Open Access Article
Open Access ArticleFacile UV-induced covalent modification and crosslinking of styrene–isoprene–styrene copolymer via Paterno–Büchi [2 + 2] photocycloaddition
Mehmet Arslan *a,
Ozgur Ceylanb,
Rabia Arslana and
Mehmet Atilla Tasdelen
*a,
Ozgur Ceylanb,
Rabia Arslana and
Mehmet Atilla Tasdelen a
a
aDepartment of Polymer Materials Engineering, Faculty of Engineering, Yalova University, 77100 Yalova, Turkey. E-mail: mehmet.arslan@yalova.edu.tr
bCentral Research Laboratory, Yalova University, 77100 Yalova, Turkey
First published on 24th February 2021
Abstract
The chemical functionalization or modification of polymers to alter or improve the physical and mechanical properties constitutes an important field in macromolecular research. Fabrication of polymeric materials via structural tailoring of commercial or commodity polymers that are produced in vast quantities especially possess unique advantages in material applications. In the present study, we report on benign chemical modification of unsaturated styrene–isoprene–styrene (SIS) copolymer using available backbone alkene groups. Covalent attachment of aldehyde functional substrates onto reactive isoprene double bond residues was conveniently carried out using UV-induced Paterno–Büchi [2 + 2] cycloaddition. Model organic compounds with different structures were utilized in high efficiency chemical modification of parent polymer chains via oxetane ring formation. Functionalization studies were confirmed via 1H NMR, FT-IR and SEC analyses. The methodology was extended to covalent crosslinking of polymer chains to obtain organogels with tailorable crosslinking degrees and physical characteristics. Considering the outstanding elastic properties of unsaturated rubbers and their high commercial availability, abundant reactive double bonds in backbone chains of these polymers offer easy to implement structural modification via proposed Paterno–Büchi photocycloaddition.
Introduction
Elastomeric rubbers are indispensable polymeric materials that enable the fabrication of strategically important products in various fields.1 Among these polymers, unsaturated polyolefin elastomers obtained from both natural and synthetic precursors are especially attractive since they carry reactive alkene units in polymer chains that can be utilized in chemical modification and crosslinking.2–5 The primary aim of covalent modification or functionalization has been to widen the structural diversity and improve properties, thus producing novel materials with technical importance. Unsaturated elastomers are adaptable to such chemical manipulations since the olefinic bonds are not only reacting with various other functional groups, but they can also activate neighboring groups.6–11 Crosslinking of unsaturated rubbers on the other hand brings profound property changes to pristine polymer. Depending on the crosslinking degree, the glass transition temperature and melt viscosity increase are associated property enhancements in low crosslinking ratios, whereas heavily crosslinking results in insoluble and infusible materials with outstanding chemical and mechanical resilience.12 Almost in all industrial applications and research studies the chemical modification and crosslinking of unsaturated rubbers rely on chain double bonds in reactions such as cycloadditions, halogenation, hydrogenation, thiol addition, epoxidation, radical induced isomerization and silylation.13 Of these chemical methods, light-triggered synthetic methods are particularly important and have been utilized exclusively in covalent tailoring.Photo-induced methodologies are valuable chemical tools in design and fabrication of precision polymers as well as large volume industrial applications.14 UV- or sunlight activated reactions usually require mild reaction conditions compared to alternative reactions that may necessitate thermal activations or toxic/expensive catalyst usage. In addition, photochemical methods enable spatial and temporal modulations over reactions offering accurate external control in polymer synthesis, modification or crosslinking.15–17 UV-sensitive polymers are used as photoresists in manufacturing of printed circuits.18 Such polymers can be used in UV-patterning and photolithography by employing a photomask to define the UV-exposure area which in return lead to fabrication of biosensors, cell engineering scaffolds and analyte detection tools.19 In conjunction with atom economic addition based chemical transformations; photochemical methods have found diverse applications in modification of industrial unsaturated elastomers.20,21
Photoaddition reactions proceeding on [2 + 2] cycloaddition route between an excited carbonyl and an alkene are referred to as Paterno–Büchi reactions and are important carbon–carbon bond forming synthetic methods in organic chemistry.22,23 Carbonyl compounds employed in these reactions are mainly aldehydes and ketones reacting preferably with electron rich alkenes to give corresponding oxetanes. Despite numerous examples of [2 + 2] cycloaddition based reactions of electron deficient alkenes in synthesis and functionalization of polymeric materials,24–28 excited carbonyl-based photochemical transformations have rarely been applied in polymer modification. In a seminal study, Junkers and coworkers reported facile tailoring of aldehyde functional ATRP polymer end groups with unactivated alkenes via [2 + 2] Paterno–Büchi cycloaddition route.29 Although complete functionalization of aldehyde bearing substrates required large molar excesses of the alkene compounds, the process has shown to be still efficient to achieve close to quantitative functionalization reactions. Same group extended the approach to modification of aldehyde bearing cellulose surfaces with alkene end group functional polymers.30 We envisioned that facile [2 + 2] photocycloaddition reaction can be utilized in grafting of carbonyl containing compounds to unsaturated rubber elastomers which carry abundant alkene groups in polymer backbones. With the possible coalescence of highly practical photocycloaddition with flow chemistry,31 Paterno–Büchi reaction could be a valuable tool for industrial high scale modification of commercial unsaturated polymers.
In this contribution we report on facile UV-induced covalent modification of a commercial unsaturated elastomer, namely styrene–isoprene–styrene copolymer. Efficient and easy to implement methodology is based on [2 + 2] Paterno–Büchi cycloaddition of various aldehyde containing compounds grafted on the available alkene groups of the middle block polyisoprene chain via oxetane formation (Scheme 1). The functionalization of copolymer with various carbonyl substrates was evidenced by 1H NMR, FT-IR and SEC analyses. The approach was extended to crosslinking of polymer chains under UV-illuminated conditions by employing a bisaldehyde functional crosslinker. Analysis of obtained organogels using rheological measurements and crosslinking density calculations via solvent absorption revealed that the properties of the materials can be fine-tuned by changing of the crosslinker stoichiometric ratios. It can be anticipated that reported benign and efficient photocycloaddition modification and crosslinking methodology can be a useful chemical tool in structural tailoring of alkene functional polymers, especially industrial unsaturated elastomers.
Experimental section
Materials and characterization
All the reagents and inorganic materials were obtained from Sigma-Aldrich (USA) and used as received. All the solvents were chromatography grade and obtained from Merck KGaA. Styrene–isoprene–styrene (SIS) copolymer with average 78% isoprene content is kindly received from Kraton Corporation (USA). Aldehyde functional compounds with triethylene glycol monomethyl ether32 and trifluoro33 groups were synthesized based on the reported procedures and the structures were confirmed with 1H and 13C NMR analysis. The size exclusion chromatography (SEC) analyses of polymers were performed by using a Shimadzu GPC analysis system using refractive index detector (RID-10A). PSS Gram separation column was calibrated with polymethyl methacrylate (PMMA) standards and as eluent N,N-dimethylacetamide (DMAc) was used at a flow rate of 1 mL min−1. Nuclear magnetic resonance data (NMR, 1H and 13C) were obtained from a Varian 400 MHz spectrometer. Fourier-transform infrared spectroscopy (FT-IR) data were obtained from a Nicolet 380 spectrometer (Thermo Fisher Scientific, Inc.). Rheological measurements were carried out using an Anton Paar MCR 302 instrument by applying 1.0% strain between 0.05–100 rad s−1 (at 25 °C). Surface morphologies of organogels were investigated by using freeze dried samples without sputtering with an ESEM XL-30 equipment (Philips, Eindhoven, The Netherlands) operating under high vacuum and 10 kV accelerating voltage.General procedure for [2 + 2] photocycloaddition modification of SIS copolymer
In a typical procedure: 100 mg of SIS copolymer (1.0 mmol of alkene groups) was placed in a Pyrex tube and dissolved in THF (2 mL). Corresponding aldehyde (0.2 mmol) and photosensitizer (benzophenone, 0.1 mg) was added to the mixture and the solution was degasses with argon for 15 min. The tube was placed in a multi-lamp photoreactor (12 × 15 W, emitting light nominally at 350 nm, with a cooling system) and stirred for 16 h with a magnetic stirrer. After the reaction, the modified polymer was purified by precipitating into methanol. The polymer was collected and dried under vacuum at 50 °C for overnight. The yields based on gravimetric calculations were near quantitative.General procedure for the crosslinking reaction
300 mg of SIS copolymer (3.0 mmol of alkene groups) and benzophenone (0.3 mg) was dissolved in 5 mL of THF. To this mixture, terephthalaldehyde (changing from 2.5% to 20.0% molar equivalent to alkene groups) was added and the resulting mixture was UV-irradiated in the photoreactor for 16 h. After the crosslinking reaction resulting gels were immersed successively in THF and toluene to remove any unbound residues. The gels were dried under vacuum at 50 °C for overnight. The gel yields based on gravimetric calculations are given in Table 1.| Entry | Gel | Crosslinker (%) | Yield (%) | dp (g cm−3) | Vp | Mc (g mol−1) | χ | ν (×10−5) (mol cm−3) |
|---|---|---|---|---|---|---|---|---|
| 1 | Gel (2.5) | 2.5 | 92 | 0.9130 | 0.0486 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 522 522 |
−0.5168 | 6.75 |
| 2 | Gel (5.0) | 5.0 | 95 | 0.9134 | 0.0594 | 9514 | −0.5207 | 9.60 |
| 3 | Gel (7.5) | 7.5 | 97 | 0.9205 | 0.0683 | 7505 | −0.5240 | 12.27 |
| 4 | Gel (15.0) | 15 | 94 | 0.9560 | 0.0781 | 6126 | −0.5277 | 15.61 |
| 5 | Gel (20.0) | 20 | 99 | 0.9918 | 0.0860 | 5348 | −0.5307 | 18.55 |
| 6 | Control | 0 | No gelation | — | — | — | — | — |
Rheological characterization
Dynamic oscillatory experiments of toluene swollen gels were performed under constant strain (1.0%) by recording the loss (G′′) and storage (G′) moduli of disk-shaped samples at 25 °C between 0.05 and 100 rad s−1 angular frequency. A solvent trap was used to equilibrate the gels and prevent drying. 8 mm diameter parallel plate with a 2.0 mm plate distance was used for measurements.Crosslink density calculations
Crosslink density calculations of organogels were conducted based on Flory–Rehner theory by absorption experiments performed in toluene. Firstly, volume fraction of polymer (Vp), described as the capacity of the gel to enable the diffusion of solvent into the network was calculated by using eqn (1):34
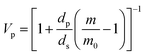 | (1) |
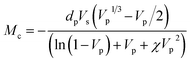 | (2) |
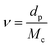 | (3) |
Results and discussion
[2 + 2] photocycloadditions usually require an activated alkene which is typically an excited state enone and an unactivated ene counterpart which can be an aliphatic alkene functionality.36 Although this type substrate combinations allow efficient synthesis of bicyclic molecules, cycloaddition reactions between unactivated alkene pairs are usually ineffective and can only be feasible in case of using special transition metal catalysts.37 Paterno–Büchi cycloaddition is a special type of photo-induced [2 + 2] cycloaddition reaction in which an excited carbonyl reacts with an alkene and this chemistry has found profound applications in organic synthesis.38 Towards our efforts to search efficient and easy-to implement chemical transformation of commercial unsaturated elastomers, Paterno–Büchi photocycloaddition has emerged as a virtual tool in tailoring of abundant alkene groups with carbonyl containing substrates.The photo-induced modification of alkene groups of SIS copolymer was studied under UV illumination with primarily benzaldehyde derivatives (Scheme 1). To better demonstrate the broad structural modification amenity of alkene groups, benzaldehyde and related compounds containing triethylene glycol and trifluorocarbon groups as well as aliphatic butyraldehyde were employed in functionalization reactions. Due to the relatively high stoichiometric ratio between alkene and aldehyde groups that an efficient Paterno–Büchi post-polymerization modification demands,29 the stoichiometry was adjusted so that 0.2 equiv. of aldehyde groups were coupled with residual alkenes on polyisoprene block of copolymer. A small amount of benzophenone as photosensitizer was also employed in photocycloaddition process. After the reactions, the modified polymers were obtained in their pure form with near quantitative yields by simple methanol precipitation. Initially, the functionalization efficiency of copolymer with triethylene glycol aldehyde (Al-TEG) under addressed conditions was studied by FT-IR, 1H NMR and SEC analyses. After the modification, the resulting copolymer SIS-g-TEG (20) (20% mol eq. aldehyde feed to alkenes) displayed characteristics proton signal at 3.37 ppm belonging to methoxy methyl protons and signals between 4.11–3.54 ppm belonging to ethylene oxide protons (Fig. 1A). Oxetane protons as stereo/regio-isomers give resonances in a relatively wide region between 5.37 ppm to 2.73 ppm.29 The functionalization efficiency was calculated as 94.5% (18.9% grafting to alkenes) based on the integrations of SIS c, c′ and a, b protons to f methoxy methyl protons after grafting (Fig. 1A and B). Considering the irregular arrangement of the structural units of polyisoprene block originated from the cis-1,4-, trans-1,4-, 3,4- and 1,2-polymerization modes of isoprene monomer, one can speculate on the regio- and stereospecific addition route of carbonyl groups. From the mechanistic understanding of Paterno–Büchi reaction, successful cycloaddition is governed by several factors including the electron richness of the alkene, relative stability of the adducts, kinetic as well as steric factors.39 Although it can be anticipated that not all alkene modes in polyisoprene block of SIS copolymer display similar reactivity towards carbonyls, results suggested that overall functionalization is still efficient to graft aldehyde bearing compounds.
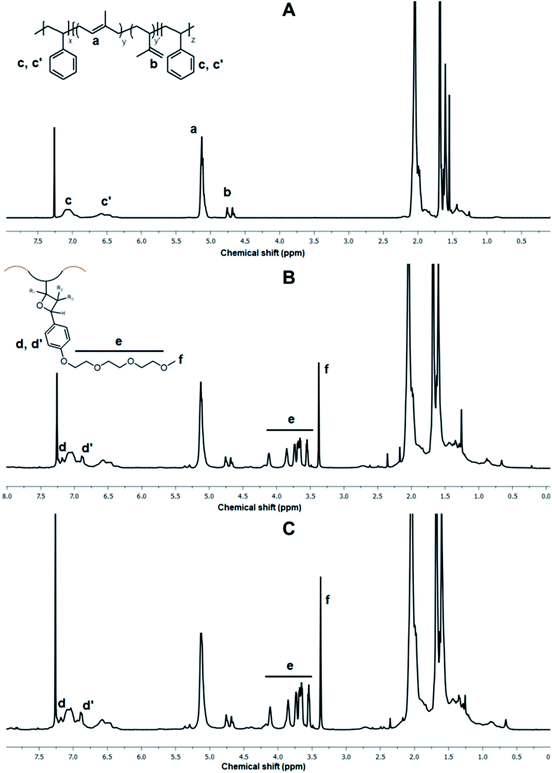 | ||
| Fig. 1 1H NMR spectrum of (A) pristine SIS copolymer, (B) SIS-g-TEG (20) and (C) SIS-g-TEG (30) functionalized copolymers. | ||
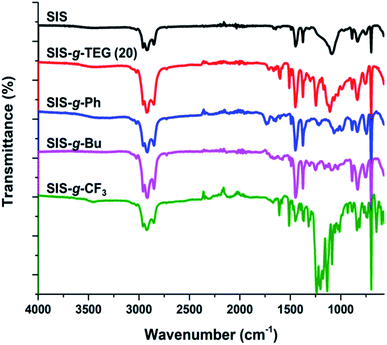
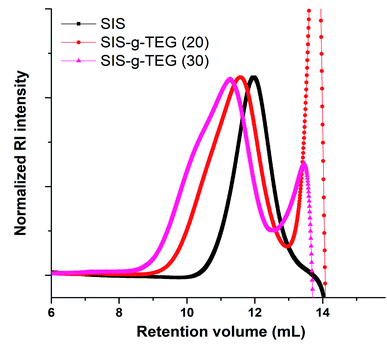
In order to investigate the effect of feed aldehyde concentration to functionalization degree, another copolymer SIS-g-TEG (30) with 30% mol eq. aldehyde to alkenes was also synthesized. 1H NMR analysis revealed 86.1% (25.8% grafting to alkenes) functionalization degree demonstrating the efficiency of the grafting reaction (Fig. 1C). Successful photocycloaddition grafting of aldehyde bearing compound Al-TEG onto residual isoprene double bonds was also studies by FT-IR analysis. As it can be seen in Fig. 2, the modified copolymer SIS-g-TEG (20) displayed characteristic absorption bands at 1247 cm−1 due to the C–O–C stretchings and enhanced bands in the region 1650–1450 cm−1 due to the grafting of aromatic benzaldehyde groups. It is important to note that UV-induced reactions were implemented in oxygen free atmosphere and no unwanted reactions or crosslinking was accounted. SEC analysis of modified polymers revealed unimodal size distributions though slightly higher molecular weight distributions were observed compared to parent SIS copolymer (Fig. 3).
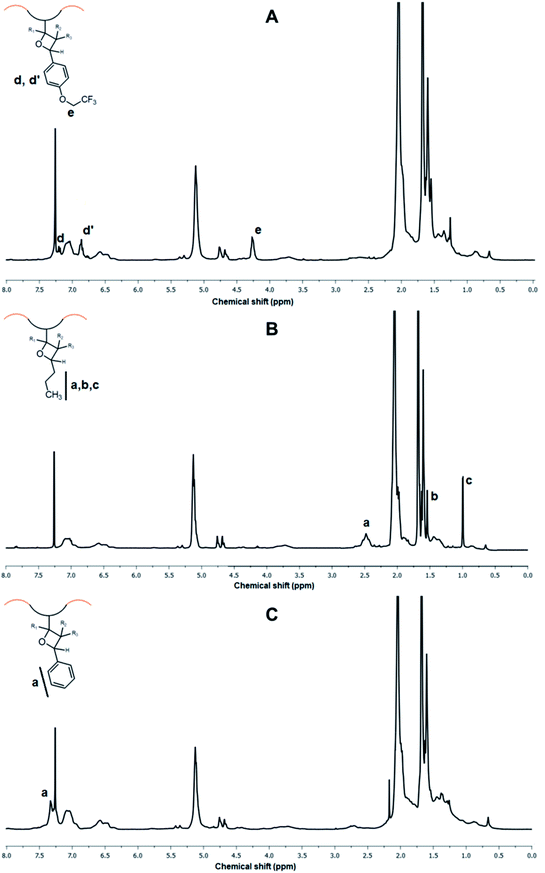
After successful and efficient UV-induced functionalization of SIS copolymer with Al-TEG, we prompted our effort to graft various other functional aldehydes to demonstrate broad structural modification amenity of alkene groups. Compounds Al–CF3, Al–Bu and Al–Ph with 20% feed ratios (20% mol eq. aldehyde feed to alkenes) were employed in [2 + 2] photocycloaddition reactions in above-mentioned reaction conditions (Scheme 1). For all the functional carbonyl compounds employed, grafting was achieved with considerably high functionalization degrees as 16.3%, 14.6% and 15.7% (for aimed 20% grafting to alkenes) for Al–CF3, Al–Bu and Al–Ph, respectively. In 1H NMR spectrum of functionalized copolymer SIS-g-CF3, covalent modification was accounted by the presence of characteristics aliphatic signal at 4.26 ppm and aromatic signals at 7.20 and 6.80 ppm (Fig. 4A). Similarly, appearance of aliphatic signals between 2.46 to 0.98 ppm of SIS-g-Bu after butyraldehyde cycloaddition (Fig. 4B) and phenyl protons at 7.33 ppm of SIS-g-Ph after benzaldehyde cycloaddition (Fig. 4C) demonstrated the successful photo-induced grafting reactions. These functionalization reactions were also followed by FT-IR analysis in which all modified polymers exhibited characteristic absorption bands of both parent SIS copolymer and grafted compounds (Fig. 2).
After demonstrating facile and effective covalent modification of unsaturated elastomer SIS with aldehyde compounds via [2 + 2] cycloaddition, we extended the approach to UV-induced crosslinking of polymer chains with a bisaldehyde containing crosslinker, namely terephthalaldehyde. Unsaturated polymers are indispensable industrial polymers that have important applications in their crosslinked insoluble form. Common drawbacks are the weak mechanical properties and low glass transition temperatures of neat unsaturated elastomers that might impede their practical use. On the other hand, abundant backbone double bonds of such polymers with both natural and synthetic origin provide utility in curing chemistries. However, the complicated curing processes that currently employed and the requirement of toxic additives might obligate more green technologies that have to be pursued. Therefore, UV-promoted, efficient and atom economic Paterno–Büchi cycloaddition could be a suitable alterative chemistry in fabrication of crosslinked rubber elastomers.
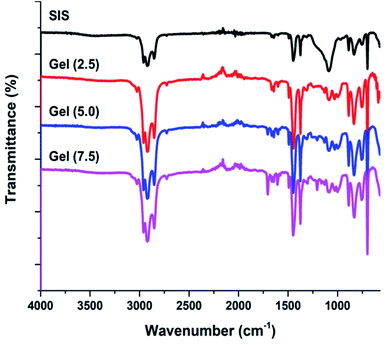
In this context, crosslinking of SIS copolymer in solution state with a bisaldehyde crosslinker was studied by employing various crosslinker ratios (Scheme 2 and Table 1). In these relatively low crosslinker ratios obtained materials are organogels with high solvent absorption profiles. After UV-induced cycloaddition crosslinking, unreacted species were removed by washing the gels with toluene. FT-IR was used to monitor the crosslinking reaction of dried gel samples by observing the variation of aromatic peaks in the region 1650–1450 cm−1. As it is seen in Fig. 5, increased crosslinker ratio resulted in increased aromatic bands in this region due to the involvement of terephthalaldehyde groups in the networks.
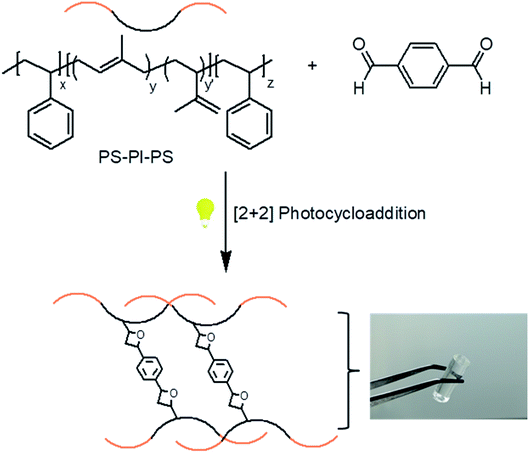 | ||
| Scheme 2 UV-induced crosslinking of SIS copolymer with a bisaldehyde crosslinker. Representative picture of transparent Gel (7.5) in toluene swollen state. | ||
In order to gather further information on the effect of crosslinker feed to the crosslinking efficiencies, the molecular weight between crosslinks (Mc) and the crosslink densities (ν) of gels were calculated from absorption experiments done in toluene. An increased crosslinking density was accounted in case of employing a higher crosslinker feed demonstrating the successful inter-chain photocycloaddition reactions (Table 1). It is important to note that the effect of the crosslinker ratio increase on crosslinking density change is less drastic when a higher crosslinker feed was employed. This can be due to the lowered stoichiometric ratio between alkenes and aldehydes when terephthalaldehyde amount increased, causing less effective cycloaddition crosslinking.29
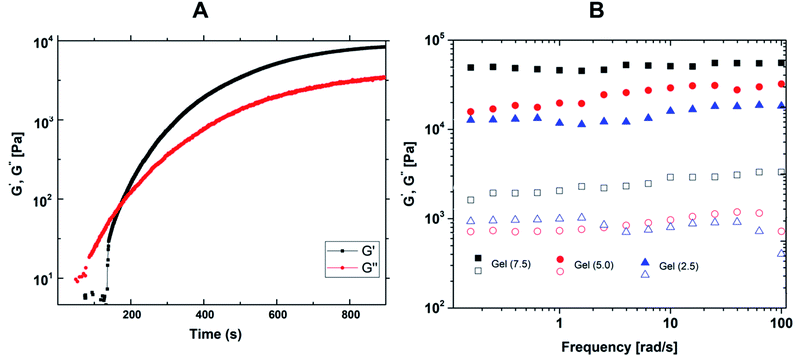
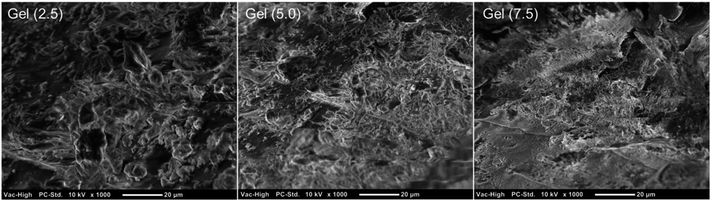
The gelation process was in situ monitored by rheology measurements of model Gel (5.0) system in time sweep test. As it is seen in Fig. 6A, the intersection point of storage (G′) and loss (G′′) moduli to reflect sol to gel transition was achieved within minutes. In dynamic rheological analysis, gels displayed increased storage modulus values by increasing crosslinker ratio (Fig. 6B). Samples exhibited permanent elastic character in 0.01–100 Hz frequency range. Relatively low oscillation frequency dependencies of G′ suggest uniform crosslinking throughout the gel networks. The damping factors (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, G′′/G′) of organogels were less than one in all crosslinking degrees representing the higher elastic character of gel networks over viscous behavior.40 Morphological characterization of organogels on freeze-dried samples revealed non-porous rubbery structures (Fig. 7).
δ, G′′/G′) of organogels were less than one in all crosslinking degrees representing the higher elastic character of gel networks over viscous behavior.40 Morphological characterization of organogels on freeze-dried samples revealed non-porous rubbery structures (Fig. 7).
In the light of obtained results, mild and efficient UV-induced Paterno–Büchi crosslinking reaction was demonstrated as a versatile chemical tool to fabricate crosslinked materials with tailorable properties. This type of organogels could be attractive materials in various applications of industrially important unsaturated elastomers.
Conclusions
In the present study, Paterno–Büchi reaction was reported as a convenient chemical reaction tool to modify unsaturated copolymer elastomers. According to all accounts, the UV-induced cycloaddition of carbonyl compounds onto double bonds of unsaturated SIS copolymer has found to be mild, efficient and easy to implement methodology for both covalent modification of polymer side chains and chemical crosslinking to fabricate organogels. By proper adjusting of the stoichiometric ratio between carbonyl and alkene reacting counterparts, structural tailoring of polymer chains can be achieved. Considering the industrial importance of the unsaturated elastomers, this chemistry could possibly be extended to other unsaturated copolymers of both natural and synthetic origin.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. A. expresses gratitudes to Bogazici University Center for Life Sciences and Technologies to access analytical equipments.References
- B. Guo, Z. Tang and L. Zhang, Prog. Polym. Sci., 2016, 61, 29–66 CrossRef CAS.
- N. Ten Brummelhuis, C. Diehl and H. Schlaad, Macromolecules, 2008, 41, 9946–9947 CrossRef CAS.
- Y. Liu, Z. Tang, Y. Chen, C. Zhang and B. Guo, ACS Appl. Mater. Interfaces, 2018, 10, 2992–3001 CrossRef CAS.
- M. Arslan, G. Acik and M. A. Tasdelen, Polym. Chem., 2019, 10, 3806–3821 RSC.
- M. Arslan and M. A. Tasdelen, Polymers, 2017, 9, 499 CrossRef.
- J. B. Williamson, S. E. Lewis, R. R. Johnson, I. M. Manning and F. A. Leibfarth, Angew. Chem., Int. Ed., 2019, 58, 8654–8668 CrossRef CAS.
- M. Arslan and M. A. Tasdelen, Chem. Afr., 2019, 2, 195–214 CrossRef.
- M. Arslan, T. N. Gevrek, R. Sanyal and A. Sanyal, Eur. Polym. J., 2015, 62, 426–434 CrossRef CAS.
- M. Arslan, D. Aydin, A. Degirmenci, A. Sanyal and R. Sanyal, ACS Omega, 2017, 2, 6658–6667 CrossRef CAS.
- M. Arslan, T. N. Gevrek, A. Sanyal and R. Sanyal, RSC Adv., 2014, 4, 57834–57841 RSC.
- I. I. Yilmaz, M. Arslan and A. Sanyal, Macromol. Rapid Commun., 2012, 33, 856–862 CrossRef CAS.
- Z. Sun, Q. Huang, Y. Wang, L. Zhang and Y. Wu, Ind. Eng. Chem. Res., 2017, 56, 1471–1477 CrossRef CAS.
- A. Brydon and G. G. Cameron, Prog. Polym. Sci., 1975, 4, 209–249 CrossRef CAS.
- S. Dadashi-Silab, S. Doran and Y. Yagci, Chem. Rev., 2016, 116, 10212–10275 CrossRef CAS.
- H. Zhao, J. Sha, X. Wang, Y. Jiang, T. Chen, T. Wu, X. Chen, H. Ji, Y. Gao, L. Xie and Y. Ma, Lab Chip, 2019, 19, 2651–2662 RSC.
- W. Xi, H. Peng, A. Aguirre-Soto, C. J. Kloxin, J. W. Stansbury and C. N. Bowman, Macromolecules, 2014, 47, 6159–6165 CrossRef CAS.
- A. Berthold, K. Sagar and S. Ndoni, Macromol. Rapid Commun., 2011, 32, 1259–1263 CrossRef CAS.
- T. Manouras and P. Argitis, Nanomaterials, 2020, 10, 1593 CrossRef CAS.
- S. Paik, G. Kim, S. Chang, S. Lee, D. Jin, K. Y. Jeong, I. S. Lee, J. Lee, H. Moon, J. Lee, K. Chang, S. S. Choi, J. Moon, S. Jung, S. Kang, W. Lee, H. J. Choi, H. Choi, H. J. Kim, J. H. Lee, J. Cheon, M. Kim, J. Myoung, H. G. Park and W. Shim, Nat. Commun., 2020, 11, 805 CrossRef CAS.
- J. Xu and C. Boyer, Macromolecules, 2015, 48, 520–529 CrossRef CAS.
- M. Tian, H. Yan, H. Sun, L. Zhang and N. Ning, RSC Adv., 2016, 6, 96190–96195 RSC.
- M. Fréneau and N. Hoffmann, J. Photochem. Photobiol., C, 2017, 33, 83–108 CrossRef.
- M. D'Auria, Photochem. Photobiol. Sci., 2019, 18, 2297–2362 CrossRef.
- T. Junkers, Eur. Polym. J., 2015, 62, 273–280 CrossRef CAS.
- G. Delaittre, N. K. Guimard and C. Barner-Kowollik, Acc. Chem. Res., 2015, 48, 1296–1307 CrossRef CAS.
- M. A. Tasdelen, B. Kiskan and Y. Yagci, Prog. Polym. Sci., 2016, 52, 19–78 CrossRef CAS.
- M. A. Tasdelen and Y. Yagci, Angew. Chem., Int. Ed., 2013, 52, 5930–5938 CrossRef CAS.
- N. Cengiz, T. N. Gevrek, R. Sanyal, R. Sanyal, A. Sanyal and A. Sanyal, Bioconjugate Chem., 2020, 31, 1382–1391 CrossRef CAS.
- M. Conradi and T. Junkers, Macromolecules, 2011, 44, 7969–7976 CrossRef CAS.
- M. Conradi, G. Ramakers and T. Junkers, Macromol. Rapid Commun., 2016, 37, 174–180 CrossRef CAS.
- M. Conradi and T. Junkers, Macromolecules, 2014, 47, 5578–5585 CrossRef CAS.
- H. S. Jung, J. Han, H. Shi, S. Koo, H. Singh, H. J. Kim, J. L. Sessler, J. Y. Lee, J. H. Kim and J. S. Kim, J. Am. Chem. Soc., 2017, 139, 7595–7602 CrossRef CAS.
- F. Camps, J. Coll, A. Messeguer and M. A. Pericàs, Synthesis, 1980, 9, 727–728 CrossRef.
- S. M. H. Bukhari, S. Khan, M. Rehanullah and N. M. Ranjha, Int. J. Polym. Sci., 2015, 4, 1–15 Search PubMed.
- H. Mark, Encyclopedia of Polymer Science and Technology, Wiley, Hoboken, New Jersey, USA, 2014, vol. 15 Search PubMed.
- K. Kokubo, H. Yamaguchi, T. Kawamoto and T. Oshima, J. Am. Chem. Soc., 2002, 124, 8912–8921 CrossRef CAS.
- J. M. Hoyt, V. A. Schmidt, A. M. Tondreau and P. J. Chirik, Science, 2015, 349, 960–963 CrossRef CAS.
- M. D'Auria and R. Racioppi, Molecules, 2013, 18, 11384–11428 CrossRef.
- B. Pinter, F. De Proft, T. Veszpremi and P. Geerlings, J. Chem. Sci., 2005, 117, 561–571 CrossRef CAS.
- H. Tan and K. G. Marra, Materials, 2010, 3, 1746–1767 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |