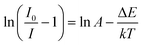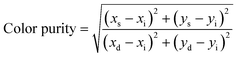DOI:
10.1039/D1RA00165E
(Paper)
RSC Adv., 2021,
11, 8282-8289
Structure and photoluminescence of Eu3+ doped Sr2InTaO6 red phosphor with high color purity†
Received
8th January 2021
, Accepted 6th February 2021
First published on 22nd February 2021
Abstract
The Eu3+-doped Sr2InTaO6 phosphors were obtained by solid-state reaction method and the phase purity of Sr2InTaO6:Eu3+ samples were investigated by the XRD patterns. The Rietveld refinements were conducted to further obtain crystal structure information and the results indicate Sr2InTaO6:Eu3+ samples crystallized in the monoclinic system with a P121 space group. The optical band gap of Sr2InTaO6 was calculated to be 3.84 eV by the diffuse reflectance spectra, which are consistent with the result (3.823 eV) of density functional theory. Under 396 nm excitation, the Sr2InTaO6:Eu3+ phosphor showed a red photoluminescence band centered at about 624 nm due to the 5D0 → 7F2 transition. Monitored at 624 nm, the phosphors showed two wide bands from 200 nm to 500 nm, which originated from the charge-transfer band of Eu3+–O2− and f–f transitions of Eu3+ ions. The optimum luminous concentration is 0.12 because of the concentration quenching determined to be a quadrupole–quadrupole interaction. The luminescence decay lifetimes of the Sr2InTaO6:Eu3+ phosphors were milliseconds. Significantly, the temperature-dependent emission spectra of Sr2InTaO6:0.12Eu3+ phosphors exhibited good thermal stability and the CIE chromaticity coordinates of Sr2InTaO6:0.12Eu3+ were (0.6265, 0.3693) with high color purity. The present work demonstrated that the Sr2InTaO6:Eu3+ red-emitting phosphors show great application in lighting technology.
1. Introduction
With the rapid advancement of contemporary society, sustainable development of energy and environmental issues has increasingly attracted extensive attention and become a significant matter for nowadays society. As is known to all, in human's daily work and life, the demand for lighting power is a very great percentage in the total power consumption. But the already invented traditional fluorescent and incandescent lamps have many shortcomings, for instance, high power consumption, short service lifespan, low energy conversion efficiency and environmental pollution, which are inconsistent with the purpose of power economy and environmental protection under the present circumstances.1–3 Over the years, luminescent materials doped with the rare-earth ions have attracted extensive attention, which has a wide range of applications in many fields especially such as a solid-state lighting system, medical treatment diagnosis, biological imaging, fiber amplifiers, solar cells, light-emitting diodes (LEDs) and so on.4–7 As a new type of solid luminescent material, white LEDs (WLEDs) have been proverbially put into use in various lighting devices on account of their superior luminescent features, such as, high fluorescent efficiency, energy conservation, light weight, long service lifetime, low heat and environmental friendliness.8,9 WLEDs are gradually replacing the traditional incandescent lamps as the next generation solid-state optical source.
Up to now, the most common form of exploiting WLEDs is the combination of blue LED chip coated with YAG:Ce3+ yellow fluorescent powder.10,11 Moreover, another way is by combining the ultraviolet (UV) chip or near-ultraviolet (NUV) chip with three basic colors of red, blue, and green (RGB) emitting materials to generate white light.12,13 While whereby, owing to the lack of efficient red emitting components, the white light would encounter low color rendering index (CRI) and high color temperature.14 Thus, it is of great significance to research the red-emitting phosphor with good spectral characteristics as novel, stable and high quantum efficiency. Until recently, red light emitting materials via doping rare-earth ions (Eu3+, La3+, Ce3+, Tb3+) and transition-metal ions (Mn4+, Cr3+, Pr3+) have aroused extensive attention on the basis of their potential applications in lighting and displays.15–17 Notably, trivalent rare-earth europium ions (Eu3+) is considered as an important red fluorescent activator which shows outstanding red emission ranging from 605 nm to 630 nm and higher quantum efficiency, corresponding to the 5D0–7F2 transition.18–20 Further exploration, the Eu3+ ions reveal pure magnetic and electric dipole transitions, which is the reason why it is a sensitive probe for the rare-earth ion site structure. As one kind of crucial light source, Eu3+ ion has been extensively studied in inorganic solid luminescence field, such as commercially available red-emitting phosphors YVO4:Eu3+, Y2O2S:Eu3+, Y2O3:Eu3+, as well as mainly reported materials LaAlGe2O7:Eu3+, SrGe4O9:Eu3+, Mg4GeO6:Eu3+, Ba2GeO4:Eu3+, Sr3La2(Ge3O9)2:Eu3+, Na4Y2Ge4O13:Eu3+ and so on.21–26 Until recently, the excellent performance of Eu3+-doped Sr2InTaO6 double perovskite red phosphors under UV or NUV excitation region has not been reported in the current study.
Hereby, we chose the double perovskite oxide Sr2InTaO6 as the host material in the present research. A cluster of Eu3+-doped red-emitting fluorescent powders were carefully depicted in this topic. Subsequently, the phase purity and crystal structure were studied by X-ray diffraction (XRD) and scanning electron microscope (SEM). In addition, the photoluminescence excitation (PLE), photoluminescence (PL), lifetime and temperature-dependent photoluminescence were systematically discussed. These results indicate that the synthesized sample Sr2InTaO6:Eu3+ in this paper is a potential red light emitting phosphor for applications of WLEDs.
2. Experimental section
2.1 Materials and syntheses
The Sr2InTaO6:xEu3+ (x = 0.04, 0.08, 0.12, 0.16, 0.20, 0.24) samples were fabricated via traditional high temperature solid state method. The Eu2O3 (99.99%), In2O3 (99.9%), Ta2O5 (99.5%) and SrCO3 (99.5%) are the raw materials. They are transferred to the crucible to pre-sinter at 600 °C for 6 h after weighting by a certain ratio relating to stoichiometry and mixing uniformly. Then the obtained samples were reground to further burn at 1300 °C for 6 h. Finally, the harvest Sr2InTaO6:Eu3+ samples were obtained to make further characterization and discussion.
2.2 Characterization
The XRD (D8 Advance, Bruker) was used to identify crystal structures of the sample by using Cu-Kα (λ = 0.15406 Å) radiation. The elementary compositions and morphology were measured by SEM (S-4800, Hitachi). Diffuse reflection spectra (DRS) were detected by the UV-Vis-NIR spectrophotometer (UH4150, Hitachi). The PL, PLE, temperature-dependent emission spectra and decay curves were precisely tested by the fluorescence spectrometer (JOBIN YVON FLuoroLog-3, HORIBA). The first principle calculation was executed by the Cambridge Sequential Total Energy Package (CASTEP) code.
3. Results and discussion
3.1 Crystal structures and phase analysis
The phase purity of Sr2InTaO6:Eu3+ samples were investigated by the XRD patterns. In Fig. 1, we presented the date of Sr2InTaO6:xEu3+ (x = 0.04, 0.08, 0.12, 0.16, 0.20, and 0.24) with a set of different Eu3+-doped concentrations in the 2θ range of 10°–80°. The detected diffraction peaks from the sample patterns exhibit similar shape and are in good agreement with the Joint Committee on Powder Diffraction Standard (JCPDS) card (No. 89-3092), even the concentration of Eu3+ is up to 0.24. This indicates the introduction of Eu3+ ions without influence the crystal structure and a pure phase is obtained for the synthesized material. With increasing Eu3+ doping concentration, the positions of the diffraction peaks are consistent with each other, which also illustrate Eu3+ ions have been successfully incorporated into this structure. When introducing Eu3+ into the Sr2InTaO6 host, the Eu3+ ions are be prior substitute for the In3+ ions owing to the similar ionic radii between Eu3+ (r = 0.947 Å, CN = 6) ions and In3+ (r = 0.800 Å, CN = 6).27
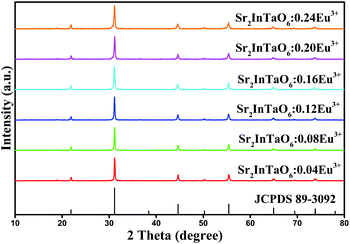 |
| | Fig. 1 XRD patterns of Sr2InTaO6:xEu3+ phosphors (x = 0.04, 0.08, 0.12, 0.16, 0.20, and 0.24) and the standard pattern for Sr2InTaO6 (JCPDS No. 89-3092). | |
In order to obtain more detailed information of crystal structure, the Rietveld refinement of Sr2InTaO6 was calculated using the general structure analysis system (GSAS) method and illustrated in Fig. 2(a). The solid red and black lines separately marked the theoretical calculation data and experimental observation pattern. The pink vertical bars refer to the sites of Bragg diffraction, and the blue line at the bottom represents the difference between theoretical and experimental data. In Table 1, more detailed crystal structure parameters of the Sr2InTaO6:0.12Eu3+ samples are provided and the reliability factors Rwp, Rp, χ2 were stable, which illustrate that the refinement results are reliable.28 From data analysis and comparison, the refinement results confirmed that the crystal belongs to the monoclinic system with a P121 space group and the cell parameters are calculated to be a = 5.74 Å, b = 5.75 Å, c = 8.12 Å, and α = γ = 90°, β = 89.96°. Moreover, the crystal structure of host Sr2InTaO6 and the around coordination environment of Sr, In and Ta atoms are also shown in Fig. 2(b). As can be seen from the diagram, each Sr atom is surrounded by eight nearest neighbor oxygen atoms, each In (or Ta) atom is surrounded by six oxygen atoms to constitute a distorted octahedron with an In–O bond length 2.112 Å and an Ta–O bond length 2.011 Å. These findings suggest that the sites of In3+ can be served as dissymmetric core for the doped ions and In3+ can be substituted by Eu3+ owing to their similar ionic radius.
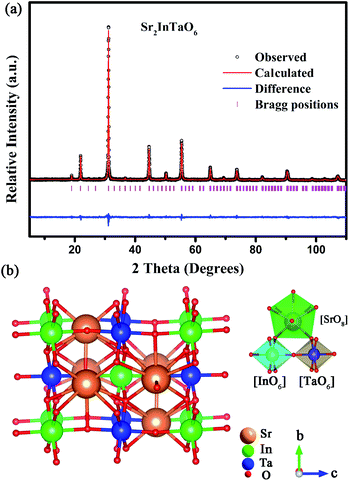 |
| | Fig. 2 (a) Rietveld refinement of Sr2InTaO6 host, (b) crystal structure of Sr2InTaO6. | |
Table 1 The crystal data and Rietveld refinement results for the Sr2InTaO6 hosta
| Atom |
X |
Y |
Z |
Occupancy |
Mult. |
| Space group: P121/n1; a = 5.74 Å, b = 5.75 Å, c = 8.12 Å, V = 268.103 Å3, α = γ = 90°, β = 89.96°, Rwp (%) = 12.1, Rp (%) = 8.94, χ2 = 1.43. |
| Sr |
0.50000 |
0.00015 |
0.25000 |
1.000 |
4 |
| In |
0.00000 |
0.00000 |
0.00000 |
0.500 |
2 |
| Ta |
0.00000 |
0.00000 |
0.50000 |
0.500 |
2 |
| O1 |
0.75023 |
0.76093 |
0.05344 |
1.000 |
4 |
| O2 |
0.74587 |
0.17600 |
0.01214 |
1.000 |
4 |
| O3 |
0.05325 |
0.12201 |
0.23431 |
1.000 |
4 |
More detailed morphological and compositional features of Sr2InTaO6:0.12Eu3+ were demonstrated through the SEM and the elemental mappings are shown in Fig. 3. It can be found that the phosphor is comprised of many agglomerate and irregular microparticles due to the high temperature reaction. And the size of Sr2InTaO6:0.12Eu3+ is about several micrometers indicating that these powders can satisfy the requirement of production.29 In addition, the elemental mapping confirms that these elements (Sr, In, Ta, Eu, and O) are homogeneously distributed, further authenticate that the Sr2InTaO6:Eu3+ phosphors with uniform composition were successfully synthesized.
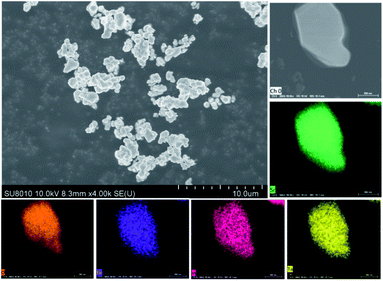 |
| | Fig. 3 The SEM diagrams of Sr2InTaO6:0.12Eu3+ phosphor and elemental mapping of the sample. | |
3.2 Electronic structure and optical band gap
In order to clarify the actual contribution on the photophysical properties of Sr2InTaO6, the first-principle theoretical calculations were carried out by the Vienna Ab initio Simulation Package (VASP).30,31 As shown in Fig. 4(a), the calculated bandgap is indirect and the calculated value is 3.823 eV. The electronic band structure and the corresponding projected density of states (PDOS) of Sr2InTaO6 host material and Sr2InTaO6:0.12Eu3+ were presented in Fig. 4(b) and S1 (ESI†). Further analysis of PDOS shows that the eigenstates around Fermi level (Ef) mainly attributes to the In and O atoms, whereas Sr and Ta atoms basically do not make contribution to the bands near Ef. This result indicates that the photophysical properties of Sr2InTaO6 are largely determined by the [InO6] unit.
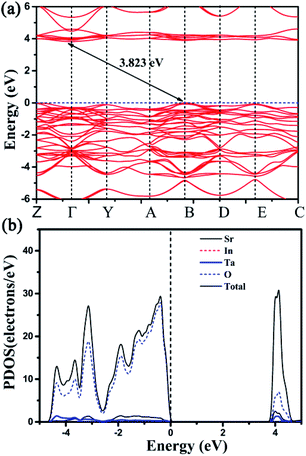 |
| | Fig. 4 (a) The calculated electronic band structure for host Sr2InTaO6. (b) The corresponding projected density of states of host Sr2InTaO6. | |
To verify the band gap experimentally, the DRS of Sr2InTaO6 host material and Sr2InTaO6:xEu3+ (x = 0.08, 0.12) samples are shown in Fig. 5. We can see that the samples show strong absorption in UV and NUV light region at about 260 nm to 350 nm. It can be ascribed to the charge-transfer band (CTB) of O2− to Eu3+, which is matching well with the excitation spectra.32 Two little faintly visible drops located at 394 nm and 468 nm are aroused from the typical f–f electron transitions of the Eu3+ ion.33 In summary, Sr2InTaO6:0.12Eu3+ showed a wide absorption band at the NUV region which indicated that these materials had applicability in the WLEDs applications. The band gap of these samples can be computed by Kubelka–Munk means via below formula:34
| | |
F(R∞hν)n = A(hν − Eg)
| (1) |
| | |
F(R∞) = (1 − R)2/2R = K/S
| (2) |
where
F(
R∞) is Kubelka–Munk function, ν denotes the photon energy,
h represents Plank constant,
A is the proportionality coefficient,
Eg denotes the bandgap value, and the index
n depends on the type of energy transition. The values of
n (2, 1/2, 3/2 or 3) are respectively for a direct transition, a indirect transition, a inhibitory direct transition and a inhibitory indirect transition.
35 The prepared fluorescent material is an indirect bandgap semiconductor as shown by previous theoretical calculation band gap value in
Fig. 4. So
n is equal to 1/2 in
eqn (1). The value of
Eg (∼3.84 eV) can be gained from linear extrapolation of [
F(
R∞)
hν]
2 = 0 (inset
Fig. 5), which is usually a little bit bigger than that calculated value of Sr
2InTaO
6 host (∼3.823 eV). And the plots for other
n indexes are also shown in Fig. S2 (ESI),
† It is attributed to the local density approximation (LDA), leading to an underestimate of the band gaps.
36
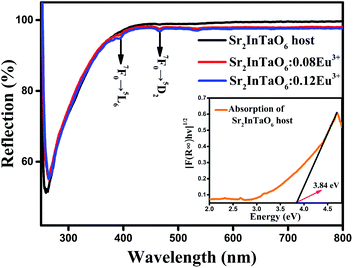 |
| | Fig. 5 Diffuse reflectance spectra of Sr2InTaO6:xEu3+ (x = 0, 0.08 and 0.12) samples. Inset: Absorption spectra of Sr2InTaO6 as calculated by the Kubelka–Munk function. | |
3.3 Detailed luminescence properties of Sr2InTaO6:Eu3+ phosphors
From the PLE spectra shown in Fig. 6(a), the first wide excitation peak from 200 to 300 nm with the largest value at approximately 246 nm is observed. It can be ascribed to the CTB from O2− to Eu3+. The other narrow excitation peaks centered at 363 nm, 384 nm, 394 nm, 417 nm, and 468 nm are respectively attributed to the characteristic 4f-4f transition (7F0–5D4, 7F0–5L7, 7F0–5L6, 7F0–5D3 and 7F0–5D2) of Eu3+.22,32 Moreover, the sharp excitation region at approximately 394 nm just yet located at the NUV region, confirming that the Sr2InTaO6:Eu3+ phosphors can be served as red fluorescent material. The PL spectra of Sr2InTaO6:xEu3+ with six doping concentrations (x = 0.04, 0.08, 0.12, 0.16, 0.20, 0.24) under 394 nm are shown in Fig. 6(b). Evidently, we can see that the shape and position of all samples exhibited similar emission profiles under different Eu3+ concentrations. At the excitation wavelength of 394 nm, the model exhibited a maximum emission at 624 nm, which is assigned to the 5D0 → 7F2 conversion of Eu3+.24,33 Additionally, the Fig. 6(b) reflected the PL strength of the sample. It was observed that the emission intensity of Eu3+ ions increases with the doping contents (x) increasing and reaches a maximum at x = 0.12, then it begins to decrease monotonously for extra Eu3+ concentration. That is to say, this phosphor appeared concentration quenching phenomenon.
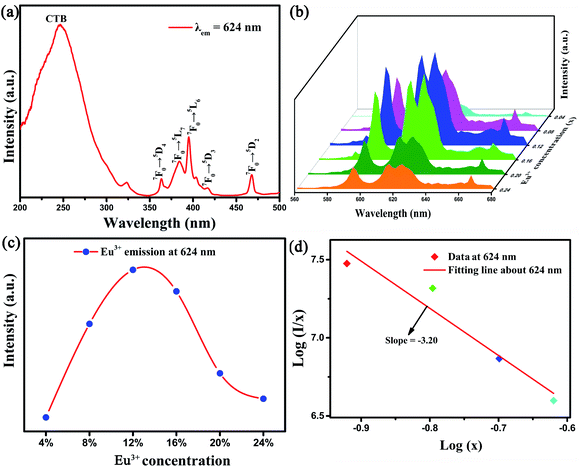 |
| | Fig. 6 (a) PLE of the Sr2InTaO6:0.12Eu3+ phosphor (b) PL of Sr2InTaO6:xEu3+ (x = 0.04, 0.08, 0.12, 0.16, 0.20, 0.24) samples. (c) The relationship of emission intensity and Eu3+ doping concentration. (d) Plot of log(I/x) versus log(x) for the 624 nm emission of Eu3+ ions in Sr2InTaO6 phosphors. | |
Concentration quenching effect is commonly ascribed to the energy transfer among the closest Eu3+–Eu3+ ions. Generally speaking, there are three ways to explain energy transfers respectively are multipole–multipole interaction, exchange interaction, and radiation reabsorption.37 To further investigate the concentration quenching effect among Eu3+ in Sr2InTaO6, the critical distance (Rc) between two activator ions (donor and acceptor) should be calculated to determine the way of energy transfer. The Rc was evaluated through this Blasse formula:38
| |
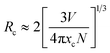 | (3) |
where
V refers to the volume of per crystal cell,
χc represents the doped-Eu
3+ critical concentration,
N denotes the number of replaced Eu
3+ ions each unit. In this case, the
V = 268.103 Å
3,
χc = 0.12 and
N = 2, separately. Consequently, the analyzed
Rc value of Eu
3+ is to be about 12.86 Å, which is greater than 5 Å. This revealed that the mode of energy transfer is mainly from electric multipolar interaction instead of the exchange interaction and radiation reabsorption. For the further investigation, the electric multipolar interaction is composed of three types: dipole–dipole (d–d), quadrupole–quadrupole (q–q), and dipole–quadrupole (d–q).
39 Based on the theory of Dexter, to further confirm the type of electric multipolar interaction in Eu
3+ ions, the equation as shown below is usually applied:
40| |
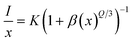 | (4) |
where
I is emission intensity for Sr
2InTaO
6:Eu
3+ phosphors,
x denotes activator concentration;
K is constant at identical excitation status. And
Q = 6, 8, 10 separately represent the d–d, d–q and q–q interaction. Subsequent analysis, the value of
Q can be gained by linear fitting with the correlation of log(
x) and log(
I/
x), as depicted in
Fig. 6(d). The detected slope is −3.20, then
Q = 9.6. The value is close to 10, suggesting the q–q interaction takes major responsibility for concentration quenching effect among Sr
2InTaO
6:Eu
3+ samples.
In order to further analyze the characteristics of fluorescence lifetime, as plotted in Fig. 7, the relaxation time at 624 nm of Sr2InTaO6:xEu3+ phosphors (x = 0.04, 0.08, 0.12, 0.16, 0.20, 0.24) under excitation 394 nm were carried out to research the luminescence dynamics. All decay curves are nicely matched with the double-exponential formula:41
| |
I(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/t1) + A2 exp(−t/t1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/t2) exp(−t/t2)
| (5) |
where
I denotes the luminescence strength,
A1 and
A2 refer to constants,
τ1 and
τ2 stand for the short and long lifetimes of exponential parts. Based on the fitting result shown in
Table 2, the lifetime was calculated to be 1.071, 0.970, 0.959, 0.926 and 0.919 ms following Eu
3+ doping ratio 0.04, 0.08, 0.12, 0.16 and 0.20, respectively. This result showed that the decay time declined from 1.071 to 0.919 ms as the Eu
3+ concentrations ascend which demonstrated by the nonradioactive energy transfer among Eu
3+.
42 The quantum efficiency (QE) of the Sr
2InTaO
6:0.12Eu
3+ sample measured at room temperature under excitation at 394 nm was presented in Fig. S3 (ESI),
† and the enlarged profile of the PL spectrum shown in the inset. The internal QE for the as-prepared sample is up to 58.4%, which is higher than that reported for several Eu
3+ doped oxide phosphors in the recent years, such as K
4BaSi
3O
9:Eu
3+ (QE: 27%), Na
3Sc
2(PO
4)
3:Eu
3+ (QE: 49%) and approaches the commercial standards for phosphor.
43,44 The high QE value implies that Sr
2InTaO
6:Eu
3+ is a promising phosphor material for application in WLEDs.
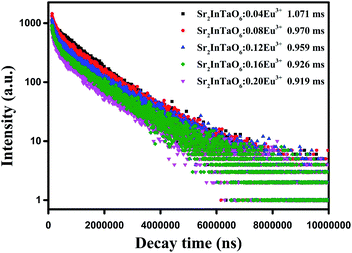 |
| | Fig. 7 The decay curves and calculated lifetimes of Sr2InTaO6:xEu3+ samples under 394 nm excitation and monitored at 624 nm. | |
Table 2 CIE chromaticity coordinates, color purity, and lifetimes of Sr2InTaO6:xEu3+ phosphors (0.04 ≤ x ≤ 0.24)
| Sr2InTaO6:xEu3+ |
x |
y |
Lifetime (ms) |
Color purity (%) |
| Sr2InTaO6:0.04Eu3+ |
0.6213 |
0.3741 |
1.071 |
93.8 |
| Sr2InTaO6:0.08Eu3+ |
0.6226 |
0.3729 |
0.970 |
96.1 |
| Sr2InTaO6:0.12Eu3+ |
0.6265 |
0.3693 |
0.959 |
96.4 |
| Sr2InTaO6:0.16Eu3+ |
0.6190 |
0.3761 |
0.926 |
95.7 |
| Sr2InTaO6:0.20Eu3+ |
0.6177 |
0.3773 |
0.919 |
96.0 |
| Sr2InTaO6:0.24Eu3+ |
0.6213 |
0.3741 |
1.071 |
93.8 |
Further study, the other important index to evaluate phosphor is the thermal stability. The temperature-dependent emission spectra of Sr2InTaO6:0.12Eu3+ samples were analyzed and shown in Fig. 8(a). It can be observed that the coordinate of emission peaks basically remained unchanged, but the emission strength gradually declined with the increment of temperature ranging from 298 to 573 K. This can be ascribed to the striking thermal quenching effect. Fig. 8(b) shows the trend about the normalized temperature-dependent emission intensity more clearly. It can be seen that the luminous intensity at 423 K decreased to 66.4% of the value at 298 K. Also, upon cooling the temperature from 573 to 298 K, the luminescence intensity at 423 K remained about 64.2% of that at the initial temperature, as presented in Fig. S4 (ESI).† These results demonstrate the prepared phosphors have good heat resistance.
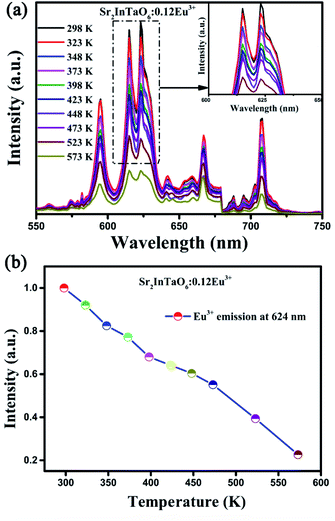 |
| | Fig. 8 (a) Temperature-dependent emission spectra of Sr2InTaO6:0.12Eu3+ samples. (b) Normalized emission intensity of Sr2InTaO6:0.12Eu3+ phosphors at different temperatures from 298 to 573 K. | |
Remarkably, the reason of thermal quenching can be explained from two aspects. One is notably ascribed to the nonradioactive transition from the excited states to ground state. The other one is the thermal excited ionization effect from 5d orbits to the charge band.45 To better understand the reduced luminous intensity, the activation energy (ΔE) as performed to illustrate the thermal quenching effect as shown in Fig. 9. The following Arrhenius formula is adopted to calculate the ΔE of thermal quenching:46
| |
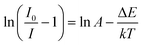 | (6) |
where
I0 represents the emission strength at initial temperature,
I denotes the strength at operated temperature
T,
k represents Boltzmann's constant with value of 8.69 × 10
5 eV K
−1, Δ
E refers to the activation energy. From
Fig. 9, the correlation between ln[(
I0/
I) − 1] and 1/
kT was well linear matched with a slope factor of −0.206, so the Δ
E for the thermal quenching equals to 0.206 eV. The comparatively higher Δ
E proved that the prepared materials had good thermal stability.
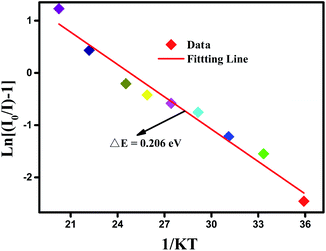 |
| | Fig. 9 The plot of ln(I0/I − 1) versus 1/kT at the corresponding temperature. | |
The color-coordinate is another important index to evaluate the performance of fluorescent materials. Therefore, according to the PL spectra, the Commission Internationale de L'Eclairage (CIE) coordinate for Eu3+-doped Sr2InTaO6 was calculated and shown in Table 2. As shown in Fig. 10, the CIE coordinates of the Sr2InTaO6:0.12Eu3+ phosphor is (0.6265, 0.3693), which are closed to the standard red chromaticity (0.67, 0.33) of National Television Standard Committee (NTSC) system.47 When illuminated with the 365 nm UV lamp, the prepared samples showed efficient red color (as shown inset). It revealed that the red-emitting phosphor Sr2InTaO6:Eu3+ is a potential candidate for display in WLEDs. Moreover, the coordinates are at the edge of the CIE diagram, demonstrating that the phosphors show good color purity. The color purity can be estimated on the basis of the following equation:48
| |
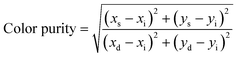 | (7) |
where
xs and
ys denote the color coordinates;
xi and
yi are the illuminant point of the 1931 CIE Standard Source C;
xd and
yd refer to color coordinates of the dominant wavelength. In this work, the (
xs,
ys), (
xi,
yi), and (
xd,
yd) are (0.6265, 0.3693), (0.310, 0.316), and (0.6322, 0.3767), respectively. The color purity of the Sr
2InTaO
6:0.12Eu
3+ phosphors is 96.4%, which indicates that the Eu
3+-activated Sr
2InTaO
6 samples have good CIE chromaticity coordinate and high color purity.
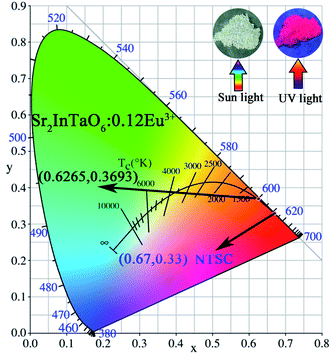 |
| | Fig. 10 CIE chromaticity coordinates diagram of Sr2InTaO6:0.12Eu3+ phosphors. | |
4. Conclusions
In Summary, series of Eu3+-doped Sr2InTaO6 double perovskite red phosphors were successfully obtained by solid state reaction method. The XRD, Rietveld refinement and SEM were executed to confirm the crystal structure and morphology information. Optical energy band gap of the host material Sr2InTaO6 was calculated by first-principles DFT with the VASP, which was consistent with the result of DRS. The PL, PLE and temperature-related emission spectra were systematically investigated to explain the advantage of doping Eu3+ ion. The optimal doping concentration value of Sr2InTaO6:xEu3+ is about 0.12 and the cause of concentration quenching can be ascribed to the q–q interaction. The decay curves of Sr2InTaO6:xEu3+ phosphors monitored at 394 nm were both displayed double exponential behavior and the lifetimes decreased from 1.071 to 0.919 ms with the increase of the Eu3+ doping concentration. Subsequently, temperature-dependent luminescence intensity spectra of Sr2InTaO6:0.12Eu3+ were also executed, in which the luminescence intensity reduced to 66.4% at 423 K and thus confirmed very good thermal stability. The mechanism of thermal quenching is explained from the non-radiative transition. In summary, the results indicated that Sr2InTaO6:Eu3+ phosphors have potential applications in the applications of WLEDs.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 21576002), Natural Science Foundation of Shandong Province, China (No. ZR2019PEM006), National College Students' Innovation and Entrepreneurship Training program (No. 201814276002).
Notes and references
- P. Pattison, J. Tsao, G. Brainard and B. Bugbee, Nature, 2018, 563, 493–500 CrossRef CAS.
- Z. G. Xia and Q. L. Liu, Prog. Mater. Sci., 2016, 84, 59–117 CrossRef CAS.
- K. Saidi and M. Dammak, RSC Adv., 2020, 10, 21867–21875 RSC.
- E. F. Schubert and J. K. Kim, Science, 2005, 308, 1274–1278 CrossRef CAS.
- P. Push, P. J. Schmidt and W. Schnick, Nat. Mater., 2015, 14, 454 CrossRef.
- Z. Xia and A. Meijerink, Chem. Soc. Rev., 2017, 46, 275–299 RSC.
- H. Guo, Z. G. Zheng, L. M. Teng, R. F. Wei and F. F. Hu, J. Lumin., 2019, 213, 494–503 CrossRef CAS.
- L. Wang, R. J. Xie, T. Suehiro, T. Takeda and N. Hirosaki, Chem. Rev., 2018, 118, 1951–2009 CrossRef CAS.
- F. W. Kang, G. H. Sun, P. Boutinaud, F. Gao, Z. H. Wang, J. Lu, Y. Y. Li and S. S. Xiao, J. Mater. Chem. C, 2019, 7, 11041 RSC.
- R. Kasuya, T. Isobe and H. Kuma, J. Alloys Compd., 2006, 408, 820–823 CrossRef.
- Q. Li, L. Gao and D. Yan, Mater. Chem. Phys., 2000, 64, 41–44 CrossRef CAS.
- D. J. Liu, X. H. Yun, G. G. Li, P. P. Dang, M. S. Molokeev, H. Z. Lian, M. M. Shang and J. Lin, Adv. Opt. Mater., 2020, 2001037 CrossRef CAS.
- Z. F. Yang, C. J. Ji, G. N. Zhang, G. M. Han, H. Wang, H. X. Bu, X. Tan, D. H. Xu and J. Y. Sun, J. Lumin., 2019, 206, 585–592 CrossRef CAS.
- X. X. Ma, L. B. Liao, Q. F. Guo, H. K. Liu, D. Yang, N. Liu and L. F. Mei, RSC Adv., 2019, 9, 35717–35726 RSC.
- P. Q. Cai, L. Qin, C. L. Chen, J. Wang, S. Bi, S. I. Kim, Y. L. Huang and H. J. Seo, Inorg. Chem., 2018, 57, 3073–3081 CrossRef CAS.
- D. P. Cui, Z. Song, Z. G. Xia and Q. L. Liu, J. Lumin., 2018, 199, 271–277 CrossRef CAS.
- D. J. Liu, X. H. Yun, P. P. Dang, H. Z. Lian, M. M. Shang, G. G. Li and J. Lin, Chem. Mater., 2020, 32, 3065–3077 CrossRef CAS.
- A. J. Fu, A. X. Guan, F. F. Gao, X. S. Zhang, L. Y. Zhou, Y. B. Meng and H. M. Pan, Opt. Laser Technol., 2017, 96, 43–49 CrossRef CAS.
- S. J. Mofokeng, L. L. Noto and M. S. Dhlamini, J. Lumin., 2020, 228, 117569 CrossRef CAS.
- C. Yue, D. C. Zhu, Q. Yan and Y. Pu, RSC Adv., 2020, 9, 26364–26372 RSC.
- K. Uematsu, A. Ochiai and K. Toda, J. Alloys Compd., 2006, 408, 860–863 CrossRef.
- J. K. Han, G. A. Hirata and J. B. Talbot, Mater. Sci. Eng., 2011, 176, 436–441 CrossRef CAS.
- S. Ekambaram, K. C. Patil and M. Maaza, J. Alloys Compd., 2005, 393, 81–92 CrossRef CAS.
- C. R. Garcia, J. Oliva and G. A. Hirata, Ceram. Int., 2017, 43, 12876 CrossRef CAS.
- S. C. Lal, V. Lalan and G. Subodh, Inorg. Chem., 2018, 57, 6226–6236 CrossRef CAS.
- F. X. Liu, Y. Z. Fang, J. S. Hou, N. Zhang and Z. F. Ma, Ceram. Int., 2014, 40, 3237–3241 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., 1976, 32, 751–767 CrossRef.
- A. R. Sharits, J. F. Khoury and P. M. Woodward, Inorg. Chem., 2016, 55, 12383–12390 CrossRef CAS.
- Y. Hua, W. Ran and J. S. Yu, J. Alloys Compd., 2020, 835, 155389 CrossRef CAS.
- Z. F. Yang, L. L. Yang, C. J. Ji, D. H. Xu, C. Q. Zhang, H. X. Bu, X. Tan, X. Y. Yun and J. Y. Sun, J. Alloys Compd., 2019, 802, 628–635 CrossRef CAS.
- D. Y. Huang, P. P. Dang, Y. Wei, B. Bai, H. Z. Lian, Q. G. Zeng and J. Lin, Mater. Res. Bull., 2020, 124, 110743 CrossRef CAS.
- P. P. Du, Q. H. Meng, X. J. Wang, Q. Zhu, X. D. Li, X. D. Sun and J. G. Li, Chem. Eng. J., 2019, 375, 121937 CrossRef CAS.
- Z. Jia, X. L. Zhang, X. Y. Hua, Y. Dong, H. L. Li, C. Q. Feng, Y. G. Wang and M. J. Xia, J. Alloys Compd., 2020, 844, 155875 CrossRef CAS.
- W. Soller, Phys. Rev., 1924, 24, 158–167 CrossRef CAS.
- Z. G. Xia, Z. H. Xu, M. Y. Chen and Q. L. Liu, Dalton Trans., 2016, 45, 11214–11232 RSC.
- G. B. Nair, H. C. Swart and S. J. Dhoble, Prog. Mater. Sci., 2020, 109, 100622 CrossRef CAS.
- M. F. Joubert, A. Remillieux, B. Jacquier, J. Mugnier, B. Boulard, O. Perrot and C. Jacoboni, J. Non-Cryst. Solids, 1995, 184, 341–345 CrossRef CAS.
- G. Blasse, J. Solid State Chem., 1986, 62, 207–211 CrossRef CAS.
- Z. F. Yang, D. H. Xu, J. N. Du, X. D. Gao and J. Y. Sun, RSC Adv., 2016, 6, 87493–87501 RSC.
- D. L. Dexter and J. H. Schulman, J. Chem. Phys., 1954, 22, 1063 CrossRef CAS.
- C. Wei, D. H. Xu, J. Li, A. C. Geng, X. Li and J. Y. Sun, J. Mater. Sci.: Mater. Electron., 2019, 30, 2864–2871 CrossRef CAS.
- X. Geng, Y. Xie, Y. Y. Ma, Y. Y. Liu, J. M. Luo, J. X. Wang, R. J. Yu, B. Deng and W. M. Zhou, J. Alloys Compd., 2020, 847, 156249 CrossRef CAS.
- D. Stefanska, M. Stefa nski and P. J. Deren, Opt. Mater., 2014, 37, 410–413 CrossRef CAS.
- H. Guo, X. Huang and Y. Zeng, J. Alloys Compd., 2018, 741, 300–306 CrossRef CAS.
- J. Y. Sun and D. P. Cui, J. Am. Ceram. Soc., 2014, 97, 843 CrossRef CAS.
- S. Bhushan and M. V. Chukichev, J. Mater. Sci. Lett., 1988, 9, 319–321 CrossRef.
- S. J. Wang, C. C. Xu and X. B. Qiao, Ceram. Int., 2020, 47, 1063–1075 CrossRef.
- H. Guo, X. Huang and Y. Zeng, J. Alloys Compd., 2018, 741, 300–306 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra00165e |
|
| This journal is © The Royal Society of Chemistry 2021 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Xuefei Caoa,
Lining Hea,
Zaifa Yang
a,
Xuefei Caoa,
Lining Hea,
Zaifa Yang *a and
Jiayue Sunc
*a and
Jiayue Sunc



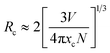
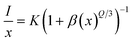
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/t1) + A2
exp(−t/t1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/t2)
exp(−t/t2)