Open Access Article
Open Access ArticleBactericidal and antioxidant bacterial cellulose hydrogels doped with chitosan as potential urinary tract infection biomedical agent
Danica Z. Zmejkoski *a,
Zoran M. Markovića,
Nemanja M. Zdravkovićb,
Dijana D. Trišić
*a,
Zoran M. Markovića,
Nemanja M. Zdravkovićb,
Dijana D. Trišić c,
Milica D. Budimira,
Sanja B. Kuzmana,
Natalia O. Kozyrovska
c,
Milica D. Budimira,
Sanja B. Kuzmana,
Natalia O. Kozyrovska d,
Iryna V. Orlovskad,
Nikol Bugárová
d,
Iryna V. Orlovskad,
Nikol Bugárová e,
Đorđe Ž. Petrovića,
Mária Kováčováe,
Angela Kleinová
e,
Đorđe Ž. Petrovića,
Mária Kováčováe,
Angela Kleinová e,
Zdeno Špitalskýe,
Vladimir B. Pavlovićf and
Biljana M. Todorović Marković*a
e,
Zdeno Špitalskýe,
Vladimir B. Pavlovićf and
Biljana M. Todorović Marković*a
aVinča Institute of Nuclear Sciences, National Institute of the Republic of Serbia, University of Belgrade, P.O.B. 522, 11001 Belgrade, Serbia. E-mail: danica@vinca.rs; biljatod@vinca.rs; biljatod@vin.bg.ac.rs; Tel: +381 113408582
bScientific Veterinary Institute of Serbia, Janisa Janulisa 14, 11107 Belgrade, Serbia
cFaculty of Dental Medicine, University of Belgrade, Dr. Subotića 8, 11000 Belgrade, Serbia
dInstitute of Molecular Biology and Genetics, National Academy of Sciences of Ukraine, 150, Zabolotnogo Str., Kyiv, 03143, Ukraine
ePolymer Institute, Slovak Academy of Sciences, Dúbravska cestá 9, 84541 Bratislava, Slovakia
fFaculty of Agriculture, University of Belgrade, Nemanjina 6, 11080 Belgrade-Zemun, Serbia
First published on 24th February 2021
Abstract
Therapy of bacterial urinary tract infections (UTIs) and catheter associated urinary tract infections (CAUTIs) is still a great challenge because of the resistance of bacteria to nowadays used antibiotics and encrustation of catheters. Bacterial cellulose (BC) as a biocompatible material with a high porosity allows incorporation of different materials in its three dimensional network structure. In this work a low molecular weight chitosan (Chi) polymer is incorporated in BC with different concentrations. Different characterization techniques are used to investigate structural and optical properties of these composites. Radical scavenging activity test shows moderate antioxidant activity of these biocompatible composites whereas in vitro release test shows that 13.3% of chitosan is released after 72 h. Antibacterial testing of BC–Chi composites conducted on Gram-positive and Gram-negative bacteria causing UTIs and CAUTIs (Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae) and encrustation (Proteus mirabilis) show bactericidal effect. The morphology analysis of bacteria after the application of BC–Chi shows that they are flattened with a rough surface, with a tendency to agglomerate and with decreased length and width. All obtained results show that BC–Chi composites might be considered as potential biomedical agents in treatment of UTIs and CAUTIs and as a urinary catheter coating in encrustation prevention.
Introduction
Urinary tract infections (UTIs) are the most common infections in humans that can be caused by bacteria, fungi and viruses.1 Rarely they involve the upper urinary tract and may be life threatening if microbes spread from the kidneys to blood. Mostly UTIs affect the lower tract (urethra and bladder) and cause pain and burning with increased frequency of urination. 80% of bacteria UTIs are caused by Escherichia coli (E. coli), the rest 20% are Proteus mirabilis (P. mirabilis), Klebsiella pneumoniae (K. pneumoniae), Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus (S. aureus) and Enterococcus faecalis (E. faecalis). Catheter associated urinary tract infections (CAUTIs) might be complicated with encrustation when bacteria produce ureases and a form crystal biofilm.2 The most potent producer of ureases is P. mirabilis. The therapy for bacteria UTIs and CAUTIs involves the use of antibiotics. Since one of the main problems of antibiotics usage is the development of bacteria resistance to them, designing a material that will fight multidrug resistance and encrustation is crucial in UTI and CAUTI treatment. The main goal in UTI and CAUTI treatment is to prevent the adhesion of bacteria and further biofilm development.Therefore, the aim of this research was to find an adequate and potential antibacterial agent that will be particularly effective against P. mirabilis, E. coli and P. aeruginosa. One of the possibilities for UTIs and CAUTIs treatment is the usage of polysaccharides and bacterial cellulose composites. Polysaccharides have diverse biological functions and their low toxicity, biocompatibility and high biodegradability are the properties that make them promising biomaterials.3 Among them are chitin, chitosan, alginate, cellulose and cellulose based antimicrobial materials used in research of cosmetics, drug delivery, food packaging, tissue engineering and wound healing.4–7
Bacterial cellulose (BC) is a natural 3D network polymer synthesized by different acetic acid- and other bacteria, mainly by Gluconacetobacter xylinus (formerly Acetobacter xylinum). In comparison with plant cellulose, BC is devoid of hemicellulose and lignin, has higher degree of polymerization and crystallinity (water content up to 99%), insoluble in water and most of organic solvents, melting point of 467 °C, good sorption of liquids, high wet strength, high chemical purity and as biocompatible material,8 it is interesting material in developing of wide range of biomedical applications.9 BC itself does not have antibacterial activity but combined with organic and inorganic materials with antibacterial activity could be used as potential biomedical agent.10
Chitosan (Chi) is linear polysaccharide provided by deacetylation of chitin with 40–50% alkaline solution. Depending of degree of deacetylation and molecular weight chitosan has different physical properties and different antimicrobial effect.11 Since it was found that low molecular weight Chi (Chi oligosaccharides higher than 10 kDa) has better antimicrobial inhibition in comparison to high molecular weight Chi (more than 250 kDa),12 in this study was used low molecular weight Chi (50–190 kDa) to test its effect on bacterial strains the most present in UTIs and CAUTIs. Lin et al.13 produced BC–Chi composites by immersing BC in Chi followed by freeze-drying. These composites showed significant growth inhibition against E. coli and S. aureus. Jia et al.14 prepared composites of Chi chloride and BC by in situ method and showed their moderate bacteriostatic activity against E. coli. Furthermore, the inhibition effect of bacteria growth was significantly enhanced by increasing the Chi content in the composites. Wahid et al.15 developed BC–Chi based semi-interpenetrating hydrogels with improved mechanical and antibacterial properties. The authors found that antibacterial properties of these hydrogels depended significantly on the ratio of BC to Chi.
Since hydrogels coatings increases hydrophilicity and establish a barrier to inhibit nonspecific bacteria protein adsorption,16 it is proposed that BC as hydrogel and good carrier of antimicrobial materials17 might be promising agent in UTIs and CAUTIs. So far, the hydrogel catheter coatings are combined with inorganic, synthetic materials and antibiotics which may cause immunogenicity or simple no effect because of the resistance to antibiotics in the coating.16 The antibacterial hydrogel catheter coatings with natural materials is promising strategy in this field.
In this work, the new designed composite hydrogels of BC and low molecular weight synthetic polymer Chi (BC–Chi) were prepared and studied in details on structural, chemical and biomedical properties. Since the urinary catheter hydrogel coatings are proposed as successful reducer of encrustation, in comparison to pure silicon,18 one of the idea was to incorporate Chi in BC matrix as potential coating for urinary catheters. Different tests have been conducted to check Chi release from BC, antioxidant activity of this composite, biocompatibility as well as its antibacterial potentials against different bacterial strains. The bacterial morphology after the application of control BC and BC–Chi composites was presented by atomic force microscopy. Since antibacterial tests included strains which cause UTIs, CAUTIs and encrustation, this composite is proposed to be potential formulation suitable for application as treatment or prevention of UTIs, CAUTIs and encrustation.
Experimental
Synthesis of bacterial cellulose (BC) and preparing of BC–Chi composites
The bacterial culture Komagataeibacter intermedius IMBG180 (the collection of microorganisms of the Institute of Molecular Biology and Genetics, Kyiv, Ukraine) was used for the cellulose-based film production. After 5 days of incubation at 30 °C in HS medium, the bacterial cellulose (BC) synthesized on the surface of the medium was harvested and purified with the method described in details by Kukharenko et al.19The low molecular weight chitosan (Chi, 50–190 kDa, 75–85% deacetylated, Aldrich, Germany) dissolved in 1% acetic acid (ZORKA Pharma-HEMIJA d.o.o., Serbia) mechanically stirred at room temperature for 3 h was used in preparation of BC–Chi composites. The composites named BC–Chi0.2 and BC–Chi2 were prepared by immersing of BC into 0.2% and 2% Chi solutions for 48 h, respectively. In order to remove the excess of solution the samples were lightly dashed on filter paper and depending of the characterization analysis samples were used as previously described or dried, on air (50 °C for 2 h) or lyophilized (−30 °C for 12 h at 10 mTorr, Mini Fast 680 laboratory freeze-dryer, Edwards Ltd, UK).
Atomic force microscopy (AFM) and scanning electron microscopy (SEM) imaging
Surface morphology measurements of control BC, composite samples and bacteria after the application of control BC and BC–Chi composites were conducted on AFM using Quesant microscope (Ambios Technology, USA) operating in tapping mode in air at room temperature, using a silicone T-shaped cantilever with a spring constant of 40 N m−1 and SEM (JEOL JSM-6390LV) in vacuum at room temperature with accelerating voltage of 15 kV. For AFM and SEM measurements were used air dried and lyophilized composite samples, respectively.For AFM images of bacteria after the application of control BC and BC–Chi composite samples, it was conducted a pilot try-out to determine the appropriate way of cell fixation (hot air, in ethanol, glutaraldehyde and Gram stain). The most suitable method was hot air drying and fixation, where the morphology of cells after the application of control BC were as typical. Surface roughness, diameters and height of control BC, composites BC–Chi0.2 and BC–Chi2 as well as height distribution, length and width of bacteria were calculated by Gwyddion software.
Raman and Fourier transform infrared (FT-IR) spectroscopy
To assess the structural differences between control BC and BC–Chi0.2 and BC–Chi2 composites, Raman and FTIR spectroscopy measurements were conducted. Raman spectra of BC and BC–Chi2 composite samples were recorded on a confocal Raman microscope (Alpha 300 R+, Witec, Ulm, Germany) in the spectral range from 100 to 2000 cm−1. The excitation laser of 785 nm (1 mW power) was focused using the 100× objective onto the sample surface. All samples were recorded in air at room temperature. The Raman spectra were acquired in five different points. FTIR measurements were performed on a Nicolet 8700 spectrometer operated in the ATR mode. The samples were air dried and measurements were performed at room temperature in the range from 400–4000 cm−1 whereas spectral resolution was 4 cm−1.X-ray diffraction (XRD)
The phase composition of lyophilized control BC, BC–Chi0.2 and BC–Chi2 composite samples were examined on Rigaku Smart Lab diffractometer operating with Cu Kα1,2 radiation at 40 mA and 30 kV. XRD data were collected in the 2θ range from 5° to 90°, counting 2° per minute in 0.02° steps. Percent crystallinity obtained by X-ray measurements was defined as the ratio of intensity from the crystalline peaks to the sum of the crystalline and amorphous intensities, by applying the equation:| Xc (%) = (Acr/(Acr + Aam)) × 100 | (1) |
X-photoelectron spectroscopy (XPS)
To check the chemical composition of control BC, BC–Chi0.2 and BC–Chi2 composites all samples were drop-casted on Al foils and XPS measurements were performed on a Thermo Scientific K-Alpha XPS system (Thermo Fisher Scientific, UK).Chi loading in BC–Chi composites
Nine replicates of unloaded BC hydrogel films were dried with filter paper, weighted and immersed in 0.2% and 2% Chi solutions at room temperature for 48 h. After 48 h the loaded hydrogels were weighed and Chi load was determined by using equation:16| Drug loading (%) = ((mt − m0)/m0) × 100 | (2) |
In order to test the statistical differences between the data of Chi loading in BC–Chi0.2 and BC–Chi2 it was used Student t-test.
In vitro release of Chi from BC–Chi composite hydrogels
In vitro release of Chi from the BC–Chi2 composite samples was monitored in 15 mL PBS, pH 7.4, at 37 °C with constant shaking at 100 rpm.20 The composite samples were cut into disks 22 mm in diameter and 1 mm average thickness. Aliquots of the sample (1 mL) were taken at predetermined time intervals (1, 2, 3, 4, 5, 6, 24, 48 and 72 h) and released Chi in dissolution media was determined spectrophotometrically (Shimadzu, Kyoto, Japan) at 290 nm. Immediately after measuring absorbance, aliquots were poured back in dissolution media, to maintain a constant volume. All experiments were performed in duplicate.Antioxidant activity of BC–Chi composites determined by DPPH˙ free radical assay
The DPPH assay was used to predict antioxidant activity. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) is a stable radical with ability to react with free radicals changing its colour from purple to yellow. The control BC and BC–Chi composite sample disks 6 mm in diameter were immersed in 1 mL 100 μM DPPH˙ ethanol solution and incubated in dark for 9 days. The absorption intensity of DPPH˙ was monitored spectrophotometrically (Shimadzu, Kyoto, Japan) at 518 nm in predetermined time intervals (30 min, 1 h, 2 h, 3rd day, 6th day and 9th day). The radical scavenging activity (RSA) of BC–Chi composites was calculated from the following formula:| RSA (%) = (Ac − ABC–Chi)/Ac × 100 | (3) |
Mitochondrial activity (MTT) assay
Human gingival tissues were obtained, and human gingival cells (HGC) were isolated as described previously by Mancic et al.21 Briefly, tissues from healthy donors that provided written consent were minced into 1 mm3 fragments and subjected to outgrowth method. Minced tissue was placed in 25 cm2 culture flasks with growth medium (Gibco Dulbecco's modified Eagle's F12 medium (DMEM/F12) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic/antimycotic (ABAM), all from Thermo Fisher Scientific), and incubated in humidified 5% CO2 atmosphere at 37 °C. The cells were regularly passaged upon reaching 80–90% confluence. The culture medium was changed every 3 days. HGCs after the third passage were used in the study.For assessment of mitochondrial activity, BC, BC–Chi0.2 and BC–Chi2 samples were prepared as described earlier. Prepared discs were placed in 96-well plates, and DMEM/F12 supplemented with 2% ABAM was added to the wells and incubated in humidified 5% CO2 atmosphere at 37 °C for 24 h. Medium was discarded, 5000 HGC cells per well were seeded onto discs in freshly prepared growth medium, and incubated. After 24 h medium was discarded, and medium containing 0.5 mg mL−1 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT, Sigma-Aldrich, St. Louis, USA) was added to each well, and incubated for 4 hours, as previous described by Castiglioni et al.22 After incubation, supernatant was discarded, and dimethyl sulfoxide (Sigma-Aldrich, St. Louis, USA) was added to each well. Plate was placed on shaker for 20 minutes, on 250 rpm, in dark, at 37 °C. Extracted coloured solutions were transferred into new 96-well plate. Optical density was measured at 540 nm using microplate reader RT-2100c (Rayto, China). Six wells without discs were used as internal control of experiment. Percentage of mitochondrial activity was calculated as difference to the control group (BC).
Antibacterial in vitro test of BC–Chi composites
The antibacterial activity of control BC and BC–Chi composite samples was examined on following bacterial strain set of Gram-positive bacteria: Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 29212), Streptococcus agalactiae – group B Streptococcus β haemolytic (SBH, ATCC 27956) as well as Gram-negative bacteria: Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 700603), Proteus mirabilis (ATCC 29906) and Pseudomonas aeruginosa (ATCC 27853). The microorganisms were obtained from Department for Bacteriology and Parasitology, Veterinary Institute of Serbia, Belgrade. Prior experiments, bacterial stocks were cultured on cation adjusted Mueller Hinton Agar (MHA, Mueller Hinton II agar, Becton, Dickinson and Company, USA) at 37 °C for 24 h. ISO 20776-1 method was used. In brief: overnight bacterial cultures were suspended in sterile saline for final cell concentration of 5 × 105 CFU mL−1 (range 2 × 105 CFU mL−1 to 8 × 105 CFU mL−1). The control BC discs (6 mm in diameter, 1 mm in height and with average weight 0.018 g) and composite BC–Chi0.2 and BC–Chi2 discs (6 mm in diameter, 1 mm in height and with average weight of 0.030 g and 0.023 g, respectively) were added to 100 μL cation adjusted Mueller Hinton Broth (MHB, Mueller Hinton II agar, Becton, Dickinson and Company, USA) followed by bacterial inoculum. The minimum inhibitory concentrations (MICs) were determined using sterile polystyrene, 96-well, U bottom, microtitre plates (Salsted, Denmark) and minimum bactericidal concentrations (MBCs) were determined after MIC in subsequent sub-cultivation of 10 μl of broth from wells without growth (i.e. obvious button or definite turbidity) to MHA. Aerobe incubation for 24 h at 37 °C was set for all plates. Exceptions were made for SHB growth media, where MHA supplemented with 5% blood, and MHB with 5% horse serum supplementation were used. The MIC recorded represents the lowest concentration of the samples that completely inhibits visible growth, while MBC represents the lowest concentration of the samples achieving 99.9% killing of the original inoculums (NCCLS 1999), thus growth of less than 5 CFU on subsequent plating indicates MBC. Experiment was performed in quadruplicate.Results and discussion
Physical and chemical characterization of BC–Chi composite hydrogels
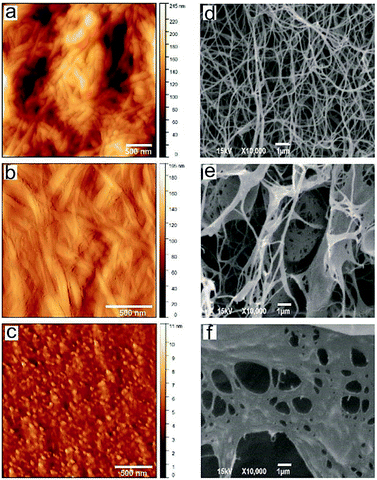
After the drying process (air dried and lyophilized, AFM and SEM respectively) samples were used for characterization. In Fig. 2 are shown top view AFM images and SEM micrographs of control BC and BC–Chi0.2 and BC–Chi2 composites.
The surface morphology of control BC and BC–Chi0.2 showed fibrous texture, whereas BC–Chi2 had granular structure. The control BC with smooth texture was consisted of the interconnected network of fairly uniform in width BC fibers organized into filaments around 120 nm in diameter. Lin et al.23 described that cellulose polymer had a network like structure consisted of glucose chains forming fibers are further aggregating in ribbons (3 nm), and these ribbons, aggregating in filaments (30–100 nm). The BC–Chi composites exhibited rough texture with average surface roughness 26.04 nm and 0.8 nm, for BC–Chi0.2 and BC–Chi2, respectively. The control BC showed the highest average roughness of 36.04 nm. The SEM images showed a Chi concentration-dependent decrease in the amount of pores in the composite samples. BC–Chi2 is almost completely covered by Chi and lacked in pores.
Chemical composition
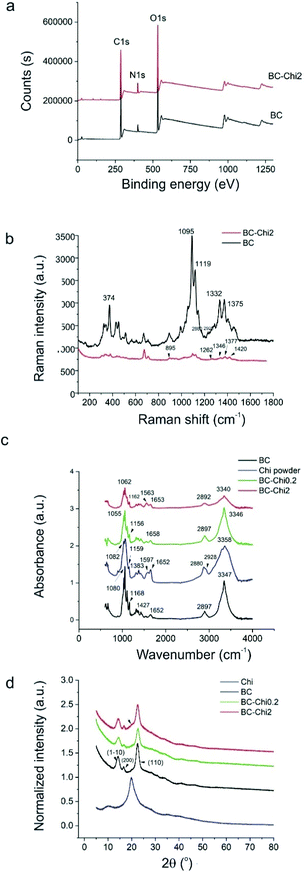
To determine chemical composition of BC, BC–Chi0.2 and BC–Chi2 composites, detailed investigation was performed by XPS, Raman and FTIR spectroscopy and XRD. Fig. 3 shows XPS, Raman and FTIR spectra of all samples as well as their XRD patterns. XPS survey spectra of BC and BC–Chi2 samples are shown in Fig. 3a. The content of detected elements in both samples is listed in Table 1.| Control BC | Binding energy (eV) | Atomic (%) | BC–Chi2 | Binding energy (eV) | Atomic (%) |
|---|---|---|---|---|---|
| O 1s | 533.0 | 36.8 | O 1s | 532.4 | 29.4 |
| C 1s | 286.8 | 59.5 | C 1s | 286–0 | 62.5 |
| N 1s | 400.2 | 3.7 | N 1s | 399.4 | 8.1 |
As can be seen from Fig. 3a, C 1s, O 1s and N 1s peaks are detected in the survey XPS spectra. After Chi incorporation in the BC the content of carbon increases for 3 Atomic% but the content of nitrogen increases for 4.4 Atomic%. The higher nitrogen content after Chi incorporation in BC indicates that this macromolecule is predominantly adhered to the surface fibrils of BC due to high viscosity.24
The Raman spectra of BC and BC–Chi2 composites are presented in Fig. 3b. After incorporation of Chi in BC the most representative peaks of BC are reduced (374, 1095, 1119, 1332, 1375 cm−1).25 The Chi characteristic peaks are overlapped with BC peaks (895, 1262, 1346, 1377, 1420 cm−1).26 There are small upshifts of these peaks (3–5 cm−1) compared to pure Chi. The Chi characteristic peaks in BC–Chi0.2 sample could not be identified due to small Chi concentration and overlapping with characteristic Raman BC peaks.
Fig. 3c presents FTIR spectra of BC, Chi powder and BC–Chi samples. In the FTIR spectra of BC we detected all peaks characteristic for BC: 3347 cm−1 (O–H vibrations), 2897 cm−1 (C–H vibrations), 1652 cm−1 stem from water molecules in the amorphous region, 1427 cm−1 (C–H vibrations), 1168 cm−1 (vibration from the β-anomeric link) and 1080 cm−1 (C–O–C stretching vibrations).24 As for peaks refers to Chi powder we detected the following peaks: 3358 cm−1 (O–H vibrations), 2928 and 2880 cm−1 (C–H stretching vibrations), 1652 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations), 1597 cm−1 (N–H vibrations), 1383 cm−1 (C–H vibrations), 1159 cm−1 and 1082 cm−1 (C–O–C vibrations).
O vibrations), 1597 cm−1 (N–H vibrations), 1383 cm−1 (C–H vibrations), 1159 cm−1 and 1082 cm−1 (C–O–C vibrations).
In the FTIR spectra of BC–Chi0.2 and BC–Chi2 composites can be observed the peaks at: 3346 and 3340 cm−1 (O–H vibrations), 2897 and 2892 cm−1 (C–H vibrations), 1658 and 1653 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations), 1563 cm−1 (N–H vibrations), 1156 and 1162 cm−1, 1055 and 1062 cm−1 (C–O–C stretching vibrations).26,27 The characteristic peak for Chi powder sample at 1597 cm−1 is down shifted for BC–Chi2 sample at 1563 cm−1. As for BC–Chi0.2 sample this peak exists but its intensity is low.28
O vibrations), 1563 cm−1 (N–H vibrations), 1156 and 1162 cm−1, 1055 and 1062 cm−1 (C–O–C stretching vibrations).26,27 The characteristic peak for Chi powder sample at 1597 cm−1 is down shifted for BC–Chi2 sample at 1563 cm−1. As for BC–Chi0.2 sample this peak exists but its intensity is low.28
XRD patterns of control BC, Chi powder, BC–Chi0.2 and BC–Chi2 composite samples are shown in Fig. 3d. Chi powder shows three peaks at 10.1°, 19.8° and 40.6° (blue curve). The control BC, BC–Chi0.2 and BC–Chi2 samples were amorphous or with disordered-crystalline phase but showed peaks at 14.2°, 16.7° and 22.5°, corresponding to the (1–10), (200) and (110) Miller indices of microbial cellulose.29 The absence of Chi characteristic peaks at 10.1° and 19.8° in BC–Chi0.2 composite sample (green curve) suggests that crystalline form of Chi is converted into amorphous form and that Chi is incorporated in BC matrix. As for BC–Chi2 sample (red curve), it can be observed the absence of peak at 10.1° but peak at 19.8° can be observed as a shoulder of BC peak (designated with black arrow in Fig. 3d – red curve). The amorphous structure of composites is essential and provides more solubility, stability and it is strong associated with drug release rate (in detail Section In vitro release of Chi from BC–Chi composites). Since antimicrobial agents require water for activity, improving the water solubility of Chi by chemical modification (alkylation, acylation, quaternization, metallization…) its biomedical application is widened.30 The Chi crystallinity depends on the degree of deacetylation.31 Namely, Chi chains with higher degree of deacetylation are more compact thus enables hydrogen bond formation, which is affected by the content of glucosamine groups. The degree of crystallinity (Xc, in percentage) for Chi powder, control BC, BC–Chi0.2 and BC–Chi2 composite samples were 99.0, 62.6, 68.6 and 68.9%, respectively. Thus the Chi incorporation into BC matrix contributed to slight improvement of BC crystallinity and showed concentration-dependent trend. The values of full width at half maximum (FWHM) for Chi powder, control BC, BC–Chi0.2 and BC–Chi2 composite samples are presented in Table 2.
| 2θ | FWHM | |
|---|---|---|
| Chi powder | 10.1 | 2.89 |
| 19.8 | 4.75 | |
| 40.6 | 7.38 | |
| Control BC | 14.2 | 1.74 |
| 16.7 | 0.92 | |
| 22.5 | 2.17 | |
| BC–Chi0.2 | 14.2 | 1.67 |
| 16.7 | 1.03 | |
| 22.5 | 2.27 | |
| BC–Chi2 | 14.2 | 1.62 |
| 16.7 | 0.79 | |
| 22.5 | 2.68 |
The broadened characteristic peak of BC at 22.5° for BC–Chi2 sample (red curve in Fig. 3d) indicates the successful incorporation of Chi into BC fibrous matrix.
By conducting different techniques to check chemical composition of control BC, BC–Chi0.2 and BC–Chi2 composites we established that Chi was found not only on the composite surface but also in the interior of BC matrix.
Chi loading in BC–Chi composites
The results of Chi loading in BC–Chi composites show that BC is loaded more with Chi from 0.2% Chi solution then from 2% Chi solution. The Chi loading in BC–Chi0.2 was 57.68% and in BC–Chi2 was 42.08%. However, statistical analysis show no differences in Chi loading between this two composites.In vitro release of Chi from BC–Chi composites
In Fig. 4a, the release profile of Chi from BC–Chi2 composites is shown. In the first hour of monitoring it is released less than 3% of Chi. During the first 24 h, the total released is 8.8% and in the next two days it is slowly released 5% more. At the end of monitoring time (72 h) it is released 13.3% of Chi. In order to characterize the mechanism of Chi release, the experiment data were fitted into different mathematical models: zero-order, first-order, second order, Higuchi model and Korsmeyer–Peppas kinetic model. The correlation coefficient (R2) was in range of 0.6536–0.9971 for mentioned formulations and the highest value (R2 = 0.997) was obtained for the Korsmeyer–Peppas model, the mathematical model for release behaviour of drug from hydrophilic polymeric matrix, with equation: Mt/M∞ = Kmtn, where Mt is an amount of drug released at time t in hours, M∞ is a total amount of drug in dosage form, Km is a kinetic constant and n is the release exponent related to the mechanism of the release. The n value (n = 0.374) was smaller than 0.5 (Fig. 4b) indicating that the release of Chi from BC–Chi composite can be characterized as a quasi-Fickian diffusion.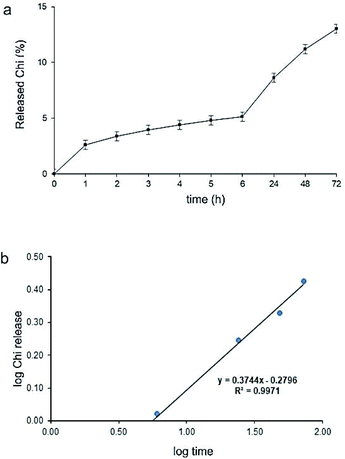 | ||
| Fig. 4 (a) Release profile of Chi from the BC–Chi composites; (b) Chi release data fitted to Korsmeyer–Peppas mathematical kinetic model. | ||
From all obtained results it is obvious that Chi was constantly released in small amount during the 72 h and that release of Chi is partially directed by diffusion from BC dependent on swellabillity of hydrogels. Insolubility of Chi in PBS and electrostatic interaction with BC might be the reason of very slow Chi releasing. Cabañas-Romero et al.32 showed also very small amount of Chi release (5%) from BC–Chi composites after 96 h incubation in aqueous medium. The release of Chi depends of its molecular weight and the pH of solution.33 The authors showed that low molecular weight Chi leakages more from composites and in solution with pH < 7.4 the releasing profile is much higher than in alkaline condition, which is in correspondence to the solubility of Chi in acidic medium. Since, pH of urine in UTIs and CAUTIs is in range of 8.5 to 9, BC–Chi composites with slow Chi release makes it suitable for maintaining antimicrobial capacity. In further studies, it will be monitored for how long Chi can diffuse from hydrogel.
Antioxidant property of BC–Chi composites – DPPH scavenging assay
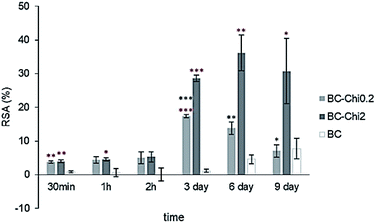
DPPH˙ (2,2-diphenyl-1-picryl-hydrazyl-hydrate) is free radical stable at room temperature which in antioxidant assay produces a violet solution in ethanol. It is used in easy and rapid antioxidant assay where in presence of an antioxidant molecule the ethanol solution become colourless and evaluation of antioxidant activity is evaluated spectrophotometrically at 518 nm.From these results it is clear that even control sample, BC itself shows low antioxidant activity, but in comparison to composite samples it is obvious that Chi shows significant role in DPPH˙ scavenging activity (Fig. 5). Concentration dependent antioxidant activity of BC–Chi0.2 and BC–Chi2 composites show peaks at 3rd and 6th day with RSA values 17% and 36%, respectively. Since the composite samples were immersed in DPPH˙ solution during the whole experiment and monitoring time of 9 days, the hydrogels swelled and depending of the Chi incorporated and/or covering the BC in composites this ability was more or less rapid. In the case of BC–Chi0.2 where the BC filaments were not totally covered by Chi (in correspondence with Fig. 2b and e), the swelling of hydrogel was faster and the maximum antioxidant activity was detected on 3rd day. The composite BC–Chi2, totally covered with Chi (in correspondence with Fig. 2c and f), swelled slower and the maximum radical scavenging activity was observed on 6th day. In many studies is already confirmed antioxidant effect of Chi,34 but for BC it was only cited in study Schönfelder et al.35 and described as little antioxidant capacity against ROS. Recently, Yin et al.36 reported weak antioxidant activity of the BC–Chi composites. But, Cabañas-Romero et al.32 claim that BC–Chi paper has very good antioxidant property due to mainly amino groups residue from Chi and secondarily to OH groups of Chi that have the capacity to scavenge radicals. Since UTIs cause oxidative stress, increase lipid peroxidation level, and lead to insufficiency of antioxidant enzymes37 the BC–Chi composites may be consider as biomedical agent in UTIs treatment.
Cytotoxicity test
The BC–Chi0.2 and BC–Chi2 composites do not significantly affect mitochondrial activity of cells seeded directly onto materials in comparison to control disks (BC), as shown in Fig. 6. After 24 h of cells direct exposure to the investigated materials, mean mitochondrial activity was over 85% for both concentrations of the tested material. The biocompatibility results are in correlation with already published literature with BC–Chi composites.13,14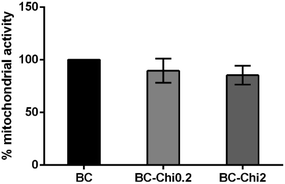 | ||
| Fig. 6 Mitochondrial activity 24 h after direct cell seeding on BC, BC–Chi0.2, and BC–Chi2 samples. One-way ANOVA revealed no statistical differences between groups, p < 0.05. | ||
Antibacterial in vitro tests
The antibacterial activity, presence of inhibition and bactericidal effect, of control BC, BC–Chi0.2 and BC–Chi2 composite samples are shown in Table 3. The both groups of BC–Chi composite samples exhibited both the inhibition and bactericidal activity on all tested Gram-positive and Gram-negative strains. The smaller dose of Chi in composites showed both inhibition and bactericidal effect on P. mirabilis, P. aeruginosa, E. faecalis and Str. beta hemo. In the study Kingkaew et al.,33 it was showed that BC–Chi films had no inhibitory effect on the growth of E. coli. The differences in response to Chi between Gram-negative and Gram-positive bacteria are showed in many studies. Wang et al.38 designed chitosan–Zn complex and showed no significant differences of the effect against Gram-negative and Gram-positive bacteria but there were many studies where was stronger antibacterial activity against Gram-negative bacteria than against Gram-positive bacteria.39 Gram-negative bacteria have more negative charge on the cell surface than Gram-positive bacteria and a lot of positively charged amine groups of Chi strongly interact with carboxyl-negative charges, change the membrane permeability, leads to its destabilization, mostly followed with leakage of intracellular substances and the cells death which is in correspondence with higher inhibitory effect of Chi against Gram-negative bacteria.40 The binding of Chi also promptly neutralizes and even reverses the surface charge of the bacteria,41 but also the chitosan-mediated chelation of metal ions combined with cell wall molecules crucial for cell wall stability are often been implicated as a possible mode of antimicrobial action.42| Bacteria | BC–Chi | Control BC | Genta, ng μL−1 | |
|---|---|---|---|---|
| 0.2% | 2% | |||
| MIC/MBCb | MIC/MBC | MIC/MBC | MIC/MBC | |
| a “+” the effect was observed; “−” the effect was not observed.b MIC – minimum inhibitory concentration; MBC – minimum bactericidal concentration. | ||||
| S. aureus | +/+a | +/+ | −/− | 0.156/0.156 |
| E. coli | +/+ | +/+ | −/− | 0.313/0.625 |
| K. pneumoniae | +/+ | +/+ | −/− | 5/5 |
| P. mirabilis | +/+ | +/+ | −/− | 0.625/0.625 |
| P. aeruginosa | +/+ | +/+ | −/− | 0.625/0.625 |
| E. faecalis | +/+ | +/+ | −/− | 5/10 |
| Str. β hemo | +/+ | +/+ | −/− | 2.5/5 |
In other studies, it was also reported good antibacterial potentials against S. aureus and E. coli36 and against P. aeruginosa and yeast Candida albicans.33
Fig. 7 presents AFM images of P. mirabilis and P. aeruginosa morphology treated with BC (control) samples and BC–Chi2 composite samples. It can be seen that both strains after the application of BC–Chi2 composites were with rough surface and agglomerated. The height distribution of P. mirabilis control and BC–Chi2 treated was almost similar, 0.18 μm and 0.22 μm, respectively. In the case of P. aeruginosa after the BC–Chi2 treatment the height distribution was decreased to 0.04 μm (for control samples the height distribution was 0.36 μm). This confirmed that after the treatment with BC–Chi2 composites P. aeruginosa was flattened. Also, both bacterial strains were shrunk after the application of composites. The length and width of P. mirabilis was decreased after the BC–Chi2 composite application (control length and width 1.92 μm and 1.15 μm; after the BC–Chi2 application length and width 0.78 μm and 0.38 μm). The length of P. aeruginosa treated with BC–Chi2 composite was also decreased to 1.14 μm (control 1.65 μm), while the width was almost similar 0.79 μm (control 0.75 μm). From all the obtained results, this new BC–Chi composites might be considered as coating for urinary catheters for prevention of encrustation in CAUTIs, since it was showed bactericidal effect on strain P. mirabilis, the strain responsible for encrustation. In the review43 authors cited the potential coatings for urinary catheters and discussed their effectiveness in preventing biofilm forming.
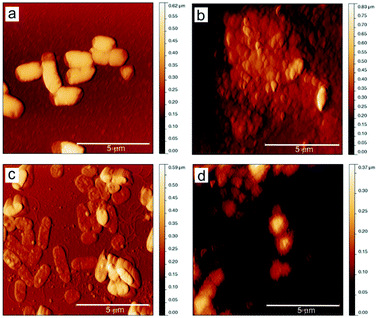 | ||
| Fig. 7 Top view AFM images of P. mirabilis and P. aeruginosa before (a and c) and after (b and d) the application of BC–Chi2 composite. | ||
The mechanism how BC–Chi composite acts against these bacteria might be by binding to the negatively charged bacterial cell wall disrupting cell membrane and resulting in leakage of intracellular components and cell death, which is in correlation with already published results.44
Conclusions
In this study, the composites of bacterial cellulose and low molecular weight synthetic polymer chitosan (BC–Chi) were prepared and characterized in detail. XPS, Raman, FTIR and XRD measurements of new designed composites show successful chitosan polymer incorporation in bacterial cellulose matrix.The composites show strong antibacterial activity against strains the most found in patients with bacterial urinary tract infections, where bactericidal effect is achieved on all other tested strains. The most challenging results are bactericidal effect on P. mirabilis, the strain responsible for encrustation in catheter associated urinary tract infections, as well as on the most frequent strains in urinary tract infections E. coli and P. aeruginosa. The AFM images of bacterial morphology after the application of BC–Chi composites show their alternation, where they are shrunk, flattened and agglomerated. The putative mechanisms of the Chi antibacterial activity are hidden in the interaction of the BC–Chi composites with the bacterial outer membrane, as well as they are associated with a number of factors that act in an orderly independent fashion. These BC–Chi composites show very good RSA activity with the peak at 36% after the 6th day of observation. In in vitro conditions, chitosan is constantly released in small amount during the 72 h and that release is partially directed by diffusion from BC-dependent swellabillity of hydrogels. Obtained results indicate high potential of these composites for successful elimination of bacteria and their promising usage in UTIs and CAUTIs treatments, as well as urinary catheter coating in prevention of encrustation.
Author contributions
Danica Z. Zmejkoski: conceptualization, methodology, formal analysis, investigation, data curation, writing – original draft. Zoran M. Marković: methodology, investigation, supervision, validation, writing – review & editing. Nemanja M. Zdravković: methodology, formal analysis. Dijana D. Trišić: methodology, formal analysis. Milica D. Budimir: methodology, data curation, visualization, formal analysis. Sanja B. Kuzman: methodology, data curation, formal analysis, software. Natalia O. Kozyrovska: resources, writing – review & editing. Iryna V. Orlovska: resources. Nikol Bugárová: methodology, formal analysis. Đorđe Ž. Petrović: methodology. Mária Kováčová: methodology. Angela Kleinová: methodology. Zdeno Špitalský: resources. Vladimir B. Pavlović: methodology, visualization. Biljana M. Todorović Marković: conceptualization, funding acquisition, investigation, project administration, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors thank for support to the Ministry of Education, Science and Technological Development of the Republic of Serbia [451-03-2/2020-14/20-0302002 and TR31079]. Author Biljana Todorović Marković also thanks for funding by a STSM grant from the COST Action CA16217 “ENIUS” and funded by COST (European Cooperation in Science and Technology). Authors Zdeno Špitalský and Mária Kováčová thank for support through VEGA project no. 2/0051/20.Notes and references
- A. L. Flores-Mireles, J. N. Walker, M. Caparon and S. J. Hultgren, Nat. Rev. Microbiol., 2015, 13, 269 CrossRef CAS.
- Y. J. Cortese, V. E. Wagner, M. Tierney, D. Devine and A. Fogarty, J. Healthc. Eng., 2018, 2018, 2986742 CrossRef.
- S. M. Mozammil Hasnain, M. S. Hasnain and A. K. Nayak, Natural Polysaccharides in Drug Delivery and Biomedical Applications, Academic Press, 2019 Search PubMed.
- M. H. Periayah, A. S. Halim and A. Z. Mat Saad, Pharmacogn. Rev., 2016, 10, 39 CrossRef CAS.
- H. Ullah, F. Wahid, H. A. Santos and T. Khan, Carbohydr. Polym., 2016, 150, 330 CrossRef CAS.
- D. Zmejkoski, D. Spasojević, I. Orlovska, N. Kozyrovska, M. Soković, J. Glamočlija, S. Dmitrović, B. Matović, N. Tasić, V. Maksimović, M. Sosnin and K. Radotić, Int. J. Biol. Macromol., 2018, 118, 494 CrossRef CAS.
- D. Zhao, T. Zhu, J. Li, L. Cui, Z. Zhag, X. Zhuang and J. Ding, Bioact. Mater., 2021, 6, 346 CrossRef.
- R. A. Pértile, S. Moreira, R. M. Gil da Costa, A. Correia, L. Guãrdao, F. Gartner, M. Vilanova and M. Gama, J. Biomater. Sci., Polym. Ed., 2012, 23, 1339 CrossRef.
- G. F. Picheth, C. L. Pirich, M. R. Sierakowski, M. A. Woehl, C. N. Sakakibara, C. F. de Souza, A. A. Martin, S. R. Da and R. A. de Freitas, Int. J. Biol. Macromol., 2017, 104, 97 CrossRef CAS.
- S. M. Fijul Kabir, P. Partha Sikdar, B. Haque, M. A. Rahman Bhuiyan, A. Ali and M. N. Islam, Prog. Biomater., 2018, 7, 153 CrossRef.
- L. Y. Zheng and J. F. Zhu, Carbohydr. Polym., 2003, 54, 527 CrossRef CAS.
- N. Liu, X. G. Chen, H. J. Park, C. G. Liu, C. S. Liu, X. H. Meng and L. J. Yu, Carbohydr. Polym., 2006, 64, 60 CrossRef CAS.
- W. C. Lin, C. C. Lien, H. Jen Yeh, C. M. Yu and S. H. Hsu, Carbohydr. Polym., 2013, 94, 603 CrossRef CAS.
- Y. Jia, X. Wang, M. Huo, X. Zhai, F. Li and C. Zhong, Nanomater. Nanotechnol., 2017, 7, 1 CAS.
- F. Wahid, X. H. Hu, L. Q. Chu, S. R. Jia, Y. Y. Xie and C. Zhong, Int. J. Biol. Macromol., 2019, 122, 380 CrossRef CAS.
- N. A. Peppas and B. D. Barr-Howell, Hydrogels in Medicine and Pharmacy, CRC Press Inc., Boca Raton, Florida, 1st edn, 1986, p. 27 Search PubMed.
- S. Li, S. Dong, W. Xu, S. Tu, L. Yan, C. Zhao, J. Ding and X. Chen, Adv. Sci., 2018, 5, 1700527 CrossRef.
- M. Talja, A. Korpela and K. Jarvi, Br. J. Urol., 1990, 66, 652 CAS.
- O. Kukharenko, J. F. Bardeau, I. Zaets, L. Ovcharenko, O. Tarasyuk, S. Porhyn, I. Mischenko, A. Vovk, S. Rogalsky and N. Kozyrovska, Eur. Polym. J., 2014, 60, 247 CrossRef CAS.
- Food and Drug Administration, Center for Drug Evaluation and Research, Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage, August 1997 Search PubMed.
- L. Mancic, A. Djukic-Vukovic, I. Djinic, M. G. Nikolic, M. D. Rabasovic, A. J. Krmpot, A. M. L. M. Costa, D. Trisic, M. Lazarevic, Lj. Mojovic and O. Milosevic, Mater. Sci. Eng., C, 2018, 91, 597 CrossRef CAS.
- S. Castiglioni, C. Caspani, A. Cazzaniga and J. A. Maier, World J. Biol. Chem., 2014, 5, 457 CrossRef.
- S. P. Lin, I. L. Calvar, J. M. Catchmark, J. R. Liu, A. Demirci and K. C. Cheng, Cellulose, 2013, 20, 2191 CrossRef CAS.
- X. Liu, Y. Wang, Z. Cheng, J. Sheng and R. Yang, Carbohydr. Polym., 2019, 214, 311 CrossRef CAS.
- Y. C. Hsieh YC, H. Yano, M. Nogi and S. J. Eichhorn, Cellulose, 2008, 15, 507 CrossRef.
- N. L. G. D. Souza, T. F. Salles, H. M. Brandão, H. G. M. Edward and F. C. de Oliveira Luiz, J. Braz. Chem. Soc., 2015, 26, 1247 CAS.
- S. C. Dey, M. Al-Amin, T. U. Rashid, M. Z. Sultan, M. Ashaduzzaman, M. Sarker and S. M. Shamsuddin, Int. J. Eng. Res. Sci. Technol., 2016, 2, 52 Search PubMed.
- K. J. Raphaël and A. Meimandipour, Iran. J. Biotechnol., 2017, 15, 111 CrossRef.
- M. W. Ullah, M. Ul-Islam, S. Khan, Y. Kim and J. K. Park, Carbohydr. Polym., 2016, 136, 908 CrossRef CAS.
- Y. J. Xie, X. F. Liu and V. Chen, Carbohydr. Polym., 2007, 69, 142 CrossRef CAS.
- C. Bangyekan, D. A. Ong and K. Srikulkit, Carbohydr. Polym., 2006, 63, 61 CrossRef CAS.
- L. V. Cabañas-Romero, C. Valls, S. V. Valenzuela, M. B. Roncero, F. I. Javier Pastor, P. Diaz and J. Martínez, Biomacromol, 2020, 21, 1568 CrossRef.
- J. Kingkaew, S. Kirdponpattara, N. Sanchavanakit, P. Pavasant and M. Phisalaphong, Biotechnol. Bioprocess Eng., 2014, 19, 534 CrossRef CAS.
- W. Yang, J. S. Owczarek, E. Fortunati, M. Kozanecki, A. Mazzaglia and G. M. Balestra, Ind. Crops Prod., 2016, 94, 800 CrossRef CAS.
- U. Schönfelder, M. Abel, C. Wiegand, D. Klemm, P. Elsner and U. C. Hipler, Biomaterials, 2005, 26, 6664 CrossRef.
- N. Yin, R. Du, F. Zhao, Y. Han and Z. Zhou, Carbohydr. Polym., 2020, 229, 115520 CrossRef CAS.
- E. B. Kurutas, P. Ciragil, M. Gul and M. Kilinc, Mediators Inflammation, 2005, 4, 242 CrossRef.
- X. H. Wang, Y. M. Du and H. Liu, Carbohydr. Polym., 2004, 56, 21 CrossRef CAS.
- Y. C. Chung, Y. P. Su, C. C. Chen, G. Jia, H. L. Wang, J. C. G. Wu and J. G. Lin, Acta Pharmacol. Sin., 2004, 25, 932 CAS.
- Y. J. Jeon, P. J. Park and S. K. Kim, Carbohydr. Polym., 2001, 44, 71 CrossRef CAS.
- C. Z. S. Chen and S. L. Cooper, Biomaterials, 2002, 23, 3359 CrossRef CAS.
- E. I. Rabea, M. E. T. Badawy, C. V. Stevens, G. Smagghe and W. Steurbaut, Biomacromolecules, 2003, 4, 1457 CrossRef CAS.
- M. J. Andersen and A. L. Flores-Mireles, Coatings, 2020, 10, 23 CrossRef CAS.
- M. Hosseinnejad and S. M. Jafari, Int. J. Biol. Macromol., 2016, 85, 46 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |