Open Access Article
Open Access ArticleDevelopment of effective potassium acetate extractant†
Maciej Zakrzewski,
Dominika Załubiniak and
Piotr Piątek *
*
Department of Chemistry, The University of Warsaw, Pasteura 1, 02-093 Warsaw, Poland. E-mail: ppiatek@chem.uw.edu.pl
First published on 16th March 2021
Abstract
Carboxylates are commonly used in the food and pharmaceutical industry and due to their extensive use, carboxylates present a significant environmental burden. In this context, valine based, heteroditopic receptor 1 was prepared and its ability to bind simultaneously potassium cation and acetate anion in water containing CH3CN solutions was demonstrated. Under liquid–liquid extraction conditions the receptor 1 was capable of extracting hydrophilic AcOK salt from aqueous solution and was proved to be nearly ten times more effective than the equimolar mixture of monotopic receptors. Furthermore, compound 1 could extract one of the most popular nonsteroidal anti-inflammatory drugs, ibuprofen (IbuOK), from relatively dilute aqueous solutions.
Introduction
Liquid–liquid extraction (solvent extraction) is one of the most important separation methods in research and industry. For example, in hydrometallurgy this process is frequently used in separation and purification of cations of transition metals (Cu, Co, Ni, Pd) as well as f-block elements (U, Pu) from aqueous solution to organic solvents.1 On the other hand, anion recognition and transport in liquid–liquid separation systems is an area of growing interest.2 This interest is mainly stimulated by the need of finding effective methods of the removal of potentially harmful anions, such as nitrate, sulfate, perchlorate or carboxylates from the environment. Molecular receptors which could bind only one kind of ion can be used to facilitate liquid–liquid extraction. However, only ions accompanied by lipophilic counterions are effectively extracted by those monotopic receptors. For example, dicyclohexyl-18-crown-6 extracts 41% of KClO4 and 16% of KI but only 0.6% of KBr from water to chloroform phase.3 To extract hydrophilic salts the use of binary mixture of monotopic receptors or one receptor combining cation and anion binding sites (heteroditopic receptor) is needed.2,4 The use of heteroditopic receptors in extraction processes has many practical advantages. First, cation–receptor–anion ternary complex is electrically neutral thus it can be directly transported across membranes in a symport process. Then mutual electrostatic interactions between co-bonded ions and allosteric conformational change of the receptor induced by binding events can cause positive cooperative effects that could substantially increase salt binding affinity.5 However, the design, synthesis and binding studies of heteroditopic receptors that display substantial positive binding synergy is not a trivial task, yet finding receptors that could facilitate liquid–liquid salt transport is even more challenging. This is because strong salt binding by a heteroditopic receptor is not the only one factor determining transport activity.2,4,6 The other crucial factor is the lipophilicity of the cation–receptor–anion complex that is transported through liquid–liquid interface.7 Therefore, the heteroditopic receptors that form less polar complexes in which cation and anion are in close proximity should be more active in salt transport. Therefore, the most effective salt extractants prepared to date have multi-macrocyclic, cryptand-like structure that ensures close spatial proximity of cation and anion binding domains.8 These extractants bind salts most likely as contact or solvent separated ion-pairs. Some very elegant multimacrocyclic salt extractants are known and proved to be able to extract KF, KCl, CsCl, NH4NO3 and LiNO2.5,8 However, due to the ‘‘closed’’ structure of these receptors it is difficult to improve their binding and extracting abilities. Unfortunately, the synthesis of these multimacrocyclic compounds is usually low yielding and requires the application of high-dilution techniques. Therefore, there is a need of simple and relatively cheap receptor which will extract potentially harmful individuals.Recently, our group has reported non-multimacrocyclic heterotopic receptor S1 which strongly binds potassium carboxylates due to high cooperativity between co-bounded ions.9 Moreover, preliminary studies showed that receptor S1 permits the extraction of hydrophilic AcOKaq into an organic phase. This unprecedented result is very important because carboxylate salts are commonly used in the chemical, pharmaceutical and food industry.10 Due to their extensive use, carboxylates present a significant environmental burden. New challenges connected with the analysis and removal of the carboxylates used as active pharmaceutical ingredients (such as nonsteroidal anti-inflammatory drugs) have been arising recently.11 Therefore, the receptors that are capable of recognizing and extracting the carboxylates may help to analyze and remove these salts. Acetate anion is the most hydrophilic unsubstituted carboxylate (ΔG0h = −373 kJ mol−1) and hence is a good model anion for testing the transport activity of molecular receptors.
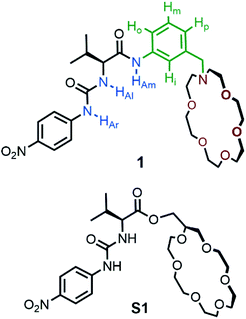
Herein, we describe the design, synthesis, binding and detailed acetate salt extraction studies of the heterotopic receptor 1. This receptor was developed thanks to the non-macrocyclic and modular structure of parent receptor S1 that can be easily modified. The main purpose of receptor S1 structure modifications was to obtain a more practical acetate receptor and extractant in terms of costs and durability. Both receptors are based on L-valine molecular scaffold and share the 4-nitrophenyl urea moiety as anion binding group. However, other groups such as thiourea or squaramide are known to interact stronger with anions. Therefore, thiourea and squaramide derivatives of 1 were prepared and studied (for structures see compounds S2 and S3 in ESI†). Unfortunately, these compounds turned out to be impractical. Specifically, the thiourea derivative (S2) was unstable in the prolongated presence of AcOKaq, whereas squaramide NHs protons of S3 were too acidic and underwent deprotonation by weak basic AcOK aqueous solution. Nevertheless, 4-nitrophenyl urea group of 1 is stable under these conditions. The strong cation coordination of S1 is ensured by the substituted 18-crown-6. However, 2-hydroxymethyl-18-crown-6 necessary for assembling of the receptor S1 is expensive and, what is more, hard to prepare. Therefore, for receptor 1 construction we used N-aza-18-crown-6 which is also commercially available but can be easily prepared in large quantities from cheap substrates.12 However, the most important reason, which forced us to search for more practical cation binding domain, was hydrolysis of S1 upon prolongated AcOKaq exposure.
The last important structural feature of the receptor 1 was the benzylaniline spacer between N-aza-18-crown-6 and valine moiety. This group ensures proper spatial arrangement of cation and anion binding domains and enables connection of receptor 1 components via chemically robust amide bond. This additional aromatic moiety can also change lipophilicity of receptor and consequently receptor·salt complex.
Results and discussion
The receptor 1 has been synthesized in three simple synthetic steps. Briefly, the HATU mediated coupling of N-Boc-L-valine with N-(3-aminobenzyl)-18-crown-6 in the presence of DIPEA gave a valine-crown ether derivative. The subsequent Boc group deprotection and acylation of the resulting TFA ammonium salt with 4-nitrophenyl-isocyanate in the presence of triethylamine led to the receptor 1 in 53% yield. The main problem at this stage was the purification of 1, which formed strong complexes with Et3N–TFA salt, drying agents, and ions present in silica gel.Initially, binding capabilities of the receptor 1 binding with acetate salts were tested using UV-Vis spectroscopic titrations in CH3CN. By fitting the titration curves to a standard 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding profile, association constants (K) corresponding to the interactions between the receptor 1 and acetate in the absence (tetra-n-butylammonium, TBA salt) and presence of one equivalent of co-bounded potassium cation could be determined (Table 1).
1 binding profile, association constants (K) corresponding to the interactions between the receptor 1 and acetate in the absence (tetra-n-butylammonium, TBA salt) and presence of one equivalent of co-bounded potassium cation could be determined (Table 1).
| CH3CN | 1% H2O/CH3CN | 3% H2O/CH3CN | |
|---|---|---|---|
| TBA+ | 580![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
970 |
| K+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3400 |
| KK+/KTBA+ | 2.93 | 4.72 | 3.51 |
In acetonitrile solution in the absence of the co-cordinated cations (TBA salt) the receptor 1 associates with acetate anions stronger than the receptor S1 (KS1AcOTBA = 252![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1). However, in the presence of potassium cations the receptor 1 binds acetate anions weaker than S1 (KS1AcOK > 10
000 M−1). However, in the presence of potassium cations the receptor 1 binds acetate anions weaker than S1 (KS1AcOK > 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1). Thus, the binding cooperativity expressed as KK+/KTBA+ is much lower for the receptor 1 compared to S1. This effect was anticipated as N-substituted-18-crown-6 associates less strongly with potassium cations than 18-crown-6.13 However, in water–organic solvent extraction process, ions are hydrated and receptor must compete efficiently with water molecules. Therefore, acetate binding was studied in solutions containing water. The addition of 1% water to acetonitrile, resulted in dramatic decrease of association constants values. Interestingly, under these condition, in the presence of potassium cations the acetate binding is almost five times stronger than in its absence. This effect is also observed in solution containing 3% of water.14 Thus, these results suggest that positive cooperation between co-bounded ions can help the receptor 1 to overcome salt and receptor hydration and therefore this represents an important step towards development of effective salt extractant.
000 M−1). Thus, the binding cooperativity expressed as KK+/KTBA+ is much lower for the receptor 1 compared to S1. This effect was anticipated as N-substituted-18-crown-6 associates less strongly with potassium cations than 18-crown-6.13 However, in water–organic solvent extraction process, ions are hydrated and receptor must compete efficiently with water molecules. Therefore, acetate binding was studied in solutions containing water. The addition of 1% water to acetonitrile, resulted in dramatic decrease of association constants values. Interestingly, under these condition, in the presence of potassium cations the acetate binding is almost five times stronger than in its absence. This effect is also observed in solution containing 3% of water.14 Thus, these results suggest that positive cooperation between co-bounded ions can help the receptor 1 to overcome salt and receptor hydration and therefore this represents an important step towards development of effective salt extractant.
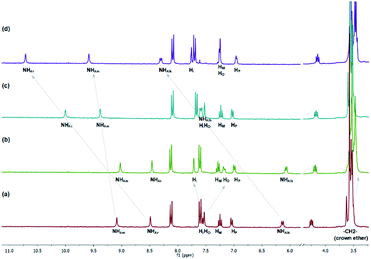
To obtain more direct experimental insights into the binding behavior of 1, 1H NMR spectroscopic titrations were performed in competitive 3% H2O/CD3CN solvent system. The addition of one equivalent of KPF6 results in upfield shifts of –N–CH2- and –O–CH2- crown ether methylene protons (Fig. 2). At the same time, a considerable downfield shift of aromatic Hi proton and upfield shift of Ho proton of the N-benzyl aromatic ring is observed. Other signals including urea and amide NH protons remain practically unaffected by the cation addition. This suggests the formation of a receptor 1·K+ inclusion complex by the crown ether domain without the involvement of urea and amide groups.
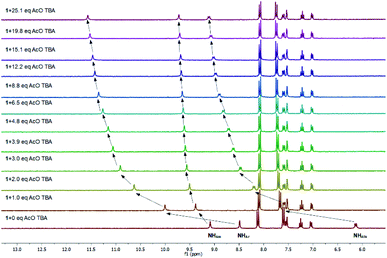 | ||
| Fig. 3 Partial 1H NMR spectra of 1 upon titrant (TBA AcO) addition, (293 K, 3% H2O/CD3CN solution, Ctitrant = 1.9 × 10−1 M, Creceptor = 3.8 × 10−3 M). | ||
As it is shown in Fig. 3, significant chemical shift changes were observed when the receptor 1 was treated with acetate anions. Specifically, upon exposure to 1 eq of TBA AcO the urea resonances at 6.1 and 8.5 ppm undergo considerable downfield shifts to 7.6 and 10.0 ppm respectively. These changes in the chemical shifts are ascribed to hydrogen bonding between the urea NHs and the acetate anion. On the contrary, the amide NH proton shifts downfield only a little, which may suggest that amide NH interacts with acetate anions and forms intramolecular bond with the oxygen atoms of the urea, simultaneously. This interaction may rigidify the conformation of 1, thus a more defined binding cavity may be created. On the other hand, protons of the N-benzyl aromatic ring do not undergo any changes upon acetate addition indicating no association of the TBA cation to the aza-crown ether moiety.
The 1H NMR spectra acquired upon the TBA AcO addition to the solution of the receptor 1 pretreated with 1 eq of KPF6 shows features characteristic for complexation of both the cation and the anion. Moreover, the chemical shifts induced by the complexation of AcO− in the presence of K+ were larger than the corresponding shifts observed upon addition of ‘‘single’’ ions, which clearly showed an increased strength of interactions. The binding constants determined from the 1H NMR titrations in the absence and presence of potassium cations was calculated to be 420 and 2060 M−1 respectively which corresponds to 4.9 binding cooperativity. Other acetate salts such as AcONa and AcONH4 were associated with 1 with binding constants of 500 and 330 M−1 respectively, which gave 1.2 and 0.8 cooperativity. Thus 1H NMR experiments validated the high cooperativity between cation and anion binding to the receptor 1 in competitive 3% H2O/CD3CN solvent system.
To assess whether the receptor 1 might be able to extract hydrophilic acetate salts from water into organic solvent, model liquid–liquid extraction studies were undertaken. Control experiments revealed no acetate transport (1H NMR detection) to CDCl3 phase from AcOK aqueous solution as well as no receptor leaking from organic into aqueous phase. Then extraction studies were carried out using 2.5 × 10−2 M solution of 1 in CDCl3 layered over 200 molar equivalent solution of AcOK in deionized water in an NMR tube. After shaking thoroughly for 1 minute and after phase separation, the 1H NMR spectrum of the organic phase was recorded. This spectrum revealed considerable changes in the resonance signals compared to the 1H NMR spectrum of 1 in wet CDCl3 (Fig. 4). Urea NHs protons signals centered at 9.5 and 7.3 ppm after extraction underwent considerable downfield shifts to 11.3 and 10.2 ppm respectively. Concurrently, the appreciable upfield shift of crown ether OCH2-resonances and shift of Hi and Ho protons of the N-benzyl aromatic ring were observed (see Fig. 1 for aromatic protons assignment).
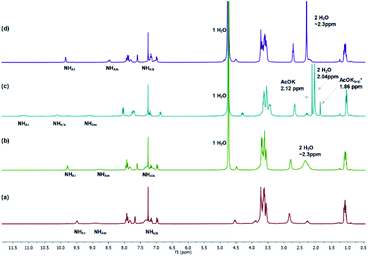 | ||
| Fig. 4 1H NMR spectra stack of extraction experiments: (a) receptor 1 dissolved in ‘dry’ CDCl3 (Creceptor = 2.5 × 10−2 M); (b) receptor 1 solution after being washed with deionized water; (c) receptor 1 solution after extraction with 50 eq AcOK aquaeus; (d) receptor 1 solution after back-extraction to deionized water *AcOK(aq) salt present in droplets of water on the NMR tubes walls (also see ESI†). | ||
This indicates the simultaneous association of the acetate anion and the potassium cation with the receptor 1 under interfacial conditions. In addition, a peak assigned to the methyl group of the acetate anion (at δ = 2.12 ppm) was observed. Based on the integration of this signal quantitative transport of AcOK from water to CDCl3 was calculated. Thus, even that the receptor 1 associates with AcOK weaker than S1 its extraction transport activity is very similar. This observation can be rationalized in terms of lipophilicity of the ternary potassium–receptor–acetate complex, which is related to the ion and counterion distance in the complex and lipophilicity of the ligand. The calculated clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for the receptors S1 and 1 are 1.0 and 2.6 respectively. Therefore, ligand 1 is much more lipophilic than S1 which may compensate its weaker binding ability.
P values for the receptors S1 and 1 are 1.0 and 2.6 respectively. Therefore, ligand 1 is much more lipophilic than S1 which may compensate its weaker binding ability.
To further test the extraction effectiveness of 1, transport of potassium acetate was measured as a function of the concentration of AcOK in the aqueous phase while keeping the extractant concentration constant (2.5 × 10−2 M) in the organic phase. Under these conditions, the receptor 1 proved to be capable of extracting efficiently potassium acetate from solutions containing up to 50 eq of AcOK (Fig. 5). For example, after extraction of 50 eq of AcOKaq with compound 1, 88% of the receptor loading was observed. Then for unit extraction operation the efficiency of 1 decreased. This result is promising in a context of practical application of 1 for separation and transport of acetate and other more lipophilic carboxylates including active pharmaceutical ingredients.
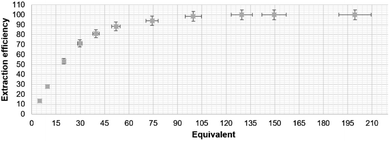 | ||
| Fig. 5 Efficiency of extraction process in function of salt concentration. Based on 1H NMR extraction experiments. | ||
Under the same conditions as described above, transport of other hydrophilic acetate salts such as AcONa and AcONH4 was studied. No appreciable changes of the receptor-based proton signals were seen when the receptor 1 was treated with AcOLiaq. Upon exposure of 1 to AcONaaq only small changes of 1H NMR spectrum were seen and 4% of acetate was detected in organic phase. On the contrary, AcONH4 extraction was effective and ∼70% of this salt was transported to the organic phase. Therefore, for a constant anion, the cation selectivity order was Li+ > Na+ > K+ ∼ NH4+. This trend is in general agreement with the Hofmeister series, in which transport of smaller, more charge-dense ions is harder because they have more unfavorable Gibbs free energy for aqueous to organic phase transfer.15
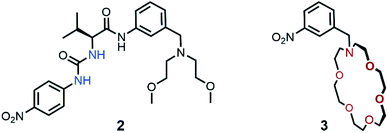
To evaluate the effect of the covalent linkage of the two binding domains in the heteroditopic receptor on salt extraction, the transport of AcOK by 1 and equimolar mixture of monotopic anion 2 and cation 3 receptors was compared (Fig. 6). Control experiments revealed that no 1H NMR changes were observed when separated monotopic receptors 2 or 3 were treated with aqueous solution containing 100 eq of AcOK (see Fig. S46–S54†). However, when 50 eq of AcOK solution was extracted with equimolar mixture of anion 2 and cation 3 receptors (2.5 × 10−2 M) small alteration of 1H NMR spectrum was observed and signal of low intensity of the methyl group of the acetate anion was observed. Based on the integration value of this signal only approximately 12% of extraction efficiency was observed for the mixture of monotopic receptors.16 The efficiency of dual receptor system can be improved by increasing salt or receptors concentration. For example, when AcOKaq concentration is increased to 100 eq, transport of 27% of acetate is observed. However, under these extraction conditions the heteroditopic receptor 1 extracts quantitative amount of AcOK. Therefore, these experiments confirmed that proper covalent connection of binding domains in one heteroditopic receptor results in superior salt extractant compared to the mixture of monotopic receptors.
Then the receptor 1 was applied to the liquid–liquid extraction of potassium salt of one of the most popular nonsteroidal anti-inflammatory drug: ibuprofen (IbuOK). Extraction of aqueous solution containing 50 eq of IbuOK induced considerable changes in the 1H NMR spectrum of 2, similar to changes caused by acetate salt. Under this condition quantitative extraction of IbuOK was observed.
As ibuprofenate is more lipophilic anion than acetate, an attempt was made to reduce the salt concentration in the aqueous phase with preservation of extraction efficiency. Series of extraction experiments revealed that IbuOK can be extracted effectively from aqueous solutions containing as low as 0.25 eq of this compound. Thus this result is very promising for practical application of extracting and detecting active pharmaceutical ingredients.
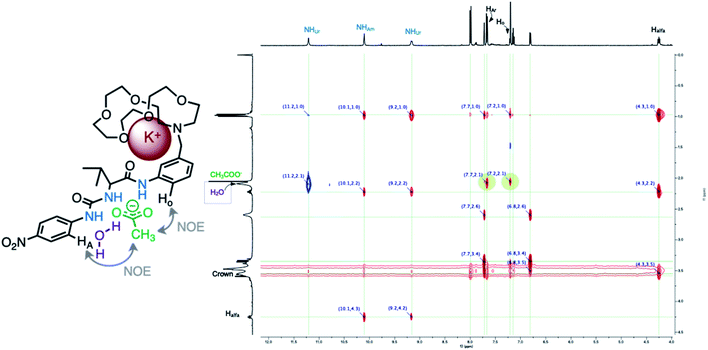
Finally, the solution structure of the complex of 1 and potassium acetate after extraction process was probed by means of 2D ROESY spectra. The through-space interactions show that all three NH-hydrogens point in the same direction. Cross-peaks between crown ether –O–CH2- methylene protons and meta-substituted aromatic ring protons as well as amino-acid alfa proton suggest that crown ether moiety is located above/below this aromatic ring (Fig. 7). To our delight, the intramolecular proton–proton contacts between acetate –CH3 group and aromatic protons HA (para-nitrophenyl ring) and Ho (meta-substituted aromatic ring) are observed. This indicates that acetate anion is positioned between these aromatic rings and the complex is strong enough to be preserved in the ROESY experiment time scale (∼200 ms). Interestingly, positive cross-peak is detected between aromatic urea NH and hydration water signal (blue cross-peak on Fig. 7). This cross-peak arises due to slow proton exchange between urea NH and complexed water molecule.17 This suggests that at least one “structural water” molecule links the acetate anion and receptor 1. Based on integration of water signal, total of five water molecules solvates 1·AcOK complex. This residual hydration may be crucial for stabilizing 1·AcOK complex and water molecule most probably bridges the co-bounded anion and cation.
The salt binding mode concluded from NMR experiments was supported by DFT-molecular calculations (see ESI†). The AcO− anion form H-bond with both urea NH as well as amide hydrogens, the average urea NH⋯O distance is 3.159 Å and angle 140°, the amide NH⋯O average distance is 3.48 Å and angle 155°. Potassium cation is positioned inside crown ether cavity (average K+⋯O distance is 2.8 Å) and interact with adjacent aromatic ring through cation π interaction (πAr⋯K+ distance is 2.871 Å).
Conclusions
In summary, non-multimacrocyclic heterotopic receptor 1 carefully designed to maximize carboxylate salt extraction was prepared in three simple synthetic steps. Analysis of UV-Vis and 1H NMR spectroscopic titrations conducted in water containing acetonitrile solutions revealed cooperative binding of potassium cation and acetate anion to the receptor 1. This effect is thought to help the receptor to overcome better salt and receptor hydration. The structure of ternary cation–receptor–anion complex was probed by means of 2D ROESY measurements and molecular calculations. When used as a extractant in liquid–liquid extraction process, the receptor 1 was capable of extracting hydrophilic AcOK salt from aqueous solution containing relatively low salt concentrations (∼1 M). In contrast, equimolar mixture of monotopic anion 2 and cation 3 receptors proved to be nearly ten times less effective. Finally, the receptor 1 could extract potassium ibuprofenate from relatively diluted aqueous solutions (CIbuOK = 6.2 mM). These results taken together will allow better understanding of the design criteria for construction of heterotopic salt receptors targeted at extraction of carboxylate salts.Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Aquilar and J. L. Cortina, Solvent Extraction and Liquid Membranes: Fundamentals and Applications in New Materials, CRC Press, Boca Raton, 2008 Search PubMed.
- N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038–8155 CrossRef CAS PubMed.
- U. Olsher, M. G. Hankins, Y. D. Kim and R. A. Bartsch, J. Am. Chem. Soc., 1993, 115(8), 3370–3371 CrossRef CAS.
- Q. He, G. I. Vargas-Zúñiga, S. H. Kim, S. K. Kim and J. L. Sessler, Chem. Rev., 2019, 119, 9753–9835 CrossRef CAS PubMed.
- (a) B. Qiao, A. Sengupta, Y. Liu, K. P. McDonald, M. Pink, J. R. Anderson, K. Raghavachari and A. H. Flood, J. Am. Chem. Soc., 2015, 137, 9746–9757 CrossRef CAS PubMed; (b) E. N. W. Howe, M. Bhadbhade and P. Thordarson, J. Am. Chem. Soc., 2014, 136, 7505–7516 CrossRef CAS PubMed; (c) L. K. S. von Krbek, C. A. Schalley and P. Thordarson, Chem. Soc. Rev., 2017, 46, 2622–2637 RSC; (d) C. Hunter, H. Anderson, Angew. Chem., Int. Ed., 2009, 48, 7488–7499 Search PubMed.
- (a) J. A. Cooper, S. T. G. Street and A. P. Davis, Angew. Chem., Int. Ed., 2014, 53, 5609–5613 CrossRef CAS PubMed; (b) H. Li, H. Valkenier, L. W. Judd, P. R. Brotherhood, S. Hussain, J. A. Cooper, O. Jurcek, H. A. Sparkes, D. N. Sheppard and A. P. Davis, Nat. Chem., 2016, 8, 24–32 CrossRef CAS PubMed; (c) S. J. Edwards, H. Valkenier, N. Busschaert, P. A. Gale and A. P. Davis, Angew. Chem., Int. Ed., 2015, 54, 4592–4596 CrossRef CAS PubMed.
- For receptors that binds salt very strongly under liquid–liquid extraction conditions binding kinetics may also be important factor determining transport activity. See ref. 6c..
- (a) J. M. Mahoney, G. U. Nawaratna, A. M. Beatty, P. J. Duggan and B. D. Smith, Inorg. Chem., 2004, 43, 5902–5907 CrossRef CAS PubMed; (b) S. K. Kim, J. L. Sessler, D. E. Gross, C.-H. Lee, J. S. Kim, V. M. Lynch, L. H. Delmau and B. P. Hay, J. Am. Chem. Soc., 2010, 132, 5827–5836 CrossRef CAS PubMed; (c) S. K. Kim, G. I. Vargas-Zúñiga, B. P. Hay, N. J. Young, L. H. Delmau, C. Masselin, C.-H. Lee, J. S. Kim, V. M. Lynch, B. A. Moyer and J. L. Sessler, J. Am. Chem. Soc., 2012, 134, 1782–1792 CrossRef CAS PubMed; (d) S. K. Kim, V. M. Lynch, N. J. Young, B. P. Hay, C.-H. Lee, J. S. Kim, B. A. Moyer and J. L. Sessler, J. Am. Chem. Soc., 2012, 134, 20837–20843 CrossRef CAS PubMed; (e) J. Romański and P. Piątek, J. Org. Chem., 2013, 78, 4341–4347 CrossRef PubMed; (f) Q. He, Z. Zhang, J. T. Brewster, V. M. Lynch, S. K. Kim and J. L. Sessler, J. Am. Chem. Soc., 2016, 138, 9779–9782 CrossRef CAS PubMed; (g) Q. He, G. M. Peters, V. M. Lynch and J. L. Sessler, Angew. Chem., Int. Ed., 2017, 56, 13396–13400 CrossRef CAS PubMed.
- M. Zakrzewski, N. Kwietniewska, W. Walczak and P. Piątek, Chem. Commun., 2018, 54, 7018–7021 RSC.
- G. Bellussi, M. Bohnet, J. Bus, K. Drauz, H. Greim, K. P. Jackel, U. Karst, A. Kleemann, G. Kreysa, T. Laird, W. Meier, E. Ottow, M. Roper, J. Scholtz, K. Sundmacher, R. Ulber and U. Wietelmann, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, 7th edn, 2011 Search PubMed.
- J. Corcoran, M. J. Winter and C. R. Tyler, Crit. Rev. Toxicol., 2010, 40, 287–304 CrossRef CAS PubMed.
- H. Maeda, S. Furuyoshi, Y. Nakatsuji and M. Okahara, Bull. Chem. Soc. Jpn., 1983, 56, 212–218 CrossRef CAS.
- M. Zakrzewski, D. Załubiniak and P. Piątek, Dalton Trans., 2018, 47, 323–330 RSC.
- UV-Vis titration technique is not suitable for determining such low Ka's, therefore these values should be treated with caution..
- M. Yizhaki, Ions in Water and Biophysical Implications: From Chaos to Cosmos, Springer Netherlands, 2012 Search PubMed.
- (a) K. Kavallieratos, A. Danby, G. J. Van Berkel, M. A. Kelly, R. A. Sachleben, B. A. Moyer and K. Bowman-James, Anal. Chem., 2000, 72, 5258–5264 CrossRef CAS PubMed; (b) S. J. Moore, M. G. Fisher, M. Yano, C. C. Tong and P. A. Gale, Chem. Commun., 2011, 47, 689–691 RSC; (c) S. J. Moore, M. G. Fisher, M. Yano, C. C. Tong and P. A. Gale, Dalton Trans., 2011, 40, 12017–12020 RSC; (d) I. Ravikumar, S. Saha and P. Ghosh, Chem. Commun., 2011, 47, 4721–4723 RSC.
- (a) N. V. Nucci, M. S. Pometun and A. J. Wand, J. Am. Chem. Soc., 2011, 133, 12326–12329 CrossRef CAS PubMed; (b) I. Deb, Ł. Popenda, J. Sarzyńska, M. Małgowska, A. Lahiri, Z. Gdaniec and R. Kierzek, Sci. Rep., 2019, 9, 16278 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra, Job's plots, UV-Vis titration spectra and the resulting binding isotherms, 1H NMR titration spectra and the resulting binding isotherms, and extraction experiment details. See DOI: 10.1039/d1ra00859e |
| This journal is © The Royal Society of Chemistry 2021 |