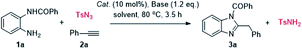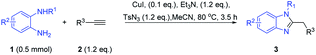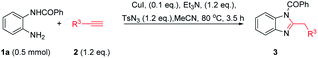Open Access Article
Open Access ArticlePreparation of 1,2-substituted benzimidazoles via a copper-catalyzed three component coupling reaction†
Weiguang Yang *abc,
Yu Zhaoa,
Zitong Zhoua,
Li Lia,
Liao Cuia and
Hui Luo*abc
*abc,
Yu Zhaoa,
Zitong Zhoua,
Li Lia,
Liao Cuia and
Hui Luo*abc
aGuangdong Key Laboratory for Research and Development of Natural Drugs, The Marine Biomedical Research Institute, Guangdong Medical University, Zhanjiang, 524023, China. E-mail: 09ywg@163.com; luohui@gdmu.edu.cn
bThe Marine Biomedical Research Institute of Guangdong Zhanjiang, Zhanjiang, Guangdong 524023, China
cSouthern Marine Science and Engineering Guangdong Laboratory (Zhanjiang), Zhanjiang, Guangdong 524023, China
First published on 25th February 2021
Abstract
1,2-Substituted benzimidazoles were prepared by simply stirring a mixture of copper catalysts, N-substituted o-phenylenediamines, sulfonyl azides and terminal alkynes. Particularly, the intermediate N-sulfonylketenimine occurred with two nucleophilic addition and the sulfonyl group was eliminated via cyclization. In a way, sulfonyl azides and copper catalysts activated the terminal alkynes to synthesize benzimidazoles.
Introduction
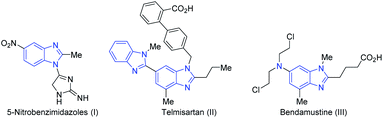
Owing to their diverse biological activity and clinical applications,1 benzimidazole derivatives are the potential candidates for a diverse set of biological activities including antiviral,2 antifungal,3 antibacterial,4 antiamoebic,5 anti-HIV,6 antiulcer,4,7 antihypertensive.8,9 One subset of such compounds are 1,2-substituted benzimidazole derivatives, such as 5-nitrobenzimidazoles (I) that exhibit antitumor activity against melanoma and breast cancer,10 telmisartan (II) that acts as AT1 receptor antagonists and tentative angiotensin receptor blocker therapeutic for COVID-19,11 and bendamustine (III) that acts as an antileukemia agent12 (Fig. 1). The observed activity depends upon the functional group attached to the moiety. In order to obtain novel effective chemotherapeutic agents, more synthetic methods and routes are required.Classical types of reactions have focused on the preparation of benzimidazole structural frameworks, such as metal catalysed reaction, metal-free catalysed/reagent-based reaction, green synthesis and photocatalyzed reaction.1a,1b The main synthesis reaction of benzimidazole drug candidates is the condensation of o-phenylenediamine with aldehydes, acyl chloride, carboxylic acids and esters.1a,1b However, most of these protocols suffer from strong acidic conditions (HCl, H2SO4, or polyphosphoric acid), readily oxidized or unstable substrate, or presence of numerous oxidative catalytic reagents. Therefore, a catalytic approach without using oxidant and stable substrate would overcome the above-mentioned disadvantages.
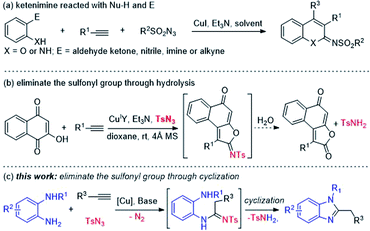
Previous studies reported that the multicomponent reactions (MCRs) of CuI-catalyzed terminal alkyne, sulfonyl azide, and nucleophiles13 were applied to synthesize numerous oxygen-containing and nitrogen-containing heterocyclic compounds.14 The ketenimine intermediate generated by copper-catalyzed terminal alkyne and sulfonyl azide could be take reaction simultaneous employing of pronucleophiles (Nu–H) and electrophiles (E) by designing the substrates. The o-hydroxy or o-amino electrophiles-containing benzene was the best strategy for the substrates, such as salicylaldehydes/o-hydroxyl-acetophenones,15 2-acetyl aniline,16 phenolic schiffs' bases,17 α-(ortho-hydroxyphenyl)-α,β-unsaturated ketones/o-hydroxy-phenylpropiolates,18 o-hydroxybenzonitrile19 (Scheme 1a) etc. However, two pronucleophiles (Nu–H) simultaneous nucleophilic addition with the ketenimine intermediate is rare.
The compounds of above MCRs contain the sulfonyl group, which is stable and difficult to eliminate. Only a few examples could eliminate the sulfonyl group, including the hydrolysis of N-sulfonyl imidates with catalytic amounts of DBU,19 or N-sulfonyl acetimid amide treated with 2% H2SO4 under reflux conditions.20 However, previous studies reported that N-sulfonyl imidates can be hydrolyzed through in situ generated H2O (Scheme 1b).21 Considering these facts, our study developed a novel strategy to eliminate the sulfonyl group through the power of cyclization reaction (Scheme 1c).
Results and discussion
We began our investigation by examining the synthesis of (2-benzyl-1H-benzo[d]imidazol-1-yl)(phenyl)methanone 3a via N-(2-aminophenyl)benzamide 1a, tosylazide and ethynylbenzene 2a. The reaction was carried out in the presence of CuI and Et3N in CHCl3 at 80 °C for 3.5 h, and 3a was isolated in 73% yield (Table 1, entry 1). Based on this finding, the reaction conditions were screened. Several other solvents were screened first, and a lower or comparable yield was obtained when toluene, THF, DMF, DCE were used as solvents, while the MeCN gave 3a the highest yield of 95% and the side product TsNH2 (Table 1, entries 2–6). Thus, the optimal solvent was determined to be MeCN. Encouraged by this promising result, numerous catalysts were screened. Among the copper catalysts used, most Cu-catalysts exhibited high catalytic reactivity in this reaction whether CuI-catalysts or CuII-catalysts (Table 1, entries 7–11). Other catalysts such as AgTFA failed to produce the desired product (Table 1, entry 12). The effects of different bases were evaluated. Screening results revealed that the use of Et3N achieved superior results than DMAP, DIPEA, tBuONa and other bases (Table 1, entries 13–16). When the reaction temperature was changed to 90 °C, the reaction yield decreased and produced side-products (Table 1, entries 17). It is worth noting that the other sulfonyl azides such as MsN3 or PhSO2N3 were also suitable for this reaction (Table 1, entries 18).| Entry | Cat. | Base | Solvent | Yieldb (%) 3a |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), Cat. (10 mol%), base (1.2 eq.) in the solvent (3 mL) was added TsN3 (1.2 eq.), 2a (1.2 eq.) stirring at 80 °C for 3.5 h.b Isolated yields.c nd = not detected the target product.d The reaction temperature was 90 °C.e MsN3 or PhSO2N3 was used instead of TsN3. | ||||
| 1 | CuI | Et3N | CHCl3 | 73 |
| 2 | CuI | Et3N | DCE | 90 |
| 3 | CuI | Et3N | Toluene | 31 |
| 4 | CuI | Et3N | MeCN | 95 |
| 5 | CuI | Et3N | THF | 84 |
| 6 | CuI | Et3N | DMF | 15 |
| 7 | CuBr | Et3N | MeCN | 92 |
| 8 | CuCl | Et3N | MeCN | 94 |
| 9 | CuBr2 | Et3N | MeCN | 90 |
| 10 | Cu(OAc)2 | Et3N | MeCN | 86 |
| 11 | Cu(OTf)2 | Et3N | MeCN | 82 |
| 12 | AgTFA | Et3N | MeCN | ndc |
| 13 | CuI | DMAP | MeCN | 15 |
| 14 | CuI | DIPEA | MeCN | 75 |
| 15 | CuI | Pyridine | MeCN | 43 |
| 16 | CuI | tBuONa | MeCN | 30 |
| 17 | CuI | Et3N | MeCN | 88d |
| 18 | CuI | Et3N | MeCN | 95e |
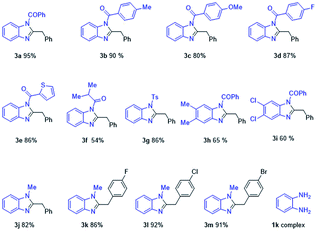
With the optimized reaction conditions obtained, the substrate diversity with the N-substituted o-phenylenediamines 1 were tested first. As shown in Table 2, the electron effects of the substituents R1 had slight influences. For example, substrates bearing 4-Me–C6H4CO–, 4-OMe–C6H4CO–, 4-F–C6H4CO–, and 2-thienyl–C6H4CO–groups were examined, and 90–86% yield of 3b–3e were obtained. The substrates R1 bearing the (CH3)2CHCO– and p-tosyl (Ts-) groups also can obtain 3f in moderate yield of 54% and 3g in good yield of 86%. Next, the scope and limitation of substrates R2 were examined by employing 3,4-dimethyl and 3,4-dichloro groups, which provided the corresponding benzimidazole derivatives, 3h and 3i, in moderate yield of 65% and 60%. It is noteworthy to mention that when R1 was changed for methyl instead of electron-withdrawing group acyl, the reaction also could smoothly obtain corresponding methyl-substituted products 3j–3m. However, interestingly, unsubstituted o-phenylenediamines 1k could not obtain the desired product and gave complex compounds.
| a Unless otherwise noted, the reaction conditions were as follow: 1 (0.5 mmol), CuI (10 mol%), Et3N (1.2 eq.) in the MeCN (3 mL) was added TsN3 (1.2 eq.), 2 (1.2 eq.) stirring at 80 °C for 3.5 h. |
|---|
 |
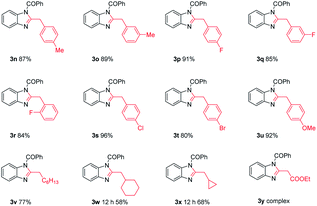
Finally, the scope and limitation of terminal alkynes 2 were examined. As shown in Table 3, the steric effects were clearly observed for two groups of products, namely 3n–3o and 3p–3r, in which both the substituents led to high yields and got influenced slightly. The electronic effects of substituents had an obvious impact on the efficiency of this transformation. The analogues R3 bearing an electron-withdrawing group (e.g., 4-Cl–C6H4– and 4-Br–C6H4–) and strong electron-donating group (e.g., –OMe) substituents produced a good yield of 3s, 3t and 3u. The aliphatic alkynes were also suitable for this reaction obtaining 3v, 3w, 3x in moderate yields of 77%, 58% and 68%, respectively. However, the other functional groups of terminal ynones such as the ethyl propiolate, propiolamide, propiolic acid made the reactions less effective, which obtained complex compounds or no corresponding desired products because the terminal ynones undergo self-condensation under the alkaline conditions.22
| a Unless otherwise noted, the reaction conditions were as follow: 1a (0.5 mmol), CuI (10 mol%), Et3N (1.2 eq.) in the MeCN (3 mL) was added TsN3 (1.2 eq.), 2 (1.2 eq.) stirring at 80 °C for 3.5 h. |
|---|
 |
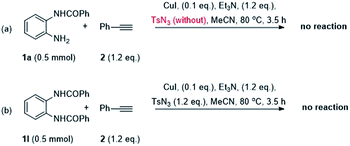
According to the above-mentioned experiments, there was no sulfonyl group in the target product and detected only the side product TsNH2. In addition, it could not obtain the desired product when test the unsubstituted o-phenylenediamines 1k (Table 2). To confirm the effects of tosylazide and elucidate the mechanism of eliminating the sulfonyl group, few control experiments were performed under the standard conditions. As shown in Scheme 2, the reaction of 1a and 2a, without tosylazide under the standard conditions was performed, and the corresponding products 3a failed to generate. Other test was carried out using the reactant of N,N′-(1,2-phenylene)dibenzamide 1l, which could not detect the target product 3a.
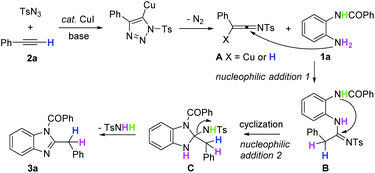
On the basis of these above experimental results, a possible reaction pathway for the synthesis of (2-benzyl-1H-benzo[d]imidazol-1-yl)(phenyl)methanone 3a was proposed (Scheme 3). According to the previous proposal, ketenimine A was generated first by the reaction of TsN3 and 2a. Then, similar to the published work by Wang,20 ketenimine A was attacked by the nucleophile to generate intermediate B. Subsequently, intermediate B underwent an intramolecular cascade addition to form intermediate C. At last, the desired product 3a and side product TsNH2 were obtained by the cyclization of intermediate C. Irrespective of change in the conditions, intermediates B and C could not be detected. Therefore, the procedure from B to 3a was fast and almost simultaneous. The sulfonyl group was eliminated via cyclization and activated the terminal alkynes to decompose into TsNH2 and N2.
Conclusions
We developed a novel and an effective three-component coupling approach to synthesize 1,2-substituted benzimidazoles in the presence of N-substituted o-phenylenediamines, terminal alkynes, copper catalyst and TsN3. TsN3 activated the terminal alkynes to generate ketenimine, took two nucleophilic addition in the process, and eliminated through cyclization. Nonetheless, we expect that this methodology could be applied to build more 1,2-substituted benzimidazole block facility.Experimental
General
All the melting points were determined on a Yanaco melting point apparatus and were uncorrected. IR spectra were recorded as KBr pellets on a Nicolet FT-IR 5DX spectrometer. All the spectra of 1H NMR (400 MHz) and 13C NMR (100 MHz) were recorded on a JEOL JNM-ECA 400 spectrometer in DMSO-d6 or CDCl3 (otherwise as indicated) with TMS was used as an internal reference and J values are given in Hz. HRMS were obtained on a Bruker micrOTOF-Q II spectrometer. All the o-phenylenediamines (1a–1j, see ESI section 1†) were prepared by previously reported methods.23Preparation and characterizations of compounds 3a–3x
The products 3b–3x were prepared by the similar procedure.
All the NMR spectra please see ESI section 3.†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support by Foundation and Applied Basic Research Fund project of Guangdong Province of China (2019A1515110918); Medical Scientific Research Foundation of Guangdong Province (A2020202); Science and Technology Planning Project of Zhanjiang (2019A01018); funds for PhD researchers of Guangdong Medical University in 2019 (B2019015); and the Science and Technology Program of Guangdong Province (2019B090905011).Notes and references
- (a) M. Faheem, A. Rathaur, A. Pandey, V. K. Singh and A. K. Tiwari, ChemistrySelect, 2020, 5, 3981 CrossRef CAS; (b) V. A. S. Pardeshi, N. S. Chundawat, S. I. Pathan, P. Sukhwal, T. P. S. Chundawat and G. P. Singh, Synth. Commun., 2021, 51, 485 CrossRef CAS; (c) A. D. Jordan, A. H. Vaidya, D. I. Rosenthal, B. Dubinsky, C. P. Kordik, P. J. Sanfilippo, W. N. Wu and A. B. Reitz, Bioorg. Med. Chem. Lett., 2002, 12, 2381 CrossRef CAS.
- (a) M. Tonelli, F. Novelli, B. Tasso, I. Vazzana, A. Sparatore, V. Boido, F. Sparatore, P. La Colla, G. Sanna, G. Giliberti, B. Busonera, P. Farci, C. Ibba and R. Loddo, Bioorg. Med. Chem., 2014, 22, 4893 CrossRef CAS; (b) M. Tonelli, M. Simone, B. Tasso, F. Novelli, V. Boido, F. Sparatore, G. Paglietti, S. Pricl, G. Giliberti, S. Blois, C. Ibba, G. Sanna, R. Loddo and P. La Colla, Bioorg. Med. Chem., 2010, 18, 2937 CrossRef CAS.
- (a) A. Ç. Karaburun, B. Kaya Çavuşoğlu, U. AcarÇevik, D. Osmaniye, B. N. Sağlık, S. Levent, Y. Özkay, Ö. Atlı, A. S. Koparal and Z. A. Kaplancıklı, Molecules, 2019, 24, 191 CrossRef; (b) Y. Shi, K. Jiang, R. Zheng, J. Fu, L. Yan, Q. Gu, Y. Zhang and F. Lin, Chem. Biodiversity, 2019, 16, e1800510 CrossRef; (c) N. T. Chandrika, S. K. Shrestha, H. X. Ngo and S. Garneau-Tsodikova, Bioorg. Med. Chem., 2016, 24, 3680 CrossRef CAS.
- (a) M. M. Sirim, V. S. Krishna, D. Sriram and O. Unsal Tan, Eur. J. Med. Chem., 2020, 188, 112010 CrossRef CAS; (b) A. M. Ganie, A. M. Dar, F. A. Khan and B. A. Dar, Mini-Rev. Med. Chem., 2019, 19, 1292 CrossRef CAS; (c) D. Song and S. Ma, ChemMedChem, 2016, 11, 646 CrossRef CAS; (d) R. Srivastava, S. K. Gupta, F. Naaz, A. Singh, V. K. Singh, R. Verma, N. Singh and R. K. Singh, Comput. Biol. Chem., 2018, 76, 1 CrossRef CAS; (e) Y. Bansal, M. Kaur and G. Bansal, Mini-Rev. Med. Chem., 2019, 19, 624 CrossRef CAS.
- (a) N. Bharti, Shailendra, M. T. Gonzalez Garza, D. E. Cruz-Vega, J. Castro-Garza, K. Saleem, F. Naqvi, M. R. Maurya and A. Azam, Bioorg. Med. Chem. Lett., 2002, 12, 869 CrossRef CAS; (b) N. Bharti, M. R. Maurya, F. Naqvi and A. Azam, Bioorg. Med. Chem. Lett., 2000, 10, 2243 CrossRef CAS.
- (a) T. Pan, X. He, B. Chen, H. Chen, G. Geng, H. Luo, H. Zhang and C. Bai, Eur. J. Med. Chem., 2015, 95, 500 CrossRef CAS; (b) S. M. Rida, S. A. El-Hawash, H. T. Fahmy, A. A. Hazzaa and M. M. El-Meligy, Arch. Pharmacal Res., 2006, 29, 826 CrossRef CAS.
- R. Radhamanalan, M. Alagumuthu and N. Nagaraju, Future Med. Chem., 2018, 10, 1805 CrossRef CAS.
- (a) M. T. Khan, M. T. Razi, S. U. Jan, M. Mukhtiar, R. Gul, IzharUllah, A. Hussain, A. M. Hashmi, M. T. Ahmad, N. A. Shahwani and I. Rabbani, Pak. J. Pharm. Sci., 2018, 31, 1067 CAS; (b) Y. Zhang, J. Xu, Y. Li, H. Yao and X. Wu, Chem. Biol. Drug Des., 2015, 85, 541 CrossRef CAS.
- (a) N. H. Bhuiyan, M. L. Varney, D. F. Wiemer and S. A. Holstein, Bioorg. Med. Chem. Lett., 2019, 29, 126757 CrossRef CAS; (b) N. Shrivastava, M. J. Naim, M. J. Alam, F. Nawaz, S. Ahmed and O. Alam, Arch. Pharm., 2017, 350 Search PubMed; (c) K. P. Barot, S. Nikolova, I. Ivanov and M. D. Ghate, Mini-Rev. Med. Chem., 2013, 13, 1421 CrossRef CAS.
- M. M. Ramla, M. A. Omar, A. M. El-Khamry and H. I. El-Diwani, Bioorg. Med. Chem., 2006, 14, 7324 CrossRef CAS.
- (a) R. P. Rothlin, H. M. Vetulli, M. Duarte and F. G. Pelorosso, Drug Dev. Res., 2020, 81, 768 CrossRef CAS; (b) K. J. McClellan and A. Markham, Drugs, 1998, 56, 1039 CrossRef CAS.
- (a) H. Mostafavi, M. R. Islami, E. Ghonchepour and A. M. Tikdari, Chem. Pap., 2018, 72, 2973 CrossRef CAS; (b) M. BogeljićPatekar, V. Milunović, K. MišuraJakobac, D. Perica, I. MandacRogulj, M. Kursar, A. Planinc-Peraica and S. OstojićKolonić, Acta. Clin. Croat., 2018, 57, 542 Search PubMed.
- (a) Review and Recent work: S. H. Kim, S. H. Park, J. H. Choi and S. Chang, Chem.–Asian J., 2011, 6, 2618 CrossRef CAS; (b) L. Xu, T. Zhou, M. Liao, R. Hu and B. Z. Tang, ACS Macro Lett., 2019, 8, 101 CrossRef CAS; (c) W. Yang, D. Huang, X. Zeng, J. Zhang, X. Wang and Y. Hu, Tetrahedron, 2019, 75, 381 CrossRef CAS; (d) W. Yang, D. Huang, X. Zeng, D. Luo, X. Wang and Y. Hu, Chem. Commun., 2018, 54, 8222 RSC; (e) M. Nallagangula and K. Namitharan, Org. Lett., 2017, 19, 3536 CrossRef CAS; (f) B. Reichart, G. Guedes de la Cruz, K. Zangger, C. O. Kappe and T. Glasnov, Adv. Synth. Catal., 2016, 358, 50 CrossRef CAS; (g) R. Kumar, S. H. Thorat and M. S. Reddy, Chem. Commun., 2016, 52, 13475 RSC; (h) D. Ramanathan and K. Pitchumani, J. Org. Chem., 2015, 80, 10299 CrossRef CAS; (i) Y. Xing, B. Cheng, J. Wang, P. Lu and Y. Wang, Org. Lett., 2014, 16, 4814 CrossRef CAS.
- S.-L. Cui, X.-F. Lin and Y.-G. Wang, Org. Lett., 2006, 8, 4517 CrossRef CAS.
- S.-L. Cui, J. Wang and Y.-G. Wang, Tetrahedron, 2008, 64, 487 CrossRef CAS.
- (a) Y. Shang, X. He, J. Hu, J. Wu, M. Zhang, S. Yu and Q. Zhang, Adv. Synth. Catal., 2009, 351, 2709 CrossRef CAS; (b) Y. Shang, K. Liao, X. He and J. Hu, Tetrahedron, 2013, 69, 10134 CrossRef CAS.
- Y. Shen, S. Cui, J. Wang, X. Chen, P. Lu and Y. Wang, Adv. Synth. Catal., 2010, 352, 1139 CrossRef CAS.
- F. Yi, S. Zhang, Y. Huang, L. Zhang and W. Yi, Eur. J. Org. Chem., 2017, 102 CrossRef CAS.
- E. J. Yoo, S. H. Park, S. H. Lee and S. Chang, Org. Lett., 2009, 11, 1155 CrossRef CAS.
- Y. Wang, J. She and Z. Jiang, Synlett, 2009, 12, 2023 CrossRef.
- D. Ramanathan, K. Namitharan and K. Pitchumani, Chem. Commun., 2016, 52, 8436 RSC.
- (a) C. Nájera, L. K. Sydnes and M. Yus, Chem. Rev., 2019, 119, 11110 CrossRef; (b) M. Nallagangula and K. Namitharan, Org. Lett., 2017, 19, 3536 CrossRef CAS; (c) C. Shao, Q. Zhang and G. Cheng, Eur. J. Org. Chem., 2013, 6443 CrossRef CAS; (d) P. V. Ramachandran, M. T. Rudd and M. V. R. Reddy, Tetrahedron Lett., 2005, 46, 2547 CrossRef CAS.
- M. Kortelainen, A. Suhonen, A. Hamza, I. Pápai, E. Nauha, S. Yliniemelä-Sipari, M. Nissinen and P. M. Pihko, Chem. - Eur. J., 2015, 21, 9493 CrossRef CAS.
- A. Puratchikody, G. Nagalakshmi and M. Doble, Chem. Pharm. Bull., 2008, 56, 273 CrossRef CAS.
- H. Jin, X. Xu, J. Gao, J. Zhong and Y. Wang, Adv. Synth. Catal., 2010, 352, 347 CrossRef CAS.
- A. Abbotto, S. Bradamante and G. A. Pagani, J. Org. Chem., 1996, 61, 1761 CrossRef CAS.
- J. She, Z. Jiang and Y. Wang, Synlett, 2009, 12, 2023 Search PubMed.
- X. Wu, A. K. Mahalingam and M. Alterman, Tetrahedron Lett., 2005, 46, 1501 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra00650a |
| This journal is © The Royal Society of Chemistry 2021 |