Open Access Article
Open Access ArticleFew-layer Bi2O2CO3 nanosheets derived from electrochemically exfoliated bismuthene for the enhanced photocatalytic degradation of ciprofloxacin antibiotic
Hangdao Qin ab,
Yingchang Yang
ab,
Yingchang Yang *a,
Wei Shia and
Yuanbin She
*a,
Wei Shia and
Yuanbin She b
b
aCollege of Material and Chemical Engineering, Tongren University, Tongren 554300, China. E-mail: yangyc612@gmail.com
bCollege of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310014, China
First published on 15th April 2021
Abstract
Few-layer two-dimensional (2D) Bi2O2CO3 nanosheets with a thickness of 4–5 nm were successfully fabricated via electrochemical exfoliation, followed by an exposure to ambient conditions. The formation process for these nanosheets was explored through ex situ X-ray diffractometer. The photocatalytic capacity of 2D Bi2O2CO3 nanosheets was investigated towards the degradation of ciprofloxacin. It was shown that 2D Bi2O2CO3 nanosheets exhibited better catalytic performance than Bi2O2CO3 nanoparticles synthesized by hydrothermal method under UV-Vis light irradiation. The enhanced photocatalytic activity is due to the larger specific surface area, as well as the lower band gap. Additionally, the radical trap experiments demonstrate that holes and hydroxyl radicals are of great importance in the degradation of ciprofloxacin. Finally, the 2D Bi2O2CO3 nanosheets show high stability in the photocatalytic degradation of ciprofloxacin, and could have a prospective application in the treatment of antibiotic wastewater.
1. Introduction
The removal of antibiotics from wastewater is significant since many antibiotics are refractory to biodegradation, and they will lead to the emergence of another global environmental pollution problem.1 Ciprofloxacin, as the second generation fluoroquinolone antibiotics, has been widely used in human treatment and animal husbandry.2 The release of ciprofloxacin into wastewater and its accumulation in the aquatic environment will bring the potential risk to aquatic ecosystems and human health.3 The development of new technologies for the degradation of such antibiotics into CO2, H2O, and other non-toxic small molecules is a great concern.Photocatalysis technology has proven to be an effective technology for the degradation of refractory organic compounds due to the highly reactive species generated in the interaction of the photocatalyst and UV light.4,5 The most widely used photocatalysts are TiO2 nanoparticles.6,7 However, due to their lower activity under visible light, many attempts have been made to look for alternatives. Recently, Bi-containing complex oxides have been found to be very effective in the degradation of organic contaminants under visible light irradiation.8,9 Among these oxides, Bi2O2CO3 has been studied by many researchers due to its layered structure, the ease of synthetic methods, the availability of precursors and nontoxic nature.10,11 Doping and coupling with other oxides can enhance the photocatalysis properties and efficiency of Bi2O2CO3.12,13 However, they will complicate the synthetics severely and increase the cost. Moreover, controlling the morphology of nanostructured Bi2O2CO3 seems to be a feasible way to improve the photocatalysis efficiency, as the morphology of nanomaterials has an impact on their catalytic performance. Bi2O2CO3 with different morphologies can be synthesized traditionally via solvothermal method by controlling the precursors, reactant ratios, and reaction temperature.14 However, to obtain ultrathin two-dimensional (2D) Bi2O2CO3 nanosheets with excellent photocatalytic activity is still a challenge. In 2013, Ag-doped Bi2O2CO3 microspheres comprising nanoplates (12 nm in thickness) were successfully fabricated through a hydrothermal method, and it showed improved photocatalytic activity towards methyl orange (MO) dye.15 Nevertheless, the synthesis process requires the addition of a polyvinylpyrrolidone template and KCl salt, and the loading of Ag nanoparticles. More recently, few-layer Bi2O2CO3 was fabricated through the electrochemical exfoliation of bulk Bi in 0.5 M Na2CO3 solution.16 This few-layer Bi2O2CO3 with a thickness of six atomic layers exhibits good activity and selectivity towards CO2 electro-reduction. In their results, Bi2O2CO3 was constructed through the reaction of oxidised exfoliated bismuth nanosheets with CO32− in the electrolyte. This method is facile and requires no surfactant. However, the Na+ intercalating agent used here is too small to exfoliate bismuth effectively, which will greatly reduce the synthesis efficiency.
In the current work, two-dimensional (2D) bismuthene nanosheets were fabricated through the electrochemical cathodic exfoliation of bulk Bi in a N,N-dimethylformamide solution of quaternary ammonium salt. These nanosheets were very unstable, even in ambient condition, as their ultrathin structures. When exposed to room air for a few days, they can be gradually transformed into ultrathin 2D Bi2O2CO3 nanosheets (4–5 nm in thickness) spontaneously. As a contrast, Bi2O2CO3 nanoparticles were synthesized by a traditionally hydrothermal method. The photocatalytic performances of these nanomaterials were evaluated in the photocatalytic degradation of ciprofloxacin in aqueous solution. To study the effect of the main active species in the degradation of ciprofloxacin, different scavengers were used to investigate the contribution of several active species. Furthermore, the stability of the as-prepared photocatalysts was evaluated.
2. Experimental
2.1. Reagents and materials
The commercial ciprofloxacin (PKU Healthcare Corp., Ltd.) was used without further purification. Lithium hexafluorophosphate (LiPF6, 98%), sodium hexafluorophosphate (NaPF6, 98%), tetramethylammonium hexafluorophosphate (TMAP, 99%), tetraethylammonium hexafluorophosphate (TEAP, 99%), tetrabutylammonium hexafluorophosphate (TBAP, 99%), tetraheptylammonium bromide (THAB, 99%), and N,N-dimethylformamide (DMF, 99.9%) were purchased from Alfa Aesar. Bismuth(III) nitrate pentahydrate ((BiNO3)3·5H2O), urea and anhydrous ethanol were obtained from Shanghai Sinopharm Chemical Reagent Co., Ltd. Titanium dioxide (TiO2, P25) was purchased from Shanghai Yien Chemical Technology Co., Ltd. Triethanolamine (TEA), p-benzoquinone (BQ) and tert-butylalcohol (t-BuOH) were purchased from Shanghai Macklin Biochemical Co., Ltd. All chemicals were at reagent or above purity, and were used without further purification. Deionized water was used throughout the experiments.2.2. Preparation of Bi2O2CO3
First, 2D bismuthene nanosheets were fabricated through electrochemically cathodic exfoliation of bulk Bi rod. The bulk bismuth rod (99.999%, 5 mm in diameter, 2 cm in length) and the platinum sheet (20 mm in length, 20 mm in width) were adopted as the cathode and anode, respectively. 0.5 M TEAP in DMF solution (50 mL) was utilized as the electrolyte. A direct current voltage of 6 V was applied for the exfoliation to proceed for 2 h. The black exfoliated bismuthene product was washed thoroughly with DMF and ethanol for six times, and dried in a vacuum oven at 60 °C. Then, the exfoliated 2D bismuthene was transformed into 2D Bi2O2CO3 (labelled as E-Bi2O2CO3) naturally when it was exposed to room temperature (25 °C). For comparison, Bi2O2CO3 nanoparticles (labelled as H-Bi2O2CO3) were obtained by a traditionally hydrothermal method.112.3. Characterization
The X-ray diffraction patterns of the samples were carried out on the Bruker X-ray diffractometer (XRD, D8 Advance) using Cu Kα. An Autosorb-iQA3200-4 sorption analyzer (Quantatech Co., USA) based on N2 adsorption/desorption was used to measure the Brunauer–Emmett–Teller specific (BET) surface area. The surface morphology and thickness of the Bi2O2CO3 samples were examined with a field emission scanning electron microscope (FESEM, Hitachi S4800), transmission electron microscope (TEM, JEM-2100F), and atomic force microscope (AFM, Dimension V, Veeco). The surface chemical valences of the Bi2O2CO3 catalyst were analysed with an ESCALAB 250 X-ray photoelectron spectroscope (XPS). The UV-visible diffuse reflectance spectra were measured with a Hitachi UV-3310. The photoluminescence (PL) spectra were collected on a Hitachi F4600 fluorescence spectrophotometer, using a Xe lamp (excitation at 365 nm) as the light source.2.4. Photocatalytic experiments
A homemade photocatalytic reactor equipped with a water-cooling device and a 300 W xenon lamp was utilized to detect the photocatalytic degradation of ciprofloxacin antibiotic. 10 mg L−1 ciprofloxacin solution (150 mL) was placed in the reactor, and then 150 mg of photocatalyst was added and stirred magnetically in the dark for 0.5 h to establish the adsorption equilibrium. The residual of ciprofloxacin after photocatalytic degradation for a certain time was taken out with a syringe, and centrifuged immediately for further concentration measurement with a high performance liquid chromatograph (Shimadzu Prominence LC-20AT, UV detector, C18 reverse phase column, 4.6 × 250 mm, 5 μm) under a wavelength of 278 nm. The mobile phase was an acetonitrile-ultrapure water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) mixed solution, and the flow rate was 0.8 mL min−1. The initial pH was adjusted by adding 0.1 mol L−1 HCl or 0.1 mol L−1 NaOH solution. The total organic carbon (TOC) was measured using a Shimadzu TOC-5000A analyser. The toxicity of ciprofloxacin and its degradation products was examined by plate count method using Escherichia coli as the reference microorganism.17
4, v/v) mixed solution, and the flow rate was 0.8 mL min−1. The initial pH was adjusted by adding 0.1 mol L−1 HCl or 0.1 mol L−1 NaOH solution. The total organic carbon (TOC) was measured using a Shimadzu TOC-5000A analyser. The toxicity of ciprofloxacin and its degradation products was examined by plate count method using Escherichia coli as the reference microorganism.17
3. Results and discussion
3.1. Preparation mechanism exploration
The stable form of bulk bismuth crystal in nature comprises parallel buckled rhombohedral layers (space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m).18 With a layered structure (Scheme 1), it provides a possibility for the intercalation of cations/anions and solvents, expansion, and exfoliation into few layers under the driven force. Herein, an electrochemical exfoliation strategy (shown in Scheme 1) was developed to peel off bulk bismuth into 2D bismuthene nanosheets in the DMF solution of (CH3CH2)4N+PF6− (TEAP). In pursuit of an ideal inserting cation, various ions with different sizes, including Li+, Na+, tetramethylammonium cation (TMA+), tetraethylammonium cation (TEA+), tetrabutylammonium cation (TBA+), and tetraheptylammonium cation (THA+) were examined. Alkali ions, such as Li+ or Na+, were expected to intercalate into bulk bismuth. However, they are too small (Li+ 0.9 Å in radius; Na+ 1.2 Å),19,20 and only intermetallic compounds (for example, Li3Bi and Na3Bi) would be formed when a negative potential of −6 V was applied for 2 h. These intermetallic compounds are stable in an inert environment, but they can be easily oxidized by proton solvents (e.g., H2O and C2H5OH). Therefore, the surface of bulk bismuth after being polarized for 2 h could be dispersed into tiny amounts of black Bi sols when placed into H2O or C2H5OH. In comparison with alkali ions such as Li+ and Na+, tetraalkylammonium cations usually display larger diameters (e.g., TMA+ 5.6 Å, TEA+ 6.7 Å, TBA+ 8.3 Å, and tetraheptylammonium bromide (THAB; d ≈ 20 Å)), and they are larger than the interlamellar space of bulk bismuth (3.95 Å).19,21 It is noted that only a thin layer of spongy black substance was generated on the surface of the bulk bismuth electrode when it was exfoliated in the electrolyte of TMA+ even under a highly negative potential of −10 V for 6 h, indicating that the TMA+ cations were not big enough to delaminate the bulk bismuth. Nevertheless, when a moderate voltage of −6 V was applied, the rapid delamination of bulk bismuth into dark sol, together with small amounts of bubbles, were observed within seconds in the electrolyte of TEA+, TBA+, or THA+. After exfoliation for 6 h, the dark sols were washed and further characterized.
m).18 With a layered structure (Scheme 1), it provides a possibility for the intercalation of cations/anions and solvents, expansion, and exfoliation into few layers under the driven force. Herein, an electrochemical exfoliation strategy (shown in Scheme 1) was developed to peel off bulk bismuth into 2D bismuthene nanosheets in the DMF solution of (CH3CH2)4N+PF6− (TEAP). In pursuit of an ideal inserting cation, various ions with different sizes, including Li+, Na+, tetramethylammonium cation (TMA+), tetraethylammonium cation (TEA+), tetrabutylammonium cation (TBA+), and tetraheptylammonium cation (THA+) were examined. Alkali ions, such as Li+ or Na+, were expected to intercalate into bulk bismuth. However, they are too small (Li+ 0.9 Å in radius; Na+ 1.2 Å),19,20 and only intermetallic compounds (for example, Li3Bi and Na3Bi) would be formed when a negative potential of −6 V was applied for 2 h. These intermetallic compounds are stable in an inert environment, but they can be easily oxidized by proton solvents (e.g., H2O and C2H5OH). Therefore, the surface of bulk bismuth after being polarized for 2 h could be dispersed into tiny amounts of black Bi sols when placed into H2O or C2H5OH. In comparison with alkali ions such as Li+ and Na+, tetraalkylammonium cations usually display larger diameters (e.g., TMA+ 5.6 Å, TEA+ 6.7 Å, TBA+ 8.3 Å, and tetraheptylammonium bromide (THAB; d ≈ 20 Å)), and they are larger than the interlamellar space of bulk bismuth (3.95 Å).19,21 It is noted that only a thin layer of spongy black substance was generated on the surface of the bulk bismuth electrode when it was exfoliated in the electrolyte of TMA+ even under a highly negative potential of −10 V for 6 h, indicating that the TMA+ cations were not big enough to delaminate the bulk bismuth. Nevertheless, when a moderate voltage of −6 V was applied, the rapid delamination of bulk bismuth into dark sol, together with small amounts of bubbles, were observed within seconds in the electrolyte of TEA+, TBA+, or THA+. After exfoliation for 6 h, the dark sols were washed and further characterized.
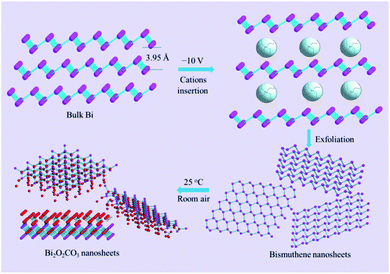 | ||
| Scheme 1 Scheme illustration for the synthesis of 2D Bi2O2CO3 nanosheets through electrochemical exfoliation, followed by exposure to ambient condition. | ||
The TEM images of the exfoliated Bi products in TBA+, THA+, and TEA+ solutions are shown in Fig. 1a–c, respectively. It is clear that both of the exfoliated Bi products in the electrolytes of TBA+ and THA+ display the features of Bi nanoparticles. Fig. 1c and d exhibit the typical TEM and HRTEM images of the exfoliated materials in the electrolyte of TEA+. The TEM image (Fig. 1a) shows that this exfoliated Bi product comprises ultrathin nanosheets. As can be seen in the HRTEM image (Fig. 1b), obvious lattice fringes with a space of 0.33 nm can be observed, which correspond to the (012) plane of the layered Bi crystal, confirming the formation of the 2D bismuthene nanosheets. These results clearly reveal that the TEA+ cation with a diameter of 6.7 Å is the best intercalating agent for the exfoliation of 2D bismuthene nanosheets. Interestingly, the as-exfoliated ultrathin 2D bismuthene nanosheets in the electrolyte of TEA+ are prone to oxidation even exposed to room air (Scheme 1), which provides a facile and feasible way for the synthesis of 2D Bi2O2CO3 at room temperature. It was found that the bismuthene nanosheets obtained in the electrolyte of TEA+ could be completely transformed into greyish white Bi2O2CO3 when exposed to room air for seven days, while it would take twenty days and a month for products to be exfoliated in the electrolyte of TBA+ and THA+, respectively.
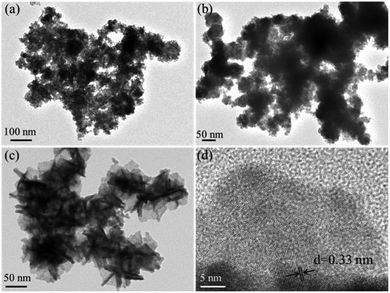 | ||
| Fig. 1 TEM images of the Bi product exfoliated in the electrolyte of TBA+ (a), THA+ (b), and TEA+ (c), and HRTEM image (d) of the exfoliated Bi product in the electrolyte of TEA+. | ||
Fig. 2a displays the XRD patterns of the as-exfoliated 2D bismuthene nanosheets and the samples that were exposed to room air for one day to seven days. The diffraction peaks for the as-exfoliated 2D bismuthene nanosheets at 22.3°, 27.1°, 37.8°, 39.5°, 48.7°, 55.9°, 64.5°, and 70.7° can be indexed to the planes of β-Bi (003), (012), (104), (110), (202), (024), (122), and (214), respectively, according to the XRD standard card for β-Bi (JCPDS card no. 44-1246).22 No diffraction peaks for other phases could be observed, illustrating that the pure material was obtained. When the 2D bismuthene sample was exposed to room air for one day to seven days, the typical characteristic diffraction peaks for bismuthene gradually disappeared, while the characteristic diffraction peaks for Bi2O2CO3 (JCPDS card no. 41-1488) increased steadily.11 Lastly, only the characteristic diffraction peaks for Bi2O2CO3 can be observed, indicating that the as-exfoliated 2D bismuthene was completely converted to Bi2O2CO3 after exposed to room air for seven days. It should be noted that increasing the temperature and air humidity could greatly reduce the reaction time.
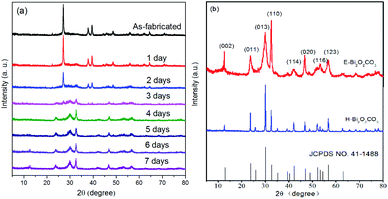 | ||
| Fig. 2 (a) XRD patterns of the as-exfoliated 2D bismuthene nanosheets, and the samples that were exposed to room air for one day to seven days; (b) XRD patterns of E-Bi2O2CO3 and H-Bi2O2CO3. | ||
3.2. Characterization of Bi2O2CO3
XRD patterns of the as-prepared E-Bi2O2CO3, H-Bi2O2CO3, and the PDF standard card of the layered Bi2O2CO3 are shown in Fig. 2b. The diffraction peaks for both samples can be clearly observed at 12.7°, 23.9°, 30.4°, 32.6°, 42.1°, 46.7°, 53.5° and 56.9°, which are consistent with the PDF standard card of tetragonal Bi2O2CO3 (JCPDS card no. 41-1488).11 Additionally, it can be found from Fig. 2b that the diffraction peaks of H-Bi2O2CO3 are sharper and stronger than those of E-Bi2O2CO3, indicating that the size of E-Bi2O2CO3 is smaller than that of H-Bi2O2CO3, according to Scherrer's equation. Fig. 3 exhibits the N2 adsorption–desorption isotherms, and the corresponding pore size distribution curves of E-Bi2O2CO3 and H-Bi2O2CO3. As can be seen, both samples exhibit the representative isotherms of type IV and hysteresis loop type H3 at high relative pressure (P/P0),23 illustrating the presence of mesopores and slit-like pores formed by the aggregated sheet-like particles.24,25 Moreover, it is seen that the area of the hysteresis loop for E-Bi2O2CO3 is much larger than that of H-Bi2O2CO3, indicating that the pore volume of E-Bi2O2CO3 is much higher than that of H-Bi2O2CO3, which can also been seen in the pore size distribution curves (Fig. 3b).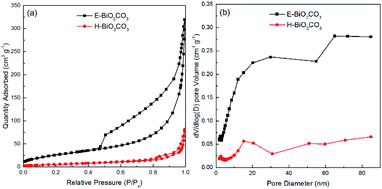 | ||
| Fig. 3 N2 adsorption–desorption isotherms (a), and the corresponding pore size distribution curves (b) of E-Bi2O2CO3 and H-Bi2O2CO3. | ||
Table 1 exhibits the textural parameters of the BET surface area (SBET) and pore volume for E-Bi2O2CO3 and H-Bi2O2CO3. It is clear that the SBET and pore volume of E-Bi2O2CO3 fabricated through electrochemical exfoliation followed by spontaneous oxidation here are much larger than those of H-Bi2O2CO3 synthesized via hydrothermal method, suggesting the advantage of this electrochemical method, which will greatly improve the photocatalytic performance of the photocatalyst.11 In comparison with the various structured Bi2O2CO3 materials prepared through hydrothermal method, the specific surface area of the few-layer E-Bi2O2CO3 nanosheets (97 m2 g−1) here is much larger than that of the flower-like Bi2O2CO3 (20.43 m2 g−1), sponge-like Bi2O2CO3 (50.60 m2 g−1) and plate-like Bi2O2CO3 (4.30 m2 g−1).26
| Samples | SBET (m2 g−1) | Pore volume (cm3 g−1) | Thickness (nm) | Band gap (eV) |
|---|---|---|---|---|
| E-Bi2O2CO3 | 97 | 0.49 | 4–5 | 2.95 |
| H-Bi2O2CO3 | 21 | 0.12 | — | 3.22 |
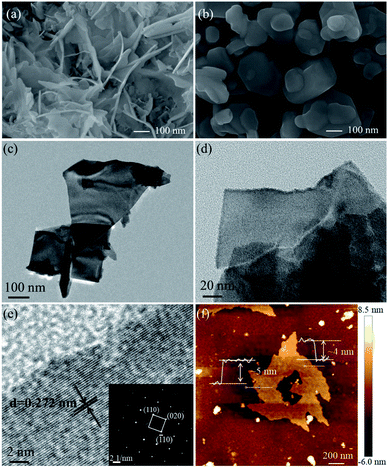
The SEM, TEM and AFM images of E-Bi2O2CO3 and H-Bi2O2CO3 are shown in Fig. 4. The SEM images in Fig. 4a and b indicate that E-Bi2O2CO3 consisted of well-defined nanosheets, and particles with other morphologies cannot be observed. In contrast, H-Bi2O2CO3 comprises irregular particles with different sizes. Moreover, it is seen from Fig. 4a and b that the size of E-Bi2O2CO3 is smaller than that of H-Bi2O2CO3, which is consistent with the result obtained by XRD. As shown in Fig. 4c–f, the morphology and microstructure of E-Bi2O2CO3 were further elucidated by TEM and AFM. The TEM images in Fig. 4c and d confirm that E-Bi2O2CO3 comprises ultrathin nanosheets. The HRTEM image (Fig. 4e) reveals a lattice spacing of 0.272 nm, agreeing well with the spacing of the (110) plane of the tetragonal Bi2O2CO3 crystal.27 In addition, the SAED pattern in the inset of Fig. 4e demonstrates an array of orderly diffraction spots indexed to the (110), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10) and (020) reflections, suggesting that the E-Bi2O2CO3 nanosheet is a well-defined single-crystal.27 Fig. 4f shows the AFM image of the E-Bi2O2CO3 nanosheet. As can be seen, the topographical profiles in Fig. 4f demonstrate that the thickness of the E-Bi2O2CO3 nanosheet is ca. 4.1–4.8 nm. According to the layer spacing of Bi2O2CO3 (∼0.68 nm), the layer number of E-Bi2O2CO3 can be assigned to be 6–7 layers.
10) and (020) reflections, suggesting that the E-Bi2O2CO3 nanosheet is a well-defined single-crystal.27 Fig. 4f shows the AFM image of the E-Bi2O2CO3 nanosheet. As can be seen, the topographical profiles in Fig. 4f demonstrate that the thickness of the E-Bi2O2CO3 nanosheet is ca. 4.1–4.8 nm. According to the layer spacing of Bi2O2CO3 (∼0.68 nm), the layer number of E-Bi2O2CO3 can be assigned to be 6–7 layers.
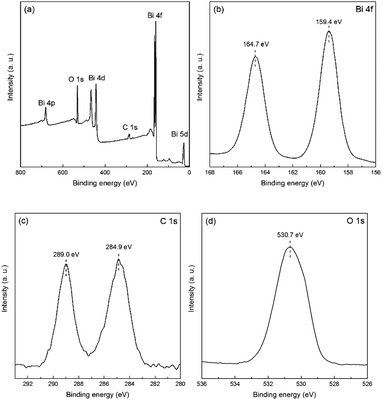
The surface composition and the chemical state of the as-fabricated few-layer E-Bi2O2CO3 nanosheets were further investigated with XPS. The survey XPS spectrum of E-Bi2O2CO3 shown in Fig. 5a suggests the presence of Bi, C and O elements. The semi-quantitative results indicate that the atomic percentage of C, O and Bi are 26.4%, 54.05% and 19.56%, respectively. The high-resolution spectra of Bi 4f, C 1s and O 1s are depicted in Fig. 5b–d, respectively. It can be clearly seen from Fig. 5b that there are two individual peaks at 164.7 eV and 159.4 eV, which can be ascribed to the binding energy of Bi 4f5/2 and Bi 4f7/2 states, respectively. The C 1s spectra display two clear peaks at 284.9 eV and 289.0 eV, which correspond to the adventitious hydrocarbon from the environment and the CO32− in the E-Bi2O2CO3 sample.28 The O 1s spectra are shown in Fig. 5d, the well-defined peak centred at 530.7 eV can be ascribed to the carbonate species and adsorbed water on the Bi2O2CO3 surface.28
As shown in Fig. 6a, the UV-Vis DRS of E-Bi2O2CO3 and H-Bi2O2CO3 nanomaterials were obtained through a UV-Vis spectrometer. It can be seen that the photoabsorption for both samples ranging from the UV light region to visible light are shorter than 450 nm. Additionally, steep absorption curves can be observed in both samples, exhibiting that the visible light absorption is ascribed to the band gap transition rather than the transition from the impurity level.26 Furthermore, E-Bi2O2CO3 shows an absorption edge at about 450 nm, while the absorption edge of H-Bi2O2CO3 blue-shifted to 390 nm, which is due to the E-Bi2O2CO3 fabricated through electrochemical exfoliation followed by spontaneous oxidation here is much thinner than that of H-Bi2O2CO3 synthesized via hydrothermal method.11 To measure the band gaps of the Bi2O2CO3, the equation αhν = A(hν − Eg)n/2 is applied,26 and the plot of (αhν)1/2 vs. energy (hν) is displayed in Fig. 6b. It is clear that the band gaps estimated from the onset of the absorption edge are about 2.95 eV and 3.22 eV for E-Bi2O2CO3 and H-Bi2O2CO3, respectively. Moreover, it was found that the band gap of the as-fabricated E-Bi2O2CO3 here is lower than that of the Bi2O2CO3 nanomaterials synthesized through hydrothermal method or solvothermal method.11,24,26 Furthermore, PL measurements can provide important information on the charge separation. As can be seen in Fig. 6c, the PL intensity of E-Bi2O2CO3 is lower than that of H-Bi2O2CO3, which implies that the ultrathin E-Bi2O2CO3 can significantly restrain the recombination of the electron–hole pairs.
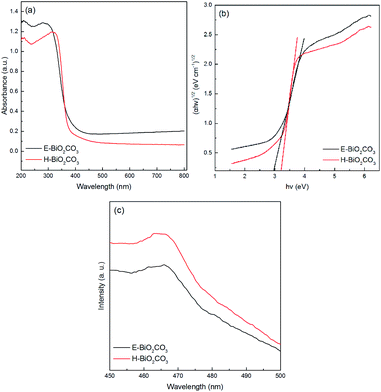 | ||
| Fig. 6 UV-Vis DRS (a), plots of the energy of absorbed light (b) and PL spectra (c) of E-Bi2O2CO3 and H-Bi2O2CO3. | ||
3.3. Photocatalytic degradation of ciprofloxacin
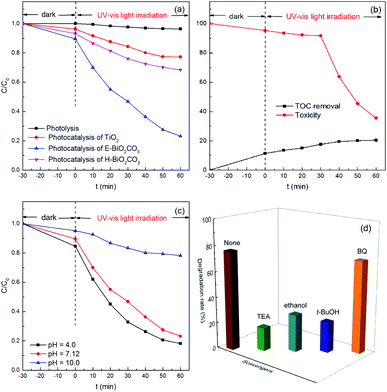
The photocatalytic performance of the Bi2O2CO3 nanomaterials was estimated in the decomposition of ciprofloxacin under UV-Vis light irradiation. The experimental parameters were set at a ciprofloxacin concentration of 10 mg L−1, pH value of 7.12, and catalyst dosage of 1.0 g L−1. As presented in Fig. 7a, a negligible removal of ciprofloxacin was observed in the absence of catalyst, which indicated that single photolysis was insufficient to degrade this antibiotic. Besides, TiO2 presented a limited catalytic activity in this study. However, E-Bi2O2CO3 exhibited the highest adsorption and degradation efficiency towards ciprofloxacin. After stirring for 30 min in the dark, 10.4% of ciprofloxacin was removed by the E-Bi2O2CO3 composite, which displayed enhanced adsorption capacity compared to H-Bi2O2CO3. The improvement could be attributed to the superior surface area (Table 1). After irradiation for 60 min with UV-Vis light, E-Bi2O2CO3 achieved 76.8% degradation of ciprofloxacin, which was remarkably high compared to that of H-Bi2O2CO3 (31.7%) and TiO2 (22.8%).Compared to H-Bi2O2CO3, E-Bi2O2CO3 showed better catalytic performance. The enhanced catalytic activity of E-Bi2O2CO3 may be ascribed to the following aspects. On the one hand, the specific surface area of E-Bi2O2CO3 was much larger than that of H-Bi2O2CO3 (Table 1). Ultrathin E-Bi2O2CO3 nanosheets could increase the contact area between the catalyst and antibiotic molecules. The good adsorption capacity of E-Bi2O2CO3 was also in favor of the reaction between the organics and active species on the surface of the catalyst.11,29 On the other hand, the band gap determined the spontaneity of a photocatalytic reaction.30,31 The band gap of E-Bi2O2CO3 was less than that of H-Bi2O2CO3 (Table 1), and therefore the photocatalysis over E-Bi2O2CO3 was more reactive under UV-Vis light irradiation.
Mineralization of ciprofloxacin by E-Bi2O2CO3 photocatalysis was estimated by measuring the TOC removal of the reaction solutions. As shown in Fig. 7b, although the removal rate of ciprofloxacin was nearly 76.8% (Fig. 7a), the TOC removal was only 20.5%. The results indicated that the aliphatic intermediates generated from the ring-opening reaction might be more resistant towards further mineralization. The toxicity at different reaction times was also presented in Fig. 7b, where the toxicity was almost unchanged in the first 30 min, and subsequently it was gradually decreased. At the end of 60 min treatment, the toxicity decreased to 35.5%, suggesting the effective elimination of the toxicity of ciprofloxacin and the intermediates by E-Bi2O2CO3 photocatalysis.
The influence of the initial pH on ciprofloxacin degradation was investigated. It can be observed from Fig. 7c that the ciprofloxacin decay was strongly pH-dependent. E-Bi2O2CO3 showed the highest photocatalytic activity at pH 4.0, while it presented the lowest photocatalytic performance at pH 10.0. These observations could be explained by the surface charge of ciprofloxacin, and E-Bi2O2CO3 would be greatly affected by the solution pH.17
To identify the active species during the photocatalytic degradation of ciprofloxacin over E-Bi2O2CO3, the trapping experiments were carried out by adding suitable radical scavengers. Undoubtedly, electrons (e−) and holes (h+) are the principal contributors to the generation of active species, and may therefore restrain the degradation of organic pollutants.32,33 In the trapping experiments, TEA and anhydrous ethanol were used as the scavengers for holes and electrons, respectively. It can be seen from Fig. 7d that the removal efficiency of ciprofloxacin decreased from 76.8% to 18.0% in the presence of TEA. The addition of ethanol also reduced the ciprofloxacin removal efficiency to 28.9%. Moreover, t-BuOH and BQ were used as the capture agents for hydroxyl radicals (˙OH) and superoxide radicals (˙O2−), respectively. As presented in Fig. 7d, only a loss of 5.4% was found for the ciprofloxacin degradation efficiency in the presence of the BQ scavenger, while the ciprofloxacin removal efficiency decreased from 76.8% to 23.8% with the t-BuOH scavenger. These results demonstrated that the existence of hydroxyl radicals, as well as holes in the system, were of great significance in the photocatalytic degradation of ciprofloxacin. However, superoxide radicals contributed the least for the ciprofloxacin degradation, which was in good agreement with the previous studies,34 such as the photodegradation of ciprofloxacin over the magnetic 3D γ-Fe2O3@ZnO core–shell catalyst and the heterogeneous Fenton degradation of three kinds of antibiotics using the magnetic core–shell MnFe2O4@C-NH2 catalyst.35
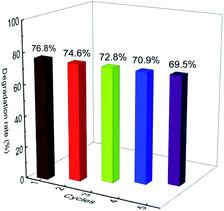
The reusability of the photocatalyst is significant to evaluate the potential of its practical application. Therefore, five consecutive cycles were performed, and the results are presented in Fig. 8. At the end of each experiment, the solid photocatalyst was filtered, washed with 50% ethanol water, and then dried in an oven at 60 °C overnight. The photocatalysis experiment was repeated with a ciprofloxacin concentration of 10 mg L−1 in the presence of the recycled catalyst at pH 7.12. It can be observed that the E-Bi2O2CO3 photocatalyst maintained a high photocatalytic activity even after the fifth cycle. No distinct deactivation was observed since there was only 7.3% loss of the ciprofloxacin degradation rate after the last cycling. A slight loss of the used catalyst was inevitable during the recovery process, which might be one of the reasons for the reduction of the photocatalysis activity. These results indicated that the E-Bi2O2CO3 photocatalyst possessed good stability and reusability.
4. Conclusions
Few-layer Bi2O2CO3 nanosheets (E-Bi2O2CO3) were successfully fabricated by a novel electrochemically cathodic exfoliation, followed by exposure to ambient condition. Compared to the Bi2O2CO3 nanoparticles synthesized by hydrothermal method (H-Bi2O2CO3), E-Bi2O2CO3 showed superior activity in the photocatalytic degradation of ciprofloxacin antibiotic under UV-Vis light irradiation. The enhanced catalytic performance of E-Bi2O2CO3 was due to the ultrathin structure, which enlarged the specific surface area and reduced the band gap. The trapping experiments confirmed that the holes, as well as hydroxyl radicals, were the major contributors for the ciprofloxacin degradation, while the superoxide radicals showed negligible contribution for ciprofloxacin decomposition. Finally, E-Bi2O2CO3 showed good reusability and had a bright application prospect in the treatment of antibiotic wastewater.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to convey their gratitude for the financial support from the National Natural Science Foundation of China (21805211, 51862033), Department of Education of Guizhou Province (QJHKYZ[2020]042, KY[2016]009), and the Science and Technology Foundation of Guizhou Province (JC[2017]1185, JC[2018]1164, ZC[2020]2Y037, PTRC[2017]5604, QKHJC-ZK[2021]YB071).Notes and references
- L. Wang, J. Yang, Y. Li, J. Lv and J. Zou, Chem. Eng. J., 2016, 284, 1058–1067 CrossRef CAS.
- N. S. Shah, J. Ali Khan, M. Sayed, Z. Ul Haq Khan, H. Sajid Ali, B. Murtaza, H. M. Khan, M. Imran and N. Muhammad, Chem. Eng. J., 2019, 356, 199–209 CrossRef CAS.
- A. S. Giri and A. K. Golder, J. Environ. Sci., 2019, 80, 82–92 CrossRef PubMed.
- S. Sood, S. K. Mehta, A. S. K. Sinha and S. K. Kansal, Chem. Eng. J., 2016, 290, 45–52 CrossRef CAS.
- Y. Zhao, X. Liang, Y. Wang, H. Shi, E. Liu, J. Fan and X. Hu, J. Colloid Interface Sci., 2018, 523, 7–17 CrossRef CAS PubMed.
- S. K. Kansal, S. Sood, A. Umar and S. K. Mehta, J. Alloys Compd., 2013, 581, 392–397 CrossRef CAS.
- A. H. Ali, S. Kapoor and S. K. Kansal, Desalin. Water Treat., 2011, 25, 268–275 CrossRef CAS.
- H. Cheng, B. Huang, P. Wang, Z. Wang, Z. Lou, J. Wang, X. Qin, X. Zhang and Y. Dai, Chem. Commun., 2011, 47, 7054–7056 RSC.
- Y. Liu, Z. Wang, B. Huang, X. Zhang, X. Qin and Y. Dai, J. Colloid Interface Sci., 2010, 348, 211–215 CrossRef CAS PubMed.
- T. Zhao, J. Zai, M. Xu, Q. Zou, Y. Su, K. Wang and X. Qian, CrystEngComm, 2011, 13, 4010–4017 RSC.
- Y. Liu, Z. Wang, B. Huang, K. Yang, X. Zhang, X. Qin and Y. Dai, Appl. Surf. Sci., 2010, 257, 172–175 CrossRef CAS.
- Y. Ao, L. Xu, P. Wang, C. Wang, J. Hou, J. Qian and Y. Li, Appl. Surf. Sci., 2015, 355, 411–418 CrossRef CAS.
- C. Yang, G. Gao, Z. Guo, L. Song, J. Chi and S. Gan, Appl. Surf. Sci., 2017, 400, 365–374 CrossRef CAS.
- G. Cheng, H. Yang, K. Rong, Z. Lu, X. Yu and R. Chen, J. Solid State Chem., 2010, 183, 1878–1883 CrossRef CAS.
- S. Peng, L. Li, H. Tan, Y. Wu, R. Cai, H. Yu, X. Huang, P. Zhu, S. Ramakrishna, M. Srinivasan and Q. Yan, J. Mater. Chem. A, 2013, 1, 7630–7638 RSC.
- Y. Zhang, X. Zhang, Y. Ling, F. Li, A. M. Bond and J. Zhang, Angew. Chem., Int. Ed., 2018, 57, 13283–13287 CrossRef CAS PubMed.
- M. Chen, J. Yao, Y. Huang, H. Gong and W. Chu, Chem. Eng. J., 2018, 334, 453–461 CrossRef CAS.
- M. Pumera and Z. Sofer, Adv. Mater., 2017, 29, 1605299 CrossRef PubMed.
- S. Yang, K. Zhang, A. G. Ricciardulli, P. Zhang, Z. Liao, M. R. Lohe, E. Zschech, P. W. Blom, W. Pisula and K. Müllen, Angew. Chem., 2018, 130, 4767–4771 CrossRef.
- F. Yang, F. Yu, Z. Zhang, K. Zhang, Y. Lai and J. Li, Chem.–Eur. J., 2016, 22, 2333–2338 CrossRef CAS PubMed.
- Z. Lin, Y. Liu, U. Halim, M. Ding, Y. Liu, Y. Wang, C. Jia, P. Chen, X. Duan, C. Wang, F. Song, M. Li, C. Wan, Y. Huang and X. Duan, Nature, 2018, 562, 254–258 CrossRef CAS PubMed.
- W. Hong, A. Wang, L. Li, T. Qiu, J. Li, Y. Jiang, G. Zou, H. Peng, H. Hou and X. Ji, Adv. Funct. Mater., 2020, 2000756 Search PubMed.
- H. Qin, R. Xiao, W. Shi, Y. Wang, H. Li, L. Guo, H. Cheng and J. Chen, RSC Adv., 2018, 8, 33972–33979 RSC.
- R. Wang, X. Li, W. Cui, Y. Zhang and F. Dong, New J. Chem., 2015, 39, 8446–8453 RSC.
- J. Zhang, J. Yu, Y. Zhang, Q. Li and J. R. Gong, Nano Lett., 2011, 11, 4774–4779 CrossRef CAS PubMed.
- Y. Zheng, F. Duan, M. Chen and Y. Xie, J. Mol. Catal. A: Chem., 2010, 317, 34–40 CrossRef CAS.
- F. Dong, J. Bian, Y. Sun, T. Xiong and W. Zhang, CrystEngComm, 2014, 16, 3592–3604 RSC.
- F. Dong, Y. Sun, M. Fu, W.-K. Ho, S. C. Lee and Z. Wu, Langmuir, 2012, 28, 766–773 CrossRef CAS PubMed.
- Y. Wang, H. Zhao, M. Li, J. Fan and G. Zhao, Appl. Catal., B, 2014, 147, 534–545 CrossRef CAS.
- S. Sood, A. Umar, S. Kumar Mehta and S. Kumar Kansal, Ceram. Int., 2015, 41, 3355–3364 CrossRef CAS.
- J. Hou, C. Yang, Z. Wang, S. Jiao and H. Zhu, Appl. Catal., B, 2013, 129, 333–341 CrossRef CAS.
- W. Li, D. Li, Y. Lin, P. Wang, W. Chen, X. Fu and Y. Shao, J. Phys. Chem. C, 2012, 116, 3552–3560 CrossRef CAS.
- R. Palominos, J. Freer, M. A. Mondaca and H. D. Mansilla, J. Photochem. Photobiol., A, 2008, 193, 139–145 CrossRef CAS.
- N. Li, J. Zhang, Y. Tian, J. Zhao, J. Zhang and W. Zuo, Chem. Eng. J., 2017, 308, 377–385 CrossRef CAS.
- H. Qin, H. Cheng, H. Li and Y. Wang, Chem. Eng. J., 2020, 396, 125304 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |