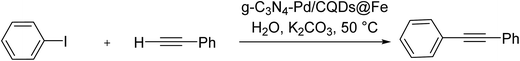Open Access Article
Open Access ArticleCopper-free Sonogashira cross-coupling reactions: an overview
Fatemeh Mohajer
 ,
Majid M. Heravi
,
Majid M. Heravi
 *,
Vahideh Zadsirjan
and
Nargess Poormohammad
*,
Vahideh Zadsirjan
and
Nargess Poormohammad
Department of Physics and Chemistry, School of Science, Alzahra University, PO Box 1993891176, Vanak, Tehran, Iran. E-mail: mmh1331@yahoo.com; mmheravi@alzahra.ac.ir; Fax: +98 21 88041344; Tel: +98 21 88044051
First published on 10th February 2021
Abstract
The Sonogashira reaction is a cross-coupling reaction of a vinyl or aryl halide with a terminal alkyne to form a C–C bond. In its original form, the Sonogashira reaction is performed with a palladium species as a catalyst while co-catalyzed by a copper species and a phosphine or amine. The reaction is conducted under mild conditions, i.e., room temperature, aqueous solutions, and the presence of mild bases. Undeniably, the Sonogashira reaction is among the most competent and efficient reactions widely used in organic synthesis. This named reaction has proved useful in many organic synthesis areas, including the synthesis of pharmaceuticals, heterocycles, natural products, organic compounds, complex molecules having biological activities, nanomaterials, and many more materials that we use in our daily lives. The presence of transition metals as a catalyst was indeed essential in the Sonogashira reaction. However, recently, the reaction has been successfully conducted without copper as a co-catalyst and phosphines or amines as bases. In this critical review, we have focused on developments in the Sonogashira reaction successfully performed in the absence of copper complexes, phosphines or amines, which could be of particular advantage in implementing green chemistry principles and making the reactions more achievable from an economic viewpoint.
1 Introduction
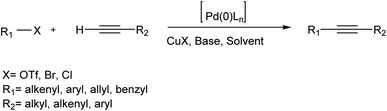
The Sonogashira reaction is unquestionably among the most competent and efficient reactions widely used in organic synthesis. This named reaction has proved useful in many areas of organic synthesis, including the synthesis of pharmaceuticals, heterocycles, natural products, organic compounds, complex molecules having biological activities, nanomaterials, and many more materials that we use in our daily lives.1 The Sonogashira reaction is among the most prominent methods for sp2-carbon–sp-carbon bond formation extensively used in organic synthesis. The original reaction includes the coupling of a terminal alkyne with an alkyl halide in the presence of a palladium species as a catalyst, a copper complex as a co-catalyst, and a phosphine or amine as a base. A considerable efficiency is afforded under ordinary conditions through this unique combination of reagents (Scheme 1).2,3 The significance of the present work lies in the issues that arise in the presence of Cu in the Sonogashira reaction,4 some of which are environmentally friendly,5,6 or solid-supported Pd catalysts.7For the first time, this cross-coupling reaction was outlined by Kenkichi Sonogashira et al. in 1975, then rapidly developed using various catalysts, additives, and ligands under different conditions.8 Since then, the Sonogashira reaction has been regularly employed in the total syntheses of all manners of natural products9 and compounds of biological activities,10,11 e.g., isocoumarins, α-pyrons and indoles,12–15 arylbenzofurans,15 dendrimers,16 conjugated polymers, and nanostructures.17 Because of its high efficiency as well as milder operational conditions, this reaction is now drawing the attention of synthetic chemists even more than before.11
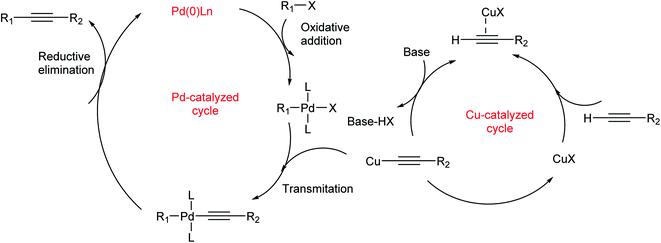
The Sonogashira reaction is often performed in the presence of a Pd compound besides CuI as the co-catalyst in different solvents.18–21 This cross-coupling reaction can also be conducted in aqueous solutions22 to provide the feasibility of biological screening of products in a direct manner. Although striking developments have been achieved and extensive attempts have been made, the mechanism for the palladium-copper catalyzed Sonogashira reaction has not been thoroughly inferred. Nonetheless, a plausible mechanism for the reaction has been proposed. As depicted in Scheme 2, the reaction probably proceeds through Pd–Cu catalytic cycles.
Therefore, the active complex, i.e., Pd(0)L2, which is obtained in situ via reduction of the Pd(II) species, first catalyzes the rapid step, i.e., oxidative addition of the vinyl or aryl halide (R–X). Upon another connection with the copper co-catalytic cycle, the RPd(–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR')L2 species is attained through a transmetalation reaction that one may consider it as the rate-determining step in the proposed mechanism. Next, as predicted for a catalyzed reaction, the desired coupled alkyne is derived through a reductive elimination reaction to reproduce the catalyst, as predicted for a catalyzed reaction. The rate of the oxidative addition of the vinyl or aryl halide widely depends upon the electronic properties of the R–X bond so that electron-withdrawing groups tend to activate the R–X bond by increasing its electron density in the order of X = I ≥ OTf ≥ Br > Cl.
CR')L2 species is attained through a transmetalation reaction that one may consider it as the rate-determining step in the proposed mechanism. Next, as predicted for a catalyzed reaction, the desired coupled alkyne is derived through a reductive elimination reaction to reproduce the catalyst, as predicted for a catalyzed reaction. The rate of the oxidative addition of the vinyl or aryl halide widely depends upon the electronic properties of the R–X bond so that electron-withdrawing groups tend to activate the R–X bond by increasing its electron density in the order of X = I ≥ OTf ≥ Br > Cl.
Quite a few Pd(II) compounds have been prepared and successfully tested to affect the Sonogashira cross-coupling reaction.23–26 However, one can consider Palladium's use without Cu and/or phosphine -or amines- co-catalysts as bases as advanced development in the organic synthesis. The Sonogashira reactions are preferred to be conducted without copper and/or phosphines as the Glaser by-products formed before the homo-coupling reaction of alkynes are undesirable,9,12–14 and copper is essentially toxic. The design and synthesis of heterogeneous palladium-containing catalysts in order to develop Sonogashira cross-coupling reactions free of Cu and/or phosphine has therefore drawn huge attention in the last decade.
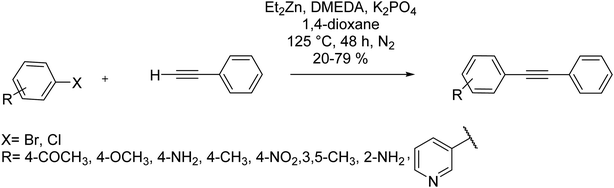
Sonogashira–Hagihara cross-coupling of a terminal alkyne with an aryl halide in the presence of Cu and catalyzed by Cd provides an easy method for the synthesis of a variety of unsymmetrical alkynes as well as enynes with biological activities. The standard reaction proceeds through catalysis by a Pd complex, which is usually Pd(PPh3)4, in the presence of CuI and also some tertiary amine in various solvents.27 A copper acetylide is formed upon the reaction of CuI with the alkyne. The resulting copper acetylide is then arylated in the presence of TMEDA or pyridine to form tolans (and benzofurans, indoles, and phthalides resulting due to subsequent heterocyclizations). The reaction is indeed a Stevens–Castro coupling reaction which dates back to 1963.28
In 1975, three different research groups working separately managed to arylate terminal acetylenes catalyzed by Pd complexes.29–31 Heck30 and Cassar29 carried out the arylation reaction without Cu that is, under the Heck conditions. On the other hand, Sonogashira and co-workers31 described a Stevens–Castro cross-coupling reaction catalyzed by Pd. The latter has become the chief method to achieve the arylation or vinylation of a terminal alkyne (the Sonogashira cross-coupling reaction). Subsequently, couplings solely catalyzed by Pd complexes with Cu absent were reported, and reaction conditions were adjusted for relatively mild reactions exclusively catalyzed by Cu complexes.
The introduction of a reaction catalyzed by palladium that results in the coupling between terminal acetylenes and aromatic/heterocyclic rings by Sonogashira and et al. dates back to 1975.31,32 It focused on furnishing an alternative for the similar process exclusively mediated by Cu, i.e., the Castro–Stevens reaction. Presently, the Sonogashira reaction does not need to use both palladium and copper as catalysts; instead, only palladium has proved quite sufficient is properly complexed by the state of art ligands much more efficient than the phosphine originally employed, i.e., Ph3P. Among the collection of coupling reactions catalyzed by palladium with a high synthetic value, the Sonogashira reaction remains unique because of the presence of an sp carbon being widely employed in the manufacture of pharmaceuticals,33,34 the total synthesis of natural products,35,36 and construction of organic compounds.37,38 However, the conditions continue to enhance, looking to improve the tolerance of the functional groups as well as yields, avoiding occasional rough reaction conditions and problematic workups, and circumvention of more costly and sensitive coupling partners (such as iodides).39 Meanwhile, it should be noted that the palladium species and the corresponding ligands, usually used in the range of 1–5 mol%, are getting more and more expensive, and to this one must add the fact that at the back end, it is necessary to remove the residual metal embedded in the final product, mainly when an API is the target of the synthesis.40 Furthermore, from an environmental viewpoint, Sonogashira coupling reactions are mostly carried out in organic solvents, without the solvent or even the expensive palladium catalyst being recycled.41 These traditional reaction parameters can affect the expected levels of both aqueous and organic waste, and our grasp of the hazardous status of platinoids.42
In parallel with our interest in the classical Sonogashira reaction in the presence of palladium(II) chloride on modified poly(styrene-co-maleic anhydride), which is an extremely active, recyclable catalyst for the Suzuki–Miyaura43 and Sonogashira reactions,2,44–47 because of the mentioned interests and equipped with the experiences mentioned above, herein, we attempt to highlight the recent developments and advances in the palladium-catalyzed Sonogashira reaction in the absence of copper as a co-catalyst or/and phosphines or amines (Fig. 1).
2 Application of the Pd-catalyzed Sonogashira reactions in the absence of Cu and/or phosphines and/or amines
As mentioned in the introduction section, nowadays, the Sonogashira cross-coupling reaction has become one of the most synthetic strategies in the formation of a C–C bond using terminal alkynes and aryl halides or triflates, leading to the synthesis of more complicated alkynes under mild and green reaction conditions. Compounds bearing carbon–carbon triple bonds are frequently present in naturally occurring compounds, pharmaceuticals, and biologically important complex molecules. Conventional Pd-catalyzed Sonogashira reaction proceeds smoothly in the presence of copper as co-catalyst and phosphines usually, in aqueous media, and in the presence of a mild base.48–50However, not only the Sonogashira reaction but in a way and in general, all Pd-catalyzed reactions are undesired in the synthesis of organic compounds due to the high cost of this particular metal. Thus, the replacement of palladium with less expensive metal has always been a big challenge for synthetic organic chemists attracting much attention that enormous attempts have been made. Several developments have been achieved, such as easy separation of catalysts by decorating it in a way for being used as a heterogeneous or magnetic catalyst, which is easily separable and can be reused several times without appreciable loss in its activity, which is also important from economic and environmental points of view. Thus, simple separability, excellent reusability, a minimum possibility for leaching, and minimum loading of Pd species have remained a scientific cumbersome synthetic organic chemists, especially those who are engaged in the R&D section of chemical industries. Nowadays, some of these problems have been circumvented by the development of and use of heterogeneous Pd-catalytic systems immobilized onto colloidal palladium species, and appropriate support (including organic, polymers, or inorganic supports), and utilization of Pd-nanoparticles.51–63 Much attempt has been made to replace palladium with any other metals such as copper. Unfortunately, such systems suffer from enormous disadvantages such as the formation of homo-coupling products, a requirement of using organic solvents, needing high temperature, a requirement of phosphine ligand, and oxidative agent or air.64,65
A classic Pd-catalyzed Sonogashira reaction, in addition to the drawbacks mentioned above, has other disadvantages, such as needing Cu as co-catalyst, phosphines and amines as a base, which makes the reaction more disadvantageous despite being conducted udder mild and green conditions. Therefore, recently much attention has been devoted to eliminating one or all co-catalysts and other additives in the Sonogashira coupling reaction63 in which several developments have been accomplished.
2.1. In the absence of the copper (copper-free)
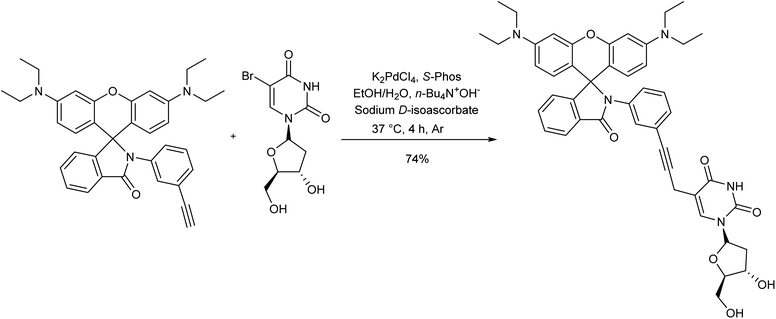
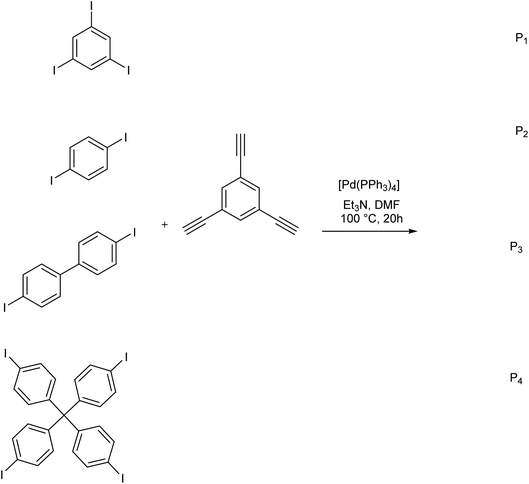
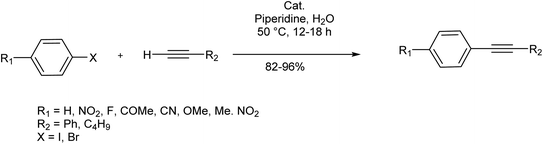
In 2018, Wang et al. developed a method for the fluorescent labeling of 5-bromo-2′-deoxyuridine (BrdU), an essential member of chemotherapeutic C5-modified uridines scaffold, using a Cu-free Sonogashira reaction in aqueous medium (Scheme 3).66 In this approach, several fluorescent dye-containing alkynyl groups were coupled with BrdU, enabling its detection in cellular contexts. The reaction was performed in the presence of water-compatible reagents like K2PdCl4 as the catalyst, n-Bu4N+OH as the base, and S-Phos as the ligand, to meet the mild biological reaction requirements. The reaction could successfully proceed in the absence of a Cu source, as the combination of the base and ligand made ionic intermediates stabilized in the catalytic reaction cycle.The same methodology was employed by Trunk et al. in the synthesis of conjugated microporous poly(arylene ethynylene) matrix (CMPs) through a copper-free Sonogashira reaction (Scheme 4).67 In this strategy, equimolar amounts of various iodoarylenes and 1,3,5-triethynylbenzene were reacted in the presence of a reduced loading of [Pd(PPh3)4] catalyst to yield the product in a controlled structure. Higher Brunauer–Emmett–Teller (BET) surface areas than other previously synthesized CMPs without any by-product was another merit of this approach.
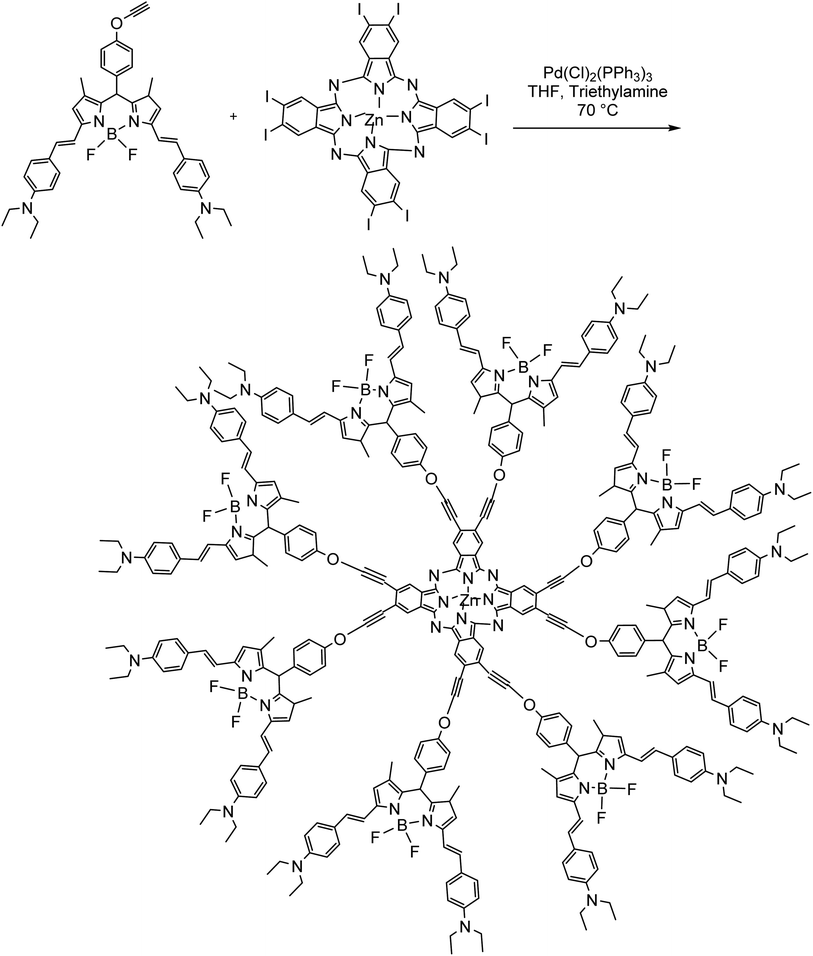
In another attempt, derivatives of 4-CHO-2-arylbenzofurans were synthesized through a microwave promoted copper-free Sonogashira reaction in the presence of PdCl2(PPh3)3 as the catalyst.68 This approach was used for the synthesis of the resveratrol dimer viniferifurans. From biologically active molecules to the microporous polymers. Durmuş et al. expanded the scope of this methodology to the synthesis of dyes, where octa-distyryl-BODIPY substituted zinc(II) phthalocyanines, a member of fluorescent BODIPY (boron-dipyrromethene) dyes scaffold, were prepared through a similar copper-free reaction.69 As shown in Scheme 5, the terminal ethynyl functionalized BODIPY derivatives were reacted with iodo zinc(II) phthalocyanines in the presence of Pd(Cl)2(PPh3)3 as the catalyst to afford the desired product.
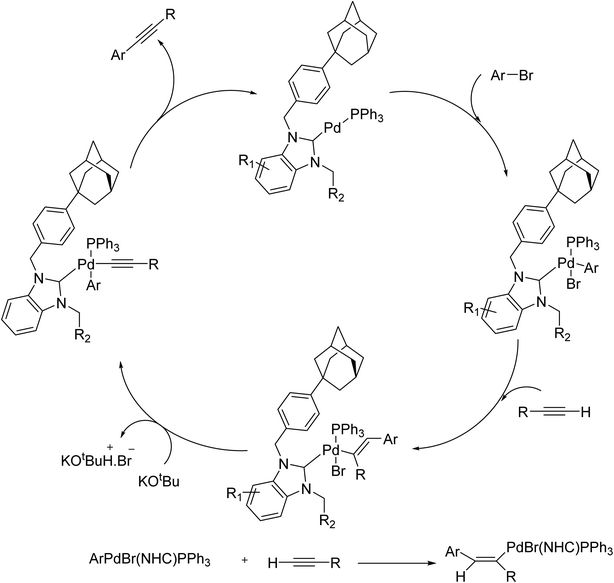
Upon discovery by Arduengo in 1991,70 metal complexes of N-heterocyclic carbenes (NHCs) bearing various substituents have been known and used as efficient catalysts in organic reactions. The versatility of NHCs complexes in catalysis is attributed to the powerful σ-donor and weak π-acceptor potential of the NHC ligand. Owing to these capabilities, a series of stable NHC–Pd-PPh3 complexes were reported in 2018 to efficiently catalyze a copper-free Sonogashira reaction of aryl bromides and phenylacetylene in DMF under aerobic conditions (Scheme 6A).71 The catalyst was obtained from the subsequent palladation, and phosphinate of benzyladamanthyl substituted NHC, prepared from bromoadamantane over a multistep procedure (Scheme 6B). In this reaction, the carbopalladation step occurred in the absence of amine, and CuI made no positive contribution. It seems that electronic and steric properties are responsible for such catalysts to efficiently perform an aerobic copper/amine-free Sonogashira coupling reaction.
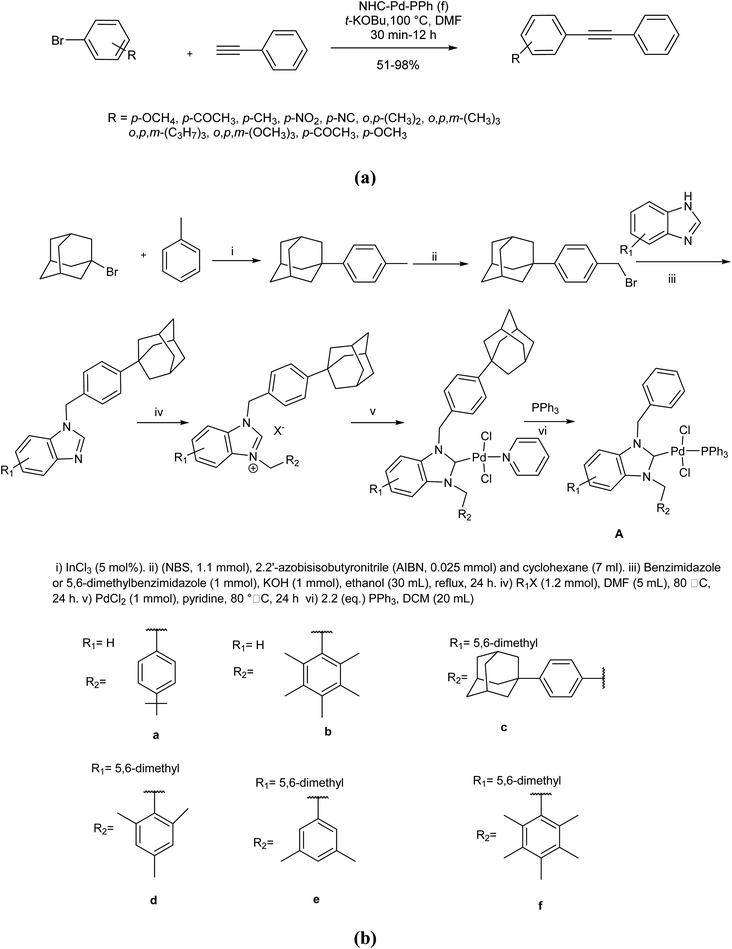 | ||
| Scheme 6 (a) Copper-free Sonogashira coupling reactions in the presence of the benzyladamanthyl substituted NHC–Pd-PPh3. (b) The preparation of benzyladamanthyl substituted NHC–Pd-PPh3. | ||
Worthy to mention that the mechanism of the Cu-free Sonogashira cross-coupling is not exactly well-known. Similar to other cross-couplings, the initial step is oxidative addition reaction of aryl halides palladium(0) complex. The second step is disputable. In this case, a carbopalladation step occurs in the absence of any amine, and can be considered as the main step. The carbopalladation is a syn addition that provides a cis-product (R1–C(PdXL2) = CH–Ar) through aryl group which is transferred to less sterically hindered position on alkyne. Other steps are the same as the well-known and recognized mechanism of Sonogashira cross-coupling reaction. The proposed mechanism based on above clarifications is exhibited in Scheme 7.72
Recently by improving the flow chemistry technique in organic synthesis,50 Routier et al. reported a copper-free Sonogashira reaction of Boc protected alkynyl(aza)indoles with 4-tolylacetylene in the presence of Silica based diphenylphosphine palladium (SiliaCat DPP-Pd) as the catalyst (Scheme 8).51 Using this strategy, the process was clean, high yielding, and completed within 3 minutes, capable of running on a pilot scale, as well.
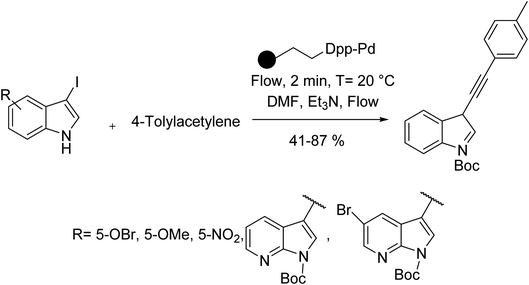 | ||
| Scheme 8 Copper-free Sonogashira coupling reactions in the presence of the silica based diphenylphosphine palladium (SiliaCat DPP-Pd). | ||
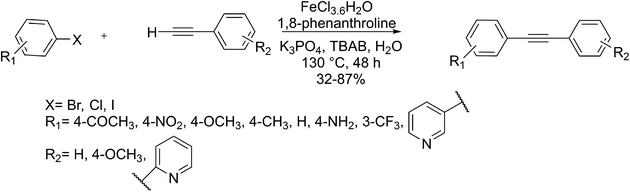
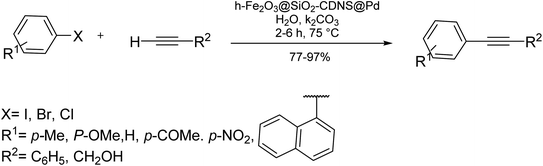
Anilkumar and co-workers in 2016 developed the first method for the formation of diaryl alkynes using the Fe-mediated Sonogashira type coupling reaction of terminal alkynes and aryl iodides under aerobic conditions in H2O as the solvent. Noticeably, this catalyst containing iron(III) chloride hexahydrate and 1,10-phenanthroline is a significant catalyst in these cross-coupling reactions. This approach is appropriate to various functionalized aryl iodides having different heteroaryl and sterically hindered substituents and terminal aryl alkynes bearing heteroaryl alkynes (Scheme 9).73
In 2016 Abi and co-workers reported the first zinc-mediated approach for the C(sp2)–C(sp) cross-coupling between terminal alkynes and aryl iodides. Various functional groups were applied in this reaction to provide different diaryl acetylenes. Under the optimal condition, the generality of this reaction was explored using various aryl halides and alkynes. The electron-rich aryl iodides afforded the corresponding products in satisfactory yield. Pleasantly, this method served well in the reaction of unsubstituted aryl iodide and phenylacetylene, giving the relevant product in moderate yield (Scheme 10).74
2.2. In the absence of the copper and amine (copper-free and amine-free)
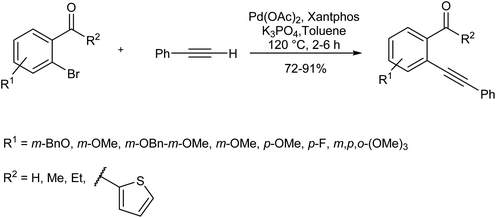
Using xantphos ligand, Satyanarayana et al. designed a copper/amine-free Sonogashira reaction for the synthesis of 2-alkynylaryl carbonyls, a useful synthon in the synthesis of heterocyclic compounds (Scheme 11).75Another example of this case is the copper-free Sonogashira cross-coupling reaction of p-tert-butyl phenyl chloride with phenylacetylene in the presence of meta-terarylphosphine palladium catalyst (Pd-Cy*phine catalyst) (Scheme 12).76
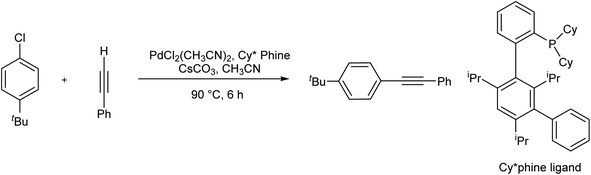 | ||
| Scheme 12 Copper-free Sonogashira coupling reactions in the presence of the meta-terarylphosphine palladium catalyst (Pd-Cy*phine catalyst). | ||
A class of air- and moisture-stable dinuclear Pd complexes having 2-benzimidazolyl ligands was developed and used as a catalyst in the Sonogashira-type coupling reactions. The conformation and rigidity of the ligand moiety were easily changed through tethering of the 2-benzimidazolyl scaffold to diamine ligands, leading to important changes in the catalytic efficiency. Based on the optimized reaction, Sonogashira-type couplings between aryl bromide derivatives were accomplished efficiently using just 0.1 mol% of catalyst in aqueous or alcohol solvents with high yield. Both electron-poor and rich para-substituted aryl bromides afforded the relevant internal alkynes in very high yields. Also, meta-substituted derivatives and heterocyclic substrates were afforded the corresponding products in high yields (Scheme 13).77
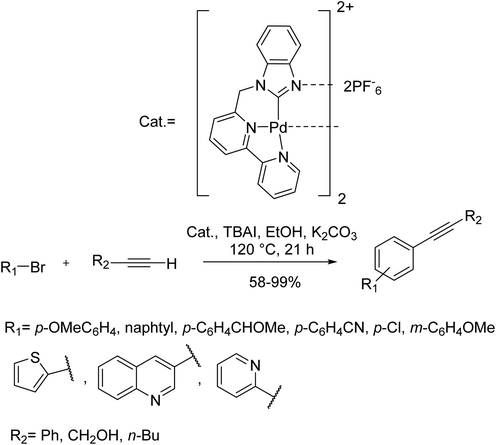 | ||
| Scheme 13 Copper-free Sonogashira coupling reactions in the presence of the benzimidazolyl palladium complexes. | ||
A Pd catalyst immobilized on a monolithic and amphipathic polystyrene-divinylbenzene polymer having acidic cation exchange functions (such as sulfonic acid scaffolds) (Pd/CM) was synthesized in 2017 by Sajiki and co-workers. This synthesized catalyst was applied efficiently in the Cu- and amine-free Sonogashira-type coupling reaction (Scheme 14).78
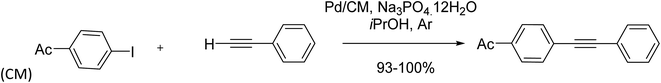 | ||
| Scheme 14 Copper-free Sonogashira coupling reactions in the presence of the polystyrene-based monolithic polymer bearing sulfonic acid moieties as cationic exchange (Pd/CM). | ||
2.3. In the absence of the copper and phosphine (copper-free and phosphine-free)
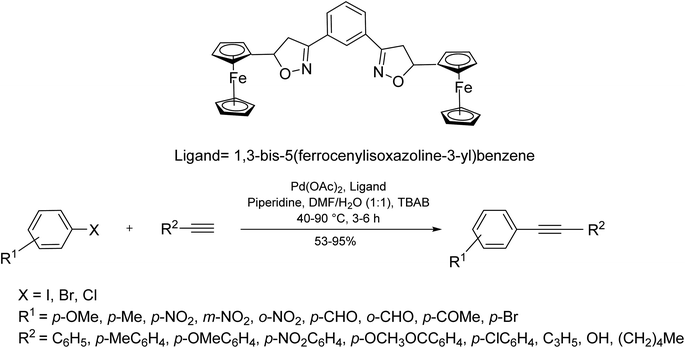
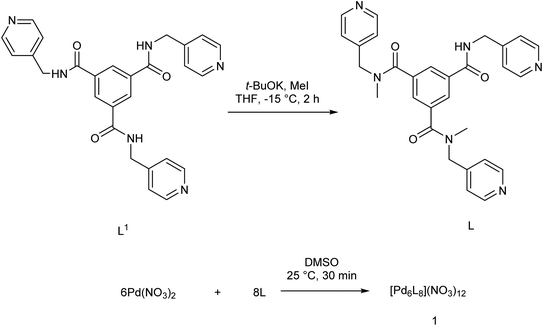
In 2017, Yu et al.79 developed an effective catalytic system employing Pd(OAc)2 and 1,3-bis(5-ferrocenylisoxazoline-3-yl)benzene as a non-phosphorous ligand-for the copper/phosphine-free Sonogashira reaction. The catalytic system benefited from high thermal stability and could efficiently react several aryl halides with substituted terminal alkynes of various electronic properties (Scheme 15).Among structurally diverse two/three dimensional supramolecular assemblies, some coordination driven Pd-assemblies of Pd6L8 type have recently found applications in catalysis C–C bond formation.43 In this structure, Pd(II) ions are used in combination with tripodal ligands possessing proper terminal groups and flexible arms (Scheme 16).80
In this category, Pd6L8 nano-ball has shown a tremendous catalytic activity over copper/phosphine-free Sonogashira coupling reaction of aryl halides with terminal alkynes in water under aerobic conditions (Scheme 17).80
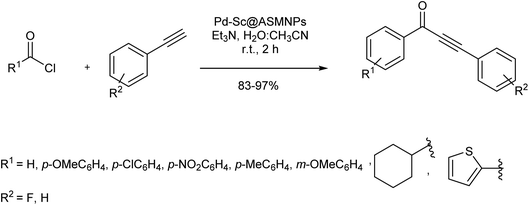
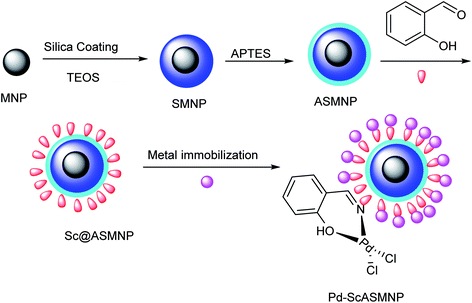
In another attempt, Sharma et al. reported a sustainable method for the synthesis of ynones, from the phosphine/copper-free Sonogashira reaction of acyl halides with terminal alkynes in the presence of stable palladium–salicylaldehyde–(3-aminopropyl)triethoxysilane-surface of magnetic silica nanoparticles (Pd–Sc@ASMNPs nanocatalyst). The catalyst could perform the reaction under aerobic conditions within a short time and afford relatively high conversion rates for a wide range of electron-rich/poor substrates (Scheme 18).81
In this method, the highly reusable Pd–Sc@ASMNPs were obtained from the reaction of ASMNPs (3-aminopropyltriethoxysilane functionalized silica magnetic nanoparticles) with salicylaldehyde (Sc) ligand, following synthesis of the initial corresponding SMNP (silica magnetic nanoparticles) (Scheme 19).81
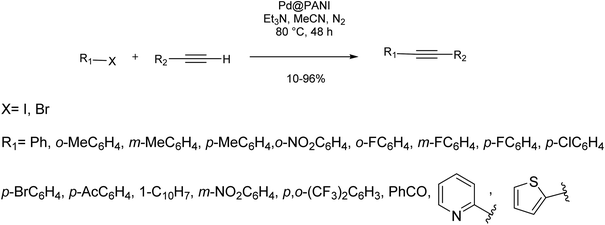
Aniline oxidative polymerization in the presence of PdCl2 and air as the oxidant affords polyaniline supported palladium (Pd@PANI) as a very stable catalyst for copper/ligand-free Sonogashira reaction (Scheme 20).82
Using this catalyst, heteroatom-containing substrates could also be tolerated since nitrogen atoms on PANI surface could act as ligands for palladium. Increasing the popularity of such heterogeneous catalysts, arylation of various aryl alkynes through a copper/phosphine free Sonogashira reaction was developed by Yu et al. using a mesoporous carbon nitride (g-C3N4) functionalized Pd catalyst (Scheme 21).82
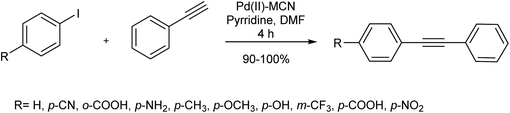
In this approach, nanoparticles of Pd(II) and Pd(0) were efficiently dispersed on mesoporous carbon nitride (MCN) polymers as the solid support, without agglomeration. The reaction proceeded well with both Pd(II) and Pd(0) catalysts, though a higher turn-over number was reported for Pd(II)–MCN (Scheme 22).83
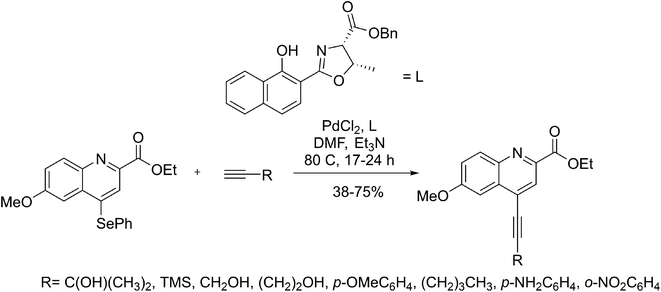
Under specific conditions, certain ligands are in charge of making a Sonogashira reaction needless of copper assistance. For instance, the copper-free reaction of phenylsilane quinolines with various alkynes was performed in the presence of PdCl2 as the catalyst and derivatives of oxazolines as ligands (Scheme 23). In the absence of this ligand, the reaction demands copper to proceed or follows with low yields.84
Using immobilized palladium nanoparticles on a magnetic nanoparticle core (ImmPd(0)-MNPs), another water-catalyzed copper-free Sonogashira reaction was developed to couple phenylacetylene with various aryl halides in satisfactory yields. According to Scheme 24, the magnetic methionine-functionalized chitosan was used as the support for this immobilization. The reusable catalyst was efficient enough to perform the reaction at room temperature with up to ten consecutive runs without significant loss inactivity.85
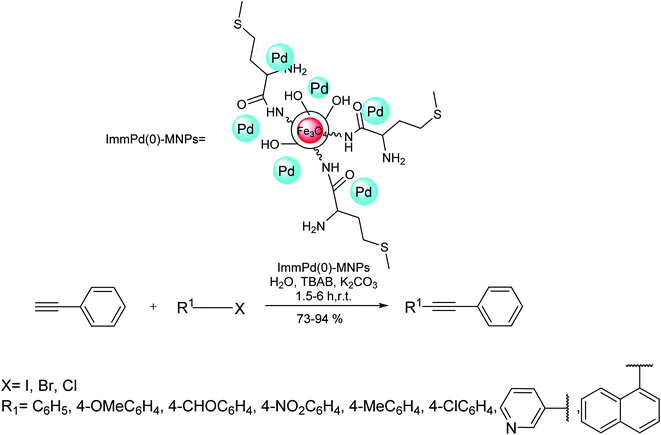 | ||
| Scheme 24 Copper-free Sonogashira coupling reactions in the presence of the immobilized palladium nanoparticles on a magnetic nanoparticle core (ImmPd(0)-MNPs). | ||
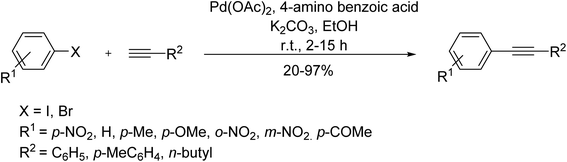
A similar green approach was reported by Das to afford various substituted 2-phenylindoles through bulky IPent (3-pentyl) groups showing the electron-withdrawing capacity of the chlorine atoms using Pd-PEPPSIIPentCl catalytic system. Taking advantage of aminobenzoic acids as a useful additive in some coupling reactions, Bora et al. explored the role of 4-aminobenzoic acid as a low-cost promoter/ligand in a copper and amine-free Sonogashira reaction to afford several phenylalkynes using Pd(OAc)2 as the catalyst (Scheme 25).86
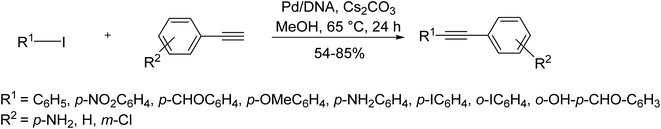
More interesting examples in this category involve the use of biomaterials as different supports in catalyzing such coupling reactions. In this regard, DNA nanotechnology has recently found applications in catalytic synthesis. DNA could be considered as a support for metal nanoparticles due to its tendency towards coordination with transition metals. In light of discovering bio nanoparticles applications, Alonso et al. reported DNA-supported palladium nanoparticles as a catalyst to catalyze the copper- and ligand-free Sonogashira reaction of aryl halides with phenylacetylenes under mild conditions (Scheme 26).87
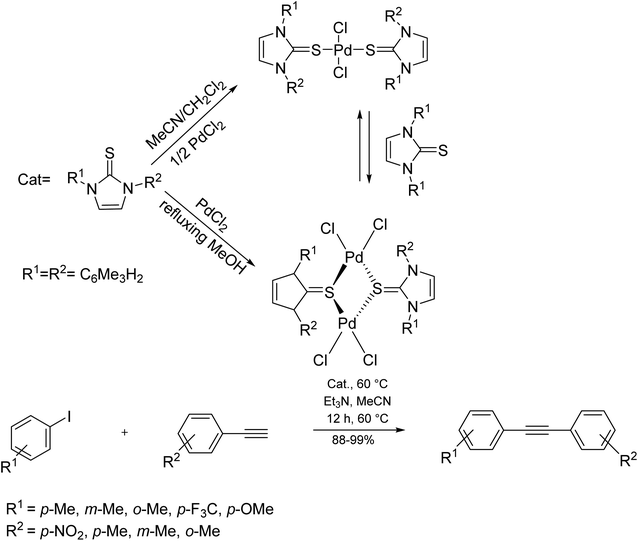
In this catalyst, palladium exists as Pd(II) and Pd(IV) oxide species. Using this system, the biodegradable and reusable catalyst could efficiently conduct the reaction in good yields under aerobic conditions. A combination of PdCl2 with N,N′-disubstituted-imidazole-2-thiones, affords a new catalytic system by which the conventional Sonogashira coupling reaction takes place under copper and phosphine-free conditions (Scheme 27).88–91
Palladium nanoparticles could also be stabilized on aluminum hydroxide Al(OH)3 as the support to introduce a new generation of catalysts in this field. For example, Li et al. employed an in situ prepared aluminum hydroxide-supported palladium nanocatalyst to catalyze a copper-free Sonogashira reaction of phenylacetylene with aryl halides, as depicted in Scheme 28.88,92
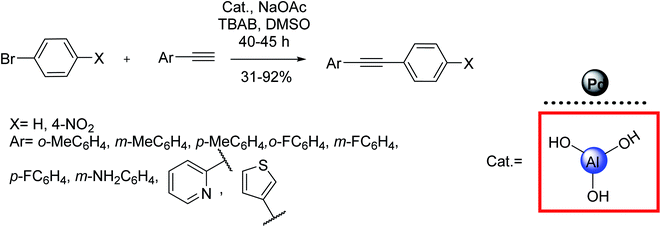 | ||
| Scheme 28 Copper-free Sonogashira coupling reactions in the presence of the palladium nanoparticles supported on in situ generated Al(OH)3. | ||
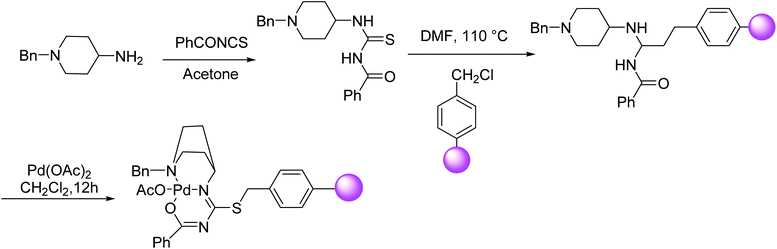
According to Thogiti et al. the polymer-supported 3-benzoyl-1-(1-benzylpiperidin-4-yl)-2-thiopseudourea-Pd(II) matrix (PS-bpt-Pd), can successfully perform a conventional Sonogashira reaction under copper and solvent-free condition.93 The catalyst was easily prepared from the reaction of the thiourea-containing molecule with chloromethylated polystyrene followed by functionalization of resin through complexation with Pd(OAc)2 (Scheme 29). For some aryl iodides, the reaction has proceeded at room temperature.
Keesara and co-workers in 2016 have demonstrated the aryl iodide coupling reactions using terminal alkynes at room temperature under copper and solvent-free conditions. It was observed moderate to good yields for aryl bromides as a coupling partner. This insoluble polystyrene supported 3-benzoyl-1-(1-benzylpiperidin-4-yl)-2-thiopseudourea-Pd(II) (PS-bpt-Pd(II)) catalyst can be easily recovered by simple filtration and reused up to five times with stable catalytic activity. In addition to this, the heterogeneous Pd(II) complex is even applicable on a gram scale for cross-coupling reaction with high catalytic efficiency (Scheme 30).93
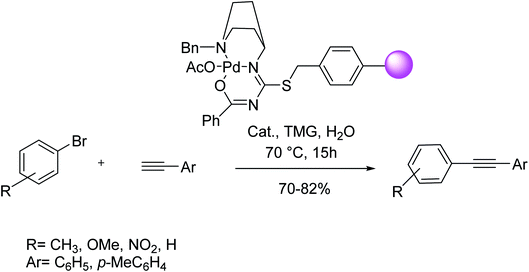 | ||
| Scheme 30 Copper-free Sonogashira coupling reactions in the presence of the 3-benzoyl-1-(1-benzylpiperidin-4-yl)-2-thiopseudourea-Pd(II). | ||
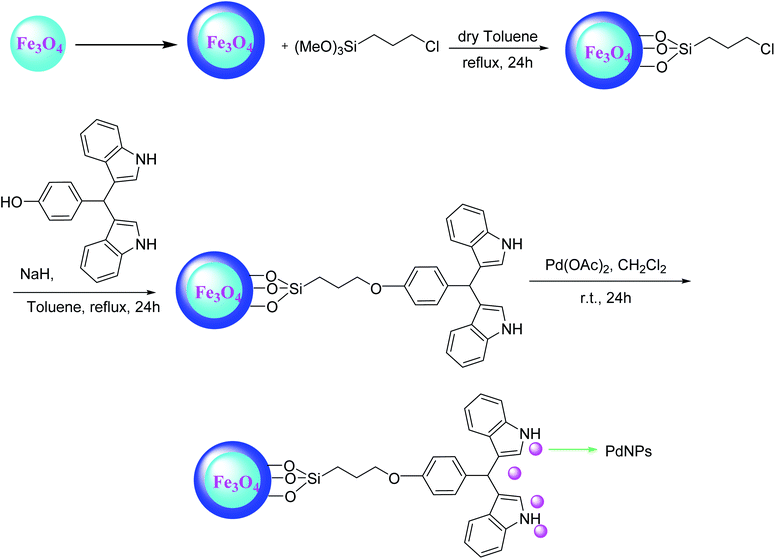
Nanoparticles of palladium supported on functionalized magnetite (Fe3O4) nanoparticles made another efficient catalyst in an aerobic copper and phosphine-free Sonogashira reaction of aryl halides with different terminal alkynes. In this approach, the catalyst was synthesized during a multistep procedure starting from silica-coated Fe3O4 nanoparticles, went on with successive functionalization by chloropropyltrimethoxysilane, 3,3′-bisindolyl(4-hydroxyphenyl) and final complexation with Pd(OAc)2 respectively (Scheme 31).94
In this method compounds having aryl iodides and aryl bromides, reacted with various terminal alkynes through alkynylation reaction at 60 °C upon phosphane and copper-free conditions using low-loading Pd nanoparticles in N,N-dimethyl acetamide as the solvent, and 1,4 diazabicyclo[2.2.2]octane (DABCO) as the base under the air atmosphere. The heterogeneous synthesized magnetic catalyst could be easily removed from the reaction media by the external magnet, and the reusability of the catalyst by the seven-run should be highlighted as positive points of view (Scheme 32).94
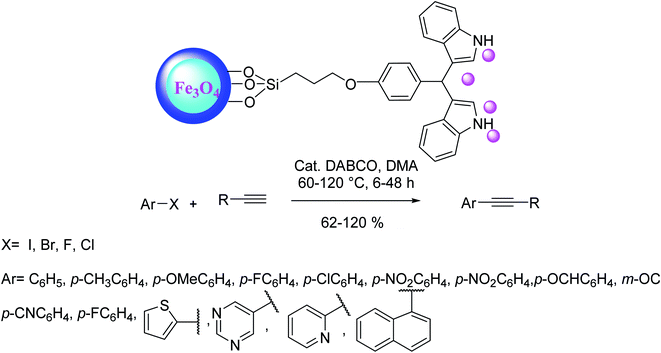 | ||
| Scheme 32 Copper-free Sonogashira coupling reactions in the presence of the Pd@bisindole@SiO2@Fe3O4. | ||
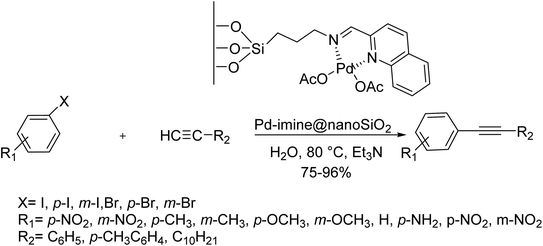
A Pd(II) complex immobilized onto nanosilica (Pd-imine@nanoSiO2) was synthesized and used as an effective catalyst for C–C triple bond activation reactions between terminal alkynes and aryl halides. Different alkyne derivatives were successfully cross-coupled with various aryl bromides and aryl iodides to give various diaryl alkynes, affording increased yields in H2O. Noticeably, this method is appropriate for various aliphatic alkynes. Worthy of mentioning that H2O (as a solvent) tolerates both aryl bromides and iodides very barely to afford moderate yield, although this catalyst exhibited high tolerance for both aryl bromides and iodides (Scheme 33).95
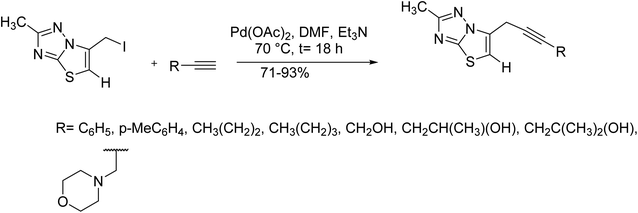
2-Functionalized thiazolo[3,2-b][1,2,4]triazoles were synthesized via Cu-free, palladium-mediated Sonogashira coupling between terminal alkynes and 6-(iodomethyl)-2-methylthiazolo[3,2-b][1,2,4]triazole at 70 °C in dimethylformamide. The Sonogashira coupling between 4-ethynyltoluene and phenylacetylene afforded the corresponding products in very good yields. Although, the Sonogashira coupling of various alkynes, for example, propargyl alcohols, 1-octyne, and propargyl amines gave the corresponding products in satisfactory yields (Scheme 34).96
Sardarian and co-workers in 2019 demonstrated the formation of a new heterogenized organometallic catalyst through coordinating Pd with polyvinyl alcohol-substituted Fe3O4@SiO2 nanospheres (Fe3O4@SiO2–TCT–PVA–Pd(0)). The synthesized Pd NPs supported on polyvinyl alcohol substituted Fe3O4@SiO2 NPs were efficiently used as a magnetically catalyst in the Sonogashira reactions in H2O. They exhibited outstanding efficacy toward aryl halides by using very low Pd loading in very high yields and also exhibited high TONs (mmol of product per mmol of catalyst). To examine the scope of this reaction, various aryl halides were treated with phenylacetylene and 1-octyne. The reaction of phenylacetylene with different heteroaryl halides having electron-donating and withdrawing groups afforded the relevant products in very high yields. Worthy of mentioning that aryl halides having electron-withdrawing groups were more reactive in comparison with electron-releasing groups (Scheme 35).97
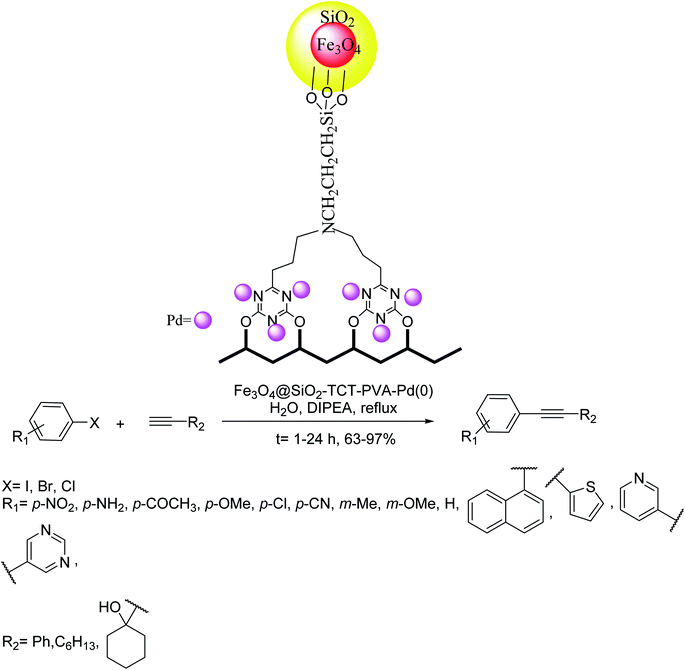 | ||
| Scheme 35 Copper-free Sonogashira coupling reactions in the presence of the magnetic silane- 2,4,6-trichloro-1,3,5-triazine-polyvinyl alcohol–palladium (Fe3O4@SiO2–TCT–PVA–Pd(0)). | ||
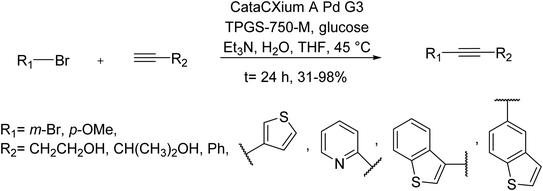
An efficient and common method for the Cu-free Sonogashira cross-coupling reaction using micellar aqueous reaction conditions was developed in 2019 by Jakobi and co-workers. By using the market purchasable CataCXium A Pd G3 and tetrahydrofuran as co-solvent, different alkyne substrates were successfully cross-coupled with various aryl halides, affording increased yields and also low catalyst loadings. Under the optimal condition, the generality of this Sonogashira reaction was examined under micellar reaction conditions. This approach affords a wide series of alkynylated arenes, heterocyclic products, and also monofunctionalized products from dihalogenated substrates with an increased selectivity (Scheme 36).98
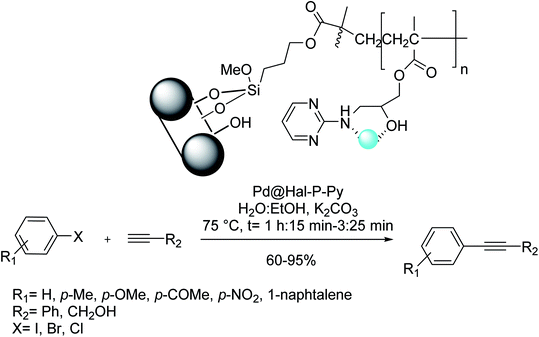
A new heterogeneous catalyst (Pd@Hal–P–Py) was synthesized via functionalization of Hal with 3-(tri-methoxysilyl) propyl methacrylate and subsequent by polymerization with glycidyl methacrylate, functionalization with 2-amino pyrimidine, and also palladium immobilization. The investigation of the catalytic efficacy of Pd@Hal–P–Py for improving the Sonogashira coupling reaction confirmed the excellent catalytic efficacy of the catalyst. The scope of this method was investigated for other aryl halide derivatives and terminal alkynes. Different substrates having electronic and steric aspects could undergo the reaction to accomplish the corresponding products in high yields. Remarkably, the reaction of aromatic alkynes was more efficient in comparison with aliphatic ones. Besides, aryl iodide resulted in increased yields in comparison with aryl bromides and chloride (Scheme 37).99
Thankachan and co-workers in 2016 reported the first zinc-catalyzed method for C(sp2)–C(sp) cross-coupling between various terminal alkynes and aryl iodides. A series of functional groups were examined in this reaction, and both functionally and structurally diverse diaryl acetylenes were synthesized successfully using this approach. In addition, this group examined the coupling reaction of aryl iodides and diverse functionalized terminal alkynes. Noticeably, this methodology is appropriate for a collection of terminal alkynes. The phenylacetylene having electron-rich MeO and Me substituents at the para-position gave the corresponding products in satisfactory yields (Scheme 38).74
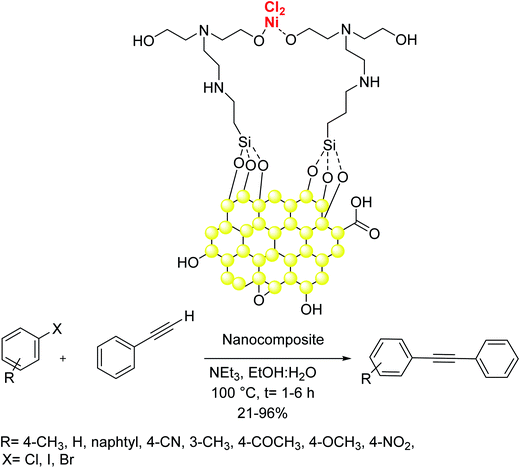
Naeimi and co-workers in 2019 reported the Sonogashira reaction for the synthesis of diarylethyne products from aryl halides derivatives and phenylacetylene in the presence of Ni complex of N,N′-bis(2-hydroxyethyl)ethylenediamine onto core–shell graphene oxide (GO@SiO2–BHED–Ni) as a catalyst. Worthy of mentioning that this heterogonous catalyst exhibited very activity and satisfactory thermal stability in the reaction. Various aryl halides were coupled efficiently using Pd and phosphine free reaction conditions. The application of this catalyst resulted in the generation of various functionalized aromatic alkynes in very high yields (Scheme 39).100
A new heterogeneous Pd catalyst was synthesized by using anchoring N-heterocyclic carbene–Pd(II) complex relied on theophylline on magnetic Fe3O4@SiO2 NPs by Sardarian and co-workers in 2018. This research group demonstrated the application of this reusable catalyst as a stable phosphine-free palladium catalyst for the Sonogashira cross-coupling of aryl halides with various terminal aliphatic and aromatic alkynes in the absence of solvent. The Sonogashira coupling reactions between electron-rich aryl iodides and phenylacetylene was performed easily to afford the relevant coupling products in very high yields. Remarkably, all Sonogashira coupling reactions were performed with satisfactory to high yields (Scheme 40).101
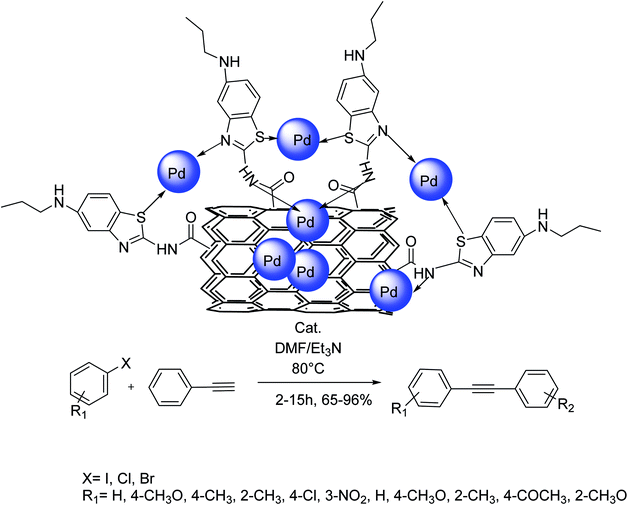
In 2016 Hekmati and co-workers demonstrated that pramipexole drug could be attached to the surface of multi-walled carbon nanotubes (MWCNTs) using the reaction of pramipexole with acylated carbon nanotubes. The synthesized pramipexole–MWCNTs were applied as an efficient nanocatalyst for the immobilization of Pd NPs. Upon identification of the final nanocomposite, the pramipexole–MWCNTs/palladium was used as a new phosphine-free reusable heterogeneous catalyst for the Sonogashira coupling reactions. Under the optimal conditions, the activity of the catalyst was investigated in the Sonogashira coupling reaction of a wide range of aryl halides with phenylacetylene. The reaction of various aryl bromides and iodides having different functional groups with phenylacetylene afforded the corresponding products in satisfactory to very high yields. In addition, this catalytic system was used in the Sonogashira reaction of diverse aryl chlorides (Scheme 41).102
Bivona and co-workers in 2017 developed a chemically effective method for the Pd-mediated Cu-free Sonogashira cross-coupling in the presence of the 3-mercaptopropyl-modified silica SBA-15 as a heterogeneous catalyst. This contains in the mixture of a Pd catalyst on a very cross-linked thiazolidine network on silica and a polystyrene-supported base. The solid catalyst/base system serves as a Pd scavenger preventing leaching of the metal. To examine a more common activity of the catalyst in the Sonogashira coupling-reaction, this method was examined using diverse iodo-substrates and various terminal alkyne derivatives in the presence of N-methylpiperazine at 90 °C in CH3CN/H2O. Based on this method, activated aryl iodides having electron-withdrawing groups treated without the construction of any side products and gave the relevant alkynes in excellent yields. Moreover, aryl iodides having electron-donating groups were applied in this Sonogashira coupling reaction and afforded the corresponding products in good yields (Scheme 42).39
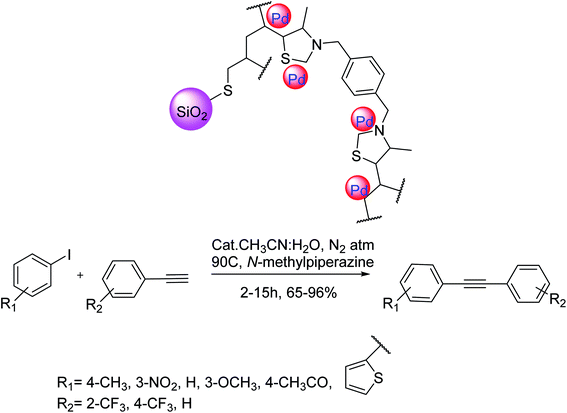 | ||
| Scheme 42 Copper-free Sonogashira coupling reactions in the presence of the thiazolidine-based heterogeneous palladium catalytic system. | ||
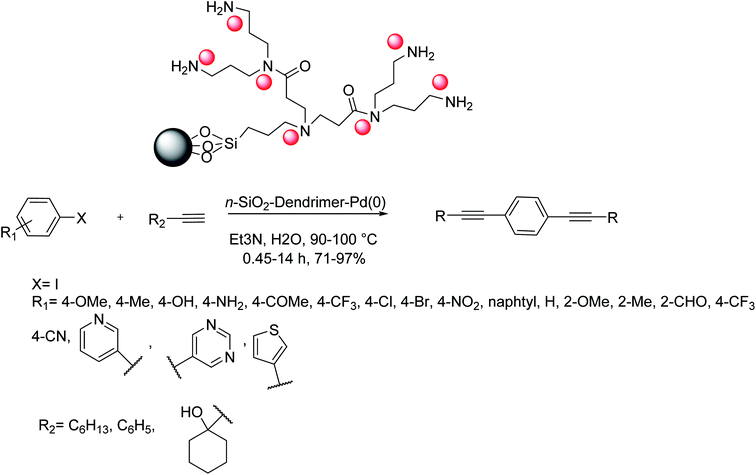
Sardarian and co-workers in 2016 reported an efficient methodology for the synthesis of porous SiO2 for catalytic usages by natural and waste material from rice husk that is found as the source of biosilica. Biogenic porous silica NPs were efficiently synthesized from rice husk (RH) biomass through a novel multistep approach. Initially, sodium silicate is removed from the rice husk. Next, cetyltrimethylammonium bromide (CTAB), hydrochloric acid, and acetic acid were added to the solution of sodium silicate, and the resulting mixture was sonicated. Upon the hydrothermal reaction, the synthesized compounds were calcinated to become silica NPs. In the following, dendrimer encapsulated palladium(0) NPs that were supported on nano-silica with surface amino substituents were fabricated. These compounds were synthesized by sequestering Pd ions into dendrimers and subsequently by reduction to afford the relevant zero-valent Pd NPs. This catalyst was used as an effective catalyst in the Sonogashira coupling of aryl halides and alkynes under Cu-free and phosphine ligand-free conditions in aqueous media.
Various aryl chlorides, bromides, and iodides having different substituents were investigated in the Sonogashira reaction using nSiO2–dendrimer–palladium(0) catalyst in aqueous media. The reaction between aryl iodides bearing electron-donating substituents was completed in longer reaction times in comparison with those having electron-withdrawing substituents. The Sonogashira coupling of phenylacetylene with aryl bromides having various electron-withdrawing and electron-releasing groups gave the corresponding products in very good yields (Scheme 43).16
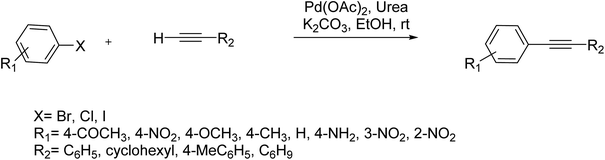
Pd(OAc)2/urea as an efficient catalyst was used in the Sonogashira cross-coupling reaction between various aryl halides and terminal alkynes at ambient temperature under Cu and amine-free conditions. The generality of this approach was investigated by using a wide range of electronically diverse aryl halogenides and various terminal acetylenes. This catalyst successfully provided polyfunctional alkyne derivatives via the Sonogashira coupling reaction. The corresponding coupled products were obtained in satisfactory to high yields. Remarkably, the reaction was effective for aryl iodides having both electron-donating and withdrawing substituents (Scheme 44).103
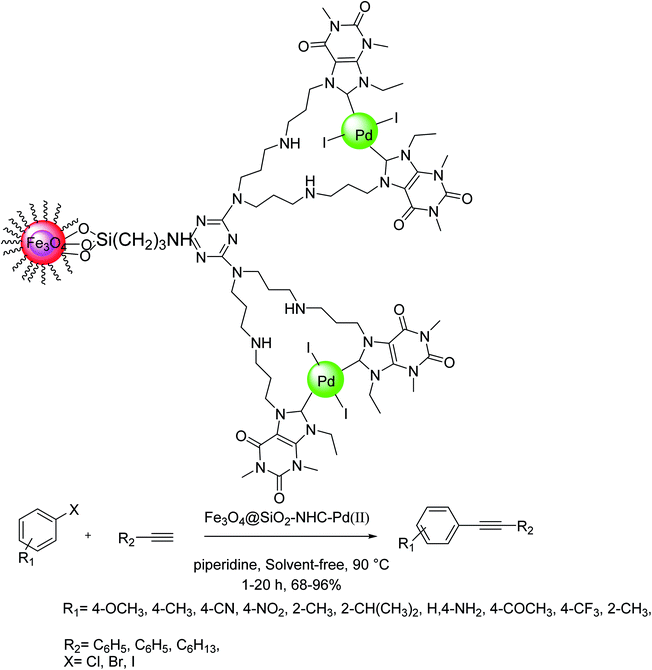
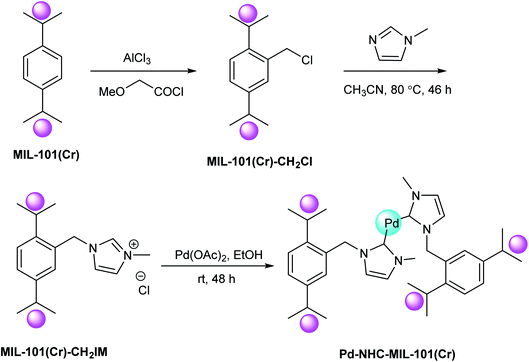
The Pd–NHC–MIL-101(Cr) catalyst was synthesized via a three-step synthetic route relied on the preceding report (Scheme 45).104 To make Pd–NHC–MIL-101(Cr) catalyst, first, the resultant MIL-101(Cr) was treated with 2-methoxyacetyl chloride in the presence of aluminium chloride that is used as an efficient reagent for the chloromethylation of benzene ring in the structure of MOFs.105 Next, the chloromethylated MOF was treated with 1-methyl-1H-imidazole in order to form imidazolium scaffolds in the structure of MOF.106 Lastly, the reaction of palladium(II) acetate with imidazolium functionalized MOF in EtOH gave Pd–NHC–MIL-101(Cr) catalyst. These results demonstrated that the used catalyst system has outstanding activity in DMF/H2O mixture solvents for the copper-free Sonogashira reactions. In this investigation, the catalytic performance of this catalyst system was examined in the copper-free Sonogashira reactions.107
Based on the optimal reaction conditions (K2CO3, 1.0 mol% Pd–NHC–MIL-101(Cr) and DMF 110 °C), the generality of this method was examined using different substrates (Scheme 46). Different aryl halides and acetylenic compound were applied in the Cu-free Sonogashira coupling reaction. Noticeably, all of the aryl iodide and aryl bromide treated with phenyl acetylene gave the corresponding coupling products in satisfactory to excellent yields, demonstrating high activity and broad substrate scope of this catalyst in Cu-free Sonogashira reaction. Aryl chlorides reacted slowly and more reaction time is required for completion of coupling process. The nature of the substituent in the aryl halide affects the yield of the reaction and for electron-donating substituent more reaction time was required and yield was lower related to electron-withdrawing substituents. Various groups such as OPh, COCH3, NO2, OMe, CHO, CN and SO2Me tolerated the reaction conditions well. It should be mentioned that there is satisfactory chemoselectivity profile for aryl halides having two different C–X bond and the product was provided from cleavage of reactive bond in the presence of Pd–NHC–MIL-101(Cr) catalyst under the optimal reaction conditions. 1,4-Bis (phenylethynyl)benzene were synthesized in the presence of this catalyst system through coupling reaction of 1,4-dibromobenzene and phenylacetylene in high yield. Worthy to mention that by combining the very high properties of MOFs as very stable solid support and high activity of Pd–NHCs in catalysis, a heterogeneous Pd catalyst by immobilization of Pd–NHCs complex on MIL-101(Cr) support using post-synthetic modification method was established. The results exhibited that, this novel heterogeneous palladium catalyst system show high performance in the Cu-free Sonogashira coupling reaction.
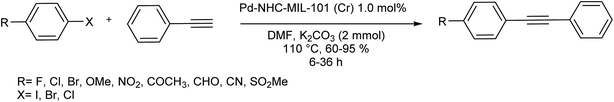 | ||
| Scheme 46 The copper-free Sonogashira coupling reaction in the presence of Pd–NHC–MIL-101(Cr) catalyst. | ||
The possibility of using chitin as an accessible and easily-modifiable support for an efficient Pd(II) catalyst was demonstrated for the first time in 2021 by Kritchenkov and co-workers. The triazole chitins showed improved metal-coordinating ability and can successfully be used as support in the imino-isonitrile Pd(II) complex [PdCl2(N (p-tol) = CPh2)(CNCy)], tuning high catalytic activity and making it reusable. The modification of chitin evading an obvious chain scission or deacetylation, is accomplished by sonochemical alkylation with 1-azido-3-chloropropan-2-ol followed by a useful azido-alkyne click reaction. The resultant polymer exhibits a very rare case of the chitin derivative soluble both in H2O and organic solvents. The reaction of that derivative with imino-isonitrile Pd(II) complex solution afforded a chitin-supported Pd(II) complex. The latter could be provided as a powder or as uniform NPs in various size ranges. The NPs having a hydrodynamic diameter of 30 nm were exhibited to be the most effective form of catalyst for the Cu- and phosphine-free Sonogashira cross-coupling in H2O. Therefore, the following reactions were accomplished using H2O as the solvent of choice and NPs having a hydrodynamic diameter of 30 nm as the optimum form of the investigated catalytic Pd(II)-chitin based complex. The NPs of the Pd(II)-based chitin complex were exhibited to be very efficient and reusable catalysts in the Sonogashira cross-coupling that needed neither anaerobic conditions nor copper-based co-catalyst and, most extraordinarily, it was performed efficiently in the greenest and most abundant solvent, i.e. water. Indeed rGO, this investigation was the first effective demonstration of a chitin-supported palladium catalyst for the cross-coupling reaction (Scheme 47).108
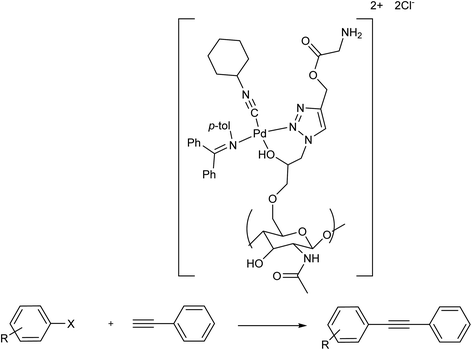 | ||
| Scheme 47 The copper-free Sonogashira coupling reaction in the presence of [PdCl2(N(p-tol) = CPh2)(CNCy)]. | ||
2.4. Miscellaneous
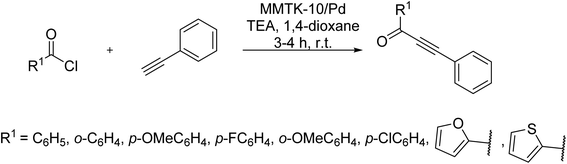
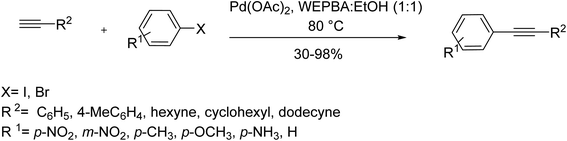
Montmorillonite K-10, an acid-activated clay with a laminar structure and high surface area, has been demonstrated that serves as suitable support in catalytic systems. In 2017, Sureshbabu et al. utilized Montmorillonite K-10 supported Pd nanoparticles for the synthesis of ynones through a copper-free Sonogashira reaction of phenyl acetylenes with an acid chloride. Moreover, the catalyst was recyclable up to seven times, capable of reacting derivatives of coumarin acid chlorides with phenylacetylene, as well (Scheme 48).109,110In 2016, Dewan et al. innovatively used water extract of waste papaya bark ash (WEPBA) in combination with ethanol to make a novel green medium for the in situ formation of Pd nanoparticles (Pd NPs).111 It seems Pd(OAc)2 salt is decomposed to nanoparticles of palladium in the reaction cycle. The WEPBA-assisted in situ-generated Pd NPs made a basic environment capable of performing a copper/amine/ligand-free Sonogashira reaction of electronically diverse substrates, in the absence of any additives or bases, under mild conditions. It is believed that potassium and sodium carbonate contents of papaya bark could act as the bases, while metal salts like palladium could likely contribute as promoters in Sonogashira reactions. There was a similar finding by Dewan's team, reporting the mild basic water extract of banana peels ash (WEB) in combination with Pd(OAc)2 as a new in situ generated catalyst for the same reaction under similar conditions; a worthy approach from an economical and green natural point of view (Scheme 49).
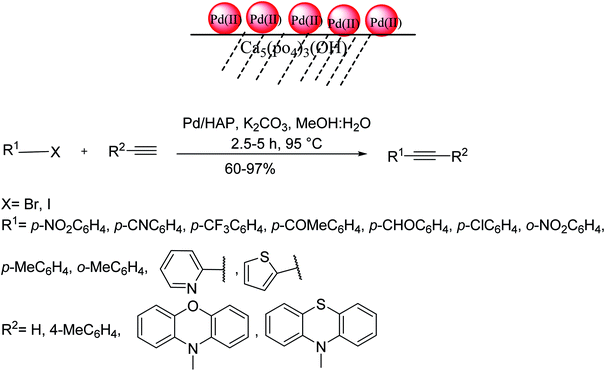
Hydroxyapatite [Ca5(PO4)3(OH)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HAP] is a naturally occurring mineral which has recently been recognized as a suitable support for several metal catalysts. In this regard, Akula et al. in 2017 employed a recyclable HAP-supported Pd catalyst for a copper/phosphine-free Sonogashira reaction (Scheme 50).112 Owing to the porous nature and base sites, HAP can improve the activation of palladium to the desired level. While the combination of Pd with supports of strong basic sites leads to the formation of homo-coupling by-products, moderate basic sites on HAP in conjugation with Pd sites that provide the desired conditions for this type of coupling reaction.
HAP] is a naturally occurring mineral which has recently been recognized as a suitable support for several metal catalysts. In this regard, Akula et al. in 2017 employed a recyclable HAP-supported Pd catalyst for a copper/phosphine-free Sonogashira reaction (Scheme 50).112 Owing to the porous nature and base sites, HAP can improve the activation of palladium to the desired level. While the combination of Pd with supports of strong basic sites leads to the formation of homo-coupling by-products, moderate basic sites on HAP in conjugation with Pd sites that provide the desired conditions for this type of coupling reaction.
This reaction firstly started by oxidative addition of alkyl halide to Pd(0) affording intermediate-I in which palladium oxidized from Pd(0) to Pd(II). Next, alkyne is coordinated to intermediate-I makes π-coordinated complex, with palladium remaining as Pd(II). In the following, the complex through deprotonation with a base, in which the palladium attached with alkyne and alkyl group (R–X) provides intermediate-II. Finally, the intermediate using reductive removal from Pd(II) to Pd(0) affords the functionalized alkyne product (Scheme 51).
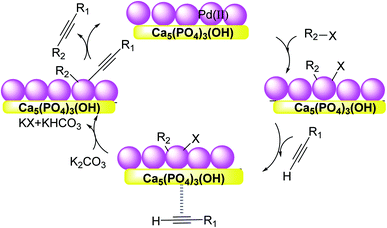 | ||
| Scheme 51 The mechanism of Copper-free Sonogashira coupling reactions in the presence of the Pd/HAP. | ||
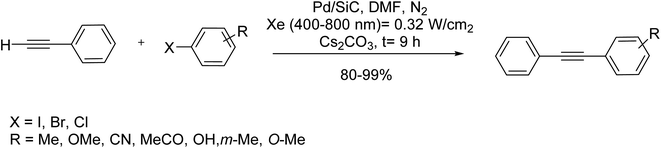
In line with controlling the reaction temperature and avoidance of thermally generated by-products, Wang et al.88 used silicon carbide supported Pd nanoparticles as a visible-light-driven heterogeneous catalyst to perform a Sonogashira reaction under copper/ligand-free conditions. Using SiC as a semiconductor, photogenerated electrons in SiC transfer the light-induced energy to Pd nanoparticles and halogen successively to ease the cleavage of carbon–halogen bond in aryl halide. Cubic SiC (β-SiC) powders were synthesized through a sol–gel and carbothermal reduction method,113 and the reaction was followed with excellent yield under Xe-lamp radiation, as shown in Scheme 52.
In 2016, Xu et al. reported that palladium supported on chitosan/silica composite core–shell microspheres could act as a novel catalyst for the copper/amine/phosphine-free Sonogashira reaction. According to their study, various aryl iodides could be efficiently coupled with terminal phenylacetylene using a minimum amount of catalyst under green conditions (Scheme 53).114
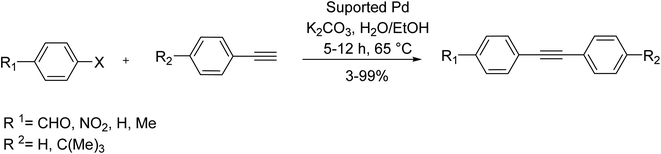 | ||
| Scheme 53 Copper-free Sonogashira coupling reactions in the presence of the chitosan/silica composite core–shell microspheres supported Pd. | ||
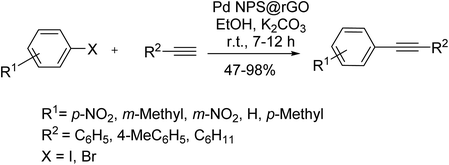
Owing to the abundant hydroxyl and amino groups in the structure, chitosan can promote chemical and electrostatic interactions with several metal ions; thus, be an efficient and stable support candidate. Possessing an oxygen-functionalized surface and fair conductivity, reduced graphene oxide (rGO) is another promising carbon-based support for stabilizing metal nanoparticles such as palladium. Deposition of Pd NPs on rGO afforded a sustainable, active catalyst for a copper/ligand-free Sonogashira reaction at room temperature. The reaction was carried out in an alcoholic solvent with satisfactory yields (Scheme 54).115
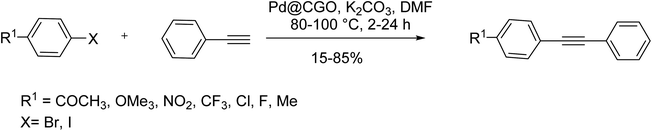
In the same category, Yadav et al. in 2016 introduced carbon graphene oxide (CGO) monolith support with improved dispersion in the aqueous phase for better transfer of reactants to the Pd catalyst layers in copper/ligand-free Sonogashira reaction (Scheme 55).116 The palladium nanoparticles supported on CGO was simply synthesized from solvothermal carbonization of glucose through an eco-friendly procedure. Due to the promising features of this catalyst, Pd leaching is decreased to the minimum level, and the system stability has highly improved.
These catalysts have recently been reported to efficiently promote this type of Sonogashira reaction due to likely inhibition of dearomative rearrangement step in the catalytic cycle. Similar reports of copper and amine-free Sonogashira reaction have also been reported to catalyze the synthesis of pyrazolo[5,1-b][1,3]oxazoles in water as well as arylation of phenylacetylene89–91 or functionalization of quinoline scaffolds88 with or without using ligands, respectively. In 2016, Karami et al. employed a heterogeneous organic–inorganic hybrid catalyst as an efficient tool in this reaction. Accordingly, the reaction of phenylacetylene with aryl halides was carried out under copper-, amine- and phosphine-free conditions in the presence of TiO2-supported Pd catalyst (Scheme 56).92
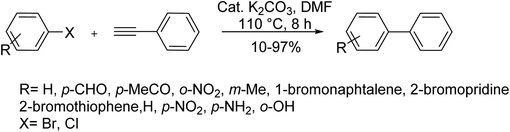 | ||
| Scheme 56 Copper-free Sonogashira coupling reactions in the presence of the TiO2 supported Pd nanocatalyst. | ||
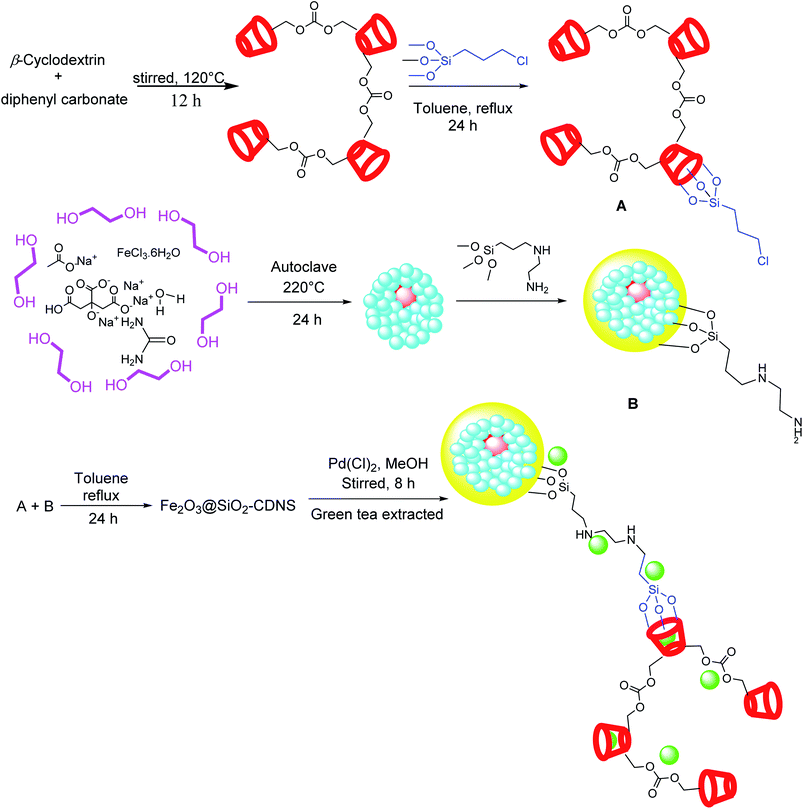
Taking advantage of halloysite nanotubes (HNTs) and cyclodextrin (CD) features, Sadjadi et al. in 2018 reported a new hybrid catalyst for copper/ligand-free Sonogashira reaction, based on the immobilization of Pd nanoparticles on covalently conjugated tosylate CD to functionalized HNTs (Pd@HNTs–T–CD).117 This group later reported Pd@h-Fe2O3@SiO2–CDNS, a similar hybrid catalytic system for the same type of reaction in an aqueous medium.118 The magnetically separable catalyst was made of Fe2O3@SiO2–CDNS, a core–shell magnetic hollow sphere, and CDNS, capable of immobilizing Pd nanoparticles (Scheme 57).
The role of thermally stable cyclodextrin nanosponge (CDNS) in such systems could be attributed to its biocompatibility and insolubility in most solvents, making it form triazole chitin derivative inclusion complexes with hydrophobic substrates and moving them into the aqueous phase, where palladium nanoparticles are in the vicinity.71 The catalyst exhibited an excellent performance and good recyclability in the reaction process. In a similar report by Sadjadi's group, Pd@HNTs–CDNS–g-C3N4, a novel ternary hybrid system, was introduced as an efficient catalyst for the same reaction under mild and green conditions.90 The g-C3N4 is a graphitic carbon nitride, which has proved to decrease palladium leaching in the reaction cycle and increases the catalyst recyclability up to ten reaction runs. Not only in the heterogeneous phase but also in homogeneous media, complexes of palladium could catalyze the same reaction under copper-free conditions. Accordingly, Bagherzadeh et al. in 2017 reported a reusable binuclear Pd(II) complex containing ONO pincer type ligand, capable of performing a green, copper-free Sonogashira reaction with moderate to excellent yields (Scheme 58).69
Worthy of mentioning that to attain the merits of the properties of carbon quantum dots and graphitic carbon nitrides, a new hybrid system was planned relied on the formation and palladation of magnetic carbon quantum dots and subsequent by hydrothermal reaction with graphitic carbon nitrides. Next, the resultant hybrid, g-C3N4–Pd/CQDs@Fe, was applied as an effective catalyst for improving ligand and co-catalyst-free Sonogashira reaction in water. Remarkably, this catalyst catalyzed the reaction of a wide series of the substrates and provided the corresponding products in high yields (Scheme 59).119
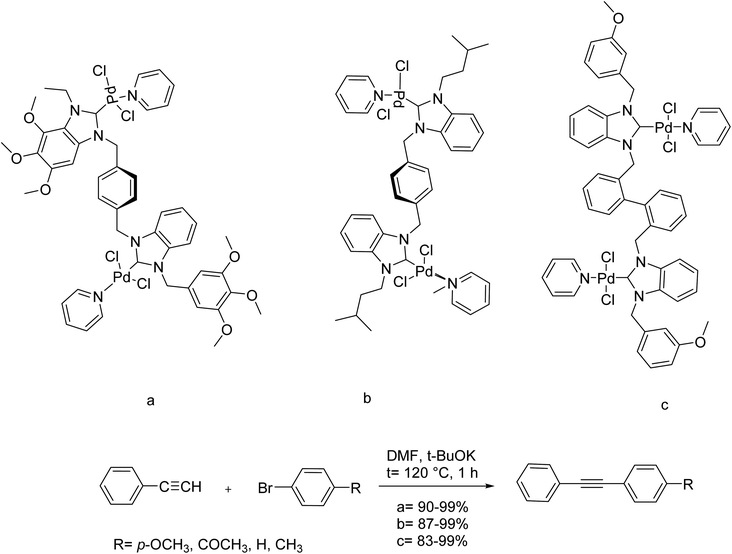
Various phenylene and biphenylene-bridged N-heterocyclic carbene (NHC) and their bimetallic NHC–Pd–PEPPSI complexes were synthesized in 2019 by Yaşar and co-workers. The N tags having different steric and electronic properties were applied to the formation of bridged bimetallic NHC–Pd–PEPPSI (PEPPSI: Pyridine Enhanced Precatalyst Preparation Stabilization Initiation) complexes. The catalytic performance of these bridged bimetallic NHC–Pd–PEPPSI complexes was explored in the Sonogashira coupling reactions between various aryl bromides and phenylacetylene. Commonly, all bridged bimetallic NHC–Pd–PEPPSI complexes exhibited good catalytic activity in this carbon–carbon coupling reaction. These reactions were performed using potassium tert-butoxide and 0.3 mol% of catalyst in dimethylformamide at 120 °C. The Sonogashira cross-coupling of aryl bromides and phenylacetylene was achieved in the presence of 2a–c as the catalyst. Commonly, all complexes demonstrated high catalytic activity with very high yields in this Sonogashira cross-coupling reaction (Scheme 60).120
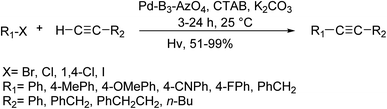
Verpoort and co-workers in 2019 demonstrated the direct harnessing of visible light energy using a new semiconducting azobenzene that relied on the colloidal porous polymer, B3-Azo4 amenable to post-synthetic palladium(II)-complexation for photo-induced Sonogashira coupling reaction through polymer-metal energy transfer approach. Completely, mesoporous nano-sized particles of the synthesized organic network manifested excellent palladium(II) coordinating capability and gave the corresponding catalytic products at room temperature in aqueous media under aerobic conditions. To examine the generality of this reaction, a mixture of various halides and terminal alkynes were exposed to the optimal condition (Scheme 61).121
A wide range of triphenylarsine and triphenylstibine-stabilized tri-heteroleptic NHC–Pd–allyl complexes [(NHC)Pd(allyl)(EPh3)]ClO4 (E = As, Sb) were synthesized and identified in 2019 by Yang et al. Palladium complexes were examined as catalysts in the Sonogashira reaction, and the synthesized complexes demonstrated satisfactory yields. Typically, electron-withdrawing substituents show an effect on improving the reactivity of substrates. The coupling reactions of differently activated aryl bromide and phenylacetylene gave the corresponding products in vary high yields (Scheme 62).122
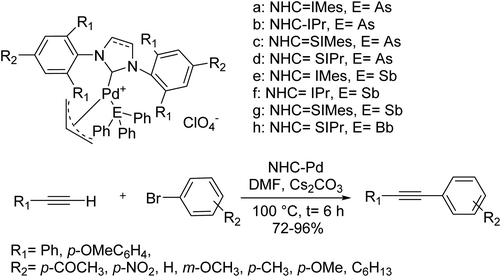 | ||
| Scheme 62 The Sonogashira reaction in the presence of the triphenylarsine and triphenylstibine-stabilized tri-heteroleptic NHC–Pd–allyl complexes. | ||
Yip and co-workers in 2019 developed a zeolite stacking structure by hydraulic pressing and programmed temperature calcination synthesis approaches. ZSM-5 type zeolites having particle sizes of nearly 100 nm, 1 mm, and 2 mm were used to provide stacking ZSM-5 with a size from 45 to 63 mm. The synthesized ZSM-5 zeolite stacking structure was applied as support to deposit Pd. The activity of the Pd/stacking ZSM-5 was explored in the Sonogashira reactions. The generality and functional group tolerance of PHZ-2 was explored. Neutral phenylacetylene was reacted successfully with various electron-deficient and electro-neutral aryl bromides based on the optimal conditions. The results demonstrated that the palladium/stacking ZSM-5 catalyst is useful in the Sonogashira coupling reactions with various substrates bearing diverse functional groups(Scheme 63).123
In this investigation, evenly distributed Pd(0) NPs supported on the eso structure of the hardwood (Pd NPs/wood) having the loading content of 3.5 wt% of Pd were synthesized through the reduction of palladium(II) chloride using lignin in the hardwood. The palladium NPs/wood exhibits an appropriate catalytic activity in the Sonogashira reactions of aryl iodide. Besides, the long channels in the wood can be applied as multiple micro-reaction vessels, permitting that the palladium-catalyzed continuous flow reactions occur in the palladium NPs/wood. The generality of this Sonogashira reaction in the presence of Pd NPs/wood as catalyst was investigated using various aryl iodides and terminal alkynes based on the optimized reaction conditions, including 3.3 mol% of Pd NPs/wood and cesium carbonate in dimethylformamide at 25 °C. Aryl iodides having steric hindered, electron-donating, and withdrawing groups demonstrated analogous activity, and the relevant internal alkyne was synthesized in satisfactory to high yields. Besides, this catalyst is effective for the reaction between aryl and alkyl alkyne. The results exhibited that palladium NPs/wood is an effective catalyst for the Sonogashira reactions of aryl iodides and terminal alkynes (Scheme 64).124
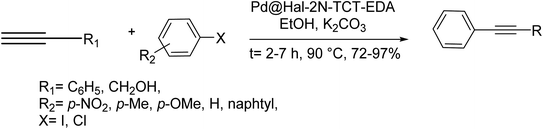
Halloysite nanoclay (Hal) was amine-functionalized and followed by reaction with ethylenediamine (EDA) and 2,4,6-trichloro-1,3,5-triazine (TCT) to offer a multi-nitrogen bearing group on the surface of halloysite nano clay. Next, the resulting surface-modified halloysite nanoclay, Hal–2N-TCT–EDA, was applied for immobilization of Pd NPs and giving a heterogeneous catalyst, Pd@Hal–2N-TCT–EDA, with utility for Cu and ligand-free Sonogashira coupling reaction of aryl halides and alkynes. The scope of this approach was demonstrated by using different alkynes and aryl halides, having electron-withdrawing and donating substituents. Worthy to mention that various aryl halides including aryl bromides and aryl chlorides and aryl iodides in the presence of Pd@Hal–2N-TCT–EDA via the Sonogashira coupling reaction provided the relevant biaryls (Scheme 65).125
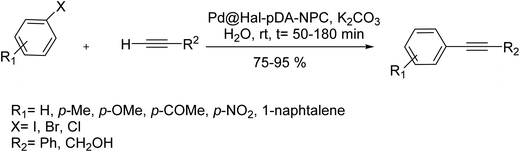
A hybrid catalyst, Pd@Hal–pDA–NPC was synthesized via two main steps and was used in the Sonogashira reactions. Initially, palladium(0) NPs were immobilized on the poly-dopamine decorated halloysite nanotubes (Hal–pDA). Next, Pd@Hal–pDA was hybridized with the layers of a new multi-N-doped porous carbon monolayer obtained from 440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400-((1,3,5-triazine-2,4,6-triyl)tris(azanediyl))tribenzonitrile. This catalyst was used in the Sonogashira coupling reaction. The results demonstrated that this novel catalyst catalyzed all the reactions successfully.126
400-((1,3,5-triazine-2,4,6-triyl)tris(azanediyl))tribenzonitrile. This catalyst was used in the Sonogashira coupling reaction. The results demonstrated that this novel catalyst catalyzed all the reactions successfully.126
Noticeably, the maximum yield of the model product was obtained in the presence of 0.5 mol% of catalyst at room temperature by using potassium carbonate as base and H2O as solvent under mild and green conditions. As a result, both electronic nature (the presence of electron-donating or withdrawing substituents on the substrate) and steric aspects of the substrate could change the yield of the relevant products (Scheme 66).126
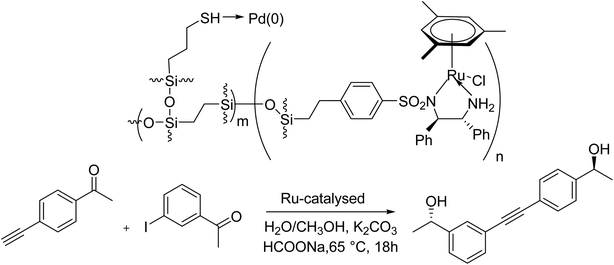
The formation of a bifunctional heterogeneous catalyst through the immobilization of Pd NPs into ethylene-bridged chiral Ru/diamine-substituted periodic mesoporous organosilica was reported in 2017 by Zhao and co-workers. As a bifunctional catalyst, it permits effective enantioselective transfer hydrogenation–Sonogashira coupling one-pot asymmetric tandem reaction in which ruthenium-catalyzed enantioselective transfer hydrogenation that followed by palladium-mediated Sonogashira coupling reaction to give a wide series of chiral conjugated alkynols in high yields and ee (Scheme 67).127
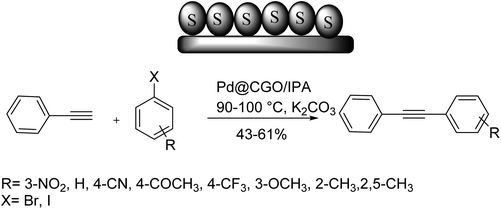
Yadav and co-workers in 2016 reported the formation of Pd NPs supported on carbon-based graphene oxide monolith (Pd@CGO monolith) via solvothermal carbonization of glucose with a small quantity of graphene oxide (GO) as a structure-directing agent. It should be mentioned that it gives a large surface for anchoring Pd NPs. CGO monolith obstructs palladium particle migration and improves the interaction between Pd NPs and support that results in increased activity. This catalyst was applied in the Sonogashira coupling reaction. The activity of Pd@CGO monolith was compared with 5% Pd@GO and 5% Pd/C in the Sonogashira reaction between iodobenzene and phenylacetylene. Worthy of mentioning that Pd@CGO monolith exhibited very high conversion with low catalyst loading under Cu-free and ligand-free conditions. The catalytic activity of the Pd@CGO monolith catalyst was investigated in other Sonogashira coupling reactions using various substituted aryl halides with phenylacetylene derivatives. The Sonogashira coupling of substrates having electron-withdrawing groups with iodobenzene occurred efficiently, with high to excellent yields of the substituted diphenylethyne as a corresponding product. The reaction between aryl iodide, having electron-releasing groups at the para- and ortho-positions with phenylacetylene afforded the corresponding products in satisfactory to high yields (Scheme 68).116
A new, biodegradable and eco-friendly magnetic cross-linked chitosan fibers and its Co complex were synthesized and identified in 2019 by Hajipour and co-workers. Based on this approach, the facile approach was used to attain the chitosan composite fibers with dimethyl malonate as a linker and ligand. Spontaneously, a cross-linking approach under mild conditions was presented that increased the solvent resistance, mechanical, and thermostability strength of the magnetic chitosan polymer. This Pd and phosphine-free catalyst, as a very active heterogeneous catalyst, exhibited outstanding stability and activity under eco-friendly condition in the Sonogashira reaction. Remarkably, the tripotassium phosphate and polyethylene glycol (PEG-200) was known as the best base and solvent, respectively (Scheme 69).128
A novel p-bis[(3,4-salicylicimino)benzophenimine] phenylenediamine palladium(II) complex Fe3O4@SiO2@Pd–Slp core–shell superparamagnetic NPs functionalized by a bis-salophen Schiff base palladium-complex was synthesized and used as an effective magnetic nanocatalyst in the Sonogashira reaction. This heterogeneous catalyst exhibited well activity in the Sonogashira coupling reaction of aryl halides and alkynes. This method provided a new magnetically bis-salophen Pd complex as a useful and recyclable nanocatalyst to perform C–C bond generation through Cu- and phosphine-free Sonogashira coupling reactions under aqueous media. Therefore, a wide series of aryl halides were reacted with phenylacetylene in water/dimethylformamide using 0.2 mol% palladium catalyst and potassium carbonate at 90 °C. This catalyst gave a very high yield of the coupling products with high TOFs via Sonogashira reaction of terminal aryl halides and acetylenes (Scheme 70).129
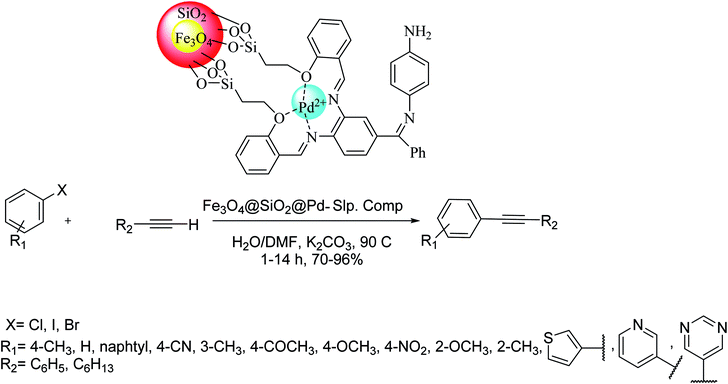 | ||
| Scheme 70 The Sonogashira reaction in the presence of the p-bis[(3,4-salicylicimino)benzophenimine] phenylenediamine palladium(II) complex (Fe3O4@SiO2@Pd–Slp). | ||
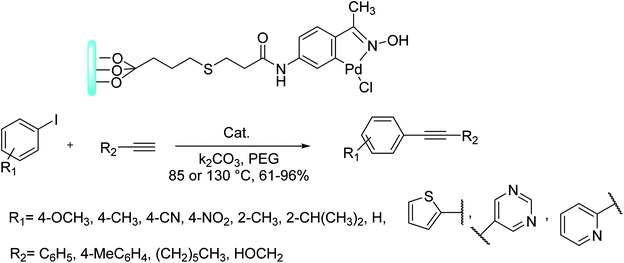
Gholinejad and co-workers in 2018 reported the formation of a new supported catalyst using an oxime-derived palladacycle supported on clay OxPdCy@clay. This Pd composite improves the Sonogashira coupling reactions between aryl chlorides, bromides, iodides, and terminal alkynes in polyethylene glycol 200 at 85 or 130 °C in the presence of 0.05–0.1 mol% of Pd loading under Cu-free and phosphine-free conditions. Remarkably, this supported palladacycle, OxPdCy@clay, exhibited superior catalytic activity in comparison with dimeric oxime-palladacycles.130
Under the optimal conditions, the Sonogashira coupling reaction of various aryl halides and terminal alkynes was investigated. The reactions of various aryl iodides bearing electron-donating substituents and aryl iodides having electron-withdrawing substituents with aryl acetylenes, 1-octyne, and propargyl alcohol were performed successfully and gave the relevant internal alkynes in very high yields. Noticeably, the results demonstrated that the reaction of sterically hindered 2-iodocumene and 2-iodotoluene with propargyl alcohol or phenylacetylene occurred in satisfactory yields (Scheme 71).130
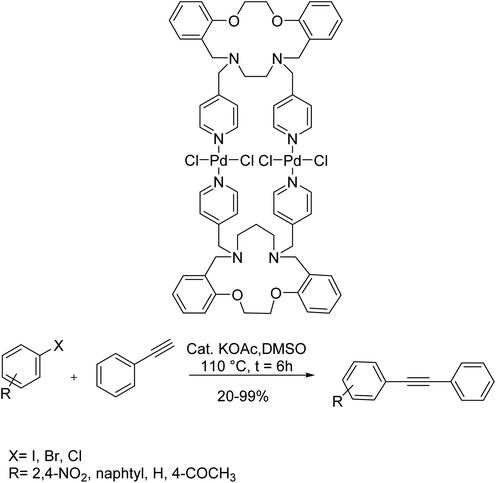
The novel 32-membered macrocyclic dinuclear Pd complex of two aza-crown macrocycles, bearing two pyridine arms, Pd2L2Cl4 was synthesized and identified in 2018 by Ghanbari and co-workers. Pd2L2Cl4 was used as a moisture/air-stable catalyst in the Sonogashira reaction through a copper-free and phosphine ligand method in dimethyl sulfoxide. Thermal stability, probable existence of tandem reactions, increased catalytic activity, as well as synergistic effects are merits aspects of Pd2L2Cl4. The activity of Pd2L2Cl4 was investigated in the Sonogashira reaction of various aryl halides and phenyl acetylene in the presence of potassium acetate at 110 °C in dimethyl sulfoxide as a solvent. The results demonstrated that the reaction between aryl bromide bearing electron-withdrawing groups gave the relevant coupled products in very good yields. Moreover, under this approach, the 1-bromo naphthalene resulted in a satisfactory yield in comparison with aryl bromide (Scheme 72).131
An eco-friendly silica-grafted nicotine-based Pd(II) complex was efficiently synthesized and used as a new and effective nanocatalyst in carbon–carbon bond-forming reactions. Grafted-nicotine in this catalytic method shows a significant role, and also as an efficient ligand and a quaternary ammonium salt exhibits an important stabilizing effect on the palladium(II) species using a synergistic effect of electrostatic and coordination interactions. Based on the optimal condition, different aryl bromides reacted with phenylacetylene and afforded the corresponding Sonogashira products (Scheme 73).132
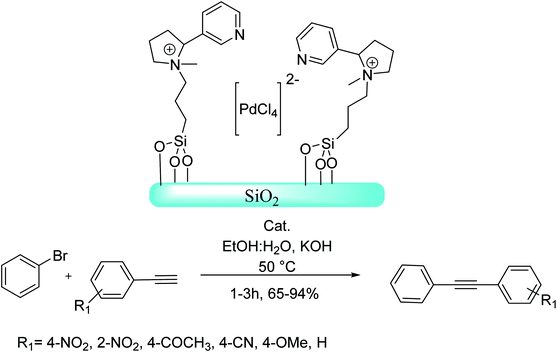 | ||
| Scheme 73 The Sonogashira reaction in the presence of the nicotine functionalized-silica palladium(II) complex catalyst. | ||
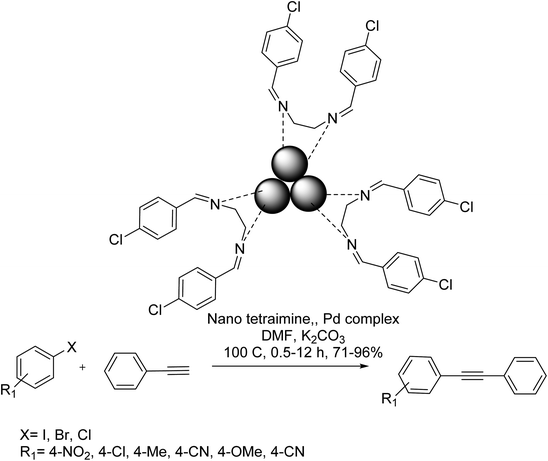
A nano tetramine palladium(0) complex catalyst was efficiently used as an effective heterogeneous catalyst in the Cu-free Sonogashira coupling reaction in DMF. Analysis of the reaction mixture demonstrated that the leaching of Pd from the catalyst was insignificant. The reactions were accomplished for aryl chlorides, bromides, and iodides. Based on the optimal conditions, the Sonogashira-type cross-coupling of various aryl halides with phenylacetylene was achieved efficiently to provide the corresponding products in satisfactory to excellent yields. Significantly, the reaction of substituted aryl iodides having electron-withdrawing substituents gave a very good yield. It should be mentioned that deactivated bromides needed longer reaction time for this reaction condition to provide good yields (Scheme 74).133
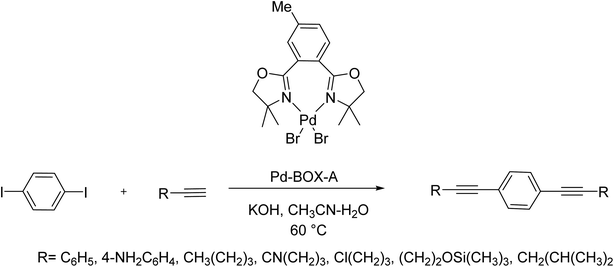
A very effective method for the Cu-free and phosphine-free Sonogashira cross-couplings between terminal alkynes and aryl iodides using aerobic conditions has been achieved and reported in 2015 by Ibrahim and co-workers. The Sonogashira cross-coupling reactions in the presence of 1 mol% of the Pd-bis(oxazoline) complex, Pd-BOX A, using potassium hydroxide, and acetonitrile/water as solvent was achieved at room temperature or 60 °C to provide the corresponding products. This novel catalyst was very active in the cross-coupling reaction of unactivated alkyl alkynes and aryl diiodo derivatives to provide different symmetrical dialkynes. In addition, the cross-coupling reaction of aryl iodides with terminal dialkynes using this catalyst provided symmetrical functionalized internal alkynes. Furthermore, it was examined the Sonogashira reaction between aryl and alkyl alkynes and diiodobenzene. Noticeably, this reaction was performed efficiently and afforded novel various internal alkynes in very yields (Scheme 75).134
The reaction of 2-(phenylthio/seleno)ethylamine and 1-naphthaldehyde afforded air and moisture insensitive Schiff bases, C10H7-1-CH = N–CH2CH2EPh (L1: E = S; L2: E = Se). They on treatment with sodium acetate and Li2PdCl4 afford palladacycles, [Pd(L1-H/L2-H)Cl] (1/2) at room temperature, in which L1/L2 ligates as an unsymmetric (C−, N, E) pincer. The reduction of >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of L1 and L2 with NaBH4 affords C10H7-1-CH2NH–CH2CH2EPh (L3: E = S; L4: E = Se). The reaction of L3/L4 at an ambient temperature similar to those of L1/L2 leads to complex [Pd(L3/L4)Cl2] (3/4) in which the ligand coordinates in a bidentate (N, E) mode. The yield of all complexes is >85%.
N bond of L1 and L2 with NaBH4 affords C10H7-1-CH2NH–CH2CH2EPh (L3: E = S; L4: E = Se). The reaction of L3/L4 at an ambient temperature similar to those of L1/L2 leads to complex [Pd(L3/L4)Cl2] (3/4) in which the ligand coordinates in a bidentate (N, E) mode. The yield of all complexes is >85%.
The catalytic activity of 1–4 was investigated in the Cu-free and amine-free Sonogashira coupling reaction under aerobic conditions. This Sonogashira coupling reaction was achieved in the presence of 0.05 mol% of catalyst to attain satisfactory conversion. Palladacycles led to satisfactory conversion. The formation of Pd bearing NPs during the coupling reaction was detected. Thus, their role in catalysis by 1–4 exists certainly.135
DMF as solvent afforded the best result. Under the optimal reaction conditions, the catalytic activity of complexes for various substrates was investigated. The 0.05 mol% of the catalyst was appropriate for activated substrates such as 4-bromonitrobenzene, 4-bromobenzaldehyde, 4-bromoacetophenone and 4-bromobenzonitrile (Scheme 76).
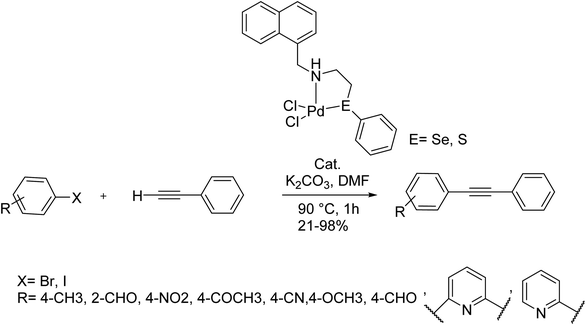 | ||
| Scheme 76 The Sonogashira reaction in the presence of the Pd(II) complexes of chalcogenated Schiff bases of 1-naphthaldehyde catalyst. | ||
3 Conclusion
In summery we tried to underscore the developments and advances of the recently Cu-free Sonogashira cross coupling reactions of different aromatic halides with various alkynes covering a plethora of recent references present in the chemical literature. Due to its huge synthetic efficacy of heterogeneous nanocatalysts, their usage were also included. Different methods with varied efficiencies for the synthesis of many target compounds via Cu-free Sonogashira cross-coupling were discussed. Different approaches have led to the design of reactions to give the desired products with fair to excellent yields in the absence of copper were summerized. Among the different strategies, there are essential motifs generated via the Sonogashira reaction. An efficient and facile synthesis these compounds are sometimes necessary for having enough material in hand for their biological screening and evaluation of their bioactivity. Their biological examination exhibited that several Sonogashira product have anti-cizmatics, anti-phobic, or even inhibiting properties to control Schizophrenia, Alzheimer's illness, etc. This review focused on the developments in the Sonogashira cross-coupling reaction being performed in the absence of copper as co-catalysts which has several merits such as being more facile, thus less complicated, avoiding complexity of undesired interaction of Cu with other components used in the classical Sonogashira reaction, complying to numbers of green chemistry principles.Conflicts of interest
There is no conflict of interest to declare.Acknowledgements
The authors acknowledge the research council of the University of Alzahra for support. M. M. H. is also thankful to Iran National Science Foundation (INSF) for granted individual research chair.References
- D. Sghairi, M. Romdhani-Younes, V. Guilloteau, J. Petrignet and J. Thibonnet, Tetrahedron Lett., 2020, 61, 151786 CrossRef CAS.
- M. M. Heravi, E. Hashemi, Y. S. Beheshtiha, S. Ahmadi and T. Hosseinnejad, J. Mol. Catal. A: Chem., 2014, 394, 74–82 CrossRef CAS.
- R. Hosseinzadeh, R. Ojani and L. Shabani, Curr. Chem. Lett., 2014, 3, 37–42 CrossRef CAS.
- A. V. Murashkina, A. Y. Mitrofanov and I. P. Beletskaya, Russ. J. Org. Chem., 2019, 55, 1445–1458 CrossRef CAS.
- R. Akhtar, A. F. Zahoor, B. Parveen and M. Suleman, Synth. Commun., 2019, 49, 167–192 CrossRef CAS.
- M. Bakherad, Appl. Organomet. Chem., 2013, 27, 125–140 CrossRef CAS.
- D. A. Alonso, A. Baeza, R. Chinchilla, C. Gómez, G. Guillena, I. M. Pastor and D. Ramón, Catalysts, 2018, 8, 202 CrossRef.
- R. R. Tykwinski, Angew. Chem., Int. Ed., 2003, 42, 1566–1568 CrossRef CAS.
- K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron lett., 1975, 16, 4467–4470 CrossRef.
- T. Hosseinnejad, M. M. Heravi and R. Firouzi, J. Mol. Model., 2013, 19, 951–961 CrossRef CAS.
- B. de Carné-Carnavalet, A. Archambeau, C. Meyer, J. Cossy, B. Folléas, J.-L. Brayer and J.-P. Demoute, Chem.–Eur. J., 2012, 18, 16716–16727 CrossRef.
- T. Yao and R. C. Larock, J. Org. Chem., 2003, 68, 5936–5942 CrossRef CAS.
- R. Venkatraman and F. R. Fronczek, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2008, 64, m577 CrossRef CAS.
- H. A. Oskooie, M. M. Heravi and F. K. Behbahani, Molecules, 2007, 12, 1438–1446 CrossRef CAS.
- C. Pan, F. Luo, W. Wang, Z. Ye and M. Liu, J. Chem. Res., 2009, 2009, 478–481 CrossRef.
- M. Esmaeilpour, A. Sardarian and J. Javidi, Catal. Sci. Technol., 2016, 6, 4005–4019 RSC.
- P. L. Golas, N. V. Tsarevsky, B. S. Sumerlin and K. Matyjaszewski, Macromolecules, 2006, 39, 6451–6457 CrossRef CAS.
- M. Feuerstein, H. Doucet and M. Santelli, Tetrahedron Lett., 2004, 45, 1603–1606 CrossRef CAS.
- J. Zhang, M. Đaković, Z. Popović, H. Wu and Y. Liu, Catal. Commun., 2012, 17, 160–163 CrossRef CAS.
- B. H. Lipshutz, D. W. Chung and B. Rich, Org. Lett., 2008, 10, 3793–3796 CrossRef CAS.
- T. Fukuyama, M. Shinmen, S. Nishitani, M. Sato and I. Ryu, Org. Lett., 2002, 4, 1691–1694 CrossRef CAS.
- A. Komáromi, G. L. Tolnai and Z. Novák, Tetrahedron Lett., 2008, 49, 7294–7298 CrossRef.
- M. M. Heravi and S. Sadjadi, Tetrahedron, 2009, 65, 7761–7775 CrossRef CAS.
- M. Daraie, M. M. Heravi and S. S. Kazemi, J. Coord. Chem., 2019, 72, 2279–2293 CrossRef CAS.
- T. Tamoradi, M. Daraie and M. M. Heravi, Appl. Organomet. Chem., 2020, 34, e5538 CAS.
- S. Sadjadi, M. Malmir, M. M. Heravi and F. Ghoreyshi Kahangi, Inorg. Chim. Acta, 2019, 488, 62–70 CrossRef CAS.
- R. F. Heck, Org. React., 2004, 27, 345–390 Search PubMed.
- C. E. Castro, E. J. Gaughan and D. C. Owsley, J. Org. Chem., 1966, 31, 4071–4078 CrossRef CAS.
- L. Cassar, J. Organomet. Chem., 1975, 93, 253–257 CrossRef CAS.
- H. A. Dieck and F. R. Heck, J. Organomet. Chem., 1975, 93, 259–263 CrossRef CAS.
- K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467–4470 CrossRef.
- C. C. C. Johansson Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef CAS.
- F. F. Wagner and D. L. Comins, J. Org. Chem., 2006, 71, 8673–8675 CrossRef CAS.
- K. Nishimura and M. Kinugawa, Org. Process Res. Dev., 2012, 16, 225–231 CrossRef CAS.
- S. López, F. Fernández-Trillo, P. Midón, L. Castedo and C. Saá, J. Org. Chem., 2006, 71, 2802–2810 CrossRef.
- F. Manoni, C. Rumo, L. Li and P. G. Harran, J. Am. Chem. Soc., 2018, 140, 1280–1284 CrossRef CAS.
- A. Idelson, C. Sterzenbach, S.-S. Jester, C. Tschierske, U. Baumeister and S. Höger, J. Am. Chem. Soc., 2017, 139, 4429–4434 CrossRef CAS.
- X. Zhuang, D. Gehrig, N. Forler, H. Liang, M. Wagner, M. R. Hansen, F. Laquai, F. Zhang and X. Feng, Adv. Mater., 2015, 27, 3789–3796 CrossRef CAS.
- V. Kozell, M. McLaughlin, G. Strappaveccia, S. Santoro, L. A. Bivona, C. Aprile, M. Gruttadauria and L. Vaccaro, ACS Sustainable Chem. Eng., 2016, 4, 7209–7216 CrossRef CAS.
- K. Königsberger, G.-P. Chen, R. R. Wu, M. J. Girgis, K. Prasad, O. Repič and T. J. Blacklock, Org. Process Res. Dev., 2003, 7, 733–742 CrossRef.
- R. Chinchilla and C. Nájera, Chem. Rev., 2007, 107, 874–922 CrossRef CAS.
- J. Kooroshy, C. Meindersma, R. Podkolinski, M. Rademaker, T. Sweijs, A. Diederen, M. Beerthuizen and S. de Goede, Scarcity of Minerals Hague Centre for Strategic Studies, Hague Centre for Strategic Studies, 2009 Search PubMed.
- F. Mohajer, G. Mohammadi Ziarani and A. Badiei, Res. Chem. Intermed., 2020, 46, 4909–4922 CrossRef CAS.
- M. M. Heravi, A. Keivanloo, M. Rahimizadeh, M. Bakavoli and M. Ghassemzadeh, Tetrahedron Lett., 2004, 45, 5747–5749 CrossRef CAS.
- M. M. Heravi, A. Kivanloo, M. Rahimzadeh, M. Bakavoli, M. Ghassemzadeh and B. Neumüller, Tetrahedron Lett., 2005, 46, 1607–1610 CrossRef CAS.
- S. Sadjadi, M. M. Heravi and M. Malmir, Carbohydr. Polym., 2018, 186, 25–34 CrossRef CAS.
- F. Mohajer, G. Mohammadi Ziarani and A. Badiei, J. Iran. Chem. Soc., 2021, 18, 589–601 CrossRef.
- R. Chinchilla and C. Nájera, Chem. Soc. Rev., 2011, 40, 5084–5121 RSC.
- T. Ren, Chem. Rev., 2008, 108, 4185–4207 CrossRef CAS.
- E.-i. Negishi and L. Anastasia, Chem. Rev., 2003, 103, 1979–2018 CrossRef CAS.
- J. D’Attoma, G. Cozien, P. L. Brun, Y. Robin, S. Bostyn, F. Buron and S. Routier, ChemistrySelect, 2016, 1, 338–342 CrossRef.
- V. Farina, Adv. Synth. Catal., 2004, 346, 1553–1582 CrossRef CAS.
- H.-U. Blaser, A. Indolese, F. Naud, U. Nettekoven and A. Schnyder, Adv. Synth. Catal., 2004, 346, 1583–1598 CrossRef CAS.
- G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644–4680 CrossRef CAS.
- B. J. Borah and D. K. Dutta, J. Mol. Catal. A: Chem., 2013, 366, 202–209 CrossRef CAS.
- A. Ohtaka, T. Yamaguchi, T. Teratani, O. Shimomura and R. Nomura, Molecules, 2011, 16, 9067–9076 CrossRef CAS.
- K. Komura, H. Nakamura and Y. Sugi, J. Mol. Catal. A: Chem., 2008, 293, 72–78 CrossRef CAS.
- J. Chung, J. Kim, Y. Jang, S. Byun, T. Hyeon and B. M. Kim, Tetrahedron Lett., 2013, 54, 5192–5196 CrossRef CAS.
- A. Cwik, Z. Hell and F. Figueras, Tetrahedron Lett., 2006, 47, 3023–3026 CrossRef CAS.
- S.-D. Oh, M.-R. Kim, S.-H. Choi, J.-H. Chun, K.-P. Lee, A. Gopalan, C.-G. Hwang, K. Sang-Ho and O. J. Hoon, J. Ind. Eng. Chem., 2008, 14, 687–692 CrossRef CAS.
- L. Zhang, X. Wang, Y. Zhao, Z. Zhu and H. Fong, Mater. Lett., 2012, 68, 133–136 CrossRef CAS.
- Y.-W. Lee, S.-B. Han, A.-R. Ko, H.-S. Kim and K.-W. Park, Catal. Commun., 2011, 15, 137–140 CrossRef CAS.
- S.-J. Kim, S.-D. Oh, S. Lee and S.-H. Choi, J. Ind. Eng. Chem., 2008, 14, 449–456 CrossRef CAS.
- A. S. Hay, J. Org. Chem., 1962, 27, 3320–3321 CrossRef CAS.
- P. Siemsen, R. C. Livingston and F. Diederich, Angew. Chem., Int. Ed., 2000, 39, 2632–2657 CrossRef CAS.
- S. Wang, Y. Gao, S. Shen, H. Wen and H. Cui, Molecules, 2018, 23, 154 CrossRef.
- M. Trunk, A. Herrmann, H. Bildirir, A. Yassin, J. Schmidt and A. Thomas, Chem.–Eur. J., 2016, 22, 7179–7183 CrossRef CAS.
- D. D. Vo and M. Elofsson, ChemistrySelect, 2017, 2, 6245–6248 CrossRef CAS.
- H. Yanık, S. Yeşilot and M. Durmuş, Dyes Pigm., 2017, 140, 157–165 CrossRef.
- A. J. Arduengo, R. L. Harlow and M. Kline, J. Am. Chem. Soc., 1991, 113, 361–363 CrossRef CAS.
- Z. I. Dehimat, S. Yaşar, D. Tebbani and İ. Özdemir, Inorg. Chim. Acta, 2018, 469, 325–334 CrossRef CAS.
- C. Amatore, S. Bensalem, S. Ghalem and A. Jutand, J. Organomet. Chem., 2004, 689, 4642–4646 CrossRef CAS.
- S. Thawarkar, N. D. Khupse and A. Kumar, ChemPhysChem, 2016, 17(7), 1006–1017 CrossRef CAS.
- A. P. Thankachan, T. G. Abi, K. S. Sindhu and G. Anilkumar, ChemistrySelect, 2016, 1, 3405–3412 CrossRef CAS.
- A. G. K. Reddy and G. Satyanarayana, Synthesis, 2017, 49, 5149–5158 CrossRef.
- A. M. Mak, Y. H. Lim, H. Jong, Y. Yang, C. W. Johannes, E. G. Robins and M. B. Sullivan, Organometallics, 2016, 35, 1036–1045 CrossRef CAS.
- J. Zhu and V. N. Lindsay, ACS Catal., 2019, 9, 6993–6998 CrossRef CAS.
- Y. Monguchi, F. Wakayama, S. Ueda, R. Ito, H. Takada, H. Inoue, A. Nakamura, Y. Sawama and H. Sajiki, RSC Adv., 2017, 7, 1833–1840 RSC.
- S. Yu, J. Wu, X. He and Y. Shang, Appl. Organomet. Chem., 2018, 32, e4156 CrossRef.
- S. Pradhan, S. Dutta and R. P. John, New J. Chem., 2016, 40, 7140–7147 RSC.
- R. K. Sharma, M. Yadav, R. Gaur, R. Gupta, A. Adholeya and M. B. Gawande, ChemPlusChem, 2016, 81, 1312–1319 CrossRef CAS.
- L. Yu, Z. Han and Y. Ding, Org. Process Res. Dev., 2016, 20, 2124–2129 CrossRef CAS.
- S. Elavarasan, B. Baskar, C. Senthil, P. Bhanja, A. Bhaumik, P. Selvam and M. Sasidharan, RSC Adv., 2016, 6, 49376–49386 RSC.
- I. M. de Oliveira, S. S. N. Vasconcelos, C. S. Barbeiro, T. C. Correra, A. Shamim, D. C. Pimenta, I. Caracelli, J. Zukerman-Schpector, H. A. Stefani and F. Manarin, New J. Chem., 2017, 41, 9884–9888 RSC.
- A. R. Hajipour and Z. Tavangar-Rizi, Appl. Organomet. Chem., 2017, 31, e3701 CrossRef.
- S. K. Das, M. Sarmah and U. Bora, Tetrahedron Lett., 2017, 58, 2094–2097 CrossRef CAS.
- A. S. Camacho, I. Martín-García, C. Contreras-Celedón, L. Chacón-García and F. Alonso, Catal. Sci. Technol., 2017, 7, 2262–2273 RSC.
- L.-M. Zhang, H.-Y. Li, H.-X. Li, D. J. Young, Y. Wang and J.-P. Lang, Inorg. Chem., 2017, 56, 11230–11243 CrossRef CAS.
- C. Wang, W. Lu, Y. Tong, Y. Zheng and Y. Yang, RSC Adv., 2014, 4, 57009–57015 RSC.
- G. E. Tyson, K. Tokmic, C. S. Oian, D. Rabinovich, H. U. Valle, T. K. Hollis, J. T. Kelly, K. A. Cuellar, L. E. McNamara, N. I. Hammer, C. E. Webster, A. G. Oliver and M. Zhang, Dalton Trans., 2015, 44, 14475–14482 RSC.
- M. Slivarichova, E. Reading, M. F. Haddow, H. Othman and G. R. Owen, Organometallics, 2012, 31, 6595–6607 CrossRef CAS.
- K. Karami, S. Abedanzadeh and P. Hervés, RSC Adv., 2016, 6, 93660–93672 RSC.
- S. Thogiti, S. P. Parvathaneni and S. Keesara, J. Organomet. Chem., 2016, 822, 165–172 CrossRef CAS.
- M. Gholinejad, A. Neshat, F. Zareh, C. Nájera, M. Razeghi and A. Khoshnood, Appl. Catal., A, 2016, 525, 31–40 CrossRef CAS.
- R. Gogoi, R. Saikia and G. Borah, J. Organomet. Chem., 2019, 897, 80–88 CrossRef CAS.
- M. Bakherad, A. Kakav Ghalenoei and A. Keivanloo, ChemistrySelect, 2019, 4, 9238–9240 CrossRef CAS.
- A. R. Sardarian, H. Eslahi and M. Esmaeilpour, Appl. Organomet. Chem., 2019, 33, e4856 CrossRef.
- M. Jakobi, F. Gallou, C. Sparr and M. Parmentier, Helv. Chim. Acta, 2019, 102, e1900024 CrossRef.
- S. Sadjadi, F. Koohestani, N. Bahri-Laleh and K. Didehban, J. Solid State Chem., 2019, 271, 59–66 CrossRef CAS.
- H. Naeimi and F. Kiani, J. Organomet. Chem., 2019, 885, 65–72 CrossRef CAS.
- M. Esmaeilpour, A. R. Sardarian and H. Firouzabadi, J. Organomet. Chem., 2018, 873, 22–34 CrossRef CAS.
- S. Abbasi and M. Hekmati, Appl. Organomet. Chem., 2017, 31, e3600 CrossRef.
- M. Sarmah, A. Dewan, A. J. Thakur and U. Bora, Tetrahedron Lett., 2016, 57, 914–916 CrossRef CAS.
- M. G. Goesten, K. B. Sai Sankar Gupta, E. V. Ramos-Fernandez, H. Khajavi, J. Gascon and F. Kapteijn, CrystEngComm, 2012, 14, 4109–4111 RSC.
- F. Zadehahmadi, S. Tangestaninejad, M. Moghadam, V. Mirkhani, I. Mohammadpoor-Baltork, A. R. Khosropour and R. Kardanpour, J. Solid State Chem., 2014, 218, 56–63 CrossRef CAS.
- G. Chen, S. Wu, H. Liu, H. Jiang and Y. Li, Green Chem., 2013, 15, 230–235 RSC.
- E. Niknam, F. Panahi and A. Khalafi-Nezhad, J. Organomet. Chem., 2021, 935, 121676 CrossRef CAS.
- A. S. Kritchenkov, A. V. Kletskov, A. R. Egorov, M. N. Kurasova, A. G. Tskhovrebov and V. N. Khrustalev, Carbohydr. Polym., 2021, 252, 117167 CrossRef CAS.
- R. K. Loganathan, S. N. Ramachandra, Shekharappa and V. V. Sureshbabu, ChemistrySelect, 2017, 2, 8059–8062 CrossRef CAS.
- J. H. Clark and D. J. Macquarrie, Chem. Soc. Rev., 1996, 25, 303–310 RSC.
- A. Dewan, M. Sarmah, U. Bora and A. Thakur, Appl. Organomet. Chem., 2017, 31, e3646 CrossRef.
- V. Bilakanti, V. Boosa, V. K. Velisoju, N. Gutta, S. Medak and V. Akula, J. Phys. Chem. C, 2017, 121, 22191–22198 CrossRef CAS.
- Y.-Y. Cai, X.-H. Li, Y.-N. Zhang, X. Wei, K.-X. Wang and J.-S. Chen, Angew. Chem., Int. Ed., 2013, 52, 11822–11825 CrossRef CAS.
- Q. Cui, H. Zhao, G. Luo and J. Xu, Ind. Eng. Chem. Res., 2017, 56, 143–152 CrossRef CAS.
- A. Mahanta, N. Hussain, M. R. Das, A. J. Thakur and U. Bora, Appl. Organomet. Chem., 2017, 31, e3679 CrossRef.
- A. V. Nakhate and G. D. Yadav, Chemistryselect, 2016, 1, 3954–3965 CrossRef CAS.
- S. Sadjadi, Appl. Organomet. Chem., 2018, 32, e4211 CrossRef.
- S. Sadjadi, M. M. Heravi and M. Malmir, J. Taiwan Inst. Chem. Eng., 2018, 86, 240–251 CrossRef CAS.
- L. Mohammadi, M. M. Heravi, S. Sadjadi and M. Malmir, ChemistrySelect, 2019, 4, 13404–13411 CrossRef CAS.
- F. İmik, S. Yaşar and İ. Özdemir, J. Organomet. Chem., 2019, 896, 162–167 CrossRef.
- I. Nath, J. Chakraborty, A. Khan, M. N. Arshad, N. Azum, M. A. Rab, A. M. Asiri, K. A. Alamry and F. Verpoort, J. Catal., 2019, 377, 183–189 CrossRef CAS.
- J. Yang, J. Organomet. Chem., 2019, 883, 35–40 CrossRef CAS.
- X. Jia, D. Jiang, D. C. W. Tsang, J. Choi and A. C. K. Yip, Microporous Mesoporous Mater., 2019, 276, 147–153 CrossRef CAS.
- M. Chao, G. Zhang, Z. Li, L. Liu, S. Yan, Y. Chen, Y. Shi, X. Yan and C. Cao, ChemistrySelect, 2019, 4, 766–773 CrossRef CAS.
- S. Sadjadi, M. M. Heravi, B. Masoumi and S. S. Kazemi, J. Coord. Chem., 2019, 72, 119–134 CrossRef CAS.
- S. Sadjadi, G. Lazzara, M. Malmir and M. M. Heravi, J. Catal., 2018, 366, 245–257 CrossRef CAS.
- Y. Zhao, R. Jin, Y. Chou, Y. Li, J. Lin and G. Liu, RSC Adv., 2017, 7, 22592–22598 RSC.
- A. R. Hajipour, Z. Khorsandi and Z. Abeshtian, Inorg. Chim. Acta, 2019, 107, 107470 CAS.
- A. R. Sardarian, M. Kazemnejadi and M. Esmaeilpour, Dalton Trans., 2019, 48, 3132–3145 RSC.
- M. Gholinejad, N. Dasvarz and C. Nájera, Inorg. Chim. Acta, 2018, 483, 262–270 CrossRef CAS.
- B. Ghanbari, L. Shahhoseini, H. Hosseini, M. Bagherzadeh, A. Owczarzak and M. Kubicki, J. Organomet. Chem., 2018, 866, 72–78 CrossRef CAS.
- A. R. Hajipour, N. S. Tadayoni and F. Mohammadsaleh, Appl. Organomet. Chem., 2016, 30, 777–782 CrossRef CAS.
- Z. Mandegani, M. Asadi and Z. Asadi, Appl. Organomet. Chem., 2016, 30, 657–663 CrossRef CAS.
- M. B. Ibrahim, S. M. Shakil Hussain, A. Fazal, M. Fettouhi and B. El Ali, J. Coord. Chem., 2015, 68(3), 432–448 CrossRef CAS.
- R. Bhaskar, A. K. Sharma, M. K. Yadav and A. K. Singh, Dalton Trans., 2017, 46, 15235–15248 RSC.
| This journal is © The Royal Society of Chemistry 2021 |