Open Access Article
Open Access ArticleThe role and effectiveness of monoculture and polyculture phytoremediation systems in fish farm wastewater†
Yin Sim Ng and
Derek Juinn Chieh Chan
and
Derek Juinn Chieh Chan *
*
School of Chemical Engineering, Universiti Sains Malaysia, 14300 Nibong Tebal, Penang, Malaysia. E-mail: chderekchan@usm.my
First published on 13th April 2021
Abstract
Phytoremediation offers a sustainable solution to aquaculture pollution, but studies with critical evaluations of the treatment performances of macrophyte systems are limited. This study intended to evaluate the roles and treatment profiles of Spirodela polyrhiza (L.) Schleid. and Lemna sp. systems in terms of ammonia, nitrate, nitrite, phosphate (NH3–N, NO3−–N, NO2−–N, PO43−), chemical oxygen demand (COD), turbidity, and total suspended solids (TSS) on fish farm wastewater and to elucidate the rationale behind the removal of the pollutants and the changes in a raceway pond rig. The nitrogen and phosphorus removal in the Spirodela polyrhiza monoculture system outperformed the other configured systems. An 81% reduction in ammonia (to 3.90 mg of NH3-N/L), and sharp declines of up to 75%, 88%, and 71% in TSS, turbidity, and COD levels were recorded within two days, while significant decreases in nitrate, nitrite, and phosphate levels were observed. This indicated that the system could inhibit nitrate and nitrite spikes in waters (nitrification) via reducing the available ammonia and limiting subsequent nitrite and nitrate conversion, while reducing TSS in algal-bloom wastewater via shading. High biomass productivity and superior protein content were observed in the macrophyte systems (S. polyrhiza + Lemna sp. polyculture system), with up to 112% and 12% increases, respectively. This study demonstrated that the S. polyrhiza monoculture system is effective at treating fish farm wastewater, lowering the levels of relevant inorganic and organic pollutants, and it could be used as a biofilter for natural waters, preserving the existing ecology.
1. Introduction
Most commercial fish raising is done in an intensive manner,1 with relatively high fish stocking and feeding rates, to ensure a high yield of fish and the growth of the fish to marketable size.2 Nevertheless, this increases the waste load of the water, leading to effluents with greater pollution potential.2 Water from fish husbandry tends to be saturated with wastes. Uneaten feed and fish faeces are the main sources of suspended solids, with each making up approximately 30% in an intensive aquaculture unit.3 Of the feed ingested by the fish, 20 to 30% of the nitrogen and 25 to 35% of the phosphorus in the feed is assimilated, while the remaining nutrients are released as waste into the water.4 In addition, the inefficient application of pond fertilisation can result in excessive nutrients in the water, uncontrolled algal blooms and increased suspended solids in the pond effluent.2 Subsequently, the decomposition of organic solids and dead algae can induce anoxic conditions in the water, which are detrimental to the fishes and aquatic life within.Frequent water exchange is therefore required to prevent fish kills and maintain fish health in the ponds, as water exchange removes toxic fish metabolites, excess algae and other wastes and simultaneously introduces oxygen into the aquaculture unit.4 The effluent from the water exchange must then be adequately treated to reduce its adverse effects towards the environment and downstream users. Inland aquaculture mainly utilises treatment technologies from conventional wastewater treatment,5 and thus huge energy requirements, sludge generation, and regular maintenance are unavoidable.6 Moreover, the adsorbents/coagulants used for treatment may not be suitable due to harmful residues, high cost and reduced treatment capacity.7 The available biological treatment systems are only designed to achieve secondary treatment standards, and the nitrogen and phosphorus levels after treatment are not low enough to be discharged into water.8,9 Therefore, further units are needed to treat nutrient-rich wastewater.8 Advanced nutrient removal technologies incur hefty costs, significant carbon footprints and high energy demand.10 On the other hand, phytoremediation represents a good option in the treatment of fish farm wastewater due to its relatively high nutrient removal performance, affordable implementation (less equipment11 and only a simple containment system are needed), minimal maintenance costs (solar-powered)12 and low energy requirements. The removal of nitrogen and phosphorus pollutants from the wastewater by the macrophytes can range from 35–98% and 45–99%, respectively, in a non-axenic phytoremediation system, including in wastewater contaminated with antibiotics, which represents a recent hot topic in wastewater study.13–16 The nutrient-richness and low toxicity of fish farm wastewater to the plants would make the growth of macrophytes and phytoremediation possible. In addition to nutrient removal, the macrophytes could simultaneously remediate other pollutants in the wastewater, such as antibiotics, heavy metals, pesticides and hormones.11 A phytoremediation system can be established without large capital outlays. It is cheaper than conventional treatment methods that rely on electricity, pumping, aeration or the addition of chemicals, which usually require large concrete/steel vessels. Since it utilises naturally occurring plants/macrophytes for remediation and is powered by sunlight, phytoremediation is sustainable and less harmful, can reduce the carbon footprint of conventional treatment systems, and does not require farmers to sacrifice much of their revenue to operate the treatment system. In addition, it is aesthetically pleasing and could provide ecological landscapes for rehabilitated areas.11 The harvested macrophytes could also potentially be used for agricultural applications such as fertiliser, compost, mulching, weed control, supplementary food for fish and poultry17,18 or biofuels19 and become an extra source of income for the farmers.
Bashyal20 studied macrophyte systems in monoculture and polyculture configurations for treating effluents; the polyculture system could induce a synergistic effect on wastewater treatment, but not many related studies were conducted. Pretreated wastewater is normally used for phytoremediation studies, but experiments using raw wastewater are limited.21,22 Additionally, data regarding phytoremediation (macrophyte) systems with a complete array of pollutant treatment profiles for fish farm wastewater and an assessment of the degree of macrophyte performance in treatment, accompanied by thorough analysis and detailed inference of the nitrogen, phosphate, and suspended solids removal, oxygen demand, and water clarity changes, as well as subsequent correlations between the tested water quality profiles are very much limited. Therefore, this research aims to address these concerns.
In this study, two of the best performing macrophytes, namely Spirodela polyrhiza (L.) Schleid. and Lemna sp., from the previous study of Ng and Chan23 were configured into monoculture and polyculture systems to evaluate their respective roles and treatment performances and profiles in treating raw fish farm wastewater in a raceway pond rig. The ammonia (NH3–N), nitrate (NO3−–N), nitrite (NO2−–N), phosphate (PO43−), total suspended solids (TSS), turbidity and chemical oxygen demand (COD) removal from the wastewater were determined throughout the study. The changes in the biomass, total carbohydrate and protein contents of the configured systems were assessed at the end of the study. The rationale behind the changes in the water quality parameters in this study was explained based on the obtained profiles. This data will reveal the fate, transformation and removal of nitrogen, phosphate and suspended solids from the fish farm wastewater by the macrophyte systems as well as indicate the causes/entities that contribute to the removal. It also provides information on the rate and degree of pollutant removal that can be achieved using the configured systems, their ability to achieve the discharge limit, and the parameters that could be further tuned and optimised in the macrophyte system to ensure high performance treatment of the studied pollutants. The best macrophyte system for fish farm wastewater can be determined and chosen for further study, experimentation or scale-up before going into final implementation and practice.
2. Materials and methods
2.1. Source of fish farm wastewater
The fish farm wastewater was collected from freshwater catfish farming ponds in Tanjong Piandang, Perak, Malaysia. The farm covers an area of approximately 3 ha with 19 ponds (approximately 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 m2). The wastewater was taken from four different ponds in which schools of catfish were observed, and water exchange was not carried out at the moment of collection; instead, the wastewater was pooled together before use to limit the water quality variation due to the age of the fish, amount of uneaten feed remaining, amount of fish excretion and faeces and the water exchange rate of the respective ponds. The quality of the fish farm wastewater is shown in ESI Table S1.† The wastewater had a relatively high ammonia level (22.61 ± 0.95 mg L−1), but low nitrate and nitrite levels. Its initial TSS, turbidity and COD values were high as well at 126 ± 10 mg L−1, 151.15 ± 17.41 mg L−1 and 185 ± 19 mg L−1, respectively, while the phosphate level was determined to be 3.26 ± 0.40 mg L−1. The recorded pH of the wastewater was 7.58 ± 0.06.
450 m2). The wastewater was taken from four different ponds in which schools of catfish were observed, and water exchange was not carried out at the moment of collection; instead, the wastewater was pooled together before use to limit the water quality variation due to the age of the fish, amount of uneaten feed remaining, amount of fish excretion and faeces and the water exchange rate of the respective ponds. The quality of the fish farm wastewater is shown in ESI Table S1.† The wastewater had a relatively high ammonia level (22.61 ± 0.95 mg L−1), but low nitrate and nitrite levels. Its initial TSS, turbidity and COD values were high as well at 126 ± 10 mg L−1, 151.15 ± 17.41 mg L−1 and 185 ± 19 mg L−1, respectively, while the phosphate level was determined to be 3.26 ± 0.40 mg L−1. The recorded pH of the wastewater was 7.58 ± 0.06.
2.2. Plant stock establishment
The macrophytes, namely, Lemna sp. and S. polyrhiza, were collected locally from water pathways at a rural area near Kerian District, Perak, Malaysia. The aseptic cultures were established according to the procedure described by Ng and Chan23 and maintained in liquid Hoagland no. 2 medium with 15 g L−1 sucrose. All cultures were then incubated at 29 ± 1 °C under fluorescent light (1500 lux) with a 16 h light:8 h dark photoperiod for 14 days.2.3. Experimental set up of the raceway pond rig system
This study was carried out in a bench-scale raceway pond rig system adapted from Ng and Chan24 with dimensions of 50 cm × 25 cm × 9 cm inside the laboratory under a controlled environment (Fig. 1(a)). 12 L of fish farm wastewater was used for the setup. A submerged pump was used to circulate the wastewater in the raceway pond rig system, which was equipped with a RMA-34-SSV flowmeter (Dwyer Instruments, USA), at a flow rate of 50 mL min−1. The rig was covered with light-absorbing material to prevent the excessive growth of algae. Four identical rigs were set up, each with different treatment conditions: one as the control (without macrophytes), one for the Spirodela polyrhiza monoculture system, another for the Lemna sp. monoculture system, and one for the polyculture system (S. polyrhiza + Lemna sp.), as shown in Fig. 1(b)–(d). Each treatment run was carried out with 72 g (fresh weight) of macrophytes distributed evenly over the wastewater surface in the raceway pond. For the polyculture system, 36 g of S. polyrhiza and 36 g of Lemna sp. were loaded onto the wastewater surface of the raceway pond, with each species occupying half of the total surface area of the pond, separated by a mesh (Fig. 1(d)).The phytoremediation study was carried out at 29 ± 1 °C under fluorescent tubes (1500 lux) with a 16 h light:8 h dark photoperiod for 14 days. A water sample (150 mL) was collected at the outlet of the pond rig every two days starting on day 0 until the end of the experiment. The water samples were analysed for ammonia (NH3–N), nitrate (NO3−–N), nitrite (NO2−–N), phosphate (PO43−), chemical oxygen demand (COD), turbidity and total suspended solids (TSS) to determine the water quality of the fish farm wastewater during the phytoremediation period. Duplication was done for the experiments. On day 14, the macrophytes from each treatment (except for the control) were harvested and blotted to determine their fresh weight. The biomasses were then subjected to biochemical analysis to evaluate their carbohydrate and protein contents.
2.4. Analytical analysis
The carbohydrate was determined through the colorimetric method described by Dubois et al.26 using sucrose as the standard. First, 0.1 mL of the supernatant was transferred into a test tube with a micropipette. Distilled water was added to make up a 2 mL solution. 1 mL of 5% phenol was added to the solution, followed by 5 mL of concentrated sulphuric acid (95%). The test tube was allowed to stand for 10 minutes at room temperature before being placed in a water bath for 15 minutes at 30 °C. The absorbance of the solution was read at 490 nm with a Shimadzu UVmini-1240 spectrophotometer, Japan. The amount of carbohydrate present was determined by reference to a sucrose standard curve constructed through a series of dilutions.
The protein was determined through the test tube procedure of the bicinchoninic acid (BCA) method outlined in the Thermo Scientific™ Pierce™ BCA Protein Assay Kit instructions with bovine serum albumin (BSA) being used as the standard. First, 0.1 mL of the supernatant was transferred into a test tube with a micropipette. 2 mL of BCA working reagent was added to the tube and mixed well with the solution. The test tube was covered and incubated in a water bath at 37 °C for 30 minutes. The tube was cooled to room temperature before the absorbance of the solution was read at 562 nm with a Shimadzu UVmini-1240 spectrophotometer, Japan. The amount of protein present was determined by reference to a BSA standard curve constructed through a series of dilutions. All the data points for the absorbance were carried out in triplicate.
2.5. Statistical analysis
The mean value and standard error were calculated for all the analysed parameters. Error bars representing the standard errors have been added to all plotted graphs. The water quality assay data of the control and treatment systems obtained over the 14 days of the experiment were subjected to one-way ANOVA with Fisher's LSD test to assess significant differences in the treated levels among the systems. The statistical significance of the changes in the biomass and biochemical content before and after the experiment for the macrophyte systems was evaluated by paired t-test. The statistical tests were performed using Minitab® version 16.2.1.3. Results and discussion
3.1. Nitrogen removal
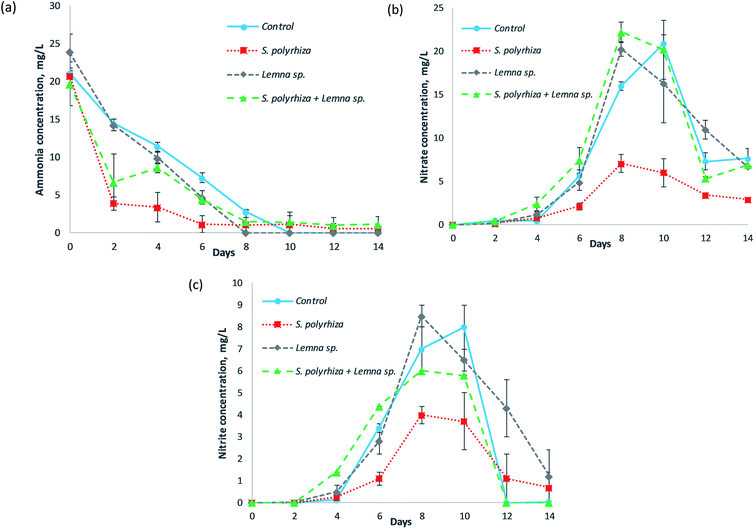
Fig. 2 shows the concentrations of the various forms of nitrogen in the fish farm wastewater as a function of the number of days of phytoremediation for the control (no macrophytes) system, S. polyrhiza monoculture system, Lemna sp. monoculture system and S. polyrhiza + Lemna sp. polyculture system. The ammonia (NH3–N) concentration profile was observed to decrease with time; however, the nitrate (NO3−–N) and nitrite (NO2−–N) levels gradually rose before dropping again. The S. polyrhiza monoculture system demonstrated the most statistically significant ammonia removal among its peer systems (Fig. 2(a) and ESI Table S2(a)†); the ammonia dropped sharply to 3.90 ± 0.90 mg L−1 at day 2 with 81% removal efficiency. The ammonia concentration eventually dropped to 1.15 ± 1.15 mg L−1 (94% ammonia removal) on day 6. The S. polyrhiza + Lemna sp. polyculture system showed a similar reduction trend to the S. polyrhiza monoculture system, except that the rate of decrease was not as substantial. The polyculture system sharply reduced the ammonia concentration to 6.70 ± 3.70 mg L−1 on day 2 before averaging at 1.25 ± 0.12 mg L−1 from day 8 onwards. Additionally, the Lemna sp. monoculture system and control system exhibited steady ammonia decrease, with the Lemna sp. system showing a slightly higher reduction in ammonia.Both the nitrate and nitrite in the all the tested systems showed a similar trend, whereby there was a gradual increase in their concentration for the first 10 days, the maximum was achieved on either day 8 or day 10, and the concentration then decreased until the end of the study. The control treatment attained a peak nitrate concentration of 20.90 mg L−1 at day 10, which dropped sharply to approximately 7.66 mg L−1 at the last day of the experiment. The Lemna sp. monoculture system and S. polyrhiza + Lemna sp. polyculture system had profiles that were almost the same as that of the control system, which suggested that the aforementioned macrophyte systems were not efficient in taking up the nitrate. The nitrate level in the S. polyrhiza monoculture system was exceptionally and significantly lower than those of the control system and the other two macrophyte systems throughout the experiment (Fig. 2(b) and ESI Table S2(b)†). The highest nitrate presence in the S. polyrhiza system was determined to be 7.05 ± 1.05 mg L−1 on day 8.
The nitrite concentrations for each of the systems were typically lower than their respective nitrate levels. The S. polyrhiza + Lemna sp. polyculture system exhibited a very similar profile to the control system, but had a lower nitrite maximum of 6.00 mg L−1. The Lemna sp. monoculture system differed from the control system only in the rate of nitrite decline; the decrease began at day 8 and continued in a significant and steady manner until the end of the run, and the recorded level was 1.20 ± 1.20 mg L−1 on the last day. The nitrite concentration in the S. polyrhiza monoculture system was still comparatively and significantly lower than those of the rest of the systems (Fig. 2(c) and ESI Table S2(c)†). The nitrite peak was observed at 4.00 ± 0.40 mg L−1 at day 8.
The ammonia, nitrate and nitrite profiles shown in the systems were interconnected to one another. They were associated with the nitrification process within the fish farm wastewater, while the removal performances of ammonia, nitrate and nitrite from the different systems were closely related to the macrophyte species employed in plant uptake. Under sterile conditions, without plants, the ammonia and nitrate levels in wastewater will remain constant since there are no organisms to initiate the removal of the mentioned nitrogen species.23 However, in a real-world scenario, indigenous microorganisms, e.g., ammonia-oxidizing bacteria (AOB), ammonia-oxidizing archaea (AOA) and nitrite-oxidizing bacteria (NOB), are present in the wastewater and responsible for carrying out nitrification.27 These nitrifiers can be found everywhere, and are found in abundant numbers in wastewater in which the ammonia level is high and the decomposition of organic nitrogen is extensive.28 The steady decline of ammonia in the control system was attributed to AOB and AOA oxidizing the available reduced nitrogen, ammonia, in the fish farm wastewater to nitrite. The ability of the macrophytes to take up ammonia enhanced the ammonia removal in the S. polyrhiza monoculture system, Lemna sp. monoculture system and S. polyrhiza + Lemna sp. polyculture system. For instance, at day 2, the removal of ammonia via plant uptake in the S. polyrhiza monoculture system enabled its ammonia concentration to be 73% lower than that of the control system (14.50 mg L−1). The ammonia removal performance of the macrophyte species in this study (Fig. 2(a)) was also found to correspond to the performance in the axenic case study.23 The higher ammonia uptake capability of S. polyrhiza compared to that of Lemna sp. allowed the S. polyrhiza monoculture system to demonstrate the highest ammonia removal in the fish farm wastewater, followed by the S. polyrhiza + Lemna sp. polyculture system and lastly the Lemna sp. monoculture system. In addition, the S. polyrhiza monoculture system was the only system capable of meeting the most stringent ammonia discharge limit (standard A for discharge into an enclosed water body) of 5 mg L−1 (ref. 29) within 2 days and in maintaining the ammonia level below this very low limit at all times. The ammonia level achieved by the S. polyrhiza monoculture system throughout the study was also far lower than the acceptable limit of 10 mg L−1 for industrial effluent release.29
However, under normal circumstances, nitrification increases the nitrite and nitrate concentrations in the wastewater;27 this was also observed in our study (Fig. 2(b) and (c)), in which the effect was most prominent in the macrophyte-free control system. The nitrification process was evident in our experiment such that when the ammonia level decreased, both the nitrite and nitrate levels increased. As soon as the ammonia in the wastewater was depleted, the nitrite and nitrate levels reached their peak. In general, the pH of the wastewater did not change much in any of the systems throughout the experiment, and ranged within 7.40 to 7.90, as shown in ESI Fig. S1.† The favorable conditions in terms of pH and temperature, as well as sufficient oxygen, supported nitrification. The pH levels and temperatures of the systems were observed to be within the optimum range for nitrification, i.e., pH 7.5–8.6 and 25–35 °C, respectively.30 With ample light illumination, the macrophyte systems produced oxygen through photosynthesis, and the oxygen would dissolve into the water column. This dissolved oxygen increased the rate of nitrification, causing the nitrate and nitrite maxima in the macrophyte systems to occur two days earlier than in the control system. Although the nitrification gave rise to the accumulation of nitrate and nitrite in the fish farm wastewater in the control system, the macrophyte systems, especially the S. polyrhiza monoculture system, were capable of suppressing the consequences of nitrification and effectively reduced the nitrate and nitrite levels in the wastewater, as presented in Fig. 2(b) and (c). The S. polyrhiza monoculture system took up a tremendous amount of ammonia (nitrification feedstock) from the wastewater (Fig. 2(a)), leading to a smaller amount of ammonia being available for conversion into nitrite and nitrate. The amount of nitrate and nitrite formed (nitrification products) were also reduced substantially (Fig. 2(b) and (c)). In addition, the nitrate and nitrite uptake capabilities of S. polyrhiza23 also assisted in removing the nitrification products, nitrite and nitrate, from the wastewater. Therefore, the nitrite and nitrate levels in the S. polyrhiza monoculture system were much lower. This strategy and concept for controlling nitrification have been reported in crops management, as evidenced in the works of Norton and Ouyang31 as well as Norton and Stark,32 although it is relatively new in phytoremediation. Some plants have also been shown to excrete root exudates capable of the inhibiting nitrification processes.31,33 Furthermore, the nitrate level in the S. polyrhiza system was always lower than the most stringent local limit29 as well as the permitted limit for drinking water,34–36 which are both set as 10 mg L−1 nitrate-N. The peak concentration was also approximately 30% lower than this limit, and most of the time, the concentration was much lower than the peak value. Additionally, the peak nitrate concentration in the S. polyrhiza system was found to be 66% lower than that in the control system. This high nitrate removal was due to the high nitrate assimilation of S. polyrhiza. S. polyrhiza had the highest nitrate uptake among the macrophytes, as revealed by Ng and Chan.23 The low nitrate level in this system was also partly associated with the high ammonia uptake, of S. polyrhiza, as mentioned earlier. The ammonia level in the wastewater after the uptake was considerably low, so less ammonia was available for conversion into nitrate or nitrite through nitrification. These are the reasons that the S. polyrhiza system managed to keep the nitrate so low during the experiment compared to the other systems. The high nitrite reduction observed was also due to similar reasons. The S. polyrhiza monoculture system can take up a substantial amount of nitrite, since nitrite is an inorganic nitrogen that is utilized by plants for growth and cellular metabolism.37 In our previous study,38 microbial biofilms formed in the rig with macrophytes after few days, but the modification made to the current rig, i.e., covering the sides with a light-impermeable material, was found to inhibit the biofilm formation, which suggested that they were photosynthetic biofilm. The microbes attached to the plant surfaces may have an influence on the nitrogen conversion, but it is believed to be negligible as the control system still exhibited significant nitrification even in the absence of macrophytes. The changes in levels of nitrogen in the macrophyte system were mainly due to plant uptake and the nitrifiers present in the wastewater in the rig. Possible approaches that could be utilized to enhance the ammonia, nitrate and nitrite treatment performance in the wastewater include increasing the density of the macrophytes on the wastewater, screening and selecting strains within the species S. polyrhiza that have higher removal of these nitrogen species (ammonia, nitrate or/and nitrite) than the current strain, and employing appropriate macrophyte species based on the wastewater characteristics. Macrophytes with superior ammonia, nitrate and nitrite uptake are highly sought, after as they would be useful for dealing with wastewater or water experiencing a dynamic nitrogen transformation/cycle, constantly being contaminated with different nitrogen pollutants, and having high biodegradable organic nitrogen waste levels. This trait makes the macrophyte system more ready and robust to withstand the circumstances that may arise. The high and rapid ammonia uptake trait of macrophytes should be further explored, optimized and utilized to treat wastewater that has elevated ammonia levels and is potentially undergoing nitrification, since it removes ammonia and inhibits increases in nitrite and nitrate in a single approach.
The decreases in the nitrate and nitrite profiles after reaching their peak values in these systems, which occurred from around day 8 to day 14 of the experiment, were believed to be due to denitrification. This is a microbial process in which nitrate is reduced and ultimately converted to nitrogen gas (N2) through a series of nitrogen oxide intermediates (including nitrite). High nitrate levels in wastewater enhance denitrification and induce larger, more robust populations of denitrifying bacteria.39 Coupled with the optimum pH of 6–8 and adequate organic carbon40 from the fish farm wastewater and macrophytes, this made the denitrification process possible.
3.2. Phosphate removal
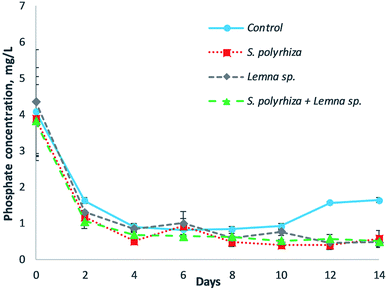
The removal of phosphate from the fish farm wastewater by the three macrophyte systems over the 14 days of the study is illustrated in Fig. 3. In general, the macrophyte and control systems showed a decrease in phosphate at the beginning of experiment, but an increase in phosphate was shown in the control system in the latter part of the experiment. The lowest concentration of phosphate for the control group was 0.82 mL L−1 at day 6, but the concentration rose to 1.64 mg L−1 at day 14. The phosphate removal within the macrophyte systems, namely, the S. polyrhiza monoculture system, Lemna sp. monoculture system, and S. polyrhiza + Lemna sp. polyculture system, was similar for all three systems, which achieved average phosphate concentrations of 0.54 ± 0.08 mg L−1, 0.69 ± 0.09 mg L−1 and 0.59 ± 0.03 mg L−1 from day 4 until the end of the experiment. Among the macrophyte systems, the phosphate levels in the S. polyrhiza monoculture system were found to be significantly lower than those in the control system (ESI Table S2(d)†).S. polyrhiza and Lemna sp. were shown to have the highest phosphate removal from the medium by Ng and Chan.23 This high phosphate absorption ability allowed the macrophyte systems to remove phosphate rapidly from the fish farm wastewater in the first four days and to exhibit higher phosphate removal than the control system. The phosphate that was taken up was subsequently incorporated into their biomass. The healthy growth of the macrophytes in the fish farm wastewater, accompanied by the high phosphate uptake, permitted the systems to retain the phosphate in the biomass, thereby maintaining a low phosphate concentration until the end of the experiment. The phosphate-P levels attained by the S. polyrhiza monoculture system were 0.16 ± 0.00 mg L−1 and 0.13 ± 0.04 mg L−1 at day 4 and day 12 respectively, both of which were very much lower than the statutory limit of 5 mg L−1.29 These levels were found to be 96.7% and 97.5% lower than the limit. For the control system, the gradual decrease in phosphate in the first four days was attributed to the presence of algae in the fish farm wastewater. The algae was capable of reducing the phosphate in the water column, as presented in the works of Griffiths41 and Delgadillo-Mirquez et al.42 As the experiment proceeded, it was found that the greenish colour of the fish farm wastewater caused by the presence of algae faded. This implied that the algae population was dying, which in turn inhibited its phosphate uptake from the wastewater. Consequently, the phosphate level in the control system remained constant from day 4 to day 10. However, decomposition and mineralization took place after the death of the algae; hence, soluble phosphate was released into the wastewater, as observed from day 10 to day 14 in the control system. In spite of this, the macrophyte system managed to take up the dissolved phosphate released from the decomposition of the algae biomass, as well as other organic substances within the wastewater, thus keeping the phosphate at a very low concentration throughout the experiment.
The monoculture and polyculture configurations of S. polyrhiza + Lemna sp. had a significant effect on ammonia, nitrate, nitrite, and phosphate removal (Fig. 2 and 3 and ESI Table S2(a)–(d)†). These data showed that the macrophytes were effective in soluble nutrient removal and the proposed configuration could affect their removal. The S. polyrhiza + Lemna sp. polyculture system did not produce any synergistic effect on nutrient removal, but the removal followed the proportional density of the macrophyte species. Thus, it had lower nutrient removal than the S. polyrhiza monoculture system, but higher removal than the Lemna sp. monoculture system, especially in the case of the ammonia and nitrite profiles since S. polyrhiza has higher nutrient removal than Lemna sp. In addition, the results also show that the macrophyte density does have an influence on the removal of the contaminants. Higher macrophyte density (or macrophyte weight) in the system would lead to a higher removal rate of contaminants, which could further decrease the level of contaminants in the wastewater, according to an in-depth study.43
3.3. Total suspended solids, turbidity, and COD profiles in the wastewater
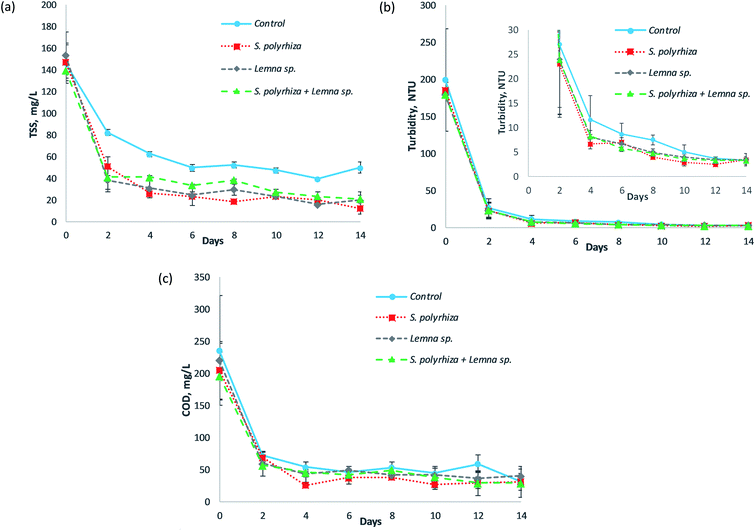
Fig. 4 illustrates the total suspended solids (TSS), turbidity and COD profiles of the fish farm wastewater treated by the S. polyrhiza monoculture system, Lemna sp. monoculture system and S. polyrhiza + Lemna sp. polyculture system throughout the 14 day experimental period. From Fig. 4(a)–(c), it was found that the TSS, turbidity and COD in the systems had similar trends in which a significant drop occurred in the first two days, followed by either a further slight decline or a constant level until the end of this study. The macrophyte systems a showed relatively higher decrease in these three parameters (TSS, turbidity and COD) than the control system, which was most obvious in the TSS profile. However, it was statistically significant only for the TSS profile, and not for the turbidity or COD profiles (ESI Table S2(e)–(g)†), suggesting that the macrophyte systems were not the main contributor towards the removal of turbidity and COD, and that other modes of removal, such as the sedimentation of organic particulates, played a role. The TSS in the macrophyte systems generally fell sharply in the first two days to values ranging from 39 ± 11 mg L−1 to 51 ± 9 mg L−1 with a removal efficiency of up to 75%. Subsequently, small gradual decreases in the TSS took place until the study ended. During this period, the TSS level could drop to 24 ± 9 mg L−1 on day 6 and as low as 13 ± 5 mg L−1 on day 14, representing 84% and 92% removal, respectively (both were achieved by the S. polyrhiza monoculture system). In the control system, the decrease in the TSS was smaller, with 82 mg L−1 of TSS on day 2. Generally, the TSS declined gradually over the first six days, and during this period the control system only managed to achieve a TSS removal between 44% and 66%. Soon after that, it maintained a TSS level of approximately 48 ± 2 mg L−1 until the end of experiment.In the case of turbidity, the difference between the macrophyte and control systems was less obvious; even so, the decline in the macrophyte system was still observed to be higher than that in the control system as demonstrated in Fig. 4(b). The macrophyte systems reduced the turbidity of the wastewater drastically from between 179.50 ± 7.50 and 186.00 ± 10.00 NTU to 22.95 ± 10.85 to 23.95 ± 11.25 NTU in just two days' time. The turbidity further declined to 6.66 ± 1.03 NTU at day 4, 3.96 ± 0.04 NTU at day 8 and 2.51 ± 0.53 NTU at day 12, in which the decline from day 4 to day 14 occurred in a slower manner. For the control system, the turbidity dropped to 27.00 ± 12.80 NTU on day 2 and 11.63 ± 4.88 NTU on day 4. The system then exhibited a slow decline in turbidity until the end of the study.
The decline of COD in the macrophyte systems was apparently higher than that in the control system, as presented in Fig. 4(c). Similarly, the macrophyte systems were able to decrease the COD sharply to 57 ± 2 mg L−1 with up to 71% removal (S. polyrhiza + Lemna sp. polyculture system) at day 2. The highest COD removal efficiency of 87% to a concentration of 27 ± 5 mg L−1 (which was the lowest COD level) was achieved on day 4. The COD in the systems then maintained an average value of 39 mg L−1 from day 4 to day 14. On the other hand, the control system only managed to reduce the COD to a level of 73 ± 1 mg L−1 on day 2 and 55 ± 8 mg L−1 on day 4. The COD in the system later fluctuated around a mean value of 48 mg L−1 until the end of the study.
The obtained total suspended solids, turbidity and COD profiles showed that these data were correlated with each other. The similarities in the decrease trends and relatively higher declines observed in the macrophyte systems compared to the control system in these three wastewater quality assays evidenced their relationship. As a large amount of TSS was removed from the wastewater in first two days, the turbidity also improved greatly, and at the same time the COD plunged to a very low level. The TSS level remained relatively steady thereafter, and the turbidity and COD displayed similar trends. Therefore, the level of TSS had a strong influence on the turbidity and COD level. The TSS was deduced to be responsible for obstructing light from penetrating through the wastewater, and also to be responsible for causing the high oxygen demand, since they are organic in nature. Most of the COD in the wastewater originated from these organic suspended solids, which were mainly composed of algae. The fish farm wastewater used for study was greenish in colour and contained flourishing algae. The reason that the TSS, turbidity and COD could be reduced steeply within two days' time was the artificial shading, i.e., the use of non-transparent materials (aluminum foil + black paper) to cover the sides of the pond rig system, preventing the light from penetrating into the wastewater. In a preliminary study,44 it was observed that it took almost 10 to 14 days for the same system to reduce the TSS to a level that can be achieved in two days' time in this study. However, the system in the preliminary study was not protected from light, while in the current system the side wall and bottom floor of the pond rig, as well as the surface of the storage tank, were overlaid with non-transparent materials. These changes to the current system actually blocked most of the light from entering the wastewater (from the sides of the pond rig and surrounding the storage tank). Additionally, part of the light that might enter the water (from the top of the pond rig) would be absorbed by the black paper, thereby preventing reflection and passage of light within the water column. As a result, the dark environment in the pond rig system prevented the algae from carrying out photosynthesis and caused it to eventually die off and sediment to the bottom of the pond rig. Since most of the organic suspended solids were algae, when a considerably high number of algae collectively died and settled out from the water column, the TSS, turbidity and COD became exceptionally low. Therefore, the non-transparent pond rig system proved to be a very effective method to reduce the TSS, turbidity and COD in the fish farm wastewater used. Yeh et al.45 also showed that artificial shading helped to control and reduce algae in eutrophic wastewater in an experiment conducted under a plastic basket, and that the suspended solids are related to the algal density. The relatively larger decreases in the TSS, turbidity and COD in the macrophyte system compared to the control system were due to several factors. Firstly, the dense mats of macrophytes on the surface of the wastewater in the raceway pond hindered light from entering the wastewater, causing more algae to die off, leading to higher settlement of the suspended algae, i.e., the TSS, to the bottom of the pond. The presence of the macrophytes also inhibited the further growth of algae46 in the water column. Secondly, the roots and fronds of the macrophytes were observed to filter and retain some of the suspended solids as well as algae assemblages. This aided somewhat in the removal of the TSS, making the wastewater become clearer. Thirdly, the macrophytes prevented the development of surface wave formations, causing the flow of wastewater in the raceway pond to be calm and quiet with reduced velocity and turbulence, thus promoting sedimentation.47,48 Moreover, the microbes that resided on the surface of the macrophytes, e.g., their roots, were capable of utilising the oxygen produced by photosynthesis of the macrophytes to metabolise organic carbon in the wastewater and transform it into carbon dioxide and water. This aerobic process was supported by the oxygen leaking from the roots of the macrophytes, as well as the oxygen supplied directly from the atmosphere via diffusion,49 and most of the soluble organic compounds could be degraded.30 Consequently, more organic suspended solids could settle and be filtered from the column of wastewater, and the degraded organic compounds led to much lower TSS, turbidity and COD readings in the macrophyte systems. Therefore, macrophytes and artificial shading could be applied to fish farm wastewater or algae-rich wastewater to effectively and rapidly reduce the suspended solids and oxygen demand and to increase the water clarity before discharging the wastewater into natural waters. The surface of the wastewater containment in the macrophyte system can be darkened and the width of the pond canal can be reduced to intensify the artificial shading effect and subsequently decrease the amount of algal suspended solids in the water column and promote the sedimentation of the solids.
The macrophyte systems were able to meet the TSS discharge limit of 50 mg L−1 (ref. 29) in just two days' time. The TSS level in the macrophyte systems (S. polyrhiza monoculture system and Lemna sp. monoculture system) complied with the Class I standard of 25 mg L−1 (ref. 35) for water supply usage from day 6 onwards, while the S. polyrhiza + Lemna sp. polyculture system achieved the standard at day 12. All the macrophyte systems met the limits faster than the control system. In terms of turbidity, all the macrophyte systems coincidently conformed to the drinking water standard of 5 NTU34–36 on day 8. The COD level in the macrophyte systems was below the statutory standards of 80 mg L−1 (ref. 29) and 120 mg L−1 (ref. 29) for effluent discharge from day 2 onwards.
3.4. Changes in biomass and biochemical content of the macrophytes
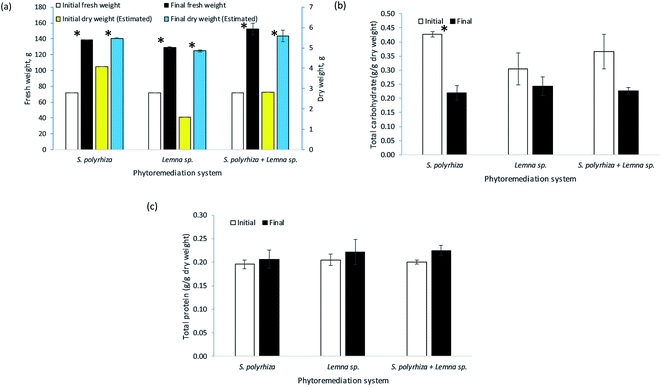
The change in fresh weight biomass of the three macrophyte systems, namely, the S. polyrhiza monoculture system, Lemna sp. monoculture system and S. polyrhiza + Lemna sp. polyculture system, over the 14 days of the study is depicted in Fig. 5(a). Generally, the macrophytes in these systems showed positive growth. All the systems were started with 72.00 ± 0.00 g fresh weight of macrophytes, but each had a different final biomass. The S. polyrhiza + Lemna sp. polyculture system showed the highest percentage increase in fresh weight among the systems, with an increase of 112% to 152.29 ± 7.32 g on day 14. The S. polyrhiza monoculture system ranked second, with a 93% increase in fresh weight to a value of 138.80 ± 0.09 g. The fresh weight increase in the Lemna sp. monoculture system was the lowest at 79% to 128.96 ± 1.24 g on the last day of the study. All three systems showed significant differences between their initial and final fresh weights.In terms of biochemical content, the change in the total carbohydrate content of the macrophytes in the systems at the end of the 14 day study is shown in Fig. 5(b). As observed, a decline in the total carbohydrate content was demonstrated in all the macrophyte systems. The initial total carbohydrate content of the macrophytes in the S. polyrhiza monoculture system was 0.4271 ± 0.0089 g g−1 DW, and the final value was 0.2195 ± 0.0251 g g−1 DW. For the Lemna sp. monoculture system, the total intracellular carbohydrate content of the macrophytes decreased from 0.3047 ± 0.0575 g g−1 DW to 0.2432 ± 0.0332 g g−1 DW, whereas the S. polyrhiza + Lemna sp. polyculture system showed a decrease from 0.3659 ± 0.0612 g g−1 DW to 0.2260 ± 0.0116 g g−1 DW. Statistically, only the carbohydrate decrease in the S. polyrhiza monoculture system was found to be significant, but this was not the case for the Lemna sp. monoculture system and S. polyrhiza + Lemna sp. polyculture system.
Fig. 5(c) illustrates the biochemical content change in the total protein of the macrophytes in the systems over the 14 day phytoremediation period. Typically, the macrophyte systems were observed to exhibit an increase in total protein content. The macrophytes in the S. polyrhiza + Lemna sp. polyculture system achieved the highest total protein content increase of 12%, whereby the initial protein level of 0.2005 ± 0.0047 g g−1 DW rose to 0.2250 ± 0.0103 g g−1 DW. The Lemna sp. monoculture system macrophytes had the second-highest total protein content increase of 8%, with the protein content increasing from 0.2052 ± 0.0124 g g−1 DW to 0.2212 ± 0.0267 g g−1 DW. The lowest increase in total protein content of 6% was found in the S. polyrhiza monoculture system, whose content increased from 0.1958 ± 0.0093 g g−1 DW to 0.2066 ± 0.0189 g g−1 DW. However, the increase in the total protein contents were not statistically significant for any of the systems.
The high increase in the fresh weight of the biomass among the systems indicated that the S. polyrhiza and Lemna sp. macrophytes grew healthily in the fish farm wastewater and that the wastewater contained sufficient nutrients to support the growth and development of the macrophytes.43,50,51 This increase was also found to be higher than that in a study carried out in synthetic medium,23 which could suggest that the fish farm wastewater used has a greater amount of nutrients and more diversified nutrients originating from the readily available inorganic nutrients and mineralised nutrients from the organic wastes, e.g., uneaten feed, dead algae, and fish excretion and faeces, and other essential plant nutrients that promoted the growth of the macrophytes. An additional analysis of the water content (moisture) of the harvested macrophytes showed that water constituted about 96% of their biomass. The increase in dry weight was the highest in the Lemna sp. monoculture system, followed by the S. polyrhiza + Lemna sp. polyculture system and finally the S. polyrhiza monoculture system, which had percentage increases of 203%, 97% and 34%, respectively. This showed that the macrophytes in all the systems were constantly fixing carbon for their growth. The rate of carbon fixation in Lemna sp. was higher than that of S. polyrhiza in this particular fish farm wastewater, and the final dry weight of Lemna sp. was roughly triple its initial weight. The relatively high biomass productivity of the three macrophyte systems, namely, the S. polyrhiza monoculture system, Lemna sp. monoculture system and S. polyrhiza + Lemna sp. polyculture system, would permit macrophyte farming, allowing the resulting biomass to be used to seed subsequent cultivation, for wastewater treatment purposes, and also as other valuable by-products, such as fertiliser. The available research on the data of percentage recovery of nutrients from the macrophyte (or duckweed) as a means of organic fertilizer and related studies are limited, which is in agreement with the report.52 Only a few relevant studies can be found.53–55 The rate of nutrient leaching by the macrophytes varied depending on the number of days after fertilizer application.53–56 The nutrients can be recovered from the macrophytes once they are fully decomposed.
In the case of biochemical analysis, the decrease in the total carbohydrate content of the macrophytes in all the systems was due to the cultivation method of the macrophyte stocks and the metabolism of the macrophytes during phytoremediation. The macrophyte stocks used in this study were cultivated by micropropagation, in which sucrose is added to the medium as a carbon source. In an enriched medium, the macrophytes tend to store the carbon source as starch grains inside their cells.57,58 Therefore, the carbohydrate content in the initial macrophytes was higher as the supply of sucrose in the medium acted as an additional organic carbon source.59 Additionally, when the macrophytes were taken out for phytoremediation, they used up their starch for growth and producing offspring,58,60 as the fronds of the macrophytes were seen to duplicate continuously, generating new biomass. In addition to carrying out cell division (plant building and producing biomass), the starch could also be used for respiration and the production of metabolic substances (protein, fat, etc.)58 which eventually lowered the carbohydrate content inside the final harvested macrophytes. In addition, this carbohydrate could either be converted into more complex, tougher forms of organic carbon, such as cellulose,58 or be disseminated or assimilated into the biochemical composition of their offspring and daughter fronds during multiplication.61
In terms of the total protein content, the differences among the macrophytes were not very significant, although the average content was determined to be higher at the end of the experiment. In spite of this, the small increase in protein content accompanied by high macrophyte biomass productivity could provide a huge amount of protein source or feedstock for fish and poultry food supplements. The elevated protein level in the macrophytes was a result of the high ability of macrophytes to take up nitrogen sources from the fish farm wastewater and convert them into proteins. Both S. polyrhiza and Lemna sp. macrophytes have high protein content and quality, and a variety of their amino acids meet the WHO nutrition recommendations.62 Their essential amino acid profile is better than that of most plant proteins and resembles animal protein more than any other plant proteins.63 They are rich in leucine, threonine, valine, isoleucine and phenylalanine64 and are comparable to alfalfa in terms of lysine and arginine, which are amino acids important in animal feed.63 Certain amino acids were found to be present in low levels in plant proteins but plentiful in the protein of duckweed.63 Haustein et al.18 reported that high levels of trace minerals and pigments, especially β-carotene and xanthophyll, were present in duckweed. Dried S. polyrhiza macrophytes could be included as up to 30% of the total diet of Nile tilapia without a significant effect on performance as compared to control meal without duckweed.65 This would help to reduce the amount spent on conventional meals, since fish feed that is rich in protein with high biological value is costly and usually locally unavailable. Eggs from Leghorn hens that were fed with 15 and 25% sewage-grown Lemna gibba had a higher protein content than control eggs and showed a significant increase in yolk pigmentation.18 However, the egg production and mean egg weights of the hens fed with the control diet and those fed with macrophyte-inclusive meals remained the same.18
4. Conclusions
The S. polyrhiza monoculture system was the most effective candidate for treating fish farm wastewater among the tested systems. It required only two days to drop below the 5 ppm ammonia standard limit (81% ammonia removal to 3.90 mg of N/L), and the wastewater never exceeded the 10 ppm nitrate-N limit during the study. Additionally, it had the lowest nitrite profile and the highest mean phosphate removal of 84% among the studied systems. This was mainly contributed to by the high uptake capabilities towards various nitrogen species and phosphate of the S. polyrhiza macrophyte; the highest removal rates of NH3–N, NO3−–N, NO2−–N, and PO43− recorded were 809 mg of NH3–N m−2 per day, 286 mg of NO3−–N m−2 per day, 116 mg of NO2−–N m−2 per day, and 131 mg of PO43− m−2 per day, respectively. Most importantly, the exceptionally high and fast ammonia, nitrate, and nitrite uptake of the system reduced the toxicity of the pollutants to freshwater animals in terms of concentration and exposure time. The S. polyrhiza + Lemna sp. polyculture system exhibited ammonia and nitrite removal levels that were intermediate between those of the S. polyrhiza monoculture system and Lemna sp. monoculture system, which corresponded to the nitrogen removal abilities of S. polyrhiza and Lemna sp., as well as the plant densities of each macrophyte species in the system.The S. polyrhiza monoculture system also achieved 92%, 99%, and 97% decreases in the TSS, turbidity, and COD, which were the highest decreases observed among the configured systems. The highest COD and TSS removal rates recorded were 6504 mg of O2 m−2 per day and 4620 mg m−2 per day, respectively. The steep decline of the TSS, turbidity, and COD during the first two days was primarily due to the sedimentation of the algae present in the wastewater. Furthermore, the filtration capacity of the suspended solids, the prevention of wave formation by the macrophytes, and the metabolic activities of the microbes residing on the macrophytes led to relatively lower TSS, turbidity, and COD readings in the macrophyte systems. Thus, effluent with dissolved nutrient, TSS, COD, and turbidity values below the relevant discharge limits was attainable, which in turn would lower the adverse effects of the effluent on the receiving water. Moreover, the high biomass productivity and superior protein levels of the macrophyte systems cultivated in the fish farm wastewater would allow their subsequent utilisation as a fish feed supplement and in poultry diets.
Other wastewater samples with similar characteristics (nutrient-rich and containing algal blooms) could also be treated using this system. It could potentially depurate bodies of water that are initially or continually polluted with nutrients, suppress surges or increases in nutrients in bodies of water (ponds/lakes), and minimise the toxicity of pollutants to eventually preserve and protect the aquatic life within and assist in ecological restoration.
Author contributions
Yin Sim Ng: methodology, validation, formal analysis, investigation, resources, data curation, writing – original draft, visualization. Derek Juinn Chieh Chan: conceptualization, resources, writing – review & editing, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Long Term Research Grant Scheme (LRGS) (203/PJKIMIA/6720015 and 4411/S01), from the Ministry of Higher Education, Malaysia. Yin Sim Ng acknowledges the award of a USM Fellowship by the Institute of Postgraduate Studies, Universiti Sains Malaysia, for supporting his study and research work.References
- C. S. Tucker, J. A. Hargreaves, C. E. Boyd, J. W. Clay, G. L. Jensen, P. W. Zajicek, S. M. Belle, C. E. Nash, G. Fornshell and J. M. Hinshaw, Aquaculture and the Environment in the United States, Environmental Best Management Practices for Aquaculture, 2008, pp. 3–54 Search PubMed.
- C. S. Tucker and J. A. Hargreaves, Environmental best management practices for aquaculture, John Wiley & Sons, 2009 Search PubMed.
- S. E. Yeo, F. P. Binkowski and J. E. Morris, Aquaculture effluents and waste by-products characteristics, potential recovery, and beneficial reuse, 2004 Search PubMed.
- C. S. Tucker, J. A. Hargreaves and C. E. Boyd, Better management practices for freshwater pond aquaculture, Wiley-Blackwell, Hoboken, NJ, USA, 2008 Search PubMed.
- S. A. Siddiqui, Wastewater treatment technology in aquaculture, World Aquaculture, 2003, pp. 49–52 Search PubMed.
- Y.-F. Lin, S.-R. Jing, D.-Y. Lee and T.-W. Wang, Nutrient removal from aquaculture wastewater using a constructed wetlands system, Aquaculture, 2002, 209(1), 169–184 CrossRef CAS.
- Y.-C. Chung, Y.-H. Li and C.-C. Chen, Pollutant Removal From Aquaculture Wastewater Using the Biopolymer Chitosan at Different Molecular Weights, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2005, 40(9), 1775–1790 CrossRef CAS PubMed.
- USEPA Nutrient Pollution – The Sources and Solutions: Wastewater, https://www.epa.gov/nutrientpollution/sources-and-solutions-wastewater, August 29 Search PubMed.
- Headworks Biological Nutrient Removal, https://www.headworksinternational.com/product/bnr-biological-nutrient-removal/, February 14 Search PubMed.
- G. T. Moore, P. Hertzler, L. Dufresne, C. Randall, J. Barnard, D. Stenseland and J. Brown, Nutrient Control Design Manual, State of Technology Review Report, Cadmus Group Inc., Watertown (MA), 2009 Search PubMed.
- H. Hu, X. Li, S. Wu and C. Yang, Sustainable livestock wastewater treatment via phytoremediation: Current status and future perspectives, Bioresour. Technol., 2020, 315, 123809 CrossRef CAS PubMed.
- D. L. LeDuc and N. Terry, Phytoremediation of toxic trace elements in soil and water, J. Ind. Microbiol. Biotechnol., 2005, 32(11–12), 514–520 CrossRef CAS PubMed.
- J. Xu and G. Shen, Growing duckweed in swine wastewater for nutrient recovery and biomass production, Bioresour. Technol., 2011, 102(2), 848–853 CrossRef CAS PubMed.
- R. A. Mohedano, R. H. Costa, F. A. Tavares and P. Belli Filho, High nutrient removal rate from swine wastes and protein biomass production by full-scale duckweed ponds, Bioresour. Technol., 2012, 112, 98–104 CrossRef CAS PubMed.
- H. Effendi, B. A. Utomo and G. M. Darmawangsa, Phytoremediation of freshwater crayfish (Cherax quadricarinatus) culture wastewater with spinach (Ipomoea aquatica) in aquaponic system, Aquaculture, Aquarium, Conservation & Legislation-International Journal of the Bioflux Society, 2015, 8(3), 421–430 Search PubMed.
- H. Hu, Q. Zhou, X. Li, W. Lou, C. Du, Q. Teng, D. Zhang, H. Liu, Y. Zhong and C. Yang, Phytoremediation of anaerobically digested swine wastewater contaminated by oxytetracycline via Lemna aequinoctialis: Nutrient removal, growth characteristics and degradation pathways, Bioresour. Technol., 2019, 291, 121853 CrossRef CAS PubMed.
- E. C. S. Little, Handbook of utilization of aquatic plants. A review of world literature, 1979 Search PubMed.
- A. Haustein, R. Gilman, P. Skillicorn, V. Vergara, V. Guevara and A. Gastanaduy, Duckweed, a useful strategy for feeding chickens: performance of layers fed with sewage-grown Lemnacea species, Poult. Sci., 1990, 69(11), 1835–1844 CrossRef.
- R. C. Baliban, J. A. Elia, C. A. Floudas, X. Xiao, Z. Zhang, J. Li, H. Cao, J. Ma, Y. Qiao and X. Hu, Thermochemical conversion of duckweed biomass to gasoline, diesel, and jet fuel: process synthesis and global optimization, Ind. Eng. Chem. Res., 2013, 52(33), 11436–11450 CrossRef CAS.
- S. Bashyal, Wastewater treatment by floating and emergent aquatic macrophytes in artificial wetland system, Doctoral dissertation, Department of Environmental Engineering, Environmental Ecology Major Graduate School, Sunmoon University, Republic of Korea, 2010.
- C. Ouellet-Plamondon, F. Chazarenc, Y. Comeau and J. Brisson, Artificial aeration to increase pollutant removal efficiency of constructed wetlands in cold climate, Ecol. Eng., 2006, 27(3), 258–264 CrossRef.
- S. Naylor, J. Brisson, M. Labelle, A. Drizo and Y. Comeau, Treatment of freshwater fish farm effluent using constructed wetlands: the role of plants and substrate, Water Sci. Technol., 2003, 48(5), 215–222 CrossRef CAS PubMed.
- Y. S. Ng and D. J. C. Chan, Phytoremediation capabilities of Spirodela polyrhiza, Salvinia molesta and Lemna sp. in synthetic wastewater: A comparative study, Int. J. Phytorem., 2018, 20(12), 1179–1186 CrossRef CAS PubMed.
- Y. S. Ng and D. J. C. Chan, Wastewater phytoremediation by Salvinia molesta, J. Water Process. Eng., 2017, 15, 107–115 CrossRef.
- C. Hoebler, J. L. Barry, A. David and J. Delort-Laval, Rapid acid hydrolysis of plant cell wall polysaccharides and simplified quantitative determination of their neutral monosaccharides by gas–liquid chromatography, J. Agric. Food Chem., 1989, 37(2), 360–367 CrossRef CAS.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. t. Rebers and F. Smith, Colorimetric method for determination of sugars and related substances, Anal. Chem., 1956, 28(3), 350–356 CrossRef CAS.
- B. B. Ward, Nitrification, in Reference Module in Earth Systems and Environmental Sciences, Elsevier, 2013 Search PubMed.
- L. W. Belser, Population ecology of nitrifying bacteria, Annu. Rev. Microbiol., 1979, 33(1), 309–333 CrossRef CAS PubMed.
- DOE, Environmental Quality (Industrial Effluent) Regulations 2009, in P.U.(A)434., NRE, Ed., PNMB, Malaysia, 2009.
- J. Vymazal, H. Brix, P. F. Cooper, R. Haberl, R. Perfler and J. Laber, Removal mechanisms and types of constructed wetlands, Constructed wetlands for wastewater treatment in Europe, 1998, pp. 17–66 Search PubMed.
- J. Norton and Y. Ouyang, Controls and Adaptive Management of Nitrification in Agricultural Soils, Front. Microbiol., 2019, 10, 1931 CrossRef PubMed.
- J. M. Norton and J. M. Stark, Chapter Fifteen – Regulation and Measurement of Nitrification in Terrestrial Systems, in Methods in Enzymology, M. G. Klotz, ed. Academic Press, 2011, vol. 486, pp. 343–368 Search PubMed.
- G. V. Subbarao, T. Yoshihashi, M. Worthington, K. Nakahara, Y. Ando, K. L. Sahrawat, I. M. Rao, J.-C. Lata, M. Kishii and H.-J. Braun, Suppression of soil nitrification by plants, Plant Sci., 2015, 233, 155–164 CrossRef CAS PubMed.
- WHO, Guidelines for drinking-water quality, WHO Press, Switzerland, 4th edn, 2011, p. 564 Search PubMed.
- DOE, Malaysia Environmental Quality Report 2014, in NRE, Ed., Misas Advertising Sdn. Bhd., Malaysia, 2015.
- USEPA Table of Regulated Drinking Water Contaminants, https://www.epa.gov/ground-water-and-drinking-water/table-regulated-drinking-water-contaminants#Inorganic, June 20 Search PubMed.
- C. Masclaux-Daubresse, F. Daniel-Vedele, J. Dechorgnat, F. Chardon, L. Gaufichon and A. Suzuki, Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture, Ann. Bot., 2010, 105(7), 1141–1157 CrossRef PubMed.
- Y. S. Ng, N. I. S. Samsudin and D. J. C. Chan, Phytoremediation Capabilities of Spirodela polyrhiza and Salvinia molesta in Fish Farm Wastewater: A Preliminary Study, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 206(1), 012084 Search PubMed.
- A. Castellano-Hinojosa, D. Correa-Galeote, P. Carrillo, E. J. Bedmar and J. M. Medina-Sánchez, Denitrification and Biodiversity of Denitrifiers in a High-Mountain Mediterranean Lake, Front. Microbiol., 2017, 8, 1911 CrossRef PubMed.
- Y. Zhang, X. Wang, W. Wang, Z. Sun and J. Li, Investigation of growth kinetics and partial denitrification performance in strain Acinetobacter johnsonii under different environmental conditions, R. Soc. Open Sci., 2019, 6(12), 191275 CrossRef CAS PubMed.
- E. W. Griffiths, Removal and utilization of wastewater nutrients for algae biomass and biofuels, 2009 Search PubMed.
- L. Delgadillo-Mirquez, F. Lopes, B. Taidi and D. Pareau, Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture, Biotechnol. Rep., 2016, 11, 18–26 CrossRef PubMed.
- Y. S. Ng and D. J. C. Chan, The enhancement of treatment capacity and the performance of phytoremediation system by fed batch and periodic harvesting, RSC Adv., 2021, 11(11), 6049–6059 RSC.
- Y. S. Ng, N. I. S. Samsudin and D. J. C. Chan, Phytoremediation Capabilities of Spirodela polyrhiza and Salvinia molesta in Fish Farm Wastewater: A Preliminary Study, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 206, 012084 CrossRef.
- T. Yeh, T. Ke and Y. Lin, Algal growth control within natural water purification systems: macrophyte light shading effects, Water, Air, Soil Pollut., 2011, 214(1–4), 575–586 CrossRef CAS.
- P. Skillicorn, W. Spira and W. Journey, Duckweed aquaculture: a new aquatic farming system for developing countries, World Bank, Washington, DC, 1993 Search PubMed.
- M. Greenway and A. Woolley, Changes in plant biomass and nutrient removal over 3 years in a constructed free water surface flow wetland in Cairns, Australia, in 7th International conference on wetland system for water pollution control, 2000, 2000 Search PubMed.
- A. Kunihiko, Importance of aquatic macrophytes in controlling water quality of shallow lakes, in Proceedings of the 11th World Lakes Conference—Proceedings, 2006, 2006, pp. 143–147 Search PubMed.
- E. Maltby and T. Barker, The Wetlands Handbook, John Wiley & Sons, United Kingdom (UK), 2009, p. 800 Search PubMed.
- Y. S. Ng, C. R. Lim and D. J. C. Chan, Development of treated palm oil mill effluent (POME) culture medium for plant tissue culture of Hemianthus callitrichoides, J. Environ. Chem. Eng., 2016, 4(4), 4890–4896 CrossRef CAS.
- Y. S. Ng, D. F. M. Farid and D. J. C. Chan, Preliminary laboratory-scale investigation of plant biomass harvesting effect on waste nutrient removal from fish farm wastewater, Eur. Chem. Bull., 2021, 10(3), 171–178 Search PubMed.
- H. van den Berg, E. van Lidth de Jeude, A. Hak, C. Mooren, R. Leen den Uijl, T. Giomi, S. Kraaijeveld and C. Barnes, Duckweed, a tiny aquatic plant with growing potential – a research on the potential applications of duckweed in urban water systems in The Netherlands, Utrecht University, KWR and Waterschap Delfland, Netherlands, 2015, p. 63 Search PubMed.
- N. Stabenau, A. Zehnsdorf, H. Rönicke, H. Wedwitschka, L. Moeller, B. Ibrahim and W. Stinner, A potential phosphorous fertilizer for organic farming: recovery of phosphorous resources in the course of bioenergy production through anaerobic digestion of aquatic macrophytes, Energy Sustain. Soc., 2018, 8(1), 16 CrossRef.
- Y. Yao, M. Zhang, Y. Tian, M. Zhao, B. Zhang, M. Zhao, K. Zeng and B. Yin, Duckweed (Spirodela polyrhiza) as green manure for increasing yield and reducing nitrogen loss in rice production, Field Crop. Res., 2017, 214, 273–282 CrossRef.
- K. Jilimane, Nitrogen and phosphorus release in soil and fertiliser value of lemna minor biomass relative to chicken litter compost, Masters thesis, University of KwaZulu-Natal, 2019.
- C.-h. Li, B. Wang, C. Ye and Y.-x. Ba, The release of nitrogen and phosphorus during the decomposition process of submerged macrophyte (Hydrilla verticillata Royle) with different biomass levels, Ecol. Eng., 2014, 70, 268–274 CrossRef.
- R. Lemoine, Sucrose transporters in plants: update on function and structure, Biochim. Biophys. Acta, Biomembr., 2000, 1465(1), 246–262 CrossRef CAS.
- BBC Photosynthesis – Uses of the sugar produced by photosynthesis, https://www.bbc.co.uk/education/guides/zcktw6f/revision/3, January 21 Search PubMed.
- V. Gollagunta, J. W. Adelberg, J. Rieck and N. Rajapakse, Sucrose concentration in liquid media affects soluble carbohydrates, biomass and storage quality of micropropagated hosta, Plant Cell, Tissue Organ Cult., 2004, 77(2), 125–131 CrossRef CAS.
- T. Ohyama, T. Ikarashi, T. Matsubara and A. Baba, Behavior of carbohydrates in mother and daughter bulbs of tulips (Tulipa gesneriana), Soil Sci. Plant Nutr., 1988, 34(3), 405–415 CrossRef.
- I. Kim, Structural Differentiation of the Connective Stalk in Spirodela polyrhiza (L.) Schleiden, Appl. Microsc., 2016, 46(2), 83–88 CrossRef.
- K.-J. Appenroth, K. S. Sree, V. Böhm, S. Hammann, W. Vetter, M. Leiterer and G. Jahreis, Nutritional value of duckweeds (Lemnaceae) as human food, Food Chem., 2017, 217, 266–273 CrossRef CAS PubMed.
- M. R. Hasan and C. Rina, Use of algae and aquatic macrophytes as feed in small-scale aquaculture: a review, Food and Agriculture Organization of the United Nations (FAO), 2009 Search PubMed.
- MOBOT Duckweed Nutritional Composition, http://www.mobot.org/jwcross/duckweed/nutritional-composition.htm#AminoAcid, August 4 Search PubMed.
- E. Fasakin, A. Balogun and B. Fasuru, Use of duckweed, Spirodela polyrrhiza L. Schleiden, as a protein feedstuff in practical diets for tilapia, Oreochromis niloticus L., Aquacult. Res., 1999, 30(5), 313–318 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Additional tables and figures. See DOI: 10.1039/d1ra00160d |
| This journal is © The Royal Society of Chemistry 2021 |