group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae), Ecol. Indic., 2013, 34, 641–647 CrossRef CAS.
- J. A. Pitt, J. S. Kozal, N. Jayasundara, A. Massarsky, R. Trevisan, N. Geitner, M. Wiesner, E. D. Levin and R. T. Di Giulio, Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio), Aquat. Toxicol., 2018, 194, 185–194 CrossRef CAS PubMed.
- Y. J. Yu, R. X. Ma, H. Qu, Y. Zuo, Z. L. Yu, G. C. Hu, Z. R. Li, H. B. Chen, B. G. Lin, B. Wang and G. Yu, Enhanced adsorption of tetrabromobisphenol a (TBBPA) on cosmetic-derived plastic microbeads and combined effects on zebrafish, Chemosphere, 2020, 248, 126067 CrossRef CAS PubMed.
- D. V. Dantas, M. Barletta and M. F. da Costa, The seasonal and spatial patterns of ingestion of polyfilament nylon fragments by estuarine drums (Sciaenidae), Environ. Sci. Pollut. Res., 2012, 19(2), 600–606 CrossRef PubMed.
- J. A. A. Ramos, M. Barletta and M. F. Costa, Ingestion of nylon threads by Gerreidae while using a tropical estuary as foraging grounds, Aquat. Biol., 2012, 17(1), 29–34 CrossRef.
- E. Fonte, P. Ferreira and L. Guilhermino, Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): Post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation, Aquat. Toxicol., 2016, 180, 173–185 CrossRef CAS PubMed.
- Y. F. Lu, Y. Zhang, Y. F. Deng, W. Jiang, Y. P. Zhao, J. J. Geng, L. L. Ding and H. Q. Ren, Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver, Environ. Sci. Technol., 2016, 50(7), 4054–4060 CrossRef CAS PubMed.
- G. L. Luís, F. Pedro, F. Elsa, O. Miguel and G. Lúcia, Does the presence of microplastics influence the acute toxicity of chromium(vi) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations, Aquatic Toxicology and Hazard Evaluation, 2015, 164, 163–174 CrossRef.
- D. Mazurais, B. Ernande, P. Quazuguel, A. Severe, C. Huelvan, L. Madec, O. Mouchel, P. Soudant, J. Robbens, A. Huvet and J. Zambonino-Infante, Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae, Mar. Environ. Res., 2015, 112, 78–85 CrossRef CAS.
- Q. Q. Chen, A. Allgeier, D. Q. Yin and H. Hollert, Leaching of endocrine disrupting chemicals from marine microplastics and mesoplastics under common life stress conditions, Environ. Int., 2019, 130, 104938 CrossRef CAS PubMed.
- A. J. Kokalj, D. Kuehnel, B. Puntar, A. Z. Gotvajn and G. Kalcikova, An exploratory ecotoxicity study of primary microplastics versus aged in natural waters and wastewaters, Environ. Pollut., 2019, 254, 112980 CrossRef PubMed.
- L. L. Lei, S. Y. Wu, S. B. Lu, M. T. Liu, Y. Song, Z. H. Fu, H. H. Shi, K. M. Raley-Susman and D. F. He, Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans, Sci. Total Environ., 2018, 619, 1–8 CrossRef PubMed.
- L. C. de Sa, L. G. Luis and L. Guilhermino, Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions, Environ. Pollut., 2015, 196, 359–362 CrossRef CAS PubMed.
- C. M. Rochman, T. Kurobe, I. Flores and S. J. Teh, Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment, Sci. Total Environ., 2014, 493, 656–661 CrossRef CAS PubMed.
- L. G. A. Barboza, L. R. Vieira, V. Branco, N. Figueiredo, F. Carvalho, C. Carvalho and L. Guilhermino, Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758), Aquat. Toxicol., 2018, 195, 49–57 CrossRef CAS PubMed.
- A. Karami, N. Romano, T. Galloway and H. Hamzah, Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus), Environ. Res., 2016, 151, 58–70 CrossRef CAS PubMed.
- A. Bartonitz, I. N. Anyanwu, J. Geist, H. K. Imhof, J. Reichel, J. Grassmann, J. E. Drewes and S. Beggel, Modulation of PAH toxicity on the freshwater organism G. roeseli by microparticles, Environ. Pollut., 2020, 260, 113999 CrossRef CAS PubMed.
- C. Peda, L. Caccamo, M. C. Fossi, F. Gai, F. Andaloro, L. Genovese, A. Perdichizzi, T. Romeo and G. Maricchiolo, Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: Preliminary results, Environ. Pollut., 2016, 212, 251–256 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |