Open Access Article
Open Access ArticleRecent advances in photocatalytic nitrogen fixation: from active sites to ammonia quantification methods
Rong Huangab,
Xiaoman Li *a,
Wanguo Gaoa,
Xu Zhanga,
Sen Liang*b and
Min Luo
*a,
Wanguo Gaoa,
Xu Zhanga,
Sen Liang*b and
Min Luo *a
*a
aState Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering, School of Chemistry and Chemical Engineering, Ningxia University, Yinchuan, Ningxia 750021, China. E-mail: martinluomin@163.com; lixm2017@nxu.edu.cn
bNingxia Key Laboratory for Photovoltaic Materials, Ningxia University, Yinchuan, Ningxia 750021, China. E-mail: liangsen@nxu.edu.cn
First published on 26th April 2021
Abstract
Photocatalytic nitrogen fixation has become a hot topic in recent years due to its mild and sustainable advantages. While modifying the photocatalyst to enhance its electron separation, light absorption and nitrogen reduction abilities, the role of the active sites in the catalytic reaction cannot be ignored because the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N nitrogen bond is too strong to activate. This review summarizes the recent research on nitrogen fixation, focusing on the active sites for N2 on the catalyst surface, classifying common active sites, explaining the main role and additional role of the active sites in catalytic reactions, and discussing the methods to increase the number of active sites and their activation ability. Finally, the outlook for future research is presented. It is hoped this review could help researchers understand more about the activation of the nitrogen molecules and lead more efforts into research on nitrogen fixation photocatalysts.
N nitrogen bond is too strong to activate. This review summarizes the recent research on nitrogen fixation, focusing on the active sites for N2 on the catalyst surface, classifying common active sites, explaining the main role and additional role of the active sites in catalytic reactions, and discussing the methods to increase the number of active sites and their activation ability. Finally, the outlook for future research is presented. It is hoped this review could help researchers understand more about the activation of the nitrogen molecules and lead more efforts into research on nitrogen fixation photocatalysts.
1. Introduction
As an important component of nitrogen fertilizers, ammonia (NH3) is significant for all life on earth. It is also a vital raw material for making chemical products, such as nitric acid and explosives. What's more, NH3 is an ideal hydrogen storage material with green and high energy hydrogen release capacity. The global ammonia output is about 15 billion tons per year. With economic development and population growth, this number is increasing.1 At present, the Haber–Bosch process is commonly used in industrial ammonia production. However, the application of the Haber–Bosch process for NH3 production must be carried out at a pressure higher than 200 bar and a temperature higher than 673 K, and it also needs to consume a large amount of H2 produced by steam reforming of fossil fuels. About 35% of the world's natural gas and 13% of the world's electric energy are usually consumed in the Haber–Bosch process.2 Therefore, the catalytic process of NH3 production without H2 at ambient pressure and temperature is very necessary for the clean and safe synthesis of NH3.3The atmosphere contains about 78% nitrogen resources,4 so if the nitrogen can be used directly under normal temperature and pressure, it will bring great energy saving. Coincidentally, photocatalysis, driven by electrons and protons excited by photons, could promote the reduction of N2 to produce NH3 under mild conditions. In 1977, Schrauzer and Guth published their first study on photocatalytic nitrogen fixation. They concluded that nitrogen (N2) could be reduced to ammonia by Fe doping TiO2 under ultraviolet radiation. Surprisingly, the energy provided by the sun per hour on the surface of the earth can meet the world's energy needs for a year.5 Photocatalytic nitrogen fixation has attracted more and more attention.
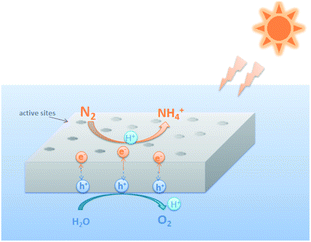
Photocatalytic nitrogen fixation is a kind of green and pollution-free mild reaction, which does not need high temperature and pressure. It can directly use solar energy to generate electrons and holes and combine with water protons to reduce N2 to NH3. The principle of photocatalysis is that the semiconductors absorb photon to generate the holes and electrons driving the oxidation–reduction reaction. When the energy of irradiated light is greater than or equal to the band gap of semiconductor nanoparticles, the electrons in the valence band will be excited to the conduction band, leaving relatively holes on the valence band, thus forming electron–hole pairs. Due to the existence of many defects and dangling bonds in nanomaterials, these defects and dangling bonds might capture electrons or holes and prevent the recombination of electrons and holes. These trapped electrons and holes diffuse to the surface of the particles respectively, resulting in a strong redox potential. Therefore, shown in Fig. 1, photocatalytic reactions generally involve three reactions: light absorption, charge carrier separation and surface reduction.6
At present, the research of metal compound catalyst and nonmetal compound catalyst is very hot. The common photocatalysts, such as g-C3N4,5,7 TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and BiOCl,9 have low nitrogen fixation efficiency because of their light absorption range and charge carrier recombination and other problems. Therefore, in recent years, there have been many studies on the modification of these materials, such as doping,10 building heterojunction,11 increasing vacancy12 and other methods to change the semiconductor band gap,13 promote photogenerated electron–hole separation,14 and expand the adsorption of N2 on the catalyst surface.15
8 and BiOCl,9 have low nitrogen fixation efficiency because of their light absorption range and charge carrier recombination and other problems. Therefore, in recent years, there have been many studies on the modification of these materials, such as doping,10 building heterojunction,11 increasing vacancy12 and other methods to change the semiconductor band gap,13 promote photogenerated electron–hole separation,14 and expand the adsorption of N2 on the catalyst surface.15
Most of the existing reviews are classified from the chemical composition of photocatalyst16 or from its structural engineering.2 In this paper, the characteristics of photocatalyst for nitrogen fixation are briefly described, and the classification of photocatalyst is described from active sites on its surface.
2. Principles of photocatalytic nitrogen fixation materials
In the development of photocatalytic nitrogen fixation, the types of catalysts are abundant. Among the metal catalysts, titanium-based catalysts are the most researched materials in the early stage, mainly TiO2.17 In recent years, there have been reports of Ti3C2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 as the catalyst precursor. In addition, iron oxides, metal sulfides,19 and bismuth oxyhalides (BiOX (X = Cl, Br, I)) are also hot materials for research. Among the non-metallic catalysts, the research on g-C3N4 has been the most popular, and its composite materials with precious metals,20 graphene, black phosphorus,21 etc. have good nitrogen fixation effects. In addition, research on single-atom catalysts22 and layered double hydroxide (LDH) catalysts23,24 has gradually increased in recent years. Photoinduced electrons from these semiconductors are used to reduce N2 to NH3. The reaction and potential of these semiconductor catalysts to reduce nitrogen to ammonia are shown in the following formula. Under the irradiation of UV visible light, the generated valence band holes can oxidize water (eqn (1)), and conduction band electrons will reduce nitrogen (eqn (2)). NH3 is finally generated by water and oxygen under light conditions (eqn (3)).25
18 as the catalyst precursor. In addition, iron oxides, metal sulfides,19 and bismuth oxyhalides (BiOX (X = Cl, Br, I)) are also hot materials for research. Among the non-metallic catalysts, the research on g-C3N4 has been the most popular, and its composite materials with precious metals,20 graphene, black phosphorus,21 etc. have good nitrogen fixation effects. In addition, research on single-atom catalysts22 and layered double hydroxide (LDH) catalysts23,24 has gradually increased in recent years. Photoinduced electrons from these semiconductors are used to reduce N2 to NH3. The reaction and potential of these semiconductor catalysts to reduce nitrogen to ammonia are shown in the following formula. Under the irradiation of UV visible light, the generated valence band holes can oxidize water (eqn (1)), and conduction band electrons will reduce nitrogen (eqn (2)). NH3 is finally generated by water and oxygen under light conditions (eqn (3)).25| 2H2O + 4h+ → O2 + 4H+ (1.23 V vs. NHE) | (1) |
| N2 + 6H+ + 6e− → 2NH3 (−0.09 V vs. NHE) | (2) |
| 1/2N2 + 3/2H2O → NH3 + 3/4O2 (Δ0 = 339 kJ mol−1) | (3) |
How to choose a suitable photocatalytic nitrogen fixation catalyst? On the one hand, it is necessary to consider the common problems of all photocatalysts, as well as the light absorption and carrier transfer. On the other hand, for the nitrogen fixation photocatalyst, the unique features are the adsorption of nitrogen molecules and the desorption of ammonia products.
2.1 Appropriate band gap energy of semiconductor
For photocatalytic nitrogen fixation, the ideal band gap value is close to 2.0 eV, the corresponding light wavelength is less than 620 nm, the value of CB should be less than overpotential of N2 (−0.092 V vs. NHE), and VB level must be greater than the oxidation potential of water (1.23 V vs. NHE).26 Some photocatalysts have insufficient response to visible light because their band gap is too wide or too narrow.27 In response to this problem, some semiconductor materials have been modified into suitable photocatalysts. Fig. 2 shows the schematic diagrams of the electronic energy bands of several common photocatalysts.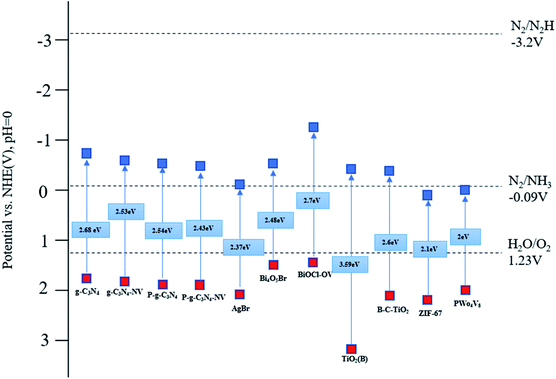 | ||
| Fig. 2 Schematic diagram of the electronic band structure of several typical semiconductor photocatalytic materials. | ||
Common modification methods include designing heterojunctions,18 doping with other elements,28 changing the structure of nanomaterials, etc. These methods can change the band gap of semiconductor materials. For example, Qin, et al. prepared Ti3C2 nanosheets by etching Ti3C2 layered precursor Ti3AlC2 (MAX phase), then grown different proportions of 0D AgInS2 nanoparticles in situ on the surface of Ti3C2 nanosheets, and finally successfully produced different quality Z-scheme heterostructure photocatalyst constructed with 0D AgInS2 nanoparticles and 2D MXene (Ti3C2) nanosheets.18 AgInS2 is a wide band gap semiconductor with a band gap of 1.62 eV, and the band gap of Ti3C2 is small, about 0.75 eV. The band gap of the heterostructure AT-30 formed by the two is about 1.00 eV (Fig. 3), and a narrow band gap semiconductor was successfully synthesized. Under visible light irradiation, the photogenerated electrons of AgInS2 are excited and transition from VB to CB, and then transfer to the Ti3C2 surface in close contact with AgInS2 to participate in the reduction reaction of nitrogen molecules by the catalyst (Fig. 3d). Its nitrogen fixation yield is 38.8 μmol g−1 h−1 under visible light irradiation. In addition to building a heterojunction between a wide band gap semiconductor and a narrow band gap material to change the band gap of the composite photocatalyst, some non-metallic atoms can be doped to change the band gap of the compound. Maimaitizi et al. synthesized flower-like hierarchical N doped MoS2 (N-MoS2) microsphere with one-step solvothermal method.29 Meantime, photo-ultrasonic reduction method was employed to loaded Pt nanoparticles on the surface of N-MoS2. It was found that the N doping could narrows the band gap and increase the response range of photocatalyst to visible light. The final yield of nitrogen reduction is 121.2 μmol gcat−1 h−1. Also, Wu et al. reported compounds containing Bi as photocatalysts.30 Bismuth chloride microchips with oxygen vacancy were prepared by surfactant assisted solvothermal method by introducing Br to replace Cl in the process of ion exchange, a cavity is formed in Br doped BiOCl-OV (Br-BiOCl-OV) crystal structure. After ion exchange, the Br heteroatom not only reduces the band gap, but also increases the top of the valence band energy level (VB), thereby obtaining a better light trapping ability and provide higher redox potential for N2 optical fixation. Ammonia production rate increased from 4.1 μmol h−1 to 6.3 μmol h−1.
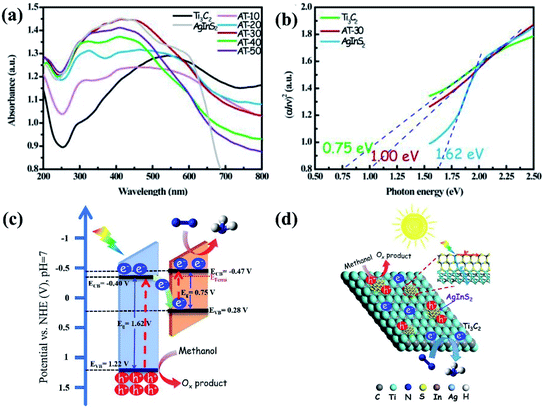 | ||
| Fig. 3 (a) UV-vis DRS of all the as-prepared samples; (b) Taucs plot of Ti3C2, AgInS2 and AT-30 for band gaps; (c) the energy band positions, and (d) scheme for spatial charge separation and transportation during photocatalytic N2 reduction over AgInS2/Ti3C2 nanosheets.18 Copyright 2019, Elsevier. | ||
In addition, reducing the size of the crystal structure of photocatalyst is also used to change the band gap. For example, Xue and his colleagues prepared the porous few-layer g-C3N4 by simple molecular self-assembly method.31 Compared with the original g-C3N4, the material is ultra-thin and the size is reduced, and its band gap has changed from 2.69 eV to 2.61 eV.
2.2 Efficiency of charge separation
Constructing a heterojunction,32 using a co-catalyst18 manufacturing surface defects,33 doping,34 etc. are all effective methods to improve the efficiency of carrier separation (Fig. 4).Using appropriate methods to increase the surface defects of the material, such as oxygen vacancies, is a common effective means to increase the efficiency of catalyst charge separation. Take the study of Wang et al. as an example.35 As the first discovered and studied photocatalyst, the electron–hole recombination rate of TiO2 is very high. Wang et al. investigated that the core defects in TiO2 nanotubes containing a lot of charge recombination centers. They used urea, dicyandiamide (DA) and cyanamide as precursors to prepare hydrogen treated TiO2 (TiO2–H2) nanotubes with less oxygen vacancies. Through a series of studies, it is found that the oxygen vacancy in metal oxides has strong fluidity. Because the thermal activation process involves oxygen diffusion and vacancy migration, these defects tend to migrate from the core to the surface after heat treatment. The rearrangement of oxygen vacancy reduces the core defect of TiO2 as charge composite center and improves the efficiency of charge separation and nitrogen reduction.
The use of promoters to improve the electron transfer efficiency of materials is also a widely used method in recent years. Qiu reported that black phosphorus is used as a co-catalyst for graphite carbon nitride, which increases the number of excited electrons and improves the separation efficiency of carriers by forming C–P covalent bonds.36 In addition, using graphene as cocatalyst can also improve the separation efficiency of electron hole pairs. According to the works of Fei and others, the hybridization of Bi 6s and O 2p orbitals of Bi2WO6 formed the valence band, while the W 5d orbitals composed the conduction band simultaneously.37 Due of this special band structure, Bi2WO6 has become an outstanding visible light catalyst with narrow band width (2.8 eV), which has better chemical properties and stability than the general monolayer catalysts. Compared with graphene nanoribbons and graphene nanosheets, the quantum confinement effect of zero-dimensional graphene quantum dots (GQD) is more obvious and the edge effect is stronger. Fei et al. found that the introduction of GQD makes the photogenerated electron–hole pair have higher separation ability (Fig. 5). In the report of Fei et al., the ammonia synthesis rate of GQD/Bi2WO6 composites prepared by hydrothermal method under visible light is 241.07 μmol g−1, which is 33.8 times and 8.88 times higher than that of GQD and raw Bi2WO6. This good result can be attributed to the effective combination of GQD and Bi2WO6. When the nitrogen molecules adsorbed on the surface of the material obtain protons from the solution, it also obtains photoelectrons from the catalyst in time, thereby generating NH4+ (Fig. 5c). In this reaction, the carrier separation efficiency of the material is high to ensure that the reaction can have a continuous supply of electrons.
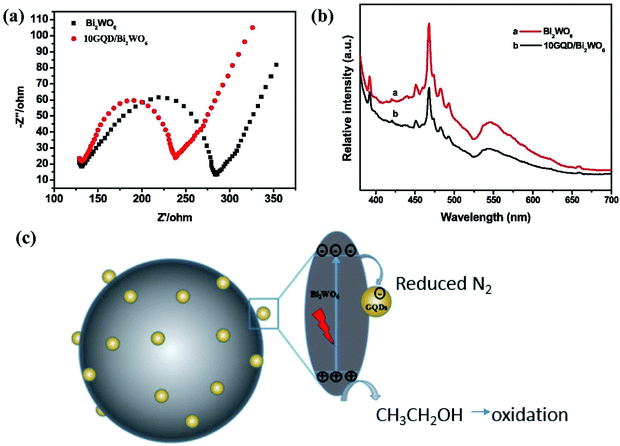 | ||
| Fig. 5 (a) The electrochemical impedance spectroscopy (EIS) measurements and (b) PL of pure Bi2WO6 and 10GQD/Bi2WO6 catalysts; (c) schematic of the possible reaction mechanism of photocatalytic nitrogen fixation procedure.37 Copyright 2019, Elsevier. | ||
Heterojunction is a widely used method to improve the charge transfer ability of catalysts. Chen et al. Constructed the Z-scheme heterojunction of AgBr and Bi4O5Br2, and used the silver nanoparticles formed in situ after AgBr decomposition as the bridge of charge transfer under illumination to improve the catalytic performance.32 In addition, doping is a common modification method for photocatalysts. Taking the report of Gao et al. in 2019 as an example,34 they doped metal Zn into δ-Bi2O3. Without changing the cubic crystal phase of Bi2O3, Zn was successfully placed in the two-dimensional thin δ-Bi2O3 material by interstitial doping to help the separation and transfer of photogenerated electrons and holes.
2.3 Chemical adsorption of nitrogen and NH3
The effective adsorption of nitrogen molecules on the surface of photocatalyst is one of the important conditions for the reduction of nitrogen under light. Many catalysts do not adsorb enough N2 due to smooth surface38 or small specific surface area,39 which results in low catalytic activity.The surface of the material is exfoliated by chemical method to increase its roughness, which can effectively increase the specific surface area of the catalyst and promote its adsorption of nitrogen molecules. A recent example, edge-rich black phosphorus nanoflakes (eBP NFs) was reported to be successful nitrogen fixation photocatalyst in ambient condition.21 The eBP NFs synthesized by Bian and his coworkers with chemical etching exfoliation method has rough surface and rich edges, and a large amount of N2 is chemically adsorbed on the edge of catalyst (Fig. 6). However, although the specific surface area of the material plays an important role in the catalytic reaction, it is more important to increase more chemical adsorption sites than to increase the specific surface area. For instance, CeCO3OH/g-C3N4/CeO2 ternary photocatalyst (2Ce–CN) was synthesized by an in situ one-pot hydrothermal method with CeCl3 and (g-C3N4) as precursor.40 It was found that the increase of specific surface area of 2Ce–CN, providing more physical adsorption sites for N2, and CeCO3OH in the ternary system provides more Ce3+ sites to enhance chemisorption of N2. In addition, Zhang et al. employed high-temperature peeling methods to achieved ultra-thin carbon nitride(g-C3N4-V).41 It's worth noting that the carbon vacancies in g-C3N4 extremely promoting the adsorption and activation of dinitrogen molecule, greatly improving the catalytic activity for the defective ultrathin g-C3N4-V photocatalyst. This phenomenon proves that the increase of active sites is more important than the increase of specific surface area.
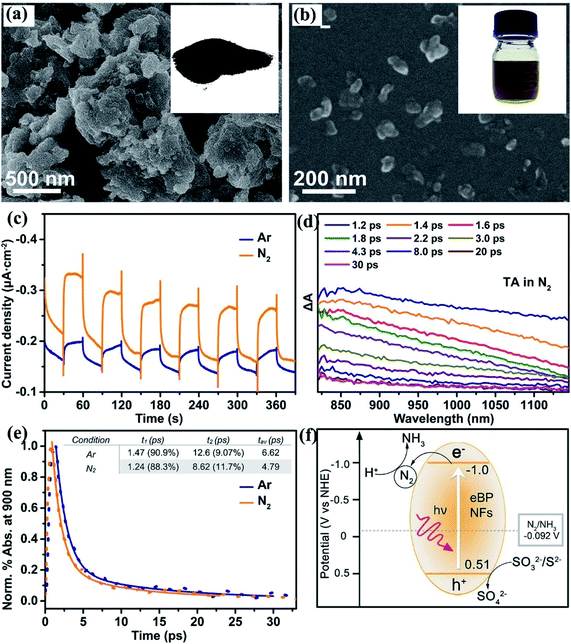 | ||
| Fig. 6 (a) SEM image and inset photograph of the bulk BP powder; (b) SEM image of eBP NFs and inset photograph of the water suspension; (c) photocurrent response of BP in different atmospheres; (d) TA decay of eBP NFs in N2 saturated water; (e) time profiles of normalized transient absorption at 900 nm; (f) schematic mechanism of semiconductor-based photocatalysis for nitrogen fixation.21 Copyright 2020, American Chemical Society. | ||
Of course, in photocatalytic nitrogen fixation, it is not enough to only pay attention to the adsorption of nitrogen molecules, but also to the desorption of ammonia. Taking Gao et al.'s report in 2021 as an example, after comparing the adsorption energies of different materials for nitrogen and ammonia, they first proposed the influence of the adsorption energy of catalytic product NH3 on the nitrogen fixation performance of the catalyst.42 In this report, they tried to improve the catalytic performance by improving the desorption of the product.
Based on the above discussion, it can be concluded that nitrogen reduction reaction photocatalyst must have appropriate band gap to absorb visible light, high separation efficiency of photogenerated electron–hole pair to ensure the continuous reduction reaction and excellent ability to adsorb and activate nitrogen molecules to achieve good catalytic activity.
3. Active sites in nitrogen fixation reactions
The active sites on the surface of photocatalyst are the key to the catalytic reaction, which can not only chemically adsorb N2, but also establish an effective channel for the transfer of photoexcited carriers from photocatalyst to adsorbed N2 molecules.43 According to the recent reports on photocatalysts for nitrogen reduction reaction, the common active sites are vacancy,44 metallic element,45,46 etc., which will be described in detail in the next part.3.1 Vacancies
With more and more photocatalytic nitrogen fixing catalysts being found, increasing the surface defects of the catalysts is an effective way to improve their activity. According to the research of Wang et al., they constructed metal nanocrystals/semiconductor oxide composites with oxygen vacancies (defect sites) as photocatalysts for nitrogen fixation.47 It was found that metal nanoparticles have small size and special extraction plane effect, which can expose a lot of large coordination unsaturated points as N2 activation center.48 At the same time, the surface defect sites are generally used as great nitrogen molecular activation sites, weakening the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond.49
N triple bond.49
Vacancy, a common surface defect, has been found to be the active site, including O,44,50–53 N,54–56 C,41 S,57 F58 and other vacancies. Oxygen vacancy refer to the vacancies formed in oxygen-containing compounds where oxygen atoms (oxygen ions) in the crystal lattice break away, resulting in oxygen deficiency. Nitrogen vacancy, that is, the vacancy formed after nitrogen in the crystal lattice of nitrogen-containing compound is separated. Table 1 summarizes the photocatalytic nitrogen fixation activity of the reported catalysts containing different vacancies. It can be clearly found that when the different vacancies are used as active sites, the difference in atmosphere, composite materials, scavengers, etc. is different for the improving the capacity of nitrogen fixation of materials.
| Types of vacancy | Samples | Light source | Hole scavenger | NH4+ generation rate | Atmosphere | Ref. | |
|---|---|---|---|---|---|---|---|
| OVs | Bi5OBr7 | Xe lamp | None | 1.38 mmol h−1 g−1 | Air | 72 | |
| H200-1.0Bi@BiOBr | 300 W point light | None | 181.2 μmol g−1 h−1 | N2 | 53 | ||
| 3% AgCl/&-Bi2O3 | Xe lamp | None | 606 μmol g−1 h−1 | N2 | 61 | ||
| 2% Co–W18O49 | Xe lamp | Ethylene glycol | 2752 μg gcat−1 | Air | 52 | ||
| Fe–SrMoO4 | Xe lamp | None | 93.1 μM g−1 h−1 | N2 | 60 | ||
| In2O3/In2S3 | Xe lamp | None | 40.04 μmol g−1 h−1 | N2 | 62 | ||
| 6% Cu–TiO2 | UV-vis | None | 78.9 μmol g−1 h−1 | Air | 63 | ||
| TiO2/ZnFe2O4 | Xe lamp | None | 88.8 μmol g−1 L−1 | N2 | 64 | ||
| WS2TiO2 | AM 1.5G | None | 1.39 mmol g−1 h−1 | N2 | 50 | ||
| TiO2 | Xe lamp | Methanol | 324.86 mmol h−1 g−1 | N2 | 74 | ||
| OV-In(OH)3/CN | Xe lamp | Triethanolamine | 3.81 mM h−1 g−1 | N2 | 71 | ||
| NVs | Nv-CN | Sodium lamp | Methanol | 5.5 mg L−1 h−1 gcat−1 | 50% O2 + 50% N2 | 86 | |
| Nv&Sd–CN | Sodium lamp | None | 6.2 mg L−1 h−1 gcat−1 | N2 | 88 | ||
| SiW12/k-C3N4 | Xe lamp | None | 353.2 μM g−1 h−1 | Air | 91 | ||
| Nv&Pd–CN | Sodium lamp | Methanol | 7.5 mg L−1 h−1 gcat−1 | N2 | 89 | ||
| Nv&Od–CN | Xe lamp | Methanol | 118.8 mg L−1 h−1 gcat−1 | N2 | 87 | ||
| Other | C | g-C3N4-V | Xe lamp | None | 32.4 mmol L−1 h−1 | N2 | 41 |
| I/g-C3N4 | Xe lamp | Methanol | 66.93 mg L−1 gcat−1 h−1 | N2 | 93 | ||
| S | CdS | Xe lamp | None | 1.7 mg L−1 h−1 | Air | 57 | |
| SV-1T-MoS-CdS | AM 1.5G | Methanol | 8220.83 μmol L−1 h−1 g−1 | N2 | 19 | ||
| F | LaF3:Yb3+, Tm3+/Pal | Xe lamp | 1 mM Na2SO3 | 43.2 mg L−1 | Air | 58 | |
| Pr3+:CeF3 | Xe lamp | Ethanol | 629.16 ± 17.62 μmol L−1 | N2 | 95 | ||
 | ||
| Fig. 7 Schematic illustration of the photocatalytic N2 fixation model in which water serves as both the solvent and proton source, as well as the reversible creation of light-induced Ovs.72 Copyright 2017, Wiley. | ||
However, too much O vacancy is not good, because oxygen vacancy can act as electron–hole recombination center, or destroy the original structure of nanocrystals, which will reduce the energy of light excited electrons.35 Wang et al. compared the photocatalytic activity and oxygen vacancy concentration of TiO2, TiO2–H2 and TiO2–H2–DA.35 In this work, an ultra-thin amorphous layer was formed on TiO2 surface in hydrogen atmosphere, which is rich in oxygen vacancy. However, with the increase of oxygen vacancy concentration, the ability of reducing nitrogen under visible light decreased. Therefore, after annealing TiO2–H2 with dicyandiamide (DA), part of the oxygen vacancies was remedied, and the catalytic activity of TiO2–H2–DA was increased to 1.2 mmol L−1 h−1. A recent example, Zhang and his coworkers demonstrated that the excess of OVs in TiO2 increased the adsorption and activation ability of N2 molecules, but also showed disappointing activity due to the decrease of charge separation efficiency.74 Excess OVs will become the recombination centers of photocarriers, which will greatly reduce the reduction ability of photogenerated electrons and have a negative impact on photocatalytic nitrogen fixation (Fig. 8). By using the reaction temperature (310–360 °C) and the reduction characteristics of NaBH4, adjusting the oxygen vacancy concentration on TiO2, the charge separation efficiency can be increased by three times under the condition of good chemisorption of N2, which has an obvious activation effect on N2. It was found that the optimum reaction temperature is 340 °C, and ammonia production is 324.86 mmol h−1 g−1.
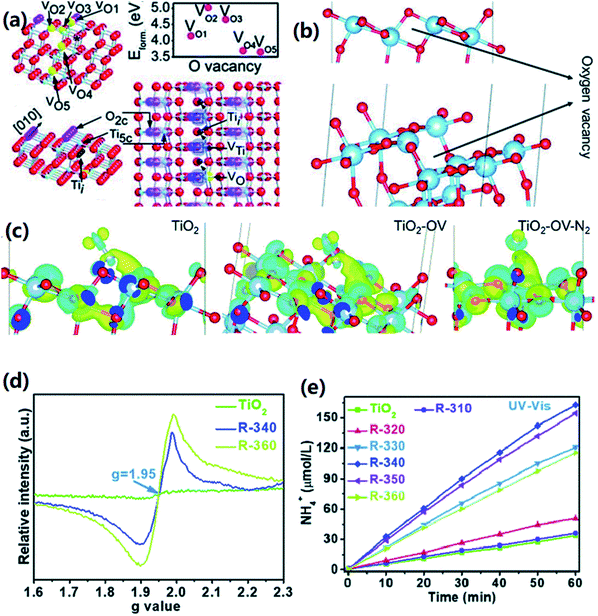 | ||
| Fig. 8 (a and b) O-vacancy configuration of the (101)-crystal facet outer layer of anatase phase TiO2; (c) Bader charge simulation and differential charge density data; (d) low temperature EPR spectra of anatase and reduced TiO2; (e) the full-spectrum N2 fixation data for anatase and reduced TiO2.74 Copyright 2020, Royal society of chemistry. | ||
There are lots of researches on photocatalytic nitrogen fixation using oxygen vacancy as active site. In recent years, some researchers have proposed that the active sites of oxygen-enriched vacancy photocatalysts are metal ions exposed after oxygen is separated from the material, such as Ti3+ species,48,75–77 Ce3+ species40 and so on. However, most studies only pointed out oxygen vacancies as active sites, and did not describe specific active substances in detail. For example, Xie et al. reported the reliable exchange–correlation functionals, combined with the periodic slab model, to explore the specific mechanism of titanium oxide catalytic reduction of N2 to NH3 under the action of H2O photolysis.76 At the Ov site of the hydroxylated surface, the inert bond of the adsorbed N2 is activated. At the same time, photogenerated electrons reduce the tetravalent Ti4+ species of the Ov site to trivalent Ti3+ species, and Ti3+ species as the active site promotes the photocatalytic nitrogen fixation. In addition, some researchers proposed that the oxygen vacancy of the catalyst itself interacts with the supported metal as the active center and active site of the catalytic reaction.78–80 For instance, in the work of Sultana et al., FeS2–FePCeO2NSs ternary nanohybrid were synthesized by simple hydrothermal method and sulfonation phosphating technology.78 They reported the Ov/Ce3+ and Fe–P act as active sites for N2 molecule adsorption and activation.
There are many ways to increase the nitrogen vacancies of intrinsic photocatalyst, such as simple KOH etching, plasma treatment, etc. For example, Xue et al. modified g-C3N4 by alkali treatment. In the relevant peninsula, they used urea-assisted heat treatment to prepare porous g-C3N4 (NC-g-C3N4) with nitrogen defects and cyano groups.43 Alkaline etching treatment can break the hydrogen bond of g-C3N4, accelerate the thermal polymerization of urea, and form nitrogen vacancies and cyano groups (Fig. 9). The presence of nitrogen vacancies expands the absorption of visible light by the catalyst, and at the same time, the cyano group helps to trap the photoelectrons generated by g-C3N4 under light and inhibits the recombination of carriers. In addition, more chemisorption sites (N vacancies) were generated to activate nitrogen molecules, which was beneficial to photocatalytic nitrogen fixation. The final activity of nitrogen fixation was 7.6 times than that of g-C3N4. In 2020, Wang and coworkers reported the method of modifying g-C3N4 with cyano and intercalated K+.85 It was proved that the cyano group in the catalyst could be regenerated under the action of intercalation K+ (N vacancies increased), and the nitrogen fixation performance reached 3.42 mmol g−1 h−1. The structure, specific surface area and morphology of catalyst will not be changed by plasma treatment, but nitrogen vacancies will be introduced into g-C3N4. For instance, Zhao et al. employed dielectric barrier discharge plasma treatment to prepared N vacancies embedded g-C3N4 (Nv-CN).86 In the atmosphere of 50% N2 and 50% O2, the nitrogen fixation rate reaches 5.5 mg L−1 h−1 gcat−1 under visible light.
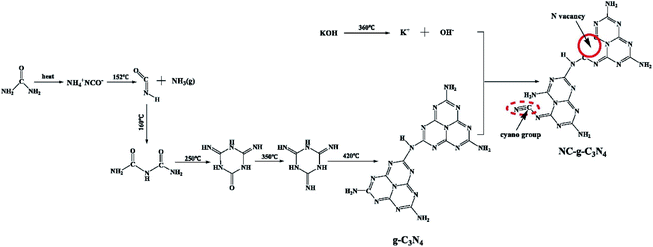 | ||
| Fig. 9 Schematic diagram for the formation of NC-g-C3N4.43 Copyright 2019, Elsevier. | ||
For a g-C3N4 photocatalyst co-doped with non-metal atoms (such as P, S, O, B, etc.), the doped non-metallic elements can increase the activity of N vacancies and further improve the nitrogen-fixing performance. Recent research, hollow porous prismatic graphitic carbon nitride with nitrogen vacancies and oxygen doping was fabricated from dicyandiamidine with a facile two-step strategy of low-temperature hydrothermal method and calcination process.87 In this work, the obtained catalyst (Nv&Od-CN) is hollow columnar and loose porous structure, which fully exposed the nitrogen vacancy as the active site with a yield of up to 118.8 mg L−1 h−1 gcat−1. Before that, nitrogen vacancy and sulfur co-doped g-C3N4,88 nitrogen vacancy and phosphorus co-doped g-C3N4 were also reported.25,89 Among them, the yield of N vacancy and S co-doped g-C3N4 (Nv&Sd-CN) produced by Li and his coworkers88 using dicyandiamide and H2S as raw materials by dielectric barrier discharge plasma treatment as high is as 6.2 mg L−1 h−1 gcat−1, which is 2.3 and 25.8 times higher than that of N doped g-C3N4 and g-C3N4, respectively. Shiraishi and coworkers made phosphor doped carbon nitride with nitrogen vacancy on the surface by a simple thermal condensation with hydroxyethyllidene diphosphonic acid (HEDP) and dicyandiamide as raw materials.25 The doped P atom promoted the oxidation of water by valence band hole formed by light, while the N vacancy promoted the reduction of N2 by conduction electron, and the ammonium ion production of catalyst under light was 4.9 μmol in 24 h. Wang et al.89 used dicyandiamide and (NH4)2HPO4 as raw materials to synthesize N vacancy and P-co-doped g-C3N4 (Nv&Pd-CN) by two-step calcination method. The yield of Co doped g-C3N4 is 7.5 mg L−1 h−1 gcat−1, which is 2.7 times and 28.8 times of that of single N vacancy-doped g-C3N4 and pure g-C3N4, respectively. In the latest example, Liang et al. made a catalyst with N vacancies and B doping.90 They found that the B dopant could not only improve the adsorption and activation ability of N2, but also maintain the high reduction ability of the catalyst.
Using the polyoxometalate (POM) is also one of the methods to increase the nitrogen fixation performance of g-C3N4 with N vacancy as the active site. For instance, Xiao et al. successfully covalently combined the polyoxometalate (POM) clusters of [H4SiO40W12] (SiW12) with KOH modified graphitic carbon nitride nanosheets (k-C3N4) through phosphate bridging strategy.91 k-C3N4 could not provide the protons needed for nitrogen fixation from water splitting, but POMs, as a cocatalyst, could enhance the ability of water oxidation and the adsorption and activation of N2. As an effective adhesive and electron transfer chain, phosphoric acid anion can covalently combine POMs with k-C3N4 by replacing the surface hydroxyl group and enhance the interaction and transport of the support. The ammonia yield of the catalyst (Nv-CN/SiW12) is 353.2 μM g−1 h−1. In addition, Li and his coworkers compared four kinds of transition metal-substituted POMs and V-g-C3N4 composites as photocatalysts for nitrogen fixation.92 The advantage of the catalyst is that the V-g-C3N4 nano sheet has a large specific surface area and rich N-defect sites, which is the main reason for the immobilization of POMs and the capture of N2. In addition, the nano-sizes POMs could accept electrons and convert into reduced POMs under light conditions. As a strong photocatalyst, reduced POMs stimulated a lot of photogenerated electrons, which can be transferred rapidly to adsorbed N2 on V-g-C3N4 nano sheet, weakening N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond.
N triple bond.
In addition to the N vacancy mentioned in the above section, there is also a C vacancy in the active site of the catalyst related to g-C3N4. For instance, Zhang et al. employed the high temperature peeling method to produce ultra-thin carbon nitride with loose structure and more carbon defects on the surface, which has a thin layer structure and more surface carbon vacancies, and can achieve effective charge separation.41 In addition, the engineering carbon vacancies greatly promotes the adsorption and activation of nitrogen molecules, and greatly improves the nitrogen fixation activity of defective ultra-thin g-C3N4-V materials (Fig. 10). It was found that the pore volume of the catalyst increased after two times of thermal oxidation. The large increase of specific surface area shows that g-C3N4-V had a loose lamellar structure and was rich in mesopores. The existence of match pores is conducive to the adsorption of nitrogen molecules to participate in the activation reaction; more active sites will be exposed around the defects. After 100 minutes, the yield of ammonium ion reached 54 mmol L−1. A recent example, the iodine-doped g-C3N4 with carbon vacancies was reported to be successful photocatalyst for nitrogen fixation in ambient condition.93 Hu and his coworkers demonstrated that the band gap of iodine doped g-C3N4 with vacancy is smaller and the average PL lifetime is longer than that of pure g-C3N4. In other words, the photocatalyst response range is enlarged and the separation efficiency of photocarriers in iodized g-C3N4 is improved. Under visible light, the nitrogen fixation yield of photocatalyst is 66.93 mg L−1 gcat−1 h−1.
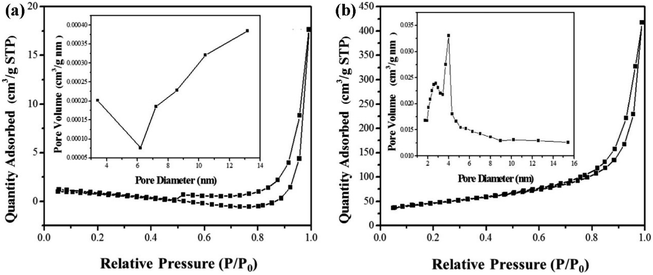 | ||
| Fig. 10 Nitrogen absorption–desorption isotherm of (a) bulk g-C3N4 and (b) g-C3N4-V.41 Copyright 2019, Elsevier. | ||
As the active site of adsorption and activation of nitrogen molecules, sulfur vacancy usually occurs in catalysts containing S elements such as CdS.19 A rencent example, using thiourea, hexaammonium heptamolybdate tetrahydrate ((NH4)6Mo7O24·4H2O), ethanol, methanol, ethylenediamine and cadmium chloride hemipentahydrate (CdCl2·2.5H2O) as raw materials, Sun and his coworkers successfully employed a simple one-step hydrothermal method to prepared the sulfur-rich oxygen doped 1T-MoS2 nano sheet (SV-1T-MoS2), and combined with CdS nano column to form a high-efficiency photocatalysis nitrogen fixing hybrid material (SV-1T-MoS2-CdS).19 In the catalyst, excessive thiourea adsorbed on the surface of the primary nano crystallite, which partially hindered the growth of the directional crystal and led to the formation of S vacancy. It was found that the conductivity of CdS is poor, which leads to slow electron transfer and long electron transfer time. However, the band gap width was reduced and negative displacement of CB occurs after adding the cocatalyst SV-1T-MoS2, which was conducive to the transfer of electrons generated by photoexcitation from CB to VB. Surprisingly, under visible light, the yield of photocatalyst nitrogen fixation is as high as 8220.83 μmol L−1 h−1 g−1. There are also many researches on CdS as photocatalyst. For example, Gao and his colleagues reported in 2019 that using NiS as cocatalyst reduced the overpotential of reducing N2 and increased charge separation. Meanwhile, the S vacancy contained on the surface of CdS nanorods was used as the active site to adsorb and activate nitrogen molecules, and the final ammonium ion yield was 1.7 mg L−1 h−1. In addition, He and his coworkers employed a selftemplated strategy to prepare In2S3 nanotubes in nitrogen atmosphere.94 The S vacancy was produced when the material was calcined in nitrogen atmosphere (Fig. 11), which was made from indium nitrate (In(NO3)3·xH2O), 1,4-benzenedicarboxylic acid (H2BDC), thiourea, N,N-dimethylformamide (DMF), anhydrous ethanol and so on. As the active site of In2S3 catalyst in photocatalytic nitrogen fixation, S vacancy could effectively adsorb and activate nitrogen molecules. Meanwhile, the presence of sulfur vacancy enhances the absorption of light and promotes the separation and migration of photocarriers. Under visible light irradiation, the rate of reducing N2 to NH3 was 52.49 mmol h−1 g−1, and the catalyst itself was stable.
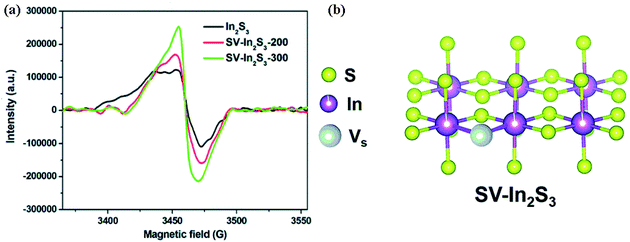 | ||
| Fig. 11 (a) ESR spectra of In2S3, SV-In2S3-200 and SV-In2S3-300; (b) a schematic drawing of the crystal structures of SV-In2S3.94 Copyright 2019, Royal Society of Chemistry. | ||
Another common vacancy as the active site of photocatalytic nitrogen fixation is F vacancy. According to He and his coworkers, they have successfully prepared palygorskite (Pal) nanocomposites (LaF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb3+, Tm3+/Pal) with sensitizer Yb3+ and activator Tm3+ co-doping LaF3 by microwave hydrothermal method.58 Due to the upconversion capability of this photocatalyst, it could be converted into visible light and ultraviolet light by near infrared, which improves the utilization of the whole solar spectrum. At the same time, LaF3:Yb3+, Tm3+ and modified Pal form an indirect Z-scheme heterostructure mediated by fluorine vacancy (FV), which is conducive to the separation of photogenerated electron–holes and the retention of high reduction oxidation potential. In addition, the F vacancy, as an active site, can effectively adsorb and activate nitrogen molecules. Under solar light irradiation, the total amount of ammonia can reach the highest of 43.2 mg L−1 under mild conditions. In 2019, Li and his coworkers successfully employed a microwave hydrothermal method to synthesized one dimensional attapulgite (ATP) mineral supported Pr3+
Yb3+, Tm3+/Pal) with sensitizer Yb3+ and activator Tm3+ co-doping LaF3 by microwave hydrothermal method.58 Due to the upconversion capability of this photocatalyst, it could be converted into visible light and ultraviolet light by near infrared, which improves the utilization of the whole solar spectrum. At the same time, LaF3:Yb3+, Tm3+ and modified Pal form an indirect Z-scheme heterostructure mediated by fluorine vacancy (FV), which is conducive to the separation of photogenerated electron–holes and the retention of high reduction oxidation potential. In addition, the F vacancy, as an active site, can effectively adsorb and activate nitrogen molecules. Under solar light irradiation, the total amount of ammonia can reach the highest of 43.2 mg L−1 under mild conditions. In 2019, Li and his coworkers successfully employed a microwave hydrothermal method to synthesized one dimensional attapulgite (ATP) mineral supported Pr3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CeF3 nanocomposite.95 It was found that the large amount of fluorine vacancies produced by the combination of some metal ions of ATP and crystal lattice of CeF3 is the active site to promote the adsorption of N2 and weaken the N
CeF3 nanocomposite.95 It was found that the large amount of fluorine vacancies produced by the combination of some metal ions of ATP and crystal lattice of CeF3 is the active site to promote the adsorption of N2 and weaken the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond. The proper doping of Pr3+ can increase the utilization of solar energy, while the Z-scheme heterojunction constructed by PR3+:CeF3 and ATP can effectively improve the recombination of photogenerated electrons and holes, and retain high redox potential to reduce nitrogen molecules. Under the irradiation of solar energy, the nitrogen fixation rate of photocatalyst detected by Nessler's reagent is about 629.16 ± 17.62 μmol L−1.
N triple bond. The proper doping of Pr3+ can increase the utilization of solar energy, while the Z-scheme heterojunction constructed by PR3+:CeF3 and ATP can effectively improve the recombination of photogenerated electrons and holes, and retain high redox potential to reduce nitrogen molecules. Under the irradiation of solar energy, the nitrogen fixation rate of photocatalyst detected by Nessler's reagent is about 629.16 ± 17.62 μmol L−1.
Therefore, based on the above discussion, this conclusion can be drawn: vacancy, as surface defect of photocatalyst, can effectively adsorb nitrogen in the atmosphere, activate nitrogen molecules and weaken N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond of N2 in the reaction of photocatalytic reduction of nitrogen. In some cases, vacancy could also adjust the energy band structure of catalyst, increase the utilization range of solar light, and transfer photoexcited electrons from catalyst to nitrogen molecules it adsorbs. The vacancy could be generated by various physical methods (such as calcination, plasma treatment, etc.) and chemical methods (such as chemical reaction with other compounds). At the same time, other atoms could be doped in the catalyst containing vacancy, so that the activation of vacancy to nitrogen is enhanced. As an active site, vacancy can adsorb nitrogen as well as other molecules. Taking oxygen vacancies on BiOBr as an example, it can adsorb both nitrogen and oxygen.96 Although the oxygen generated in situ competes with nitrogen for surface adsorption, it can be removed from the reaction mixture in time under continuous N2 purge, thereby avoiding further oxidation of NH3 to NO3−. Oxygen vacancies can still provide a lot of nitrogen molecules for the photocatalytic nitrogen fixation of BiOBr. In addition, the concentration of vacancies is very important for the yield of photocatalytic nitrogen fixation. The more vacancies, the more active sites can participate in the reaction, the higher the nitrogen fixation effect. However, when there are too many vacancies, as discussed in Section 3.1.1, some vacancies may become the centers of charge recombination, which will inhibit the photocatalytic activity.
N triple bond of N2 in the reaction of photocatalytic reduction of nitrogen. In some cases, vacancy could also adjust the energy band structure of catalyst, increase the utilization range of solar light, and transfer photoexcited electrons from catalyst to nitrogen molecules it adsorbs. The vacancy could be generated by various physical methods (such as calcination, plasma treatment, etc.) and chemical methods (such as chemical reaction with other compounds). At the same time, other atoms could be doped in the catalyst containing vacancy, so that the activation of vacancy to nitrogen is enhanced. As an active site, vacancy can adsorb nitrogen as well as other molecules. Taking oxygen vacancies on BiOBr as an example, it can adsorb both nitrogen and oxygen.96 Although the oxygen generated in situ competes with nitrogen for surface adsorption, it can be removed from the reaction mixture in time under continuous N2 purge, thereby avoiding further oxidation of NH3 to NO3−. Oxygen vacancies can still provide a lot of nitrogen molecules for the photocatalytic nitrogen fixation of BiOBr. In addition, the concentration of vacancies is very important for the yield of photocatalytic nitrogen fixation. The more vacancies, the more active sites can participate in the reaction, the higher the nitrogen fixation effect. However, when there are too many vacancies, as discussed in Section 3.1.1, some vacancies may become the centers of charge recombination, which will inhibit the photocatalytic activity.
3.2 Metal doping site
Most of the metals acted as active sites in the photocatalyst of reducing nitrogen reaction are transition metals. Due of their high charge/radius ratio and space-d orbital for bonding, transition metals are easy to form stable coordination compounds with various ligands. In photocatalytic nitrogen fixation, the transition metal atoms (ions) in the catalyst can chemisorb nitrogen molecules and capture photoelectrons in time.Iron is the main active site in nitrogenase, thus Fe-containing97 catalysts have been studied in photocatalytic nitrogen fixation, and Fe ions can act as active sites in photocatalytic nitrogen fixation. For instance, Yao and his coworkers compared the nitrogen reduction performance of the heterojunction photocatalyst constructed by g-C3N4 and Fe2O3 (g-C3N4/Fe2O3) with the composite photocatalyst of chelating Fe3+ ion and g-C3N4 nano sheet (CNNs) by EDTA (Fe-EDTA-CNNs).98 They think that N2 has lone pair electrons, and the empty d orbital of Fe atom can accept lone pair electrons. In addition, Fe atom has independent d electrons, which can be supplied to the antibinding orbital to strengthen N–Fe bond and weaken N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond. It was found that the Fe active sites are highly dispersed and stable in 2D CNNs due to the complexation of EDTA. Under the irradiation of visible light, Fe-EDTA-CNNs photocatalyst generates photogenerated electrons and holes. Under the electric traction effect of Fe-EDTA group, photoexcited electrons accumulate on Fe-EDTA, which are transferred to adsorbed N2 by Fe3+, and finally combine with protons in water to form ammonium ions (Fig. 12). Impressively, compared with other reported g-C3N4 catalysts, Fe-EDTA-CNNs photocatalyst showed a much higher activity for N2 fixation (49.252 mmol L−1 h−1).
N triple bond. It was found that the Fe active sites are highly dispersed and stable in 2D CNNs due to the complexation of EDTA. Under the irradiation of visible light, Fe-EDTA-CNNs photocatalyst generates photogenerated electrons and holes. Under the electric traction effect of Fe-EDTA group, photoexcited electrons accumulate on Fe-EDTA, which are transferred to adsorbed N2 by Fe3+, and finally combine with protons in water to form ammonium ions (Fig. 12). Impressively, compared with other reported g-C3N4 catalysts, Fe-EDTA-CNNs photocatalyst showed a much higher activity for N2 fixation (49.252 mmol L−1 h−1).
 | ||
| Fig. 12 Possible mechanism for photocatalytic nitrogen fixation of Fe-EDTA-CNNS.98 Copyright 2019, Royal Society of Chemistry. | ||
Transition metal Ru exhibits a lower N2 reduction overpotential than Fe. Therefore, in the experiment of studying photocatalytic nitrogen fixation, Ru-containing photocatalyst is the choice of some researchers. Liu and his coworkers demonstrated that the monoatomic metal dispersed on the support showed the homogeneity of catalytic active sites, the maximum efficiency of metal utilization and the low coordination environment of metal atoms.99 The Ru–TiO2 Ns catalyst was obtained by mixing the ultrasonic dispersed TiO2 in ethanol with ruthenium chloride ethanol solution. Under the irradiation of sunlight, the photocatalyst loaded with 1 wt% of Ru reduced N2 to ammonia at the highest rate of 56.3 μg h−1 gcat−1. After a series of analysis, they thought that Ru atoms deposited on the O vacancy of TiO2 surface is the center of nitrogen molecules adsorption and activation, and could improve the separation of charge carriers, thus promoting the performance of photocatalysis. According to reports by Awati and his coworkers, they used organic solvent extraction to extract the fine graphite-like carbon layer in coal, and obtained metals containing Ca2+, Ti4+, Ti3+,100 Fe2+, Al3+, Si4+, C, N, O, etc. The coal-based carbon nanosheets (CNs), and finally ruthenium (Ru) nanoparticles were loaded in situ from RuCl3·3H2O onto the prepared catalyst to prepare a Ru/CNs composite catalyst.101 In this work, metal Ru provides more active sites for CNS, promotes the adsorption and activation of nitrogen molecules on the catalyst, and increases the ammonia yield to 55.325 μmol L−1 h−1. A recent example, using Ti3AlC2 as the precursor, Hao and coworkers synthesized MXene by HF etching, and completed the growth of RuO2 nanoparticles and TiO2 on the substrate MXene by hydrothermal reaction.102 It was found that the ultrafine RuO2 nanoparticles with a large number of metal Ru(0) centers can effectively reduce the reduction barrier and increase the ammonia yield as the active site of reducing nitrogen. Although most studies have shown that metal atoms are evenly loaded on the layered material structure, and better play its role as an active site, there are exceptions in everything. Liu and his coworkers reported an interesting discovery, they demonstrated that even dispersion of atoms on the support surface is not necessarily the best way to improve the efficiency of photocatalytic nitrogen fixation.103 They compared the catalytic performance of bulk carbon nitride (B-g-C3N4) and exfoliated carbon nitride (E-g-C3N4) catalysts supported with K and Ru. After a series of analysis, they concluded that although Ru–K/E-g-C3N4 has larger specific surface area and more effective electron hole separation than Ru–K/B-g-C3N4, and Ru is evenly dispersed on the surface of E-g-C3N4, the catalytic nitrogen fixation activity of Ru–K/B-g-C3N4 is better than that of Ru–K/E-g-C3N4. The reason is that Ru is distributed on the edge steps of Ru–K/B-g-C3N4. Due to the strong combination between Ru cluster and support, the interaction between N2 and Ru cluster is reduced. The energy barrier of Ru–K/B-g-C3N4 is low. In the photocatalytic synthesis of ammonia, Ru cluster can not only enhance the charge generated by light adsorption, but also provide an active center for proton reduction and ammonia synthesis. Therefore, Ru–K/B-g-C3N4 has stronger photocatalytic nitrogen fixation ability.
In addition to the above mentioned Fe,104 Ru metals as active sites, Mo,105 Co,20,106,107 Li,108 Ag,109 Ce110 and Te84 are also common as active centers for photocatalytic nitrogen fixation. For instance, a novel ternary MoS2/C–ZnO composite synthesized by Xing and his colleagues through hydrothermal method and photodeposition method. In the photocatalytic reduction of nitrogen, Mo acts as the active site, receives photoelectrons transmitted from the carbon layer, and transfers electrons to the adsorbed nitrogen fraction.111 Guo and coworkers prepared polyatomic Mo doped carbon nitride with urea and Na2MoO4·2H2O as raw materials.112 The isolated Mo centers were fixed by coordination with two N donors on the in situ formed polymeric carbonitride, forming two coordinated MoN2 species. As an active site, the low coordination Mo center strongly adsorbs nitrogen molecules through end-on configuration, which shortens the bond length and weakens the bond energy of N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond. When ethanol is used as a hole cleaner, the ammonia yield under visible light is as high as 830 μmol gcat−1 h−1. Wang and his coworkers prepared three-dimensional cobalt doped graphite carbon nitride by molten salt assistant method.106 On this catalyst, cobalt exists in the form of Co–N coordination bond (Fig. 13). As an active site, it can chemically adsorb and activate N2 molecule, and promote the electron transfer from catalyst to nitrogen molecules. In this work, they carried out density functional theory (DFT) simulation, and found that cobalt doping can not only prolong the activation of N
N bond. When ethanol is used as a hole cleaner, the ammonia yield under visible light is as high as 830 μmol gcat−1 h−1. Wang and his coworkers prepared three-dimensional cobalt doped graphite carbon nitride by molten salt assistant method.106 On this catalyst, cobalt exists in the form of Co–N coordination bond (Fig. 13). As an active site, it can chemically adsorb and activate N2 molecule, and promote the electron transfer from catalyst to nitrogen molecules. In this work, they carried out density functional theory (DFT) simulation, and found that cobalt doping can not only prolong the activation of N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond on N2 molecule, but also capture photoelectrons and improve the efficiency of electron hole separation of g-C3N4. The d orbital of cobalt can be used as a channel for transition metal to transfer electrons to N2 molecule, which improves the optical fixation ability of N2 molecule. Under visible light irradiation, the NH4+ yield of Co doped g-C3N4 is 5.8 mg L−1 h−1 gcat−1, 24 times higher than that of bulk g-C3N4. In the same study, Li doped g-C3N4 catalyst made by Gu et al. was used for photocatalytic nitrogen fixation.108 It was found that Li existed in the form of Li–N bond. As an active site in photocatalytic nitrogen fixation, Li chemically adsorbed and activated nitrogen molecules, and transferred photoelectrons from the catalyst to nitrogen gas. Ag, as a kind of precious metal, could improve the activity of the catalyst after plasma treatment. Xing and his coworkers synthesized Ag/KNbO3 photocatalyst by photodeposition and hydrothermal deposition.113 Ag nanoparticles (Ag NPs) could capture electrons from the surface of photocatalyst and increase the separation of photogenerated carriers. At the same time, due to the surface plasmon resonance effect of silver nanoparticles, it could promote the absorption of visible light. With the help of Ag NPs as the active site, the ammonia yield of photocatalyst Ag/KNbO3 containing 0.5% Ag is 385.0 μmol L−1 g−1 h−1, which is 4 times of that of KNbO.
N bond on N2 molecule, but also capture photoelectrons and improve the efficiency of electron hole separation of g-C3N4. The d orbital of cobalt can be used as a channel for transition metal to transfer electrons to N2 molecule, which improves the optical fixation ability of N2 molecule. Under visible light irradiation, the NH4+ yield of Co doped g-C3N4 is 5.8 mg L−1 h−1 gcat−1, 24 times higher than that of bulk g-C3N4. In the same study, Li doped g-C3N4 catalyst made by Gu et al. was used for photocatalytic nitrogen fixation.108 It was found that Li existed in the form of Li–N bond. As an active site in photocatalytic nitrogen fixation, Li chemically adsorbed and activated nitrogen molecules, and transferred photoelectrons from the catalyst to nitrogen gas. Ag, as a kind of precious metal, could improve the activity of the catalyst after plasma treatment. Xing and his coworkers synthesized Ag/KNbO3 photocatalyst by photodeposition and hydrothermal deposition.113 Ag nanoparticles (Ag NPs) could capture electrons from the surface of photocatalyst and increase the separation of photogenerated carriers. At the same time, due to the surface plasmon resonance effect of silver nanoparticles, it could promote the absorption of visible light. With the help of Ag NPs as the active site, the ammonia yield of photocatalyst Ag/KNbO3 containing 0.5% Ag is 385.0 μmol L−1 g−1 h−1, which is 4 times of that of KNbO.
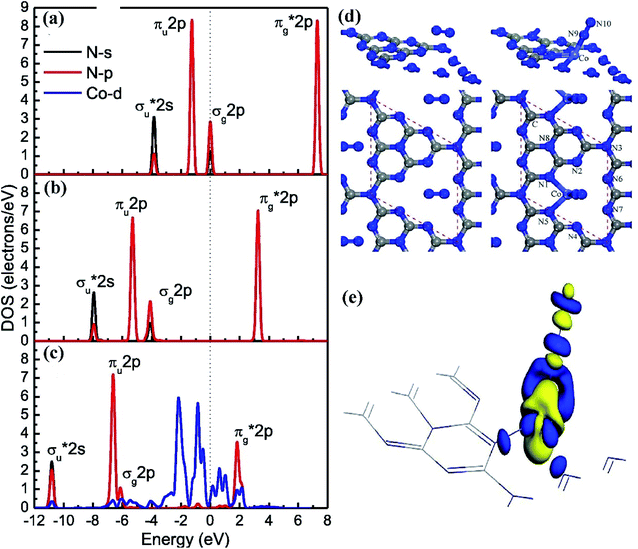 | ||
| Fig. 13 Density of states of (a) free N2, (b) N2 adsorbed on bulk g-C3N4 and (c) N2 adsorbed on Co-GCN(1); (d) the charge density difference of the N2 molecule adsorbed on Co-GCN(1); (e) the optimal N2 adsorption models on bulk g-C3N4 (left) and Co-GCN(1) (right).106 Copyright 2019, Elsevier. | ||
To sum up, in the photocatalytic nitrogen fixation, the active sites on the catalyst play the role of adsorbing and activating nitrogen molecules, transferring photoelectrons from the catalyst to the adsorbed nitrogen molecules. Some of them are the vacancies generated by the catalyst itself through various treatments as active sites, and some of them are the foreign elements doped through various measures as the active site. While these vacancies or metal elements adsorb and activate nitrogen molecules, they can sometimes adjust the band gap of the catalyst and increase the light absorption range. It is immensely helpful to fully understand the active site of photocatalyst in nitrogen fixation reaction for improving the activity of photocatalyst in nitrogen fixation. However, at present, many studies have not really indicated the active site in the catalyst, and the detection of the active site has not been unified. That because the determination of the active site is basically directly based on DFT calculation and some researchers indirectly prove the existence of the active site using N2 absorption–desorption isotherm, the temperature programmed desorption of N2 (N2-TPD) and in situ FTIR spectroscopy. It's still needs to develop more intuitive and simple methods to determine the active site of nitrogen. There is still a long way to go in the research of photocatalyst in nitrogen fixation.
4. Method for determination of ammonia content
The accurate determination of ammonia yield is especially important to explain the nitrogen fixation performance of photocatalyst. At present, almost all the researches on photocatalytic reduction of nitrogen use the following three methods to test the amount of ammonia: ion chromatography,48 Nessler's reagent114 and indophenol blue method.115 Moreover, a more advanced method for quantitative ammonia measurement has now been discovered firstly, which is gas chromatographic method for in situ ammonia quantification.116 Here is a brief analysis of three common methods, namely the indophenol blue method, Nessler's reagent method and ion chromatography. Among them, Nessler's reagent and indophenol blue method can be attributed to spectrophotometry. Compared with the other too methods, ion chromatography has higher efficiency (multiple components and cations can be detected at the same time in a short time), higher sensitivity (the concentration can be from several to hundreds of mg L−1), high selectivity (the inorganic cations and organic cations can be quantified), better stability and compatibility (the pH stability of ion chromatography column packing is higher and the application scope is wider),117 but its price is expensive and there will be large errors in the measurement of ammonia when there is sacrificial agent. The Nessler's reagent method and indophenol blue method are used more economically, but the ammonia content measured with indophenol is very inaccurate when the acid condition and ammonia concentration are higher than 500 g L−1.117Therefore, when measuring the rate of reducing nitrogen by photocatalyst under light irradiation, we need to consider all kinds of situations: whether there is sacrificial agent, pH value, approximate concentration of ammonia and economic conditions. In one work, it is sometimes considered to use a variety of ammonia measurement methods to ensure the accuracy of experimental data. So far, some studies have used indophenol blue method and ion chromatography,102 some have used indophenol blue method and Nessler's reagent method at the same time,60 some have even used three methods to explore nitrogen fixation efficiency.69 For example, Meng and his coworkers tested the ammonia production of the Fe-doped Bi2MoO6 photocatalyst in the solution after reaction under light with Nessler's reagent, indophenol blue method and ion spectrum method, and finally confirmed that the data obtained by the three methods were almost the same(Fig. 14).118
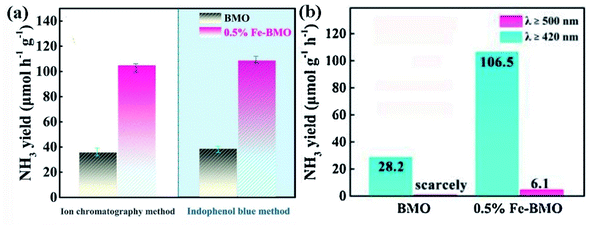 | ||
| Fig. 14 Photocatalytic nitrogen fixation of BMO and 0.5% Fe-BMO with (a) indophenol blue method and ion chromatography method, (b) Nesslers reagent.118 Copyright 2019, Elsevier. | ||
The biggest advantage of gas chromatographic method is that it can detect the real-time concentration of ammonia in the reaction process online. In addition, the sample processing and preparation time is greatly reduced, which reduces the working time of the experimenter.116 Unfortunately, there are not many applications of this method at present, and there are too few related documents. But because of its unique advantages, it is expected to have more applications in the future.
In addition, the synthesis of many photocatalysts involves the use of nitrogen-containing compounds. Some photocatalysts are also rich in nitrogen. In the process of photocatalytic reaction, some nitrogen may be lost to the reaction medium, which makes the quantitative complexity of ammonia. Therefore, isotope labeling is widely used to detect the rate of photocatalytic nitrogen fixation. For example, in the study of Yuan et al., For the prepared graphite carbon nitride nanosheets (Ru-vs-cos/CN) photocatalysts, they used 1H nuclear magnetic resonance (1H NMR) spectroscopy to further confirm that only 14N2 or 15N2 can be used as a nitrogen source to feed gas NH3 is produced. The signal intensity of 15NH4+ or 14NH4+ produced is positively correlated with the reaction time under irradiation (Fig. 15), which made the quantification of ammonia simple and clear.20
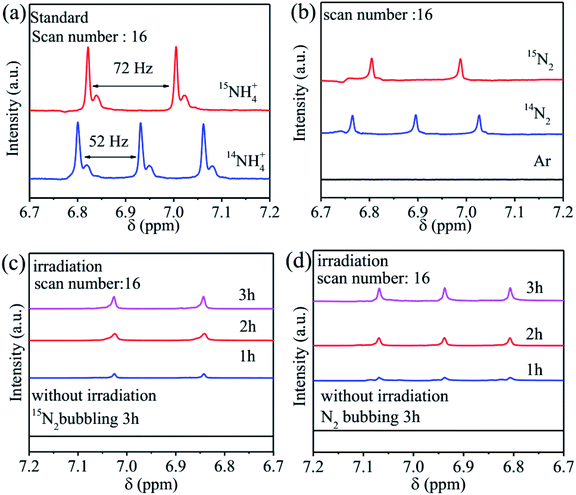 | ||
| Fig. 15 (a) 1H nuclear magnetic resonance (NMR) spectra were calibrated using dimethyl sulphoxide (DMSO) as an internal standard; (b) the chemical shifts in the spectra were obtained for the 10 vol% methanol solution with 15N2, 14N2, or Ar as the feeding gas, respectively; the chemical shifts in the spectra were obtained for the vol% methanol solution with 15N2 (c) and 14N2 (d) as the feeding gas.20 Copyright 2019, Wiley. | ||
5. Conclusions and outlook
It has been a hundred years since the discovery and use of the Haber–Bosch process. Scientists have been exploring new, milder methods nitrogen fixation reactions. Photocatalytic nitrogen fixation can use sunlight to reduce nitrogen molecules in the air under mild conditions. In recent years, with the efforts of generations of researchers, photocatalytic nitrogen fixation technology has achieved good results, but it is still far from being used in industrial production. In the photocatalytic nitrogen reduction reaction, the photocatalyst must simultaneously meet three conditions: high utilization rate of light, high separation rate of photogenerated electron–holes, and high adsorption and activation ability of nitrogen molecules. Increasing material surface defects, loading precious metal atoms, doping various atoms or ions, constructing heterojunctions, and supporting auxiliary catalysts, etc. are all effective methods to improve the above three conditions. These methods, in the final analysis, have a common effect, which increases the active sites on the photocatalyst surface. The active site plays an important role in adsorbing and activating N2 molecules and transferring photogenerated electrons to the adsorbed nitrogen molecules in the photocatalytic nitrogen fixation reaction. Common active sites are various vacancies and metal dopants. O vacancies are the most common active sites. In many photocatalysts that use O vacancies as active sites, the presence of O vacancies also enhances the light absorption range of the catalyst, but the concentration of O vacancies cannot be too much. Excessive O vacancies will become the electron–hole recombination center, thereby inhibiting the reduction of nitrogen. In g-C3N4-based photocatalysts, N vacancies is developed as active sites. Increasing the number of N vacancies is one of the ways to improve the ability of catalytic nitrogen fixation. To achieve non-metal (P, S, O, etc.) and N vacancies Co-doping is also an effective method to enhance the activity of nitrogen fixation. Doped nonmetals can effectively enhance the activity of N vacancies. In recent years, photocatalytic nitrogen fixation catalysts with metal doped bodies as active sites have become very popular. The transition metal Ru, Fe, Mo, Co, etc., the coordinated unsaturated bond of the rare earth element Ce, the Li–N bond formed by metal Li, etc. The electronic feedback can weaken the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond, activate the N2 molecule, and promote the reduction of N2 to NH3.
N triple bond, activate the N2 molecule, and promote the reduction of N2 to NH3.
Besides aforementioned achievements, to improve the reducing ability of the photocatalyst for nitrogen under sunlight, much attention needs to be paid to improving the number and performance of active sites on the surface of the catalyst. Thus, many aspects need to be studied and explored. First, in the subsequent nitrogen fixation studies, it may be possible to consider combining multiple active sites for nitrogen fixation testing, such as the exploration of nitrogen fixation in the presence of nitrogen vacancies and oxygen vacancies, oxygen vacancies and metal sites or metal and metal sites. Secondly, nitrogen has low solubility in water. Thus, regarding the existing aqueous photocatalytic nitrogen fixation reaction system, some improvements could be considered, such as the ratio of catalyst to water, the use of ionic liquids, developing the gas–solid system and the use of heat for light conversion instead of cooling water only. In addition, in future research, the calculation of the active site and the in situ test may be used to calculate the catalytic reaction process, and a suitable catalyst could be designed according to the calculation results.
Disclaimer
The author cannot accept liability for any errors in the experimental data referenced in this article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was sponsored by National Natural Science Foundation of China (Grant No. 21802078 and 21965027) and Natural Science Foundation of Ningxia, China (2018AAC03053).References
- C. X. Guo, J. R. Ran, A. Vasileff and S. Z. Qiao, Energy Environ. Sci., 2018, 11, 45–56 RSC.
- M. H. Vu, M. Sakar, S. A. Hassanzadeh-Tabrizi and T. O. Do, Adv. Mater. Interfaces, 2019, 6, 1900091 CrossRef.
- H. Mou, J. Wang, D. Yu, D. Zhang, W. Chen, Y. Wang, D. Wang and T. Mu, ACS Appl. Mater. Interfaces, 2019, 11, 44360–44365 CrossRef CAS PubMed.
- L. Ye, C. Han, Z. Ma, Y. Leng, J. Li, X. Ji, D. Bi, H. Xie and Z. Huang, Chem. Eng. J., 2017, 307, 311–318 CrossRef CAS.
- Y. Ren, D. Zeng and W.-J. Ong, Chin. J. Catal., 2019, 40, 289–319 CrossRef CAS.
- Y. Liao, J. Lin, B. Cui, G. Xie and S. Hu, J. Photochem. Photobiol., A, 2020, 387, 112100 CrossRef CAS.
- S. Cao, H. Chen, F. Jiang and X. Wang, Appl. Catal., B, 2018, 224, 222–229 CrossRef CAS.
- B. M. Comer and A. J. Medford, ACS Sustainable Chem. Eng., 2018, 6, 4648–4660 CrossRef CAS.
- X. Rong, Y. Mao, J. Xu, X. Zhang, L. Zhang, X. Zhou, F. Qiu and Z. Wu, Catal. Commun., 2018, 116, 16–19 CrossRef CAS.
- C. Tian, W. Sheng, H. Tan, H. Jiang and C. Xiong, ACS Appl. Mater. Interfaces, 2018, 10, 37453–37460 CrossRef CAS PubMed.
- H. Mou, J. Wang, D. Zhang, D. Yu, W. Chen, D. Wang and T. Mu, J. Mater. Chem. A, 2019, 7, 5719–5725 RSC.
- C. Mao, J. Wang, Y. Zou, H. Li, G. Zhan, J. Li, J. Zhao and L. Zhang, Green Chem., 2019, 21, 2852–2867 RSC.
- H. Diarmand-Khalilabad, A. Habibi-Yangjeh, D. Seifzadeh, S. Asadzadeh-Khaneghah and E. Vesali-Kermani, Ceram. Int., 2019, 45, 2542–2555 CrossRef CAS.
- S. Liu, S. Wang, Y. Jiang, Z. Zhao, G. Jiang and Z. Sun, Chem. Eng. J., 2019, 373, 572–579 CrossRef CAS.
- X. Gao, Y. Shang, L. Liu and K. Gao, J. Alloys Compd., 2019, 803, 565–575 CrossRef CAS.
- C. Guo, J. Ran, A. Vasileff and S.-Z. Qiao, Energy Environ. Sci., 2018, 11, 45–56 RSC.
- M. Cheng, C. Xiao and Y. Xie, J. Mater. Chem. A, 2019, 7, 19616–19633 RSC.
- J. Qin, X. Hu, X. Li, Z. Yin, B. Liu and K.-h. Lam, Nano Energy, 2019, 61, 27–35 CrossRef CAS.
- B. Sun, Z. Liang, Y. Qian, X. Xu, Y. Han and J. Tian, ACS Appl. Mater. Interfaces, 2020, 12, 7257–7269 CrossRef CAS PubMed.
- J. Yuan, X. Yi, Y. Tang, M. Liu and C. Liu, Adv. Funct. Mater., 2019, 30, 1906983 CrossRef.
- S. Bian, M. Wen, J. Wang, N. Yang, P. K. Chu and X.-F. Yu, J. Phys. Chem. Lett., 2020, 11, 1052–1058 CrossRef CAS PubMed.
- X. Niu, Q. Zhu, S. Jiang and Q. Zhang, J. Phys. Chem. Lett., 2020, 11, 9579–9586 CrossRef CAS PubMed.
- S. Zhang, Y. Zhao, R. Shi, C. Zhou, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung and T. Zhang, Adv. Energy Mater., 2020, 10, 1901973 CrossRef CAS.
- Y. Zhao, L. Zheng, R. Shi, S. Zhang, X. Bian, F. Wu, X. Cao, G. I. N. Waterhouse and T. Zhang, Adv. Energy Mater., 2020, 10, 2002199 CrossRef CAS.
- Y. Shiraishi, S. Shiota, Y. Kofuji, M. Hashimoto, K. Chishiro, H. Hirakawa, S. Tanaka, S. Ichikawa and T. Hirai, ACS Appl. Energy Mater., 2018, 1, 4169–4177 CrossRef CAS.
- X. Cui, C. Tang and Q. Zhang, Adv. Energy Mater., 2018, 8, 1800369 CrossRef.
- A. Shi, H. Li, S. Yin, Z. Hou, J. Rong, J. Zhang and Y. Wang, Appl. Catal., B, 2018, 235, 197–206 CrossRef CAS.
- X.-M. Gao, Y.-Y. Shang, L.-B. Liu and W. Nie, J. Inorg. Mater., 2019, 34, 967–973 Search PubMed.
- H. Maimaitizi, A. Abulizi, T. Zhang, K. Okitsu and J.-J. Zhu, Ultrason. Sonochem., 2020, 63, 104956 CrossRef CAS PubMed.
- D. Wu, R. Wang, C. Yang, Y. An, H. Lu, H. Wang, K. Cao, Z. Gao, W. Zhang, F. Xu and K. Jiang, J. Colloid Interface Sci., 2019, 556, 111–119 CrossRef CAS PubMed.
- Y. J. Xue, X. K. Kong, Y. C. Guo, Z. Q. Liang, H. Z. Cui and J. Tian, J. Materiomics, 2020, 6, 128–137 CrossRef.
- Y. Chen, C. Zhao, S. Ma, P. Xing, X. Hu, Y. Wu and Y. He, Inorg. Chem. Front., 2019, 6, 3083–3092 RSC.
- X. Yan, D. Dai, K. Ma, S. Zuo, W. Liu, X. Li and C. Yao, Front. Mater. Sci., 2019, 14, 43–51 CrossRef.
- N. I. E. Wei, L. I. U. Li-Bo, S. Yan-Yan and G. A. O. Xiao-Ming, J. Inorg. Mater., 2019, 34, 967–973 Search PubMed.
- J. Wang, W. Lin, Y. Ran, J. Cui, L. Wang, X. Yu and Y. Zhang, J. Phys. Chem. C, 2020, 124, 1253–1259 CrossRef CAS.
- P. Qiu, C. Xu, N. Zhou, H. Chen and F. Jiang, Appl. Catal., B, 2018, 221, 27–35 CrossRef CAS.
- T. Fei, L. Yu, Z. Liu, Y. Song, F. Xu, Z. Mo, C. Liu, J. Deng, H. Ji, M. Cheng, Y. Lei, H. Xu and H. Li, J. Colloid Interface Sci., 2019, 557, 498–505 CrossRef CAS PubMed.
- W. Chen, X.-H. Li, P. He, T. Wang, X.-W. Zhang and Y.-G. Li, ChemSusChem, 2020, 13, 2769–2778 CrossRef PubMed.
- H. Wang, R. Zhao, J. Qin, H. Hu, X. Fan, X. Cao and D. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 44249–44262 CrossRef CAS PubMed.
- X. Feng, H. Chen, F. Jiang and X. Wang, Catal. Sci. Technol., 2019, 9, 2849–2857 RSC.
- Y. Zhang, J. Di, P. Ding, J. Zhao, K. Gu, X. Chen, C. Yan, S. Yin, J. Xia and H. Li, J. Colloid Interface Sci., 2019, 553, 530–539 CrossRef CAS PubMed.
- W. Gao, X. Li, S. Luo, Z. Luo, X. Zhang, R. Huang and M. Luo, J. Colloid Interface Sci., 2020, 585, 20–29 CrossRef PubMed.
- Y. Xue, Y. Guo, Z. Liang, H. Cui and J. Tian, J. Colloid Interface Sci., 2019, 556, 206–213 CrossRef CAS PubMed.
- J. Li, H. Li, G. Zhan and L. Zhang, Acc. Chem. Res., 2017, 50, 112–121 CrossRef CAS PubMed.
- S. H. Wang, W. Wei, X. S. Lv, B. B. Huang and Y. Dai, J. Mater. Chem. A, 2020, 8, 1378–1385 RSC.
- C. Xiao, H. Wang, L. Zhang, S. Sun and W. Wang, Chemcatchem, 2019, 11, 6467–6472 CrossRef CAS.
- S.-X. Wang, H. Maimaiti, B. Xu, Y. Guo, P.-s. Zhai and H.-z. Zhang, J. Phys. Chem. C, 2019, 123, 31119–31129 CrossRef CAS.
- H. Hirakawa, M. Hashimoto, Y. Shiraishi and T. Hirai, J. Am. Chem. Soc., 2017, 139, 10929–10936 CrossRef CAS PubMed.
- H.-P. Jia and E. A. Quadrelli, Chem. Soc. Rev., 2014, 43, 547–564 RSC.
- L. Shi, Z. Li, L. Ju, A. Carrasco-Pena, N. Orlovskaya, H. Zhou and Y. Yang, J. Mater. Chem. A, 2020, 8, 1059–1065 RSC.
- Q. Wang, W. Wang, L. Zhong, D. Liu, X. Cao and F. Cui, Appl. Catal., B, 2018, 220, 290–302 CrossRef CAS.
- J. Ge, J. Xu, Y. Liu, L. Zhang, L. Wang and D. Wu, Nano, 2019, 14, 1950143 CrossRef CAS.
- C. Hua, X. Dong, Y. Wang, N. Zheng, H. Ma and X. Zhang, J. Mater. Sci., 2019, 54, 9397–9413 CrossRef CAS.
- Y. Shiraishi, K. Chishiro, S. Tanaka and T. Hirai, Langmuir, 2020, 36, 734–741 CrossRef CAS PubMed.
- D. Vidyasagar, A. Balapure, S. G. Ghugal, A. G. Shende and S. S. Umare, Phys. Status Solidi A, 2019, 216, 1900212 CrossRef CAS.
- R. Ding, S. Cao, H. Chen, F. Jiang and X. Wang, Colloids Surf., A, 2019, 563, 263–270 CrossRef CAS.
- X. Gao, L. An, D. Qu, W. Jiang, Y. Chai, S. Sun, X. Liu and Z. Sun, Sci. Bull., 2019, 64, 918–925 CrossRef CAS.
- C. He, X. Li, X. Chen, S. Ma, X. Yan, Y. Zhang, S. Zuo and C. Yao, Appl. Clay Sci., 2020, 184, 105398 CrossRef CAS.
- P. Li, Z. Zhou, Q. Wang, M. Guo, S. Chen, J. Low, R. Long, W. Liu, P. Ding, Y. Wu and Y. Xiong, J. Am. Chem. Soc., 2020, 142, 12430–12439 CrossRef CAS PubMed.
- J. Luo, X. Bai, Q. Li, X. Yu, C. Li, Z. Wang, W. Wu, Y. Liang, Z. Zhao and H. Liu, Nano Energy, 2019, 66, 104187 CrossRef CAS.
- X. Gao, Y. Shang, L. Liu and F. Fu, J. Catal., 2019, 371, 71–80 CrossRef CAS.
- H. Xu, Y. Wang, X. Dong, N. Zheng, H. Ma and X. Zhang, Appl. Catal., B, 2019, 257, 117932 CrossRef CAS.
- Y. Zhao, Y. Zhao, R. Shi, B. Wang, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung and T. Zhang, Adv. Mater., 2019, 31, 1806482 CrossRef PubMed.
- X. Rong, H. Chen, J. Rong, X. Zhang, J. Wei, S. Liu, X. Zhou, J. Xu, F. Qiu and Z. Wu, Chem. Eng. J., 2019, 371, 286–293 CrossRef CAS.
- M. Song, M. Du, Q. Liu, F. Xing, C. Huang and X. Qiu, Catal. Today, 2019, 335, 193–199 CrossRef CAS.
- X. Xue, R. Chen, C. Yan, Y. Hu, W. Zhang, S. Yang, L. Ma, G. Zhu and Z. Jin, Nanoscale, 2019, 11, 10439–10445 RSC.
- P. Guan, D. Xu, W. Fan and W. Shi, Chem. Eng. J., 2019, 362, 349–356 CrossRef.
- N. Zhang, L. Li, Q. Shao, T. Zhu, X. Huang and X. Xiao, ACS Appl. Energy Mater., 2019, 2, 8394–8398 CrossRef CAS.
- T. Hou, R. Guo, L. Chen, Y. Xie, J. Guo, W. Zhang, X. Zheng, W. Zhu, X. Tan and L. Wang, Nano Energy, 2019, 65, 104003 CrossRef CAS.
- X. Gao, Y. Shang, K. Gao and F. Fu, Nanomaterials, 2019, 9, 781 CrossRef CAS PubMed.
- J. Fan, M. Zuo, Z. Ding, Z. Zhao, J. Liu and B. Sun, Chem. Eng. J., 2020, 396, 125263 CrossRef CAS.
- S. Wang, X. Hai, X. Ding, K. Chang, Y. Xiang, X. Meng, Z. Yang, H. Chen and J. Ye, Adv. Mater., 2017, 29, 1701774 CrossRef PubMed.
- J. Di, J. Xia, M. F. Chisholm, J. Zhong, C. Chen, X. Cao, F. Dong, Z. Chi, H. Chen, Y.-X. Weng, J. Xiong, S.-Z. Yang, H. Li, Z. Liu and S. Dai, Adv. Mater., 2019, 31, 1807576 CrossRef PubMed.
- G. Zhang, X. Yang, C. He, P. Zhang and H. Mi, J. Mater. Chem. A, 2020, 8, 334–341 RSC.
- Q. Liu, L. Ai and J. Jiang, J. Mater. Chem. A, 2018, 6, 4102–4110 RSC.
- X.-Y. Xie, P. Xiao, W.-H. Fang, G. Cui and W. Thiel, ACS Catal., 2019, 9, 9178–9187 CrossRef CAS.
- S. Wu, X. Tan, K. Liu, J. Lei, L. Wang and J. Zhang, Catal. Today, 2019, 335, 214–220 CrossRef CAS.
- S. Sultana, S. Mansingh and K. M. Parida, J. Mater. Chem. A, 2019, 7, 9145–9153 RSC.
- X. Gao, Y. Shang, L. Liu and F. Fu, J. Colloid Interface Sci., 2019, 533, 649–657 CrossRef CAS PubMed.
- J. Yang, Y. Guo, R. Jiang, F. Qin, H. Zhang, W. Lu, J. Wang and J. C. Yu, J. Am. Chem. Soc., 2018, 140, 8497–8508 CrossRef CAS PubMed.
- G. Wu, L. Yu, Y. Liu, J. Zhao, Z. Han and G. Geng, Appl. Surf. Sci., 2019, 481, 649–660 CrossRef CAS.
- N. H. Kwon, S.-J. Shin, X. Jin, Y. Jung, G.-S. Hwang, H. Kim and S.-J. Hwang, Appl. Catal., B, 2020, 277, 119191 CrossRef CAS.
- Y. Guo, J. Yang, D. Wu, H. Bai, Z. Yang, J. Wang and B. Yang, J. Mater. Chem. A, 2020, 8, 16218–16231 RSC.
- C. Ren, Y. Zhang, Y. Li, Y. Zhang, S. Huang, W. Lin and K. Ding, J. Phys. Chem. C, 2019, 123, 17296–17305 CrossRef CAS.
- W. Wang, H. Zhang, S. Zhang, Y. Liu, G. Wang, C. Sun and H. Zhao, Angew. Chem. Int. Ed., 2019, 58, 16644–16650 CrossRef CAS PubMed.
- Y. Zhao, E. Wang and R. Jin, Diamond Relat. Mater., 2019, 94, 146–154 CrossRef CAS.
- T. Huang, S. Pan, L. Shi, A. Yu, X. Wang and Y. Fu, Nanoscale, 2020, 12, 1833–1841 RSC.
- Z. Li, G. Gu, S. Hu, X. Zou and G. Wu, Chin. J. Catal., 2019, 40, 1178–1186 CrossRef CAS.
- H. Wang, Y. Bu, G. Wu and X. Zou, Dalton Trans., 2019, 48, 11724–11731 RSC.
- C. Liang, H.-Y. Niu, H. Guo, C.-G. Niu, D.-W. Huang, Y.-Y. Yang, H.-Y. Liu, B.-B. Shao and H.-P. Feng, Chem. Eng. J., 2020, 396, 125395 CrossRef CAS.
- C. Xiao, L. Zhang, K. Wang, H. Wang, Y. Zhou and W. Wang, Appl. Catal., B, 2018, 239, 260–267 CrossRef CAS.
- X.-H. Li, W.-L. Chen, P. He, T. Wang, D. Liu, Y.-W. Li, Y.-G. Li and E.-B. Wang, Inorg. Chem. Front., 2019, 6, 3315–3326 RSC.
- X. Hu, W. Zhang, Y. Yong, Y. Xu, X. Wang and X. Yao, Appl. Surf. Sci., 2020, 510, 145413 CrossRef CAS.
- Z. He, Y. Wang, X. Dong, N. Zheng, H. Ma and X. Zhang, RSC Adv., 2019, 9, 21646–21652 RSC.
- X. Li, C. He, S. Zuo, X. Yan, D. Dai, Y. Zhang and C. Yao, Sol. Energy, 2019, 191, 251–262 CrossRef CAS.
- H. Li, J. Li, Z. Ai, F. Jia and L. Zhang, Angew. Chem. Int. Ed., 2018, 57, 122–138 CrossRef CAS PubMed.
- T. Hou, H. Peng, Y. Xin, S. Wang, W. Zhu, L. Chen, Y. Yao, W. Zhang, S. Liang and L. Wang, ACS Catal., 2020, 10, 5502–5510 CrossRef CAS.
- C. Yao, R. Wang, Z. Wang, H. Lei, X. Dong and C. He, J. Mater. Chem. A, 2019, 7, 27547–27559 RSC.
- S. Liu, Y. Wang, S. Wang, M. You, S. Hong, T.-S. Wu, Y.-L. Soo, Z. Zhao, G. Jiang, J. Qiu, B. Wang and Z. Sun, ACS Sustain. Chem. Eng., 2019, 7, 6813–6820 CrossRef CAS.
- H. Huang, X.-S. Wang, D. Philo, F. Ichihara, H. Song, Y. Li, D. Li, T. Qiu, S. Wang and J. Ye, Appl. Catal., B, 2020, 267, 118686 CrossRef CAS.
- A. Awati, H. Maimaiti, S. Wang and B. Xu, Sci. Total Environ., 2019, 695, 133865 CrossRef CAS PubMed.
- C. Hao, Y. Liao, Y. Wu, Y. An, J. Lin, Z. Gu, M. Jiang, S. Hu and X. Wang, J. Phys. Chem. Solids, 2020, 136, 109141 CrossRef CAS.
- H. Liu, P. Wu, H. Li, Z. Chen, L. Wang, X. Zeng, Y. Zhu, Y. Jiang, X. Liao, B. S. Haynes, J. Ye, C. Stampfl and J. Huang, Appl. Catal., B, 2019, 259, 118026 CrossRef CAS.
- T. Hou, L. Chen, Y. Xin, W. Zhu, C. Zhang, W. Zhang, S. Liang and L. Wang, ACS Energy Lett., 2020, 5, 2444–2451 CrossRef CAS.
- X. H. Li, W. L. Chen, H. Q. Tan, F. R. Li, J. P. Li, Y. G. Li and E. B. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 37927–37938 CrossRef CAS PubMed.
- K. Wang, G. Gu, S. Hu, J. Zhang, X. Sun, F. Wang, P. Li, Y. Zhao, Z. Fan and X. Zou, Chem. Eng. J., 2019, 368, 896–904 CrossRef CAS.
- H. Zhang, X. Li, H. Su, X. Chen, S. Zuo, X. Yan, W. Liu and C. Yao, J. Sol-Gel Sci. Technol., 2019, 92, 154–162 CrossRef CAS.
- G. Gu, K. Wang, N. Xiong, Z. Li, Z. Fan, S. Hu and X. Zou, Dalton Trans., 2019, 48, 5083–5089 RSC.
- M. Nazemi and M. A. El-Sayed, Nano Energy, 2019, 63, 103886 CrossRef CAS.
- C. Zhang, Y. Xu, C. Lv, X. Zhou, Y. Wang, W. Xing, Q. Meng, Y. Kong and G. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 29917–29923 CrossRef CAS PubMed.
- P. Xing, P. Chen, Z. Chen, X. Hu, H. Lin, Y. Wu, L. Zhao and Y. He, ACS Sustainable Chem. Eng., 2018, 6, 14866–14879 CrossRef CAS.
- X.-W. Guo, S.-M. Chen, H.-J. Wang, Z.-M. Zhang, H. Lin, L. Song and T.-B. Lu, J. Mater. Chem. A, 2019, 7, 19831–19837 RSC.
- P. Xing, S. Wu, Y. Chen, P. Chen, X. Hu, H. Lin, L. Zhao and Y. He, ACS Sustainable Chem. Eng., 2019, 7, 12408–12418 CAS.
- L. Guo, X. Han, K. Zhang, Y. Zhang, Q. Zhao, D. Wang and F. Fu, Catalysts, 2019, 9, 729 CrossRef CAS.
- J. Liu, R. Li, X. Zu, X. Zhang, Y. Wang, Y. Wang and C. Fan, Chem. Eng. J., 2019, 371, 796–803 CrossRef CAS.
- R. Zaffaroni, D. Ripepi, J. Middelkoop and F. M. Mulder, ACS Energy Lett., 2020, 5, 3773–3777 CrossRef CAS.
- Y. Zhao, R. Shi, X. Bian, C. Zhou, Y. Zhao, S. Zhang, F. Wu, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung and T. Zhang, Adv. Sci., 2019, 6, 1802109 CrossRef.
- Q. Meng, C. Lv, J. Sun, W. Hong, W. Xing, L. Qiang, G. Chen and X. Jin, Appl. Catal., B, 2019, 256, 117781 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |