Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tin dioxide-based nanomaterials as anodes for lithium-ion batteries
Minkang Wanga,
Tianrui Chenb,
Tianhao Liaoa,
Xinglong Zhanga,
Bin Zhua,
Hui Tang *a and
Changsong Dai
*a and
Changsong Dai *b
*b
aSchool of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, 611731, China. E-mail: tanghui@uestc.edu.cn
bSchool of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin 150001, P. R. China. E-mail: changsd@hit.edu.cn
First published on 4th January 2021
Abstract
The development of new electrode materials for lithium-ion batteries (LIBs) has attracted significant attention because commercial anode materials in LIBs, like graphite, may not be able to meet the increasing energy demand of new electronic devices. Tin dioxide (SnO2) is considered as a promising alternative to graphite due to its high specific capacity. However, the large volume changes of SnO2 during the lithiation/delithiation process lead to capacity fading and poor cycling performance. In this review, we have summarized the synthesis of SnO2-based nanomaterials with various structures and chemical compositions, and their electrochemical performance as LIB anodes. This review addresses pure SnO2 nanomaterials, the composites of SnO2 and carbonaceous materials, the composites of SnO2 and transition metal oxides, and other hybrid SnO2-based materials. By providing a discussion on the synthesis methods and electrochemistry of some representative SnO2-based nanomaterials, we aim to demonstrate that electrochemical properties can be significantly improved by modifying chemical composition and morphology. By analyzing and summarizing the recent progress in SnO2 anode materials, we hope to show that there is still a long way to go for SnO2 to become a commercial LIB electrode and more research has to be focused on how to enhance the cycling stability.
1. Introduction
Lithium-ion batteries (LIBs) have been widely used in modern electronic equipment, such as laptops, mobile phones, and mechanical devices for their high energy densities, long cycle life, and environmental friendliness.1–6 As an important component of LIBs, electrode materials are responsible for energy storage and cycling life of LIBs.7–11 Currently, graphite is the main anode in commercial LIBs owing to its good stability.12–14 However, it cannot fully meet the increasing energy demand for batteries due to its low theoretical specific capacity (372 mA h g−1).15–18 In order to solve this problem, much work has been focused on exploring alternative materials with high specific capacity.19–26Potential candidates like metal oxides have been widely researched due to their high theoretical specific capacities, such as MnO,27–30 MnO2,31–36 Mn3O4,37–40 Fe2O3,41–45 Fe3O4,46–50 Co3O4,51–54 and SnO2.55–57 Among these materials, SnO2 has attracted significant attention due to its low cost, natural abundance, and high theoretical specific capacity (782 mA h g−1 of bulk SnO2). SnO2 is superior to other metal oxides as it has a low charge and discharge potential, i.e., an average charge and discharge potential of 0.5 V and 0.3 V vs. Li/Li+, respectively,58 resulting in LIBs with higher energy density. However, the commercial use of SnO2 as an anode material is still hindered by poor cycling stability and inferior rate performance, which is attributed to the electrochemical reaction mechanism of SnO2 during lithiation/delithiation. For SnO2-based anode material for LIBs, the electrochemistry includes two steps, shown as follows:59–61
| SnO2 + 4Li+ + 4e− → Sn + 2Li2O | (1) |
| Sn + xLi+ + xe− ↔ LixSn (0 ≤ x ≤ 4.4) | (2) |
In the first reaction, SnO2 reacts with Li+ and electrons to generate Sn and Li2O. It is believed as an irreversible process, and this is the main reason why SnO2 suffers severe capacity deterioration in the initial lithiation process.62 In the second reaction, Sn obtained from the first step reacts with Li+ and electrons to reversibly generate LixSn alloys. The alloying and dealloying processes represent discharging and charging processes of SnO2-based anode material, respectively.63 However, Li-alloying anode materials like LixSn and LixSi, possess the disadvantages of limited cycle life and severe capacity loss because of large volume changes, pulverization, and continuous formation of solid electrolyte interphase (SEI) during the alloying/dealloying process. Therefore, owing to this irreversible phase transformation process during lithiation/delithiation, the commercial use of SnO2 is largely hampered.
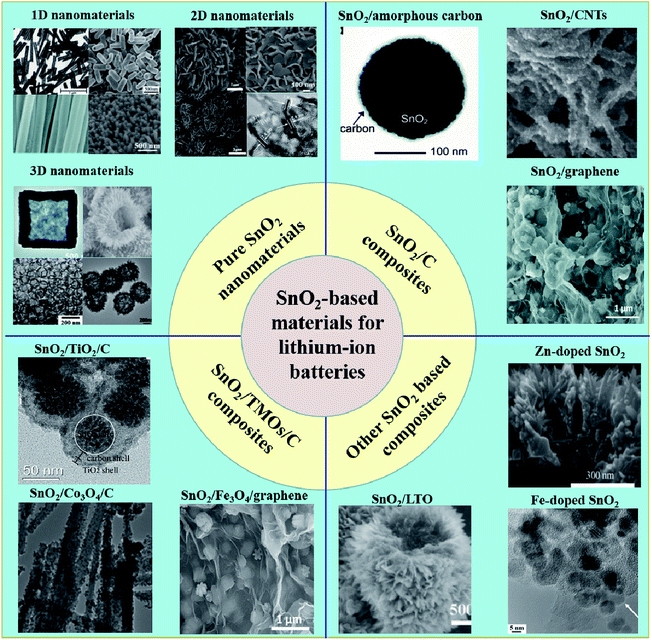
In order to solve these problems and to improve the electrochemical performance of SnO2, researchers have synthesized many SnO2-based anode materials with various well-designed architectures. These SnO2-based anode materials can be classified into four types, according to their chemical composition. The first category includes pure nanostructured SnO2 materials, such as one-dimensional (1D) nanorods (NDs), nanotubes (NTs)64–68 and nanowires (NWs),69–74 two-dimensional nanobelts,75,76 nanosheets77–82 and nanoplates,83–85 three-dimensional (3D) hollow nanostructures,86–92 and hierarchical nanostructures.93 Nanosized materials shorten transmission distance for electrons and Li+ and also help to reduce the extent of volume changes during the electrochemical process. In addition, it has been reported that the reduction reaction of SnO2 nanomaterials becomes reversible or partly reversible, which improves the lithium storage capacity and reduces the capacity loss during the charge/discharge process.94 So the theoretic capacity of SnO2 nanomaterials can be increased up to 1495 mA h g−1. The second category includes the composites of SnO2 and carbonaceous materials,95–97 such as SnO2/carbon nanotubes (CNTs),98–101 SnO2/hollow carbon spheres,102–104 SnO2/graphene105–110 and SnO2/amorphous carbon. Carbonaceous materials improve the conductivity of the composites and also provide abundant nanosized voids as buffers to decrease the effect of large volume changes during the charge/discharge process.111–113 The third category includes the composites of SnO2, transition metal oxides, and carbonaceous materials (SnO2/TMOs/C). Various composites of SnO2 and transition metal oxides (SnO2/TMOs) have been synthesized in the past 20 years, such as SnO2/Fe2O3,114–117 SnO2/Co3O4,118,119 SnO2/TiO2,120–122 SnO2/ZnO123 and SnO2/MoO3 (ref. 124) and they showed enhanced lithium storage capacity compared to pure SnO2 anode material.125 It has been reported that the introduction of TMOs, such as Fe2O3 (ref. 126) and Co3O4,127 can effectively enhance the capacity because the transition metal nanoparticles in the composite can reversibly convert the extra Li2O into Li+; thus, influencing the charge/discharge processes. However, cycling stability and rate performance of the composites still need to be further improved. Therefore, based on SnO2/TMOs materials, much work has been done to synthesize the composites of SnO2, TMOs, and carbonaceous materials (SnO2/TMOs/C),128–131 like Fe3O4/SnO2/rGO,132 SnO2@C@Fe3O4 (ref. 133) and SnO2/MoO3/C.134,135 SnO2/TMOs/C materials more effectively alleviate the impact of volume changes and improve conductivity, leading to better electrochemical performance as an anode, compared to the SnO2/TMOs materials.136,137 The fourth category includes some other tin dioxide-based compounds, such as heteroatom-doped SnO2 (ref. 138 and 139) (Fe-doped SnO2,140 Zn-doped SnO2 (ref. 141)), Li4Ti5O12/SnO2 (ref. 142) and SnO2/C3N4.143
In this article, we provide the recent progress in the research of these four major types of SnO2-based anode material, as mentioned above (Scheme 1). For the following sections, we will introduce the various SnO2-based nanomaterials as well as their corresponding synthesis methods and electrochemical performance. We hope this review article will serve as a good reference for further research.
2. Pure nanostructured SnO2 materials
2.1 1D nanomaterials
Many 1D nanostructured SnO2 materials, such as nanorods,144–146 nanotubes147–150 and nanowires151–153 have been synthesized in recent years. 1D SnO2 nanomaterials usually exhibit superior specific discharge capacity since they can offer extra channels and pathways for electron transmission compared to bulk SnO2 and SnO2 powders.154–156| Sn4+ + 4OH− → Sn(OH)4 → SnO2 + 2H2O | (3) |
First, Sn(OH)4 forms by the hydrolysis of Sn-based salts in aqueous medium. During the hydrothermal treatment, Sn(OH)4 tends to convert into SnO2 and subsequently grows along the [001] direction.160,161 Early in 2003, Zhang et al. fabricated uniform SnO2 nanorods with diameters of about 8–15 nm and lengths of about 150–200 nm by a one-step procedure under mild conditions.148 They dissolved sodium dodecyl sulfate and Sn(OH)62− salt in a solution consisting of heptane and hexanol by stirring. Then, the homogeneously dispersed solution was transferred into a Teflon-lined autoclave and heated to 200 °C for 18 h. The as-prepared SnO2 nanorods displayed a crystalline rutile structure. Zhang et al. also discovered that the concentration of Sn(OH)62− ions and the ratio of NaOH and SnCl4 determined the shape of the SnO2 nanorods. It was found that on increasing the concentration of Sn(OH)62− from 0.2 M to 0.3 M, the number of nanorods significantly decreased; additionally, on increasing the molar ratio of NaOH to SnCl4 from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 30
1 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the aspect ratio of SnO2 nanorods increased.162
1, the aspect ratio of SnO2 nanorods increased.162
In 2004, Cheng et al. investigated a large-scale hydrothermal method to synthesize single-crystalline SnO2 nanorods with lengths of 15–20 nm and diameters of 2.5–5 nm (Fig. 1A). Sn4+ precursor was dissolved in a mixture of water and alcohol, and pH was adjusted 12 and the solution was then heated at 150 °C for 24 h.163 Based on Zhang and Cheng's work, many groups have synthesized SnO2 nanorods via hydrothermal methods in other different systems. Guo et al. synthesized SnO2 nanorods with diameter in the range of 120–260 nm and length up to 2–3 μm by using hexadecyltrimethylammonium bromide as a template (Fig. 1B).58 Chen et al. synthesized single crystalline SnO2 nanorods with diameters of 4–15 nm and lengths of 100–200 nm.145 Xi et al. investigated a new synthesis method of ultrathin SnO2 nanorods with an average diameter of 2 ± 0.5 nm.164 Therefore, various SnO2 nanorods with distinct morphology can be synthesized by controlling hydrothermal conditions.
 | ||
| Fig. 1 (A) Transmission electron microscopy (TEM) images of the as-prepared SnO2 nanorods (adapted with permission from ref. 163 copyright 2004 American Chemical Society). (B) Scanning electron microscopy (SEM) images of long SnO2 nanorods (adapted with permission from ref. 58 copyright 2004 Elsevier). (C) SEM images, (D) high-resolution TEM (HRTEM) images, (E) FFT patterns of the HRTEM images, and (F) cycling performance of SnO2 nanorods (adapted with permission from ref. 154 copyright 2009 Royal Society of Chemistry). | ||
SnO2 nanorods can be applied as anode materials in LIBs.165 Liu et al. synthesized SnO2 nanorods arrays on a flexible alloy substrate via hydrothermal method. The nanorods arrays have average diameter and length of 60 and 670 nm, respectively (Fig. 1C). Observed by HRTEM images (Fig. 1D) and FFT pattern of HRTEM images (Fig. 1E), SnO2 nanorod array was growing on substrate along [001] direction, since (001) plane is more loosely packed and has a relatively high surface energy compared to {110} planes.166 As-collected hierarchical array structure can directly be used as a binder-free electrode for LIBs, which shows good energy performance, including high discharge capacity (the first discharge is 1918 mA h g−1) and good cycling stability (580 mA h g−1 after 100 cycles at 0.1C, with coulombic efficiency of nearly 100%) (Fig. 1F).154
Early in 2006, Lai et al. reported the preparation of SnO2 nanotubes with a thickness of 10 nm and a length of about 0.4–1.4 μm via the electrodeposition method (Fig. 2A). They first electroplated the SnO2 nanoparticles on a gold electrode which was modified with a porous PC membrane. Then, the SnO2 particles were annealed at 650 °C in ambient conditions. The shape and size of the as-prepared nanotubes could be easily controlled by monitoring the charge passed.66 Meanwhile, Lai et al. also reported that the SnO2 nanotubes possessed better crystallinity and uniformity in terms of length and width.
 | ||
| Fig. 2 (A) TEM image of SnO2 nanotubes (adapted with permission from ref. 66 copyright 2006 Royal Society of Chemistry). (B) HRTEM image of SnO2 nanotubes for measuring thickness, (C) FESEM image of the side view, and (D) FESEM images of the top view of SnO2 nanotubes (adapted with permission from ref. 68 copyright 2005 American Chemical Society). (E) TEM image, (F) HRTEM image, and (G) discharge capacity vs. cycle number of SnO2 nanotube arrays on Ti substrates (adapted with permission from ref. 64 copyright 2011 American Chemical Society). | ||
The AAO and PC membrane-template methods have been widely used in the synthesis of metal oxide nanotubes. Wang et al. fabricated uniform polycrystalline SnO2 nanotubes via this template.68 They found that the diameter, thickness, length, and texture of nanotubes can be controlled with template structure, pristine particle size, and heating-rate temperature. As shown in Fig. 2B–D, nanotube walls, with a thickness of about 10–25 nm, were composed of abundant nanocrystallites of 6–15 nm. Furthermore, the SnO2 nanotube electrode showed superior charge/discharge performance compared to SnO2 nanoparticles. The specific capacity of the SnO2 nanotube electrode can be 525 mA h g−1 after 80 cycles.68 They summarized that the improved cycling performance resulted from the following factors, (1) cavities in the nanotubes which provide space and reduce the effect of volume change during lithiation/delithiation process; (2) SnO2 nanotubes provide more active sites for Li+ intercalation and deintercalation; and (3) compared to SnO2 nanoparticles, the SnO2 nanotubes are less movable and result in less agglomeration.68 Si and SiO2 have been commonly used in the synthesis of hollow nanostructures, including nanotubes. Morphology and shapes of SnO2 nanotubes can be well designed by controlling the shape of the Si templates and the hydrothermal conditions.168 Ye et al. also discovered that the shorter SnO2 nanotubes showed superior electrochemical performance, since the hollow structure in the short nanotubes can alleviate the volume changes.147 ZnO is another promising sacrificial template used for synthesizing SnO2 nanotube arrays as ZnO can be conveniently synthesized and removed. The as-prepared nanotubes exhibited a diameter and thickness of about 100–300 nm and about 10–20 nm, respectively, composed of nanoparticles with diameters of about 2–5 nm (Fig. 2E and F). The material also showed high capacity and improved cycling performance, i.e., 750–800 mA h g−1 after 20 cycles at 0.1C, as shown in Fig. 2G.64
Researchers have synthesized SnO2 nanowires via many methods. For example, Ko et al. synthesized SnO2 nanowires on the current collector via thermal evaporation at (600 °C). The as-prepared SnO2 nanowires exhibited a highly-ordered single-crystalline phase with a thin diameter of 40–50 nm and length of more than 1 μm (Fig. 3A and B). The SnO2 nanowire-based anode exhibited high specific discharge capacity and good cycling performance, i.e., 2140 mA h g−1 at the first cycle and 510 mA h g−1 at the 50th cycle at 1C (Fig. 3C).72 Ding et al. reported a facile strategy for the synthesis of SnO2 nanowire arrays using SBA-15 nanorods as a template by infiltrating molten SnCl2 into the channels of the SBA-15 nanorods followed by calcination and removal of the template.69 Han et al. investigated the synthesis of porous SnO2 nanowire bundles with a high yield via solution-based approaches. This hierarchical nanostructure is made up of SnO2 nanowires with an overall diameter of 80–120 nm and an average length of 6 μm (Fig. 3D). Moreover, the as-prepared nanowires have a highly porous structure composed of numerous nanocrystals.170 Ren et al. synthesized 3D hierarchical SnO2 nanowire arrays on carbon cloth by first, a CVD method for SnO2 nanowires, followed by a Plasma Enhanced-CVD (PECVD) method for Si thin film coating. The SnO2@Si nanowire arrays can directly serve as a flexible and binder-free anode for LIBs. In this unique structure, SnO2 nanowires act as a lithium storage material and a conductive matrix to support Si; in addition, the thin Si layer acts as a buffer for SnO2 to reduce the effect of volume changes (Fig. 3F). Anodes based on such novel hierarchical structures showed excellent electrochemical performance with discharge capacity of 2.13 mA h cm−2 in the first cycle and 1.386 mA h cm−2 after 50 cycles, i.e., 65% of the first cycle (Fig. 3G).73 Thus, in this section, different synthesis methods for SnO2 nanowires, corresponding morphologies, and electrochemical properties have been presented, demonstrating that SnO2 nanowires are promising anode materials for LIBs.
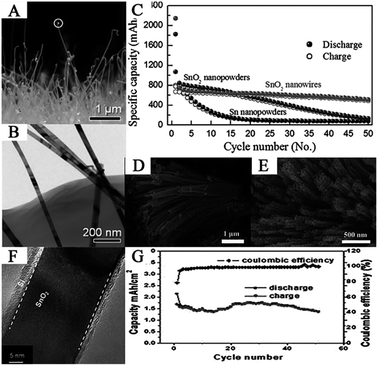 | ||
| Fig. 3 (A) Cross-sectional FESEM image, (B) TEM image, and (C) comparative cycling performance of the SnO2 nanowires (adapted with permission from ref. 72 copyright 2009 IOP Publishing). (D and E) SEM images of SnO2 porous nanowire bundles (adapted with permission from ref. 170 copyright 2011 Royal Society of Chemistry). (F) HRTEM image, and (G) cycling performance of the SnO2@Si nanowires (adapted with permission from ref. 73 copyright 2013 Royal Society of Chemistry). | ||
2.2 2D nanomaterials
Due to the difficulty of synthesizing well-defined 2D SnO2 nanomaterials, obtaining a large variety of 2D SnO2 nanostructures is significantly harder than 1D and 3D nanomaterials. The anisotropic crystal growth of SnO2, in the formation process, limits the synthesis of 2D nanomaterials.82 In order to overcome this problem, in situ growth of SnO2 nanosheets on physical or chemical substrates has been widely investigated.171,172 Among these fabrication processes, SnO2 nanosheets grown on physical substrates, such as metal foam,49,173–175 foil,77,154,176–178 carbon cloth78,179 and graphite paper180–183 can be directly used as binder-free and current-collector free electrode in LIBs due to excellent conductive and supportive properties of such substrates. Meanwhile, some organic macromolecules can be used as chemical templates to fabricate SnO2 nanosheets. These substrates could provide a growth plane for SnO2 nanosheets and inhibit self-aggregation during the lithiation/delithiation processes.184 Moreover, SnO2 nanosheets have a tendency to assemble into complex structures, such as nanosheet arrays, clusters, and spheres, which usually exhibit superior lithium storage capacities due to their highly interconnected and hierarchical nanostructures.184–187Zhao et al. fabricated SnO2 nanosheet arrays on Ni foam with a thickness of 20 nm and a length of 500 nm (Fig. 4A) via a hydrothermal method. Nickel foam, i.e., a 3D macroporous conductive network, was used as a supportive substrate to fabricate SnO2 nanosheet arrays, which can be directly used as a binder-free anode in LIBs. Due to the high electroactive surface area, ultrathin sheets, and shorter electron transport pathways, the nanosheets exhibited excellent electrochemical properties. The novel anode showed an initial discharge capacity of approximately 1800 mA h g−1 and remained at 674.9 mA h g−1 after 50 cycles at 0.5C (Fig. 4B).79 Moreover, interconnected single and double layer SnO2 nanosheets were also fabricated on three different conductive substrates, i.e., Ti, Cu foil, and flexible graphite paper, as integrated binder-free electrodes for LIBs. The nanosheets were interconnected with each other to form a hierarchical network, and the thickness of a single SnO2 nanosheet layer was about 250 nm (Fig. 4C and D). The electrodes delivered high specific capacity, excellent cycling stability, and good rate capability.77,79 Zhu et al. reported an ultra-rapid, low-cost, and simple microwave-assisted synthesis of ultrathin SnO2 nanosheets with a thickness of less than 5 nm (Fig. 4E). The ultrathin SnO2 nanosheets exhibited significantly enhanced electrochemical lithium storage properties with a high reversible capacity of 757.6 mA h g−1 at a current density of 200 mA g−1 up to 40 cycles. The ultrathin 2D nanosheets can significantly reduce the ion diffusion paths and allow faster phase transitions; furthermore, the sufficient external surface interspace and porous configuration successfully accommodate the large volume changes.77,78,80
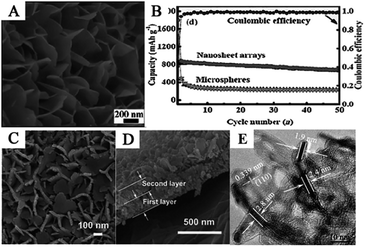 | ||
| Fig. 4 (A) SEM image, and (B) cycling performance of hierarchical SnO2 nanosheet arrays on nickel foam (adapted with permission from ref. 79 copyright 2014 Wiley). (C) Top view, and (D) cross-sectional FESEM images of SnO2 nanosheets double layer on Ti foil (adapted with permission from ref. 77 copyright 2014 Royal Society of Chemistry). (E) HRTEM image of ultrathin SnO2 nanosheets of less than 5 nm (adapted the permission from ref. 80 copyright 2015 American Chemical Society). | ||
Many complex hierarchical SnO2 nanostructures were also assembled by 2D SnO2 nanosheets. Flower-like SnO2 assembled by many SnO2 nanosheets, with an average thickness of 100 nm, have been synthesized on a flexible carbon cloth using CVD at 750 °C.78 SEM images (Fig. 5A) show that as-prepared flower-like SnO2 nanostructures were formed by an assembly of numerous nanosheets with a thickness of 100 nm. Ding et al. synthesized hollow spheres assembled from SnO2 nanosheets (Fig. 5B), using sulfonated polystyrene hollow spheres (sPSHSs) as a template; the surface of the sPSHS is covered with –SO3– functional groups. Therefore, the Sn2+ ions can easily interact with these templates via electrostatic forces and subsequently grow into SnO2 nanosheets, assisted by mercaptoacetic acid. Moreover, sPSHSs templates are beneficial for minimizing the gas outflux during the template removal process, which helps the retention of the final structure.188–191 The as-prepared samples showed a superior cycling capacity retention compared to other SnO2 nanoflowers assembled by SnO2 nanosheets as well as SnO2 nanoparticles, indicating the positive effect of the unique nanostructure (Fig. 5C).87 Wei et al. fabricated nanoporous SnO2 nanosheets via a simple one-step ultrasonic-assisted chemical precipitation strategy with polyvinylpyrrolidone (PVP) as a soft template. Due to the porous nanosheet structure, it exhibited high capacity, i.e., an initial discharge capacity of 2231 mA h g−1 at 0.2 A g−1, and excellent cycling performance, i.e., an initial discharge capacity of 710 mA h g−1 and 606 mA g−1 at 1.6 A g−1 and 4 A g−1, respectively, which remained at 497 mA h g−1 and 280 mA h g−1 after 60 cycles, respectively (Fig. 5D).81
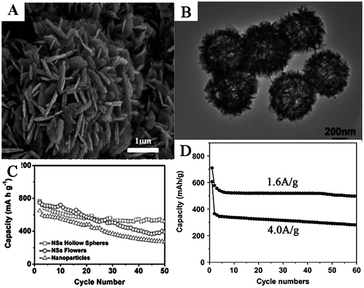 | ||
| Fig. 5 (A) SEM images of flower-like SnO2 nanosheets (adapted with permission from ref. 78 copyright 2014 Elsevier). (B) TEM image, and (C) cycling performance of SnO2 nanosheets hollow spheres (adapted the permission from ref. 87 copyright 2011 Royal Society of Chemistry). (D) Cycling performance of SnO2 nanosheets at 1.6 A g−1 and 4.0 A g−1 (adapted the permission from ref. 81 copyright 2017 Elsevier). | ||
2.3 3D nanomaterials
In recent years, 3D SnO2 structures have attracted significant attention.86 Various 3D nanostructures have been synthesized, such as hollow spheres91,192–194 and microboxes.195–197 Furthermore, many new synthetic methods to prepare SnO2 hollow structures, including Ostwald ripening,198–200 Kirkendall effect,201–204 removable templates195 and chemically induced self-assembly192,205 have been reported.In 2006, Lou et al. investigated a one-pot template-free synthesis of hollow SnO2 nanostructures, based on an unusual inside-out Ostwald ripening mechanism. The synthesis process was performed in an ethanol–water (EtOH–H2O) mixed solvent with K2SnO3 as the precursor, followed by a hydrothermal treatment. Lou et al. discovered that the concentration of the precursor and the ratio of EtOH in the mixed solvent determined both the particle size and morphology of the product. Moreover, the addition of urea or thiourea in the synthetic mixtures was also found to increase the product yield, morphological yield to nearly 100%, and a well-dispersed monodispersity (Fig. 6A–C). Such hollow SnO2 nanosphere-based anode material exhibited high discharge capacity and good cycling performance, i.e., an initial discharge capacity of 1140 mA h g−1, which is comparable to the theoretical capacity of graphite after more than 40 cycles.86
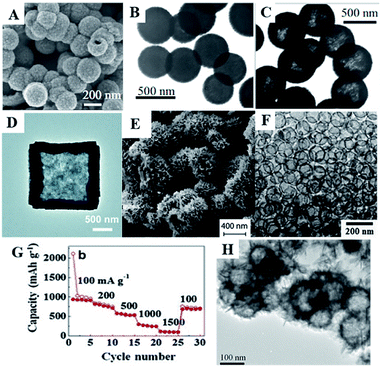 | ||
Fig. 6 (A) FESEM images of SnO2 hollow nanosphere prepared in the solvent of EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio of 1 H2O ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. (B) TEM images of SnO2 hollow nanosphere prepared with the value of EtOH in the solvent of EtOH–H2O = 37.5%. (C) TEM images of SnO2 hollow nanosphere prepared with the value of EtOH in the solvent EtOH–H2O = 37.5% and 0.1 M urea (adapted the permission from ref. 86 copyright 2006 WILEY). (D) TEM images of SnO2 microboxes (adapted the permission from ref. 195 copyright 2016 Royal Society of Chemistry). (E) SEM images of SnO2 hierarchical hollow microspheres (adapted the permission from ref. 205 copyright 2017 Royal Society of Chemistry). (F) TEM images, and (G) rate performance of SnO2 hollow spheres (adapted with permission from ref. 91 copyright 2012 Elsevier). (H) TEM image of the hierarchical SnO2 (adapted with permission from ref. 210 copyright 2010 American Chemical Society). 1. (B) TEM images of SnO2 hollow nanosphere prepared with the value of EtOH in the solvent of EtOH–H2O = 37.5%. (C) TEM images of SnO2 hollow nanosphere prepared with the value of EtOH in the solvent EtOH–H2O = 37.5% and 0.1 M urea (adapted the permission from ref. 86 copyright 2006 WILEY). (D) TEM images of SnO2 microboxes (adapted the permission from ref. 195 copyright 2016 Royal Society of Chemistry). (E) SEM images of SnO2 hierarchical hollow microspheres (adapted the permission from ref. 205 copyright 2017 Royal Society of Chemistry). (F) TEM images, and (G) rate performance of SnO2 hollow spheres (adapted with permission from ref. 91 copyright 2012 Elsevier). (H) TEM image of the hierarchical SnO2 (adapted with permission from ref. 210 copyright 2010 American Chemical Society). | ||
Porous hollow SnO2 micro-boxes have been synthesized via a selective leaching strategy using ZnSn(OH)6 as the precursor. ZnSn(OH)6 micro-boxes were first formed through a modified one-pot co-precipitation method and subsequently, the Zn species were removed via a selective leaching strategy. The TEM image (Fig. 6D) shows that the thickness of the shell was about 100 nm.195 Hu et al. used a template-and additive-free hydrothermal route to prepare a uniquely shaped SnO2 material that comprised of a hollow spherical morphology with uniform diameter and very thin petal-like nanosheets grown perpendicularly on the surface of the spheres, resembling a “chestnut cupule” (Fig. 6E). In contrast to conventional SnO2 materials, this unique morphology significantly improved the storage capacity and cycling performance of SnO2 as an anode material for lithium and sodium ion batteries.206
In addition, 3D hierarchical SnO2 nanomaterials can be assembled by 0D, 1D, and 2D SnO2 nanomaterials. For example, 0D crystalline SnO2 nanoparticles were successfully assembled into a high-order nanostructure of hollow core–shell. First, SnCl4 was dissolved in a mixture of DI water and ethanol to form a homogeneous solution which was heated to 180 °C for 24 h. Then, the collected sediments were calcined in air to remove carbon by oxidation. FESEM showed that the meso-spheres had an overall dimension of 1–3 μm, and the surface of the spheres was formed by small aggregated SnO2 nanoparticles (11 nm). The shell was estimated to be approximately 200 nm in thickness. This unique SnO2 nanostructure could store an exceedingly large amount of Li+ and cycled well for a pure phase SnO2 anode.207–209 Hollow urchin-like SnO2 nanospheres have been fabricated using ultrathin nanorods via a solvothermal route. The diameters of urchin-like nanospheres and nanorods are about 300 and 100 nm, respectively. The as-obtained hollow urchin-like SnO2 nanospheres with ultrathin 1D nanorods exhibited high capacity and excellent rate discharging performance. The 1st, 2nd, 20th, and 50th discharge capacities were 1881 mA h g−1, 1090 mA h g−1, 781 mA h g−1, and 719 mA h g−1, respectively, at a current density of 100 mA g−1. Upon changing the discharge–charge rates to 0.2, 0.4, 0.8, 1, 2, and 0.4C, the capacities of urchin-like SnO2 were maintained at 815, 687, 601, 446, 282, and 520 mA h g−1, respectively, while for commercial SnO2, the capacities were only 828, 677, 512, 386, 246, 133, and 375 mA h g−1, respectively. The retention of the reversible capacity of the hollow nanosphere electrodes was better than that of commercial SnO2 samples.210 Furthermore, SnO2 hollow nanospheres can also be synthesized by sol–gel methods.91 The size of the hollow spheres is controlled by using different-sized templates. As-prepared SnO2 shells are almost amorphous and exhibit a rutile phase after annealing at 600 °C. The size of the SnO2 hollow spheres ranges from 25 to 100 nm (Fig. 6F), and the thickness of the shell is constantly 5 nm despite the size of the hollow spheres. Due to the nanosized hollow sphere and thin shell thickness, SnO2 hollow spheres show excellent electrochemical performance. The smallest hollow sphere of SnO2 (25 nm) exhibited a high reversible capacity of 750 mA h g−1 as well as good rate performance, i.e., 700 mA h g−1 at 0.2 A g−1 and 530 mA h g−1 at 0.5 A g−1 (Fig. 6G).91 Hierarchical SnO2 hollow nanostructures can also be assembled by 2D nanosheets. As shown in Fig. 6H, the hierarchical SnO2 shows high capacities and excellent cycle performance as an anode material for LIBs. The improved electrochemical properties could be ascribed to the large surface area, enhanced structure stability, and short diffusion length for both lithium ions and electrons.211
3. Composites of SnO2 and carbonaceous materials
3.1 SnO2 with carbon nanotubes (CNTs)
Nanocomposites of SnO2 and CNTs reportedly exhibit improved lithium storage capacities compared to pure SnO2 materials.212 This can be attributed to the flexible nature and superior conductivity of the CNTs, which alleviates the internal stress caused during the charge/discharge process213–215 and enhances the electron transportation. With increased conductivity and surface area, such nanocomposites show enhanced lithium storage capability.216,217Wen et al. reported an in situ synthesis of mesoporous SnO2 on the surface of multi-walled CNTs (MWCNTs) through a hydrothermal method utilizing CTAB as the structure-directing agent. The MWCNTs/SnO2 hybrid electrodes showed great electrochemical performance and cycling stability. This can be attributed to the synergistic effects of the unique combination of properties including their one-dimensional hollow structure, high-strength with flexibility, excellent electric conductivity, and large surface area, which helped alleviate the effect of volume expansion, shorten the distance of Li+ diffusion, and contribute to the transmission of electrons.100,218–221
In another hydrothermal system, Du et al. synthesized SnO2/MWCNT composites by a simple solvothermal method and subsequent heat treatment at 360 °C with SnCl2 and CNTs as reactants. The distribution of SnO2 nanocrystals can be controlled by changing the molar ratio of Sn2+ and CNTs in the precursor. For SnO2/MWCNTs composites prepared with a molar ratio of Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C = 0.3
C = 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a uniform layer of SnO2 nanocrystals, with a crystal size of about 5 nm, was deposited on the surface of the MWCNTs (Fig. 7A).222 These composites showed very stable cycling retention, of up to 100 cycles, because of nanosized materials and the introduction of CNTs.201,223 Jin et al. prepared SnO2/MWCNTs electrodes via a hydrothermal method at 150 °C for 24 h. The SnO2 nanoparticles with diameters of less than 3 nm were uniformly loaded onto the surface of MWCNTs. The MWCNT/SnO2 nanocomposites exhibited a high reversible capacity of 420 mA h g−1 even after 100 cycles.137,224
1, a uniform layer of SnO2 nanocrystals, with a crystal size of about 5 nm, was deposited on the surface of the MWCNTs (Fig. 7A).222 These composites showed very stable cycling retention, of up to 100 cycles, because of nanosized materials and the introduction of CNTs.201,223 Jin et al. prepared SnO2/MWCNTs electrodes via a hydrothermal method at 150 °C for 24 h. The SnO2 nanoparticles with diameters of less than 3 nm were uniformly loaded onto the surface of MWCNTs. The MWCNT/SnO2 nanocomposites exhibited a high reversible capacity of 420 mA h g−1 even after 100 cycles.137,224
 | ||
Fig. 7 (A) FESEM image of SnO2/CNT composites prepared with MSn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C = 0.3 C = 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (adapted with permission from ref. 221 copyright 2009 Elsevier). (B) SEM image, and (C) rate performance of SnO2@C/MWCNTs-LiF (adapted with permission from ref. 224 copyright 2019 Elsevier). (D) TEM image and (E) cycling performance of CNT@void@SnO2@C composite (adapted the permission from ref. 225 copyright 2015 Elsevier). (F) SEM image, and (G) rate performance of SnO2/CNTH composites (adapted with permission from ref. 226 copyright 2019 American Chemical Society). 1 (adapted with permission from ref. 221 copyright 2009 Elsevier). (B) SEM image, and (C) rate performance of SnO2@C/MWCNTs-LiF (adapted with permission from ref. 224 copyright 2019 Elsevier). (D) TEM image and (E) cycling performance of CNT@void@SnO2@C composite (adapted the permission from ref. 225 copyright 2015 Elsevier). (F) SEM image, and (G) rate performance of SnO2/CNTH composites (adapted with permission from ref. 226 copyright 2019 American Chemical Society). | ||
In order to further improve electrochemical properties of the SnO2/CNTs hybrid nanomaterials, many groups have synthesized various SnO2/CNTs-based anode materials. For example, Liang et al. synthesized SnO2@C/MWCNTs-lithium fluoride (LiF) composite nanomaterials (Fig. 7B). Carbon-coated SnO2 (SnO2@C) was prepared by a spray drying method, with water-soluble asphalt as the carbon source and MWCNTs as the conductive agent. The conductivity was significantly enhanced, and the extent of volume expansion of the SnO2 was reduced. Compared to SnO2/CNTs, the presence of LiF enhanced the stability of the SEI film and improved the coulombic efficiency and capacity retention rate of the electrode. After 200 cycles, the SnO2@C/MWCNTs-LiF anode still maintained 70.1% of the capacity retention rate and the specific capacity was held at 274 mA h g−1 at 2400 mA g−1, compared with 136 mA h g−1 for the SnO2@C/MWCNTs anode (Fig. 7C).225 Tian et al. fabricated a tube-in-tube nanostructure, denoted as CNT@void@SnO2@C, by a facile hydrothermal method and subsequent carbonation with polysaccharide as the carbon source, SiO2 as the sacrificial template, and NH4F as the etchant. The CNT@void@SnO2@C exhibited one-dimensional nanostructures with average diameters of about 100–150 nm and hollow structures filled with abundant voids (Fig. 7D). Tian et al. interpreted the formation mechanism as follows: (1) the crystallization and deposition of SnO2 could occur prior to the polycondensation of glucose; (2) the formation rate of SnO2@polysaccharide was faster than the etching rate of SiO2 by NH4F due to the slow etching process of SiO2 by NH4F solution under hydrothermal condition; and (3) the large void-space between the SnO2@polysaccharide and CNT, formed after the SiO2 coating layer, was etched away completely. The CNT@void@SnO2@C exhibited good electrochemical properties, delivering a reversible capacity of 702.5 mA h g−1 at 200 mA g−1 even after 350 cycles (Fig. 7E). This indicated that the unique tube-in-tube nanostructure, as well as the one-dimensional void space, which formed between the inner CNT and outer SnO2@C nanotubes, contributed significantly to the electrochemical performance.226 Liu et al. synthesized ultrafine SnO2 (6–7 nm)/carbon nanotube hairball (SnO2/CNTH) composites with a 3D hierarchical structure (Fig. 7F), which was prepared by spray drying and a solvothermal method. Fig. 7G shows that SnO2/CNTH exhibited superior electrochemical performance and improved the lithium storage capacity compared to conventional SnO2/CNT. The improved electrochemical performance can be attributed to the increased conductivity and enhanced electrode reactivity due to the 3D hierarchical cross-linked structure. This structure can also address the large volume changes upon cycling.227
3.2 SnO2 with amorphous carbon
Hydrothermal treatment followed by carbonization is the most common method for synthesizing SnO2/amorphous carbon nanomaterials.228–230 There are mainly two synthesis routes of SnO2/amorphous carbon; one is to treat Sn2+ or Sn4+ salt with the precursor of carbon,231–236 and the other is to deposit a carbon layer on the as-prepared SnO2 nanostructures.237,238 Conventional amorphous carbon can be derived from glucose,239,240 sucrose241,242 and many organic compounds.243 The morphology and shapes of the nanostructures can be adjusted by the hydrothermal and annealing conditions.Various SnO2/C nanomaterials have been synthesized in recent years, such as carbon-coated SnO2 NPs,244–247 carbon-coated SnO2 nanorods,248,249 carbon-coated SnO2 nanowires151,217 and carbon-coated SnO2 nanotubes.57 Different structures and morphologies of SnO2 are known to lead to different electrochemical performance. In 2008, Lou et al. prepared SnO2/C composite hollow spheres. The mesoporous SnO2 hollow spheres were embedded in 3D carbon networks (Fig. 8A). The carbon networks act as a physical buffering cushion for the intrinsic large volume change and electronically conducting pathways. Compared to SnO2 hollow spheres and graphite, these SnO2/carbon hollow spheres were able to deliver a reversible lithium storage capacity of 473 mA h g−1 after 50 cycles (Fig. 8B).99 In 2009, Lou et al. also synthesized a thin layer of carbon-coated SnO2 nano-colloids (Fig. 8C) and coaxial SnO2@carbon hollow nanospheres (Fig. 8D) by a simple hydrothermal method followed by carbonization; both exhibited improved electrochemical performance.57,250 Courtel et al. reported an in situ synthesis of SnO2 nanoparticles (5–10 nm)/carbon composite materials using the polyol method by oxidizing SnCl2·2H2O in the presence of a carbon matrix. The TEM image (Fig. 8E) show that the SnO2 nanoparticles were uniformly embedded in the carbon matrix. Based on the nanostructure, the as-obtained composites showed an improved lithium storage capacity of 370 mA h g−1 at 200 mA g−1 and a lower capacity fading compared to commercial SnO2 (50 nm).251
 | ||
| Fig. 8 (A) TEM image, and (B) cycling performance of SnO2/carbon composites hollow spheres (adapted with permission from ref. 99 copyright 2008 American Chemistry Society). (C) TEM image of carbon-coated SnO2 nanocolloids obtained after carbonization at 550 °C (adapted with permission from ref. 239 copyright 2009 American Chemical Society). (D) TEM image of SnO2@carbon hollow nanospheres (adapted with permission from ref. 240 copyright 2009 Wiley). (E) TEM image of nanosized SnO2/carbon composites (adapted with permission from ref. 248 copyright 2009 Elsevier). (F) TEM image, and (G) HRETM image of SnO2/RHPC composite materials (adapted with permission from ref. 231 copyright 2019 Elsevier). (H) The fabrication schematic of SnO2@non-smooth carbon. (I) TEM image of SnO2 quasi-nanocubes@non-smooth carbon (adapted with permission from ref. 255 copyright 2019 Elsevier). | ||
While the morphology and structure of SnO2 influence the electrochemical properties of the SnO2/C composites, different carbonaceous materials also influence the performance of the composite. Xu et al. prepared composites of SnO2/ordered mesoporous carbon (SnO2/OMC) through a hydrolysis process. OMC, a novel kind of carbon material, has been widely used in LIBs due to its large surface area, high conductivity, and highly porous structure; it promotes the diffusion of lithium ions and electrolyte. The SnO2/OMC composites delivered a good cycling performance with a reversible capacity of 395.6 mA h g−1 for up to 50 cycles.252 Shi et al. synthesized SnO2/rice-husk-based porous carbon composites (RHPC) via a simple melt-impregnation method with a heat treatment route. RHPC can be easily and cheaply obtained by convenient carbonization and activation of rice husk. The TEM images (Fig. 8F and G) show that the SnO2 nanoparticles with the average size of 4 nm can be loaded on the RHPC matrix. The cycling measurements showed that the discharge capacity of the RHPC/SnO2 anode at the current density of 100 mA g−1, at the 50th cycle, was 550 mA h g−1, which demonstrated that in contrast to pure SnO2 anodes, the cycling performance of the RHPC/SnO2 anode was remarkably enhanced by the introduction of the RHPC matrix. It also demonstrates that biomass-sourced carbonaceous materials like RHPC253–255 have promising applications in LIBs due to their low cost and porous structure.233 Tian et al. fabricated non-smooth carbon-coated SnO2 quasi-nanocubes. Generally, in SnO2/C nanocomposites, nanostructured SnO2 is usually coated with smooth carbon, which is easily fabricated from organically sourced carbon via hydrothermal or CVD methods.90,256 However, it is believed that SnO2-coated 3D non-smooth carbon usually shows better lithium storage properties owing to the substantial free space and larger surface area of the 3D structure.257 In this work, Tian et al. synthesized the hybrid nanostructures via multiple hydrothermal and calcination methods (Fig. 8H). As shown by the TEM images (Fig. 8I), the porous SnO2 quasi-nanocubes were coated by a carbon layer with a rough surface. The introduction of 3D non-smooth carbon can reduce the transmission length of electrons and Li+ and increase the electrochemical reaction sites. Based on such 3D porous structures, the SnO2@C anode displayed extraordinary cycling performance and outstanding rate capability, maintaining a capacity of 1089.5 mA h g−1 at 200 mA g−1, even after 400 cycles, as well as 479.2 mA h g−1 at 3000 mA g−1.257
It is worth mentioning that even though the amorphous carbon layer can enhance the cycling stability and conductivity of the composites, it has a relatively low lithium storage capacity compared to SnO2. Therefore, the ratio of the carbon layer and the SnO2 nanomaterial determines the capacity and cycle life of the composites.
3.3 SnO2/graphene
As an important 2D carbon material, graphene has quickly gained importance in material science in recent years. Due to its excellent mechanical properties and superior conductivity, graphene has been widely studied in LIBs.258–260 Compared to CNTs and amorphous carbon, graphene exhibits higher specific discharge capacity, high surface area, good mechanical properties, and high chemical stability;261–263 thus, SnO2/graphene, as an anode material in LIBs, shows better electrochemical performance.264–268Graphene can be used as a supporting substrate for the synthesis of hierarchical SnO2 nanostructures. For example, Ding et al. fabricated 2D SnO2 nanosheets grown on a graphene oxide (GO) support via a facile hydrothermal method. The SnO2 nanosheets were uniformly embedded in the GO support, approximately 100 nm in length and 5–10 nm in thickness (Fig. 9A). This unique SnO2/GO hybrid structure exhibited enhanced lithium storage properties with high reversible capacities, i.e., an initial discharge capacity of 1666 mA h g−1, and good cycling performance, i.e., 518 mA h g−1 after 50 cycles at 400 mA h g−1.171 Additionally, due to its mechanical properties and electronic conductivity, graphene can serve as the carbon matrix to accommodate the SnO2 nanoparticles. In 2017, Shi et al. investigated a facile microwave-assisted hydrothermal method to synthesize a composite of SnO2 and graphene, which took only 30 min and did not require any chelating agents. As shown by TEM images (Fig. 9B) and the nitrogen adsorption–desorption isotherms (Fig. 9C), Shi et al. found that ultra-small SnO2 nanoparticles were well dispersed on the surface of the graphene, with an average particle size of about 3–8 nm. It also showed superior lithium storage capability. The charge/discharge capacity of this material was 969.4/978.6 mA h g−1 after 100 cycles at 200 mA g−1.269 Binder-free multilayered SnO2/graphene on Ni foam was fabricated via a dip-coating method. SnO2 nanoparticles and grapheme were alternatively coated on to the Ni foam to obtain a sandwich-like structure. As shown in SEM images in Fig. 9D and E, SnO2 nanoparticles were uniformly distributed on the surface of the Ni foam and wrapped tightly in GN. Such a multilayered nanostructure showed superior electrochemical performance due to the following factors: (1) 3D porous Ni foam serves as a conductive network and binder-free current which is beneficial for electron and ion diffusion; (2) the graphene layer improves the conductivity of anode material and buffers the SnO2 volume changes; and (3) the extent of volume change of the SnO2 nanoparticles is lower than that of bulk SnO2. Owing to such porous Ni foam frameworks and sandwich-like structures, the SnO2/graphene composites exhibited good rate performance and excellent cycling stability. High capacities, i.e., 708 and 609 mA h g−1 were achieved at current densities 1 and 2 A g−1, respectively (Fig. 9F). Furthermore, the SnO2/GN electrode delivered a high capacity of 757 mA h g−1 after 500 cycles at 1 A g−1.174
 | ||
| Fig. 9 (A) SEM image of SnO2 nanosheets/graphene (adapted with permission from ref. 170 copyright 2011 Royal Society of Chemistry). (B) TEM image and (C) nitrogen adsorption–desorption isotherms of SnO2 nanoparticles/graphene (adapted with permission from ref. 169 copyright 2017 Elsevier). (D and E) SEM images and (F) rate performance of SnO2/GN on Ni foam (adapted with permission from ref. 267 copyright 2018 Elsevier). (G) HRTEM image and (H) rate performance of SnO2NC@N-RGO (adapted with permission from ref. 173 copyright 2013 Wiley). (I) SEM images of SnO2−x/N-rGO (adapted with permission from ref. 280 copyright 2018 Royal Society of Chemistry). (J) Cycling stability of SnO2QDs@S-rGO (adapted with permission from ref. 277 copyright 2018 Elsevier). (K) Cycling performance and (L) rate performance of FTO/rGO (adapted with permission from ref. 109 copyright 2015 American Chemical Society). | ||
Moreover, it is worth mentioning that atom-doped graphene can also be used as a carbon matrix for SnO2, which effectively enhances the electrochemical performance of the composites. Heteroatoms in graphene can act as anchor sites that prevent aggregation and exfoliation of the SnO2 anchored on graphene; this helps improve the cycling stability of such anode materials.270–274 Liu et al. synthesized SnO2 nanoparticles anchored on chlorinated graphene (SnO2@rGO-Cl) as binder-free electrodes that exhibit a long cycling life, of up to 400 cycles, with a discharge capacity of 1008 mA h g−1 via a facile strategy using a one-step heat treatment at low temperature.275 Liu et al. found that Cl-doping can enhance the electrical conductivity of graphene and the Cl–Sn bonds can prevent the exfoliation of SnO2 nanoparticles during the charge/discharge process; thus, improving the electrochemical properties of SnO2-based hybrid nanomaterials. SnO2/nitrogen-doped graphene (N-rGO) was also applied as an anode in LIBs. It is believed that N-doped carbonaceous materials enhance the electronic conductivity and SEI film stability.276–278 Zhou et al. fabricated SnO2@N-rGO via a hydrazine monohydrate vapor reduction approach for anchoring SnO2 nanocrystals uniformly into N-rGO (Fig. 9G). Due to the bond formed between SnO2 and graphene, and the void pores dispersed in N-rGO, the as-prepared hybrid materials displayed superior mechanical properties and lithium storage capacity. SnO2@N-rGO anode showed a reversible charge capacity of 1346 mA h g−1 after 500 cycles; furthermore, as the current density increased from 0.5 to 1, 2, 5, 10, and 20 A g−1, their discharge capacity varied from 1074 to 994, 915, 782, 631, and 417 mA h g−1, respectively (Fig. 9H).279 Wu et al. synthesized the SnO2−x/N-rGO hybrid material through electrostatic adsorption-induced self-assembly together with a thermal reduction process. This treatment induced the generation of the oxygen vacancies on the surface of SnO2 hollow nanospheres; thus, building up a long-range and bi-continuous transfer channel for rapid electron and ion transport. Meanwhile, SnO2−x hollow spheres are well-wrapped by graphene sheets; thus, enhancing the conductivity of the anode material (Fig. 9I). Due to these structural advantages, the as-obtained SnO2−x/N-rGO electrode exhibited excellent robust cycling stability, i.e., about 912 mA h g−1 after 500 cycles at 0.5 A g−1 and 652 mA h g−1 after 200 cycles at 1 A g−1, and superior rate capability, i.e., 309 mA h g−1 at 10 A g−1.280 Sulfur-doped graphene (S-rGO) also proved to be a feasible anode material for LIBs.281 Compared to the C atom, the S atom has a larger volume and lower electronegativity, which is beneficial for the diffusion of Li+ and electrons. For instance, Wu et al. successfully loaded SnO2 quantum dots (QDs) on sulfur-doped reduced graphene oxide (S-rGO), and it exhibited excellent lithium storage with a high specific capacity of 897 mA h g−1 and a long cycling stability with 88% capacity retention after 500 cycles (Fig. 9J).282 The abovementioned results demonstrate that heteroatoms can tailor the electronic structure of carbon and create topological defects in the carbon lattice.281,283
Apart from doped-graphene, atom-doped SnO2, such as fluorine-doped tin oxide138 and antimony-doped tin oxide122 can also be used with pristine graphene as an anode material.284–287 For example, Xu et al. has successfully fabricated composites of fluorine-doped tin oxide (FTO) and rGO from a colloidal solution containing FTO nanocrystals and rGO by a hydrothermal treatment; the FTO nanocrystals were tightly embedded in the RGO nanosheets. As an anode material, the FTO/RGO composite showed high structural stability during the lithiation and delithiation processes. The conductive FTO nanocrystals help form stable and thin SEI films. Moreover, the FTO/RGO composite retains a discharge capacity as high as 1439 mA h g−1 after 200 cycles at 100 mA g−1 (Fig. 9J), and 1148 mA h g−1 at 1000 mA g−1 (Fig. 9K).109
3.4 Complex SnO2/C nanostructure
Compared to 1D or 2D carbonaceous materials, complex 3D carbonaceous matrices usually show better electrochemical properties due to their high specific surface area and porous structure, which is beneficial in suppressing volume changes.288 In recent years, various SnO2/C complex hybrid nanomaterials have been reported, such as the combination of CNTs and amorphous carbon,288 and CNTs and graphene.283,289,290 These complex hierarchical SnO2/C nanostructures often show superior electrochemical properties, making them an important research subject.A sandwich structure with carbon nanofiber, SnO2, and nanofiber bundle for carbon-coating (C@SnO2@C) has been fabricated by using collagen fiber (CF), which is a typical fibrous protein extracted from cattle skin and is used as a bio-template as well as the carbon source. FESEM image (Fig. 10A) and TEM image (Fig. 10B) show that the average diameter of the SnO2 nanofiber bundle was about 5–10 μm and a layer of SnO2 was sandwiched between the carbon nanofiber and the carbon coating layer. Such hierarchical architectures of the C@SnO2@C nanofiber bundle guaranteed a good balance between electron transport and Li+ diffusion kinetics. Thus, efficient ambipolar diffusion and reduced volume changes of SnO2 were obtained to ensure structural integrity with high cycling stability.162
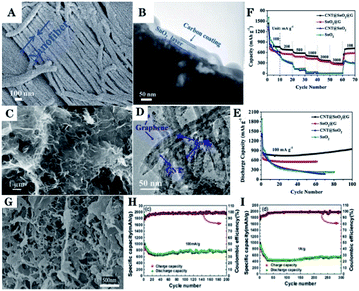 | ||
| Fig. 10 (A) FESEM image, and (B) TEM image of C@SnO2@C nanofiber bundle (adapted with permission from ref. 162 copyright 2016 Royal Society of Chemistry). (C) FESEM image, (D) TEM image, (E) cycling performance, and (F) rate performance of CNT@SnO2@G (adapted with permission from ref. 263 copyright 2017 Elsevier). (G) SEM image, (H) cycling performance, and (I) rate performance of SnO2@G-SWCNT materials (adapted with permission from ref. 289 copyright 2017 American Chemical Society). | ||
A porous 3D core–shell structured CNT@SnO2 composite, with a graphene coating (CNT@SnO2@G), has been synthesized via a two-step hydrothermal method. The first step consists of the synthesis of CNT@SnO2, and then, CNT@SnO2@G is formed in the subsequent step. FESEM image (Fig. 10C) and TEM image (Fig. 10D) show that CNT@SnO2 (about 20–40 nm in width) particles was distributed across the graphene sheets and was encased in the graphene coating, which suppressed the formation of SEI layers on the surface of the CNT@SnO2@G. The as-prepared CNT@SnO2@G electrode exhibited outstanding lithium storage capability, including a large specific capacity, remarkable cycling stability, and excellent rate capability (Fig. 10E and F).265
SnO2 nanoparticles anchored on an aerogel based on 3D graphene-single walled carbon nanotube (SnO2@G-SWCNT), as shown in Fig. 10G, has been fabricated by a hydrothermal self-assembly process.291 The 3D G-SWCNT matrix provides a flexible conductive matrix and a more porous network to support SnO2. This is beneficial for facilitating electronic and ionic transportation and mitigating the volume changes of the SnO2 during lithiation/delithiation; thus, leading to enhanced electrochemical performance of the SnO2 anodes for LIBs. The discharge capacity remained 758 mA h g−1 at 100 mA g−1 after 200 cycles (Fig. 10H) and 537 mA h g−1 at 1 A g−1 after 300 cycles (Fig. 10I).291
4. SnO2/TMOs/C
The composites of SnO2 and transition metal oxides (TMOs) have been considered as promising anode materials for LIBs.292–294 By the introduction of metal oxides, the reversible decomposition of Li2O can be increased because TMOs can convert extra Li2O into Li+;295–297 thus, enhancing their cycling stability, rate performance, and rate capability. However, the electrochemical performance of SnO2/TMOs is unsatisfactory because of low conductivity and large volume expansion, leading to poor cycling stability.136 In order to overcome these problems, much work has been focused on the fabrication of SnO2/TMOs/C materials in recent years. Supported and coated by carbonaceous material and TMOs, the structural stability and conductivity of SnO2 can be significantly improved. This section will cover synthesis methods, morphology, and electrochemical performance of SnO2/TMOs/C.298–3024.1 SnO2/Fe2O3/C
Fe2O3 has been considered as another promising anode material for LIBs due to its large theoretical capacity (1007 mA h g−1) and low cost.303,304 However, the short carrier diffusion length inhibits lithiation/delithiation processes. It has been reported that SnO2/Fe2O3 showed an improvement in photocatalysis, energy storage, and gas sensing.303,305–307 Furthermore, the SnO2/Fe2O3/C electrode showed better lithium storage capability.308 In this composite, SnO2 possesses a high intrinsic conductivity and a shortened charge diffusion distance, while Fe2O3 facilitates the reversible decomposition of Li2O and prevents Sn aggregation during charging and discharging.309In 2014, Wu et al. proposed a facile hydrothermal method to synthesize a ternary phased SnO2/Fe2O3/SWCNTs composite. As shown in Fig. 11A, the composites of SnO2 and Fe2O3 nanoparticles were well distributed and firmly anchored on to SWCNTs, which serve as a buffer and conductive matrix. Nanosized Fe2O3/SnO2 composites can suppress the effect of volume changes and particle agglomeration. The SnO2/Fe2O3/SWCNTs electrode showed a high reversible capacity, superior cycle performance, and high rate capability. It delivered a capacity of 692 mA h g−1 at 200 mA g−1 after 50 cycles. Even at a rate as high as 2000 mA g−1, this composite could still maintain its capacity at 656 mA h g−1 (Fig. 11B).310
 | ||
| Fig. 11 (A) TEM image, and (B) rate performance of SnO2/Fe2O3/SWCNTs (adapted with permission from ref. 308 copyright 2014 Elsevier). (C) TEM images, and (D) schematic illustration of the synthesis of γ-Fe2O3@SnO2@C core–shell nanorods (adapted with permission from ref. 115 copyright 2013 Royal Society of Chemistry). | ||
The growth of SnO2 on Fe2O3 and subsequent carbon coating on SnO2/Fe2O3 is another common synthesis route for SnO2/Fe2O3/C. Du et al. synthesized γ-Fe2O3@SnO2@C porous core–shell nanorods. They first formed FeOOH nanorods via a hydrothermal process, which served as a template for the subsequent SnO2 deposition process in another hydrothermal system. After the deposition of SnO2 on the FeOOH nanorods, the as-synthesized FeOOH@SnO2 nanorods were coated with a carbon layer via another hydrothermal process and carbonized under N2 at 500 °C for 2 h (Fig. 11C). The TEM image (Fig. 11D) shows that SnO2 can successfully grow on the Fe2O3 nanostructure with well-defined interfaces. The thickness of the SnO2 layer is 5–10 nm, which is composed of SnO2 nanoparticles with a diameter of about 3–5 nm, and the core nanorod is highly porous. Such a porous core–shell hybrid nanorod-based electrode showed good cycling and rate performance due to the improvement of conductivity and structural stability by the introduction of carbon and Fe2O3.115
4.2 SnO2/Co3O4/C
Many studies have concentrated on improving electrochemical properties of SnO2 and Co3O4 by designing and synthesizing unique nanostructures. Like most transition metals, the presence of Co can improve the reversibility of the reduction reaction of Li2O and further enhance the reversible capacity.311–315 As shown in Fig. 12A, Co3O4@SnO2@C core–shell nanorods have been synthesized via a hydrothermal method followed by carbonization. Fig. 12B and C show that SnO2 nanoparticles uniformly coated the surface of Co3O4 nanorods and that the coating was smooth and uniform, with a thickness of 5–10 nm. Such materials exhibited improved cycling performance and higher specific capability as an anode material for LIBs (860 mA h g−1 after 50 cycles at 0.2 A g−1). This result demonstrated that the combination of SnO2 and Co3O4, into an integrated core–shell nanorod structure, exhibited a better and more elegant synergistic effect during the charge/discharge processes.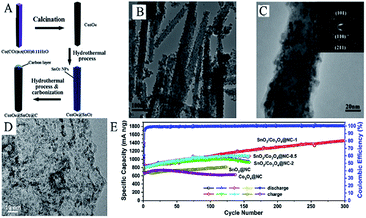 | ||
| Fig. 12 (A) Schematic illustration of the fabrication of Co3O4@SnO2@C core–shell nanorods. (B) TEM image, and (C) HRTEM images of Co3O4@SnO2@C core–shell nanorods (adapted with permission from ref. 313 copyright 2012 Royal Society of Chemistry). (D) HRTEM image and, (E) cycling performance of SnO2/Co3O4@NC-1 nanoflakes (adapted with permission from ref. 119 copyright 2019 Elsevier). | ||
SnO2/Co3O4@N-doped carbon (NC) usually shows better lithium storage properties compared to most SnO2/Co3O4/C materials.316 Wang et al. successfully designed and fabricated SnO2/Co3O4/NC nanoflakes via a combined strategy of CVD and template synthesis.119,317 HRTEM images (Fig. 12D) show that SnO2/Co3O4 nanoparticles were uniformly distributed on the NC nanoflakes. By adjusting the ratio of the precursor and reaction conditions, they found that the SnO2/Co3O4@NC (RSn/Co = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanoflakes-based electrode demonstrated excellent lithium storage capability, i.e., a discharge capacity of 1450.3 mA h g−1 after 300 cycles at 200 mA h g−1 (Fig. 12E). The superior lithium storage of such materials may result from the synergistic effect between the combination of SnO2 and Co3O4 and better conductivity caused by the N-doped carbon matrix.119
1) nanoflakes-based electrode demonstrated excellent lithium storage capability, i.e., a discharge capacity of 1450.3 mA h g−1 after 300 cycles at 200 mA h g−1 (Fig. 12E). The superior lithium storage of such materials may result from the synergistic effect between the combination of SnO2 and Co3O4 and better conductivity caused by the N-doped carbon matrix.119
Therefore, in SnO2/Co3O4/C composites, SnO2 and Co3O4 display a synergistic enhancement effect. It can provide additional active sites for lithium storage and shorten the lithium diffusion distance. Additionally, the carbon layer can greatly improve the electrode conductivity and restrain volume changes during the lithiation/delithiation processes.
4.3 SnO2/TiO2/C
SnO2, as one of the most extensively investigated anode material, which offers a high specific capacity. However, poor cycling stability, due to large volume expansion and subsequent pulverization during Li+ insertion/extraction processes, greatly restricts its application as an anode material.130,318–320 In contrast, TiO2 exhibits negligible volume change (less than 4%) and stable electrochemical properties, but its application is limited due to its low specific capacity.321–325 Furthermore, SnO2 and TiO2 have complementary characteristics in LIBs, i.e., Sn4+ and Ti4+ possess similar radii and the lattice matching of SnO2 and TiO2 is good.326–328 Considering the facts mentioned above, in order to promote cycling stability of SnO2, various SnO2/TiO2 based hybrid materials have been synthesized.329–334Mesoporous SnO2@C@TiO2 nanochains have been synthesized by first fabricating SnO2@C core–shell nanochains via a hydrothermal method and subsequent continuous mechanical stirring of the solution of SnO2@C and tetrabutyl titanate (C16H36O4Ti). It is noticeable that the SnO2 core is composed of SnO2 nanoparticles with a diameter of 2–6 nm and is coated by a thin carbon layer (2–6 nm) as well as a TiO2 layer (about 8 nm) (Fig. 13A). In this 3D hierarchical structure, the carbon layer and the TiO2 layer can effectively improve cycling stability and discharge capacity, i.e., the initial discharge capacity of 807 mA h g−1 and 369 mA h g−1 after 100 cycles at 100 mA h g−1.298
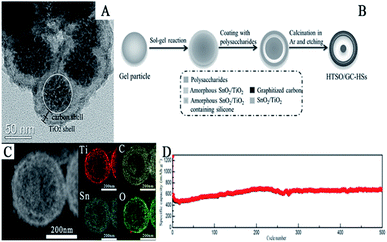 | ||
| Fig. 13 (A) TEM images of SnO2@C@TiO2 nanochains (adapted with permission from ref. 296 copyright 2015 Elsevier). (B) Schematic illustration of the synthesis, (C) STEM image, and (D) cycling performance of HTSO/GC-HSs (adapted with permission from ref. 299 copyright 2017 Wiley). | ||
Xie et al. reported the synthesis of hierarchical TiO2/SnO2 hollow spheres coated with graphitized carbon (HTSO/GC-HSs) by a multi-step approach. As shown in Fig. 13B, titanate-silicone gel particles were first reacted with SnCl2 via a sol–gel process to obtain core–shell hybrid structures, which were further coated with a polysaccharide via a hydrothermal process and subsequent carbonization in Ar atmosphere. As shown in Fig. 13C, the as-prepared mesoporous HTSO/GC-HSs had a yolk–shell structure and elements Sn, Ti, O and C were found to be uniformly distributed in this hierarchical hollow nanostructure. Additionally, they also found that due to the uniform distribution of SnO2, TiO2, and the carbon layer, a solid solution was formed which could effectively suppress the effect of volume changes during the charge/discharge process. The specific discharge capacity remained at about 680 mA h g−1 at 1 A g−1 after 500 cycles, which demonstrates the excellent cycling performance of HTSO/GC-HSs at high current densities (Fig. 13D).301
SnO2/TiO2/C combines the high capacity of SnO2 with the long cycle life and high rate capability of TiO2.335,336 Furthermore, carbonaceous materials can inhibit agglomeration and pulverization of SnO2 and enhance the conductivity of TiO2.337,338 Therefore, SnO2/TiO2/C present an important area of research for the future.
4.4 SnO2/TMOs/graphene
In the previous sections, we have discussed the synthesis methods, morphologies, and electrochemical performance of some representative SnO2/TMOs/C anode materials. We classified them into different categories according to metal oxides because different TMOs greatly influence the physical and chemical properties of such complex materials due to different reaction mechanisms. In addition, different carbonaceous materials used in SnO2/TMOs/C also influence the electrode performance in LIBs. Among these complex materials, much work has been focused on the fabrication of SnO2/TMOs/graphene materials in recent years. Compared to amorphous carbon and CNTs, graphene shows higher lithium storage capacity.339–342 Additionally, graphene, as a supportive matrix and conductive network, displays excellent mechanical properties and electronic conductivity, which is conducive to improvement in cycling stability and rate performance of SnO2. Therefore, effectively combining SnO2, TMOs, and graphene, to synthesize high-performance and practical anode material, has been a popular research subject in recent years.46,275,343–345Wang et al. synthesized a Fe3O4/SnO2/rGO (FSG) composite via a facile hydrothermal method. SEM images (Fig. 14A) and elemental mapping show that FSG consists of SnO2 and Fe3O4 nanoparticles with diameters of about 10–100 nm, uniformly distributed on the surface of the rGO. In this composite, rGO served as a conductive and robust matrix to prevent aggregation of Fe3O4 and SnO2 nanoparticles on rGO. As shown in Fig. 14B, the nanocrystallites of Fe3O4 and SnO2 tend to link with each other, which is beneficial for suppressing both, i.e., the formation of the SEI film and the volume changes. Such a novel nanostructure can effectively shorten the transport path of Li+ and electrons and helps improve electrolyte penetration. The FSG nanocomposite exhibited a reversible capacity of 947 mA h g−1 at a current density of 200 mA g−1 in the first cycle and maintained a capacity of 831 mA h g−1 after 200 cycles (Fig. 14C).132
 | ||
| Fig. 14 (A) SEM image, (B) HRTEM image, and (C) cycling performance of FSG (adapted with permission from ref. 132 copyright 2016 Elsevier). (D) SEM image, (E) TEM image from top and side view of CNT/S, and (F) cycling performance of FNT/S/RGO (adapted with permission from ref. 136 copyright 2017 Elsevier). | ||
Lee et al. have synthesized hollow nanostructured α-Fe2O3 nanotubes/SnO2/rGO (FNT/S/G) via a microwave-assisted hydrothermal method. As shown in Fig. 14D, SnO2 nanorods grow on the surface of FNT, and FNT/SnO2 are uniformly anchored on rGO sheets. The diameter and length (Fig. 14E) of FNT/S are about 230 nm and 660 nm, respectively. Lee et al. also compared the electrochemical performance of FNT, FNT/S, and FNT/S/G. As shown in Fig. 14F, FNT/S/G exhibited the highest discharge capacity under all tested current densities. FNT/S was the second best, demonstrating that the introduction of SnO2 and rGO can effectively enhance the lithium storage capability and cycling stability of the anode material. The specific discharge capacity of FNT/S/RGO remained at 629 mA h g−1 at 1 A g−1 after 1000 cycles.136,346
Other SnO2/TMOs/graphene composites like SnO2/TiO2/GN,338 SnO2/CuO/GN347 and SnO2/In2O3/GN348 also exhibit improved electrochemical performance. The introduction of TMOs and graphene effectively improve the lithium storage capacity of SnO2. Graphene serves as a supportive and conductive matrix that inhibits agglomeration and pulverization of SnO2 and TMOs during the charge/discharge process.
5. Other SnO2-based compounds
Li4Ti5O12 (LTO) has been considered as a promising anode material for LIBs due to its excellent cycling stability during lithiation/delithiation.349,350 However, like TiO2, LTO possesses a lower theoretical capacity than other anode materials (175 mA h g−1) and shows poor rate performance.351,352 Therefore, in order to improve lithium storage of LTO and cycling performance of SnO2, an effective strategy is to synthesize composites of SnO2 and LTO nanostructures. Ding et al. synthesized hierarchical yolk–shell LTO–SnO2 structures via a two-step hydrothermal method. They first synthesized SnO2 microspheres at 180 °C for 12 h in a hydrothermal system and then, coated LTO on the surface of the SnO2 in another hydrothermal system, followed by calcination at 600 °C for 3 h. SEM images (Fig. 15A) and TEM images (Fig. 15B) clearly show that the yolks are SnO2 microspheres composed of SnO2 nanoparticles, while the shells consist of LTO nanosheets. Hence, this hierarchical coating structure can effectively improve structural stability and suppress the volume changes of SnO2 microspheres; thus, improving the cycling performance and showing high rate capacity.142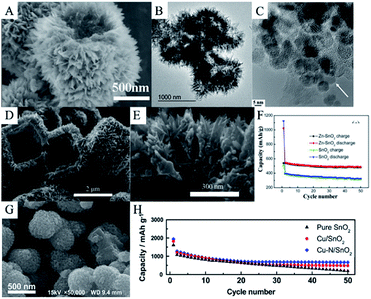 | ||
| Fig. 15 5(A) SEM image, and (B) TEM image of LTO–SnO2 composites (adapted with permission from ref. 142 copyright 2018 Elsevier). (C) HRTEM image of carbon-coated Fe-doped SnO2 nanoparticles (adapted with permission from ref. 351 copyright 2015 Elsevier). (D and E) SEM images, and (F) cycling performance of Zn-doped SnO2 nanomaterials (adapted with permission from ref. 355 copyright 2014 Elsevier). (G) SEM image, and (H) cycling performance of Cu/N-doped SnO2 (adapted with permission from ref. 139 copyright 2014 Elsevier). | ||
Heteroatom-doped SnO2 reveals better electrochemical performance compared to pure SnO2. The presence of a dopant may favor the reversible formation of Li2O and improve the conductivity of SnO2; thus, enhancing the specific capacity of the anode material.353 Various heteroatom-doped SnO2 nanomaterials have been synthesized and tested as anode materials in LIBs.354–356 For example, Mueller et al. have synthesized carbon-coated Fe-doped SnO2 nanoparticles (Fig. 15C). The discharge capacity of such materials is about 1726 mA h g−1 after 10 cycles at 50 mA g−1, which is around twice the theoretical capacity of pure SnO2.353 Zn-doped SnO2 hierarchical cube-like nanomaterials were fabricated via hydrothermal method. As shown in Fig. 15D and E, the cube is about 2 μm in length and composed of assembled nanorods which are 20–40 nm in diameter. The discharge capacity of Zn-doped SnO2 composites is 488.3 mA h g−1 after 50 cycles at 10 mA g−1 (Fig. 15F).357 Wan et al. fabricated Cu/N-doped SnO2 nanocomposites, as shown in Fig. 15G. They found that with the introduction of Cu/N, the average diameter of SnO2 became smaller, and the surface became rough, which is beneficial for ion diffusion and suppressing volume expansion. Cu/N-doped SnO2 electrode materials are known to deliver a discharge capacity of 1939 mA h g−1 in the first cycle and remain at 664 mA h g−1 after 50 cycles at 0.1C.139
6. Conclusions
In this review, we summarized various SnO2-based nanomaterials as anode materials for lithium-ion batteries (Table 1). By optimizing the structure and composition of the materials, i.e., by synthesizing nanostructured SnO2 and making composites with carbonaceous materials or transition metal oxides (TMOs), the surface area and reaction sites of the anode materials can be effectively increased, improving the electrochemical properties of SnO2-based anode materials. The introduction of carbonaceous materials or TMOs can effectively buffer the internal stresses caused by large volume changes, reduce the irreversible capacity loss, and improve the cycle performance. These aspects lay the foundation for the development and commercialization of high-performance LIBs in the future. Since lithium storage in SnO2 is accompanied by repeatedly inserting and removing lithium ions, volume changes and irreversible capacity loss of SnO2 electrode cannot be completely avoided. Significant research on nanosized SnO2-based anode materials has been carried out, and while these materials display superior lithium storage capacity and cycling stability, critical issues like volume expansion and pulverization of SnO2 remain to be solved. Further extensive research is needed before new, non-carbonaceous anode materials, such as SnO2-based nanomaterials, can be commercialized.| Materials | Morphology | Preparation approach | Voltage window (V) | Current density (A g−1) | Cycle number | Specific capacity (mA h g−1) | Reference | |
|---|---|---|---|---|---|---|---|---|
| SnO2 nanorods | Diameter of 60 nm and length of 670 nm | Hydrothermal | 0.005–2.5 | 0.078 | 100 | 580 | 154 | |
| SnO2 nanotube arrays | Diameter of 100–300 nm and thickness of 10–20 nm | Solvothermal and annealing | 0.005–2 | 0.078 | 20 | 750–800 | 64 | |
| SnO2 nanowires | Diameter of 6 nm and length of >3 μm | Solvothermal and annealing | 0–1.2 | 0.156 | 50 | 773 | 71 | |
| SnO2 nanosheet arrays | Thickness of 20 nm and length of 500 nm | Hydrothermal | 0.01–1.2 | 0.391 | 50 | 674.9 | 79 | |
| Hollow SnO2 nanospheres | Size of 100–250 nm | Solvothermal and hydrothermal | 0.01–2 | 0.391 | 40 | 1140 | 86 | |
| Urchin-like SnO2 nanospheres | Diameter of 300 nm | Solvothermal | 0.01–3 | 0.1 | 50 | 719 | 210 | |
| SnO2 nanocrystals/MWCNT composites | Crystal size of 5 nm | Solvothermal and heat treatment | 0.01–3 | 0.1 | 100 | 402 | 223 | |
| CNT@void@SnO2@C | Tube in tube diameter of 100–150 nm | Spray drying | 0.01–3 | 0.2 | 350 | 702.5 | 226 | |
| SnO2/C composite hollow spheres | Size of 150–400 nm | Hydrothermal and carbonation | 0–2.5 | 0.16 | 50 | 473 | 99 | |
| SnO2 nanoparticles/carbon composite | Nanoparticles of 5–10 nm | Polyol method | 0.005–1.5 | 0.2 | 100 | 370 | 251 | |
| SnO2 nanosheets/GO | Length of 100 nm and thickness of 5–10 nm | Hydrothermal | 0.01–1.2 | 0.4 | 50 | 518 | 171 | |
| SnO2 nanoparticles/graphene | Particle size of 3–8 nm | Microwave-assisted hydrothermal | 0.01–3 | 0.2 | 100 | 978.6 | 269 | |
| SnO2@rGO-Cl | Nanoparticle size of 5 nm | Heat treatment | 0.01–3 | 0.2 | 400 | 1008 | 275 | |
| CNT@SnO2@G | Width of 20–40 nm | Hydrothermal | 0.01–3 | 0.1 | 100 | 947 | 265 | |
| SnO2@G-SWCNT | Diameter of 3–5 nm and particle size of 6–8 nm | Hydrothermal | 0.01–3 | 1 | 300 | 537 | 291 | |
| SnO2–Fe2O3/SWCNTs nanocomposite | Particle size of 10–50 nm | Hydrothermal | 0.01–3 | 0.2 | 50 | 692 | 310 | |
| Co3O4@SnO2@C core–shell nanorods | Thickness of 5–10 nm | Hydrothermal | 0.01–2.5 | 0.2 | 50 | 860 | 313 | |
| SnO2@C@TiO2 nanochains | SnO2 for 2–6 nm; thickness of 2–6 nm and 8 nm for carbon and TiO2 | Hydrothermal and subsequent mechanical stirring | 0.01–3 | 0.1 | 100 | 369 | 298 | |
| Fe2O3 nanotubes/SnO2/rGO | Diameter of 230 nm and length of 660 nm | Microwave-assisted hydrothermal | 0.01–3 | 1 | 1000 | 629 | 136 | |
| Yolk–shell LTO–SnO2 | Diameter of 1.0–1.5 μm | Hydrothermal and calcination | 0.01–3 | 0.175 | 200 | 253.2 | 142 | |
| Fe-doped SnO2 nanoparticles | Diameter of 15 nm | Hydrothermal and calcination | 0.01–2 | 0.05 | 10 | 1726 | 351 | |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Key Research and Development Project (Grant No. 2020YFB1506001), Major Project of Education Department in Sichuan (Grant No. 18ZB0229), Sichuan Province Science and Technology Support Program (Grant No. 18ZDYF2973).References
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS.
- N. Nitta, F. Wu, J. T. Lee and G. Yushin, Mater. Today, 2015, 18, 252–264 CrossRef CAS.
- M. V. Reddy, G. V. S. Rao and B. V. R. Chowdari, Chem. Rev., 2013, 113, 5364–5457 CrossRef CAS.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243–3262 RSC.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28–E62 CrossRef CAS.
- M.-S. Kim, B. Fang, J. H. Kim, D. Yang, Y. K. Kim, T.-S. Bae and J.-S. Yu, J. Mater. Chem., 2011, 21, 19362–19367 RSC.
- W. Li, L. Zhao, Y. Tian, Y. Gong, X. Huang, Z. Cui and R. Zeng, Electrochim. Acta, 2014, 147, 603–609 CrossRef CAS.
- J. Cabana, L. Monconduit, D. Larcher and M. Rosa Palacin, Adv. Mater., 2010, 22, E170–E192 CrossRef CAS.
- A. Magasinski, P. Dixon, B. Hertzberg, A. Kvit, J. Ayala and G. Yushin, Nat. Mater., 2010, 9, 353–358 CrossRef CAS.
- B. Scrosati, J. Hassoun and Y.-K. Sun, Energy Environ. Sci., 2011, 4, 3287–3295 RSC.
- B. Fang, M.-S. Kim, J. H. Kim, S. Lim and J.-S. Yu, J. Mater. Chem., 2010, 20, 10253–10259 RSC.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587–603 CrossRef CAS.
- J. Jiang, Y. Li, J. Liu, X. Huang, C. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166–5180 CrossRef CAS.
- K. Chang and W. Chen, ACS Nano, 2011, 5, 4720–4728 CrossRef CAS.
- Y. Sun, Q. Wu and G. Shi, Energy Environ. Sci., 2011, 4, 1113–1132 RSC.
- Z.-S. Wu, G. Zhou, L.-C. Yin, W. Ren, F. Li and H.-M. Cheng, Nano Energy, 2012, 1, 107–131 CrossRef CAS.
- C. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488–1504 CrossRef CAS.
- N. Liu, Z. Lu, J. Zhao, M. T. McDowell, H.-W. Lee, W. Zhao and Y. Cui, Nat. Nanotechnol., 2014, 9, 187–192 CrossRef CAS.
- J. B. Goodenough and K.-S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS.
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J.-M. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS.
- Y. Deng, C. Fang and G. Chen, J. Power Sources, 2016, 304, 81–101 CrossRef CAS.
- L. Lu, X. Han, J. Li, J. Hua and M. Ouyang, J. Power Sources, 2013, 226, 272–288 CrossRef CAS.
- J. W. Choi and D. Aurbach, Nat. Rev. Mater., 2016, 1, 16013 CrossRef CAS.
- W.-B. Hua, X.-D. Guo, Z. Zheng, Y.-J. Wang, B.-H. Zhong, B. Fang, J.-Z. Wang, S.-L. Chou and H. Liu, J. Power Sources, 2015, 275, 200–206 CrossRef CAS.
- Y. Xing, S. Wang, B. Fang, Y. Feng and S. Zhang, Microporous Mesoporous Mater., 2018, 261, 237–243 CrossRef CAS.
- W. Hua, Y. Wang, Y. Zhong, G. Wang, B. Zhong, B. Fang, X. Guo, S. Liao and H. Wang, Chin. J. Chem., 2015, 33, 261–267 CrossRef CAS.
- F. Cheng, Y. Chen, A. Sun, X. Zhou, J. Yang and J. Tang, Ceram. Int., 2019, 45, 13556–13560 CrossRef CAS.
- J. Jia, X. Hu and Z. Wen, Nano Res., 2018, 11, 1135–1145 CrossRef CAS.
- T. Wang, H. Li, S. Shi, T. Liu, G. Yang, Y. Chao and F. Yin, Small, 2017, 13, 1604182 CrossRef.
- G. Xu, L. Zhang, C. Guo, L. Gu, X. Wang, P. Han, K. Zhang, C. Zhang and G. Cui, Electrochim. Acta, 2012, 85, 345–351 CrossRef CAS.
- H. Xia, M. Lai and L. Lu, J. Mater. Chem., 2010, 20, 6896–6902 RSC.
- A. L. M. Reddy, M. M. Shaijumon, S. R. Gowda and P. M. Ajayan, Nano Lett., 2009, 9, 1002–1006 CrossRef CAS.
- J. Zhu and J. He, ACS Appl. Mater. Interfaces, 2012, 4, 1770–1776 CrossRef CAS.
- H. Xu, X. Hu, H. Yang, Y. Sun, C. Hu and Y. Huang, Adv. Energy Mater., 2015, 5, 1401882 CrossRef.
- B. Sun, Z. Chen, H.-S. Kim, H. Ahn and G. Wang, J. Power Sources, 2011, 196, 3346–3349 CrossRef CAS.
- D. Kong, J. Luo, Y. Wang, W. Ren, T. Yu, Y. Luo, Y. Yang and C. Cheng, Adv. Funct. Mater., 2014, 24, 3815–3826 CrossRef CAS.
- S. Zhu, J. Li, X. Deng, C. He, E. Liu, F. He, C. Shi and N. Zhao, Adv. Funct. Mater., 2017, 27, 1605017 CrossRef.
- H. Wang, L.-F. Cui, Y. Yang, H. S. Casalongue, J. T. Robinson, Y. Liang, Y. Cui and H. Dai, J. Am. Chem. Soc., 2010, 132, 13978–13980 CrossRef CAS.
- J. Gao, M. A. Lowe and H. D. Abruna, Chem. Mater., 2011, 23, 3223–3227 CrossRef CAS.
- Z. Cai, L. Xu, M. Yan, C. Han, L. He, K. M. Hercule, C. Niu, Z. Yuan, W. Xu, L. Qu, K. Zhao and L. Mai, Nano Lett., 2015, 15, 738–744 CrossRef CAS.
- X. Zhu, Y. Zhu, S. Murali, M. D. Stollers and R. S. Ruoff, ACS Nano, 2011, 5, 3333–3338 CrossRef CAS.
- L. Zhang, H. B. Wu, S. Madhavi, H. H. Hng and X. W. Lou, J. Am. Chem. Soc., 2012, 134, 17388–17391 CrossRef CAS.
- X. Xu, R. Cao, S. Jeong and J. Cho, Nano Lett., 2012, 12, 4988–4991 CrossRef CAS.
- Z. Wang, D. Luan, S. Madhavi, Y. Hu and X. W. Lou, Energy Environ. Sci., 2012, 5, 5252–5256 RSC.
- M. V. Reddy, T. Yu, C.-H. Sow, Z. X. Shen, C. T. Lim, G. V. S. Rao and B. V. R. Chowdari, Adv. Funct. Mater., 2007, 17, 2792–2799 CrossRef CAS.
- G. Zhou, D.-W. Wang, F. Li, L. Zhang, N. Li, Z.-S. Wu, L. Wen, G. Q. Lu and H.-M. Cheng, Chem. Mater., 2010, 22, 5306–5313 CrossRef CAS.
- W.-M. Zhang, X.-L. Wu, J.-S. Hu, Y.-G. Guo and L.-J. Wan, Adv. Funct. Mater., 2008, 18, 3941–3946 CrossRef CAS.
- W. Wei, S. Yang, H. Zhou, I. Lieberwirth, X. Feng and K. Muellen, Adv. Mater., 2013, 25, 2909–2914 CrossRef CAS.
- J. Luo, J. Liu, Z. Zeng, C. F. Ng, L. Ma, H. Zhang, J. Lin, Z. Shen and H. J. Fan, Nano Lett., 2013, 13, 6136–6143 CrossRef CAS.
- C. He, S. Wu, N. Zhao, C. Shi, E. Liu and J. Li, ACS Nano, 2013, 7, 4459–4469 CrossRef CAS.
- X. Wang, X.-L. Wu, Y.-G. Guo, Y. Zhong, X. Cao, Y. Ma and J. Yao, Adv. Funct. Mater., 2010, 20, 1680–1686 CrossRef CAS.
- X. W. Lou, D. Deng, J. Y. Lee, J. Feng and L. A. Archer, Adv. Mater., 2008, 20, 258–262 CrossRef CAS.
- Y. Li, B. Tan and Y. Wu, Nano Lett., 2008, 8, 265–270 CrossRef CAS.
- W. Y. Li, L. N. Xu and J. Chen, Adv. Funct. Mater., 2005, 15, 851–857 CrossRef CAS.
- W. M. Zhang, J. S. Hu, Y. G. Guo, S. F. Zheng, L. S. Zhong, W. G. Song and L. J. Wan, Adv. Mater., 2008, 20, 1160–1165 CrossRef CAS.
- X. W. Lou, Y. Wang, C. L. Yuan, J. Y. Lee and L. A. Archer, Adv. Mater., 2006, 18, 2325–2329 CrossRef CAS.
- X. W. Lou, C. M. Li and L. A. Archer, Adv. Mater., 2009, 21, 2536–2539 CrossRef CAS.
- C. Guo, M. Cao and C. Hu, Inorg. Chem. Commun., 2004, 7, 929–931 CrossRef CAS.
- B. Liu, J.-G. Zhang and W. Xu, Joule, 2018, 2, 833–845 CrossRef CAS.
- I. A. Courtney, J. Electrochem. Soc., 1997, 144, 2045 CrossRef CAS.
- X. Zhou, L.-J. Wan and Y.-G. Guo, Adv. Mater., 2013, 25, 2152–2157 CrossRef CAS.
- H. Kim, G. O. Park, Y. Kim, S. Muhammad, J. Yoo, M. Balasubramanian, Y.-H. Cho, M.-G. Kim, B. Lee, K. Kang, H. Kim, J. M. Kim and W.-S. Yoon, Chem. Mater., 2014, 26, 6361–6370 CrossRef CAS.
- M.-S. Park, G.-X. Wang, Y.-M. Kang, D. Wexler, S.-X. Dou and H.-K. Liu, Angew. Chem., Int. Ed., 2007, 46, 750–753 CrossRef CAS.
- J. Wang, N. Du, H. Zhang, J. Yu and D. Yang, J. Phys. Chem. C, 2011, 115, 11302–11305 CrossRef CAS.
- Y. Wang, M. Wu, Z. Jiao and J. Y. Lee, Nanotechnology, 2009, 20, 345704 CrossRef.
- M. Lai, J. A. Gonzalez Martinez, M. Grätzel and D. J. Riley, J. Mater. Chem., 2006, 16, 2843–2845 RSC.
- N. Du, H. Zhang, B. Chen, X. Ma and D. Yang, Chem. Commun., 2008, 3028–3030 RSC.
- Y. Wang, J. Y. Lee and H. C. Zeng, Chem. Mater., 2005, 17, 3899–3903 CrossRef CAS.
- S. J. Ding, Z. Y. Wang, S. Madhavi and X. W. Lou, J. Mater. Chem., 2011, 21, 13860–13864 RSC.
- Y. T. Han, X. Wu, Y. L. Ma, L. H. Gong, F. Y. Qu and H. J. Fan, CrystEngComm, 2011, 13, 3506–3510 RSC.
- H. Kim and J. Cho, J. Mater. Chem., 2008, 18, 771–775 RSC.
- Y. D. Ko, J. G. Kang, J. G. Park, S. Lee and D. W. Kim, Nanotechnology, 2009, 20, 455701 CrossRef.
- W. Ren, C. Wang, L. Lu, D. Li, C. Cheng and J. Liu, J. Mater. Chem. A, 2013, 1, 13433–13438 RSC.
- Q. Wan, E. N. Dattoli and W. Lu, Appl. Phys. Lett., 2007, 90, 222107 CrossRef.
- Q. Tian, L. Li, J. Chen, L. Yang and S.-i. Hirano, J. Power Sources, 2018, 376, 1–10 CrossRef CAS.
- X. Zhu, H. Shi, J. Yin, H. Zhu, Y. Zhou, Y. Tang, P. Wu and T. Lu, RSC Adv., 2014, 4, 34417–34420 RSC.
- L. Zhang, H. B. Wu and X. Wen Lou, Mater. Horiz., 2014, 1, 133–138 RSC.
- S. Zhang, B. Yin, Y. Jiao, Y. Liu, F. Qu and X. Wu, Appl. Surf. Sci., 2014, 305, 626–629 CrossRef CAS.
- X. Zhao, B. Liu, C. Hu and M. Cao, Chemistry, 2014, 20, 467–473 CrossRef CAS.
- Y. Zhu, H. Guo, H. Zhai and C. Cao, ACS Appl. Mater. Interfaces, 2015, 7, 2745–2753 CrossRef CAS.
- W. Wei, P. Du, D. Liu, H. Wang and P. Liu, J. Colloid Interface Sci., 2017, 503, 205–213 CrossRef CAS.
- B. Kumar, D. H. Lee, S. H. Kim, B. Yang, S. Maeng and S. W. Kim, J. Phys. Chem. C, 2010, 114, 11050–11055 CrossRef CAS.
- G. D. Park, J.-K. Lee and Y. C. Kang, Adv. Funct. Mater., 2017, 27, 222107 Search PubMed.
- J. H. Kim, K. M. Jeon, J.-S. Park and Y. C. Kang, J. Power Sources, 2017, 359, 363–370 CrossRef CAS.
- J.-S. Park, Y. J. Oh, J. H. Kim and Y. C. Kang, Mater. Charact., 2020, 161, 222107 CrossRef.
- X. W. Lou, Y. Wang, C. Yuan, J. Y. Lee and L. A. Archer, Adv. Mater., 2006, 18, 2325–2329 CrossRef CAS.
- S. Ding and X. Wen Lou, Nanoscale, 2011, 3, 3586–3588 RSC.
- H. Wang, F. Fu, F. Zhang, H.-E. Wang, S. V. Kershaw, J. Xu, S.-G. Sun and A. L. Rogach, J. Mater. Chem., 2012, 22, 2140–2148 RSC.
- Y. Wang, T. Su, H. Chen, W. Liu, Y. Dong and S. Hu, Mater. Lett., 2014, 137, 241–244 CrossRef CAS.
- J. Qin, N. Zhao, C. Shi, E. Liu, F. He, L. Ma, Q. Li, J. Li and C. He, J. Mater. Chem. A, 2017, 5, 10946–10956 RSC.
- W.-S. Kim, Y. Hwa, J.-H. Jeun, H.-J. Sohn and S.-H. Hong, J. Power Sources, 2013, 225, 108–112 CrossRef CAS.
- J. Zhang, H. Ren, J. Wang, J. Qi, R. Yu, D. Wang and Y. Liu, J. Mater. Chem. A, 2016, 4, 17673–17677 RSC.
- L.-Y. Jiang, X.-L. Wu, Y.-G. Guo and L.-J. Wan, J. Phys. Chem. C, 2009, 113, 14213–14219 CrossRef CAS.
- H.-X. Zhang, C. Feng, Y.-C. Zhai, K.-L. Jiang, Q.-Q. Li and S.-S. Fan, Adv. Mater., 2009, 21, 2299–2304 CrossRef CAS.
- W. Dong, J. Xu, C. Wang, Y. Lu, X. Liu, X. Wang, X. Yuan, Z. Wang, T. Lin, M. Sui, I. W. Chen and F. Huang, Adv. Mater., 2017, 29, 1700136 CrossRef.
- C. Zhang, X. Peng, Z. Guo, C. Cai, Z. Chen, D. Wexler, S. Li and H. Liu, Carbon, 2012, 50, 1897–1903 CrossRef CAS.
- W. Long, B. Fang, A. Ignaszak, Z. Wu, Y.-J. Wang and D. Wilkinson, Chem. Soc. Rev., 2017, 46, 7176–7190 RSC.
- L. Noerochim, J.-Z. Wang, S.-L. Chou, H.-J. Li and H.-K. Liu, Electrochim. Acta, 2010, 56, 314–320 CrossRef CAS.
- X. W. Lou, D. Deng, J. Y. Lee and L. A. Archer, Chem. Mater., 2008, 20, 6562–6566 CrossRef CAS.
- Z. Wen, Q. Wang, Q. Zhang and J. Li, Adv. Funct. Mater., 2007, 17, 2772–2778 CrossRef CAS.
- D. Guan, J. Li, X. Gao and C. Yuan, RSC Adv., 2015, 5, 58514–58521 RSC.
- X. Zhou, W. Liu, X. Yu, Y. Liu, Y. Fang, S. Klankowski, Y. Yang, J. E. Brown and J. Li, ACS Appl. Mater. Interfaces, 2014, 6, 7434–7443 CrossRef CAS.
- J. S. Chen, Y. L. Cheah, Y. T. Chen, N. Jayaprakash, S. Madhavi, Y. H. Yang and X. W. Lou, J. Phys. Chem. C, 2009, 113, 20504–20508 CrossRef CAS.
- X. Zhao, Z. Zhang, F. Yang, Y. Fu, Y. Lai and J. Li, RSC Adv., 2015, 5, 31465–31471 RSC.
- J. Cheng, H. Xin, H. Zheng and B. Wang, J. Power Sources, 2013, 232, 152–158 CrossRef CAS.
- H. Song, N. Li, H. Cui and C. Wang, J. Mater. Chem. A, 2013, 1, 7558–7562 RSC.
- H. Liu, J. Huang, X. Li, J. Liu, Y. Zhang and K. Du, Appl. Surf. Sci., 2012, 258, 4917–4921 CrossRef CAS.
- L. Fan, X. Li, B. Yan, X. Li, D. Xiong, D. Li, H. Xu, X. Zhang and X. Sun, Appl. Energy, 2016, 175, 529–535 CrossRef CAS.
- H. Xu, L. Shi, Z. Wang, J. Liu, J. Zhu, Y. Zhao, M. Zhang and S. Yuan, ACS Appl. Mater. Interfaces, 2015, 7, 27486–27493 CrossRef CAS.
- J. Liu, J. Huang, L. Hao, H. Liu and X. Li, Ceram. Int., 2013, 39, 8623–8627 CrossRef CAS.
- H. Xu, J. Chen, D. Wang, Z. Sun, P. Zhang, Y. Zhang and X. Guo, Carbon, 2017, 124, 565–575 CrossRef CAS.
- B. Fang, J. H. Kim, M.-S. Kim and J.-S. Yu, Acc. Chem. Res., 2013, 46, 1397–1406 CrossRef CAS.
- Y. Xing, Y. Wang, C. Zhou, S. Zhang and B. Fang, ACS Appl. Mater. Interfaces, 2014, 6, 2561–2567 CrossRef CAS.
- J. Lu, D. Qi, C. Deng, X. Zhang and P. Yang, Nanoscale, 2010, 2, 1892–1900 RSC.
- N. Du, Y. Chen, C. Zhai, H. Zhang and D. Yang, Nanoscale, 2013, 5, 4744–4750 RSC.
- F. Li, G. Luo, J. Yu, W. Huang, D. Xu, W. Chen, X. Huang, S. Yang, Y. Fang and X. Yu, J. Alloys Compd., 2019, 773, 778–787 CrossRef CAS.
- X. Wu, W. Wu, Y. Zhou, X. Huang, W. Chen and Q. Wang, Powder Technol., 2015, 280, 119–123 CrossRef CAS.
- M. Huang, X. L. Zhao, F. Li, W. Li, B. Zhang and Y. X. Zhang, J. Mater. Chem. A, 2015, 3, 12852–12857 RSC.
- J. Wang, H. Wang, T. Yao, T. Liu, Y. Tian, C. Li, F. Li, L. Meng and Y. Cheng, J. Colloid Interface Sci., 2020, 560, 546–554 CrossRef CAS.
- D. Deng, M. G. Kim, J. Y. Lee and J. Cho, Energy Environ. Sci., 2009, 2, 818–837 RSC.
- Z. Yang, G. Du, Z. Guo, X. Yu, Z. Chen, T. Guo and R. Zeng, Nanoscale, 2011, 3, 4440–4447 RSC.
- A. Ansari and D. Nematollahi, Appl. Catal., B, 2020, 261, 118226 CrossRef CAS.
- B. Zhao, F. Mattelaer, J. Kint, A. Werbrouck, L. Henderick, M. Minjauw, J. Dendooven and C. Detavernier, Electrochim. Acta, 2019, 320, 134604 CrossRef CAS.
- L. L. Si, Z. Q. Yuan, J. W. Liang, L. Hu, Y. C. Zhu and Y. T. Qian, J. Mater. Chem. A, 2014, 2, 9784–9791 RSC.
- L.-J. Xue, Y.-F. Xu, L. Huang, F.-S. Ke, Y. He, Y.-X. Wang, G.-Z. Wei, J.-T. Li and S.-G. Sun, Electrochim. Acta, 2011, 56, 5979–5987 CrossRef CAS.
- H. El-Shinawi, A. S. Schulze, M. Neumeier, T. Leichtweiß and J. Janek, J. Phys. Chem. C, 2014, 118, 8818–8823 CrossRef CAS.
- Q. Tian, Y. Chen, F. Zhang, W. Zhang, Z. Sui and L. Yang, Appl. Surf. Sci., 2020, 511, 145625 CrossRef CAS.
- M. Jayalakshmi, M. M. Rao, N. Venugopal and K.-B. Kim, J. Power Sources, 2007, 166, 578–583 CrossRef CAS.
- Q. Guo, Z. Sun, M. Gao, Z. Tan, B. Zhang and D. S. Su, J. Energy Chem., 2013, 22, 347–355 CrossRef CAS.
- D. Wei, S. Zhong, H. Zhang, X. Zhang, C. Zhu, J. Duan, L. Li, Z. Chen, P. Liu, G. Zhang and H. Duan, Electrochim. Acta, 2018, 290, 312–321 CrossRef CAS.
- G. Wen, H. Liu, T. Liang, Y. Ouyang, L. Tan, R. Hu, J. Liu, Y. Zhang and M. Zhu, Electrochim. Acta, 2020, 334, 135640 CrossRef CAS.
- Y. Wang, H. Zhang, R. Hu, J. Liu, T. van Ree, H. Wang, L. Yang and M. Zhu, J. Alloys Compd., 2017, 693, 1174–1179 CrossRef CAS.
- W. Guo, Y. Wang, F. Zhang, S. Rao, P. Mao and D. Wang, Energy Fuels, 2020, 34, 2462–2470 CrossRef CAS.
- L. Si, Z. Yuan, J. Liang, L. Hu, Y. Zhu and Y. Qian, J. Mater. Chem. A, 2014, 2, 9784–9791 RSC.
- D. Cao, H. Wang, B. Li, C. Li, S. Xie, A. L. Rogach and C. Niu, Electrochim. Acta, 2016, 216, 79–87 CrossRef CAS.
- K. Lee, S. Shin, T. Degen, W. Lee and Y. S. Yoon, Nano Energy, 2017, 32, 397–407 CrossRef CAS.
- X. Liu, F. Liu, Q. Sun, A. M. Ng, A. B. Djurisic, M. Xie, C. Liao, K. Shih and Z. Deng, ACS Appl. Mater. Interfaces, 2014, 6, 13478–13486 CrossRef CAS.
- H.-S. Oh, H. N. Nong and P. Strasser, Adv. Funct. Mater., 2015, 25, 1074–1081 CrossRef CAS.
- N. Wan, P. Yu, S. Sun, Q. Wu, T. Li and Y. Bai, Mater. Lett., 2014, 133, 168–170 CrossRef CAS.
- X. Liu, G. Zhou, S. W. Or and Y. Sun, RSC Adv., 2014, 4, 51389–51394 RSC.
- A. Vignesh, A. S. Siddarth, O. S. Gokul and B. R. Babu, Int. J. Environ. Sci. Technol., 2014, 11, 1669–1678 CrossRef CAS.
- M. Ding, H. Liu, J. Zhu, X. Zhao, L. Pang, Y. Qin and L. Deng, Appl. Surf. Sci., 2018, 448, 389–399 CrossRef CAS.
- V. Vo, X. D. Nguyen Thi, Y.-S. Jin, G. Ly Thi, T. T. Nguyen, T. Q. Duong and S.-J. Kim, Chem. Phys. Lett., 2017, 674, 42–47 CrossRef CAS.
- J. Liu, Y. Li, X. Huang, R. Ding, Y. Hu, J. Jiang and L. Liao, J. Mater. Chem., 2009, 19, 1859–1864 RSC.
- Y. J. Chen, X. Y. Xue, Y. G. Wang and T. H. Wang, Appl. Phys. Lett., 2005, 87, 233503 CrossRef.
- W. Xu, K. Zhao, C. Niu, L. Zhang, Z. Cai, C. Han, L. He, T. Shen, M. Yan, L. Qu and L. Mai, Nano Energy, 2014, 8, 196–204 CrossRef CAS.
- J. Ye, H. Zhang, R. Yang, X. Li and L. Qi, Small, 2010, 6, 296–306 CrossRef CAS.
- D. F. Zhang, L. D. Sun, J. L. Yin and C. H. Yan, Adv. Mater., 2003, 15, 1022–1025 CrossRef CAS.
- C. Yang, G. Zhang, L. Zhang and L. Ma, in Advances In Composites, Pts 1 and 2, ed. J. L. Bu, Z. Y. Jiang and S. Jiao, 2011, vol. 150–151, pp. 1387–1390 Search PubMed.
- C. Li, W. Wei, S. Fang, H. Wang, Y. Zhang, Y. Gui and R. Chen, J. Power Sources, 2010, 195, 2939–2944 CrossRef CAS.
- C. Guan, X. Wang, Q. Zhang, Z. Fan, H. Zhang and H. J. Fan, Nano Lett., 2014, 14, 4852–4858 CrossRef CAS.
- J. Y. Huang, L. Zhong, C. M. Wang, J. P. Sullivan, W. Xu, L. Q. Zhang, S. X. Mao, N. S. Hudak, X. H. Liu, A. Subramanian, H. Fan, L. Qi, A. Kushima and J. Li, Science, 2010, 330, 1515–1520 CrossRef CAS.
- R. Thomas and G. Mohan Rao, J. Mater. Chem. A, 2015, 3, 274–280 RSC.
- J. Liu, Y. Li, X. Huang, R. Ding, Y. Hu, J. Jiang and L. Liao, J. Mater. Chem., 2009, 19, 1859–1864 RSC.
- X. W. Lou and H. C. Zeng, Chem. Mater., 2002, 14, 4781–4789 CrossRef CAS.
- J. S. Chen, Y. L. Cheah, S. Madhavi and X. W. Lou, J. Phys. Chem. C, 2010, 114, 8675–8678 CrossRef CAS.
- X. Wang, W. Liu, H. Yang, X. Li, N. Li, R. Shi, H. Zhao and J. Yu, Acta Mater., 2011, 59, 1291–1299 CrossRef CAS.
- W. Zhang, L. L. Feng, H. Y. Chen and Y. Y. Zhang, Nano, 2019, 14, 1950109 CrossRef CAS.
- F. Paraguay-Delgado, W. Antunez-Flores, M. Miki-Yoshida, A. Aguilar-Elguezaba, P. Santiago, R. Diaz and J. A. Ascencio, Nanotechnology, 2005, 16, 688–694 CrossRef CAS.
- S. Ding, J. S. Chen and X. W. Lou, Chem.–Asian J., 2011, 6, 2278–2281 CrossRef CAS.
- N. Du, H. Zhang, B. Chen, X. Ma, X. Huang, J. Tu and D. Yang, Mater. Res. Bull., 2009, 44, 211–215 CrossRef CAS.
- X. Wang, J. Li, Z. Chen, L. Lei, X. Liao, X. Huang and B. Shi, J. Mater. Chem. A, 2016, 4, 18783–18791 RSC.
- B. Cheng, J. M. Russell, W. S. Shi, L. Zhang and E. T. Samulski, J. Am. Chem. Soc., 2004, 126, 5972–5973 CrossRef CAS.
- G. Xi and J. Ye, Inorg. Chem., 2010, 49, 2302–2309 CrossRef CAS.
- L. Tan, M. S. Wang, Y. J. Liu, X. C. Xiao, L. Z. Fan and Y. D. Wang, Mater. Technol., 2013, 27, 191–195 CrossRef.
- Z.-S. Wu, W. Ren, L. Wen, L. Gao, J. Zhao, Z. Chen, G. Zhou, F. Li and H.-M. Cheng, ACS Nano, 2010, 4, 3187–3194 CrossRef CAS.
- Q. Zhao, L. Ma, Q. Zhang, C. Wang and X. Xu, J. Nanomater., 2015, 2015, 850147 Search PubMed.
- J. Ye, H. Zhang, R. Yang, X. Li and L. Qi, Small, 2010, 6, 296–306 CrossRef CAS.
- V. Kumar, J. H. Kim, C. Pendyala, B. Chernomordik and M. K. Sunkara, J. Phys. Chem. C, 2008, 112, 17750–17754 CrossRef CAS.
- Y. Han, X. Wu, Y. Ma, L. Gong, F. Qu and H. Fan, CrystEngComm, 2011, 13, 3506–3510 RSC.
- S. Ding, D. Luan, F. Y. Boey, J. S. Chen and X. W. Lou, Chem. Commun., 2011, 47, 7155–7157 RSC.
- D. Wang, X. Li, J. Wang, J. Yang, D. Geng, R. Li, M. Cai, T.-K. Sham and X. Sun, J. Phys. Chem. C, 2012, 116, 22149–22156 CrossRef CAS.
- B. Zhao, S.-Y. Huang, T. Wang, K. Zhang, M. M. F. Yuen, J.-B. Xu, X.-Z. Fu, R. Sun and C.-P. Wong, J. Power Sources, 2015, 298, 83–91 CrossRef CAS.
- L. Zhan, X. Zhou, J. Luo and X. Ning, Ceram. Int., 2019, 45, 6931–6936 CrossRef CAS.
- S. Hao, Y. Sun, Y. Liu, Y. Zhang and G. Hu, J. Alloys Compd., 2016, 689, 587–592 CrossRef CAS.
- S. Ramasamy, P. Nagamony and V. Chinnuswamy, Mater. Lett., 2018, 218, 295–298 CrossRef CAS.
- L.-L. Xing, Y.-Y. Zhao, J. Zhao, Y.-X. Nie, P. Deng, Q. Wang and X.-Y. Xue, J. Alloys Compd., 2014, 586, 28–33 CrossRef CAS.
- G. Z. Xing, Y. Wang, J. I. Wong, Y. M. Shi, Z. X. Huang, S. Li and H. Y. Yang, Appl. Phys. Lett., 2014, 105, 143905 CrossRef.
- L. S. Fan, Z. K. Guo, Y. Zhang, X. Y. Zhang, M. X. Wang, Y. Y. Yin, N. Q. Zhang and K. N. Sun, Mater. Today Energy, 2019, 14, 6 Search PubMed.
- D. Y. A. Esquivel, R. K. Brown, S. Knohl and U. Schroeder, ChemElectroChem, 2020, 7, 1851–1859 CrossRef.
- Y. Fu and A. Manthiram, Nano Energy, 2013, 2, 1107–1112 CrossRef CAS.
- X. Meng, J. Zhang, Y. Wang and H. Liu, Acta Chim. Sin., 2012, 70, 812–816 CrossRef CAS.
- L. Jabbour, M. Destro, C. Gerbaldi, D. Chaussy, N. Penazzi and D. Beneventi, J. Mater. Chem., 2012, 22, 3227–3233 RSC.
- L. Chang, Z. Yi, Z. Wang, L. Wang and Y. Cheng, Appl. Surf. Sci., 2019, 484, 646–654 CrossRef CAS.
- M.-S. Wang, Z.-Q. Wang, Z.-L. Yang, Y. Huang, J. Zheng and X. Li, Electrochim. Acta, 2017, 240, 7–15 CrossRef CAS.
- R. Jia, J. Yue, Q. Xia, J. Xu, X. Zhu, S. Sun, T. Zhai and H. Xia, Energy Storage Mater., 2018, 13, 303–311 CrossRef.
- L. Fan, Z. Guo, Y. Zhang, X. Zhang, M. Wang, Y. Yin, N. Zhang and K. Sun, Mater. Today Energy, 2019, 14, E28–E62 Search PubMed.
- L. Vayssieres, Adv. Mater., 2003, 15, 464–466 CrossRef CAS.
- W.-w. Zhan, Q. Kuang, J.-z. Zhou, X.-j. Kong, Z.-x. Xie and L.-s. Zheng, J. Am. Chem. Soc., 2013, 135, 1926–1933 CrossRef CAS.
- J. Liu, S. Z. Qiao, S. B. Hartono and G. Q. Lu, Angew. Chem., Int. Ed., 2010, 49, 4981–4985 CrossRef CAS.
- J. Liu, T. Yang, D.-W. Wang, G. Q. Lu, D. Zhao and S. Z. Qiao, Nat. Commun., 2013, 4, 2798 CrossRef.
- H. X. Yang, J. F. Qian, Z. X. Chen, X. P. Ai and Y. L. Cao, J. Phys. Chem. C, 2007, 111, 14067–14071 CrossRef CAS.
- C.-Y. Chang-Chien, C.-H. Hsu, T.-Y. Lee, C.-W. Liu, S.-H. Wu, H.-P. Lin, C.-Y. Tang and C.-Y. Lin, Eur. J. Inorg. Chem., 2007, 2007, 3798–3804 CrossRef.
- Y. Yin, S. Xin, L. Wan, C. Li and Y. Guo, Sci. China: Chem., 2012, 55, 1314–1318 CrossRef CAS.
- L. Zhang, H. B. Wu, B. Liu and X. W. Lou, Energy Environ. Sci., 2014, 7, 1013–1017 RSC.
- Y. Liu, H. Dong and M. L. Liu, Adv. Mater., 2004, 16, 353–356 CrossRef CAS.
- T. Wang, H. Xu, Y. Wang, Y. Zeng and B. Liu, Mater. Lett., 2020, 264, 127320 CrossRef CAS.
- L. Zhou, J. Zhang, Y. Wu, W. Wang, H. Ming, Q. Sun, L. Wang, J. Ming and H. N. Alshareef, Adv. Energy Mater., 2019, 9, 1902194 CrossRef CAS.
- J. S. Chen, C. M. Li, W. W. Zhou, Q. Y. Yan, L. A. Archer and X. W. Lou, Nanoscale, 2009, 1, 280–285 RSC.
- S. Liu, M. Xie, Y. Li, X. Guo, W. Ji, W. Ding and C. Au, Sens. Actuators, B, 2010, 151, 229–235 CrossRef CAS.
- Y.-Z. Wu, S. Brahma, S.-C. Weng, C.-C. Chang and J.-L. Huang, J. Alloys Compd., 2020, 818, 152889 CrossRef CAS.
- G. D. Park, J.-K. Lee and Y. C. Kang, Adv. Funct. Mater., 2017, 27, 1603399 CrossRef.
- G. D. Park, J. H. Kim and Y. C. Kang, Nanoscale, 2018, 10, 13531–13538 RSC.
- J. S. Cho and Y. C. Kang, Small, 2015, 11, 4673–4681 CrossRef CAS.
- L. Liu, F. Xie, J. Lyu, T. Zhao, T. Li and B. G. Choi, J. Power Sources, 2016, 321, 11–35 CrossRef CAS.
- H. Hu, L. Wu, P. Gebhardt, X. Zhang, A. Cherevan, B. Gerke, R. Pöttgen, A. Balducci, S. Passerini and D. Eder, CrystEngComm, 2017, 19, 6454–6463 RSC.
- W. Tian, C. Zhang, T. Zhai, S.-L. Li, X. Wang, M. Liao, K. Tsukagoshi, D. Golberg and Y. Bando, Chem. Commun., 2013, 49, 3739–3741 RSC.
- Y. Kang, Z. Li, K. Xu, X. He, S. Wei and Y. Cao, J. Alloys Compd., 2019, 779, 728–734 CrossRef CAS.
- S. Ding, J. S. Chen, G. Qi, X. Duan, Z. Wang, E. P. Giannelis, L. A. Archer and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 21–23 CrossRef CAS.
- J. Deng, Y. Chen, J. Ma, E. Zhang and T. Wang, J. Nanosci. Nanotechnol., 2013, 13, 4297–4301 CrossRef CAS.
- X. M. Yin, C. C. Li, M. Zhang, Q. Y. Hao, S. Liu, L. B. Chen and T. H. Wang, J. Phys. Chem. C, 2010, 114, 8084–8088 CrossRef CAS.
- S. P. Kim, M. Y. Choi and H. C. Choi, Appl. Surf. Sci., 2015, 357, 302–308 CrossRef CAS.
- Z. Lu and H. Wang, CrystEngComm, 2014, 16, 550–555 RSC.
- H. Song, N. Li, H. Cui and C. Wang, Electrochim. Acta, 2014, 120, 46–51 CrossRef CAS.
- J. Deng, Y. Dai, Z. Xiao, S. Song, H. Dai, L. Li and J. Li, Nanomaterials, 2020, 10, 249 CrossRef CAS.
- Q. H. Tian, Y. B. Chen, W. Zhang, Z. Y. Sui and L. Yang, J. Alloys Compd., 2020, 820, 8 Search PubMed.
- X. Zhou, L. Yu and X. W. Lou, Nanoscale, 2016, 8, 8384–8389 RSC.
- A. Yang, X. Tao, R. Wang, S. Lee and C. Surya, Appl. Phys. Lett., 2007, 91, 133110 CrossRef.
- X.-H. Li, H.-C. Huang, L. Ling, X.-Y. Wang, J.-R. Zhang and L. Gao, Chin. J. Inorg. Chem., 2011, 27, 781–784 Search PubMed.
- M. Xia, H.-Y. Guo and B. Yang, Fullerenes, Nanotubes, Carbon Nanostruct., 2018, 26, 76–79 CrossRef.
- P. Wu, N. Du, H. Zhang, J. Yu, Y. Qi and D. Yang, Nanoscale, 2011, 3, 746–750 RSC.
- G. Du, C. Zhong, P. Zhang, Z. Guo, Z. Chen and H. Liu, Electrochim. Acta, 2010, 55, 2582–2586 CrossRef CAS.
- K. Liu, S. Zhu, X. Dong, H. Huang and M. Qi, Adv. Mater. Interfaces, 2020, 7, 1901916 CrossRef CAS.
- Y.-H. Jin, K.-M. Min, S.-D. Seo, H.-W. Shim and D.-W. Kim, J. Phys. Chem. C, 2011, 115, 22062–22067 CrossRef CAS.
- G. Liang, X. Sun, J. Lai, C. Wei, Y. Huang and H. Hu, J. Electroanal. Chem., 2019, 853, 113401 CrossRef CAS.
- Q. Tian, Y. Tian, Z. Zhang, L. Yang and S.-i. Hirano, J. Power Sources, 2015, 291, 173–180 CrossRef CAS.
- M. Liu, S. Zhang, H. C. Dong, X. Chen, S. Gao, Y. P. Sun, W. H. Li, J. Q. Xu, L. W. Chen, A. B. Yuan and W. Lu, ACS Sustainable Chem. Eng., 2019, 7, 4195–4203 CrossRef CAS.
- Q. Liu, Y. Dou, B. Ruan, Z. Sun, S. L. Chou and S. X. Dou, Chemistry, 2016, 22, 5853–5857 CrossRef CAS.
- Y. Wan, Y. Sha, W. Deng, Q. Zhu, Z. Chen, X. Wang, W. Chen, G. Xue and D. Zhou, Electrochim. Acta, 2015, 167, 69–74 CrossRef CAS.
- F. R. He, Q. Xu, B. P. Zheng, J. Zhang, Z. G. Wu, Y. J. Zhong, Y. X. Chen, W. Xiang, B. H. Zhong and X. D. Guo, RSC Adv., 2020, 10, 6035–6042 RSC.
- L. Yuan, K. Konstantinov, G. X. Wang, H. K. Liu and S. X. Dou, J. Power Sources, 2005, 146, 180–184 CrossRef CAS.
- H. Liu, D. Long, X. Liu, W. Qiao, L. Zhan and L. Ling, Electrochim. Acta, 2009, 54, 5782–5788 CrossRef CAS.
- J. Shi, N. Lin, D. Liu, Y. Wang and H. Lin, J. Electroanal. Chem., 2020, 857, 113634 CrossRef CAS.
- X. Sun, J. Liu and Y. Li, Chem. Mater., 2006, 18, 3486–3494 CrossRef CAS.
- H. Qiao, Z. Zheng, L. Zhang and L. Xiao, J. Mater. Sci., 2008, 43, 2778–2784 CrossRef CAS.
- X. Tao, Q. Tian, L. Yang and Y. Xiang, Mater. Lett., 2017, 202, 107–110 CrossRef CAS.
- P. Wu, N. Du, H. Zhang, C. Zhai and D. Yang, ACS Appl. Mater. Interfaces, 2011, 3, 1946–1952 CrossRef CAS.
- Y. Chen, Q. Z. Huang, J. Wang, Q. Wang and J. M. Xue, J. Mater. Chem., 2011, 21, 17448–17453 RSC.
- M.-S. Wang, M. Lei, Z.-Q. Wang, X. Zhao, J. Xu, W. Yang, Y. Huang and X. Li, J. Power Sources, 2016, 309, 238–244 CrossRef CAS.
- Z.-G. Wu, J.-T. Li, Y.-J. Zhong, J. Liu, X.-D. Guo, L. Huang, B.-H. Zhong and S.-G. Sun, J. Alloys Compd., 2015, 620, 407–412 CrossRef CAS.
- X. Wang, H. Fan, P. Ren and M. Li, RSC Adv., 2014, 4, 10284–10289 RSC.
- X. Zhao, T. Wen, J. Zhang, J. Ye, Z. Ma, H. Yuan, X. Ye and Y. Wang, RSC Adv., 2017, 7, 21678–21685 RSC.
- J. Yuan, C. Chen, Y. Hao, X. Zhang, B. Zou, R. Agrawal, C. Wang, H. Yu, X. Zhu, Y. Yu, Z. Xiong, Y. Luo, H. Li and Y. Xie, J. Alloys Compd., 2017, 691, 34–39 CrossRef CAS.
- C. Ma, W. Zhang, Y. S. He, Q. Gong, H. Che and Z. F. Ma, Nanoscale, 2016, 8, 4121–4126 RSC.
- S.-L. Yang, B.-H. Zhou, M. Lei, L.-P. Huang, J. Pan, W. Wu and H.-B. Zhang, Chin. Chem. Lett., 2015, 26, 1293–1297 CrossRef CAS.
- Yiliguma, Z. Wang, C. Yang, A. Guan, L. Shang, A. M. Al-Enizi, L. Zhang and G. Zheng, J. Mater. Chem. A, 2018, 6, 20121–20127 RSC.
- S. Nam, S. Kim, S. Wi, H. Choi, S. Byun, S.-M. Choi, S.-I. Yoo, K. T. Lee and B. Park, J. Power Sources, 2012, 211, 154–160 CrossRef CAS.
- X. Ji, X. Huang, J. Liu, J. Jiang, X. Li, R. Ding, Y. Hu, F. Wu and Q. Li, Nanoscale Res. Lett., 2010, 5, 649–653 CrossRef CAS.
- L. Yu, D. Cai, H. Wang and M.-M. Titirici, RSC Adv., 2013, 3, 17281–17286 RSC.
- X. W. Lou, J. S. Chen, P. Chen and L. A. Archer, Chem. Mater., 2009, 21, 2868–2874 CrossRef CAS.
- F. M. Courtel, E. A. Baranova, Y. Abu-Lebdeh and I. J. Davidson, J. Power Sources, 2010, 195, 2355–2361 CrossRef CAS.
- G.-L. Xu, S.-R. Chen, J.-T. Li, F.-S. Ke, L. Huang and S.-G. Sun, J. Electroanal. Chem., 2011, 656, 185–191 CrossRef CAS.
- W. Zhang and H. Liu, Neurocomputing, 2017, 247, 183–191 CrossRef.
- C. Hernández-Rentero, V. Marangon, M. Olivares-Marín, V. Gómez-Serrano, Á. Caballero, J. Morales and J. Hassoun, J. Colloid Interface Sci., 2020, 573, 396–408 CrossRef.
- Y. Dou, X. Liu, K. Yu, X. Wang, W. Liu, J. Liang and C. Liang, Diamond Relat. Mater., 2019, 98, 107514 CrossRef CAS.
- L.-L. Hu, L.-P. Yang, D. Zhang, X.-S. Tao, C. Zeng, A.-M. Cao and L.-J. Wan, Chem. Commun., 2017, 53, 11189–11192 RSC.
- Q. Tian, F. Zhang, W. Zhang and L. Yang, Electrochim. Acta, 2019, 307, 393–402 CrossRef CAS.
- Z. Rujia, Z. Zhang, L. Jiang, K. Xu, Q. Tian, S. Xue, J. Hu, Y. Bando and D. Golberg, J. Mater. Chem., 2012, 22, 19196–19201 RSC.
- W. Zhang, M. Li, X. Xiao, X. Huang, Y. Jiang, X. Fan and L. Chen, J. Alloys Compd., 2017, 727, 1–7 CrossRef CAS.
- Z. S. Wu, Y. Sun, Y. Z. Tan, S. Yang, X. Feng and K. Mullen, J. Am. Chem. Soc., 2012, 134, 19532–19535 CrossRef CAS.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- A. H. Castro Neto, F. Guinea, N. M. R. Peres, K. S. Novoselov and A. K. Geim, Rev. Mod. Phys., 2009, 81, 109–162 CrossRef CAS.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS.
- S.-y. Zuo, Z.-g. Wu, S.-k. Li, D. Yan, Y.-h. Liu, F.-y. Wang, R.-f. Zhuo, B.-s. Geng, J. Wang and P.-x. Yan, RSC Adv., 2017, 7, 18054–18059 RSC.
- D. Zhou, X. Li, L.-Z. Fan and Y. Deng, Electrochim. Acta, 2017, 230, 212–221 CrossRef CAS.
- M. Zhang, Z. Sun, T. Zhang, D. Sui, Y. Ma and Y. Chen, Carbon, 2016, 102, 32–38 CrossRef CAS.
- Z. Li, G. Wu, S. Deng, S. Wang, Y. Wang, J. Zhou, S. Liu, W. Wu and M. Wu, Chem. Eng. J., 2016, 283, 1435–1442 CrossRef CAS.
- J. Han, D. Kong, W. Lv, D. M. Tang, D. Han, C. Zhang, D. Liu, Z. Xiao, X. Zhang, J. Xiao, X. He, F. C. Hsia, C. Zhang, Y. Tao, D. Golberg, F. Kang, L. Zhi and Q. H. Yang, Nat. Commun., 2018, 9, 402 CrossRef.
- S. Shi, T. Deng, M. Zhang and G. Yang, Electrochim. Acta, 2017, 246, 1104–1111 CrossRef CAS.
- Z.-S. Wu, W. Ren, L. Xu, F. Li and H.-M. Cheng, ACS Nano, 2011, 5, 5463–5471 CrossRef CAS.
- D. D. Liu, Z. Y. Wei, B. Zhong, L. M. Liu, T. Zhang, X. M. Duan, M. Chen, H. T. Wang and X. X. Huang, Appl. Surf. Sci., 2020, 519, 5 CrossRef.
- Y. Huang, R. Yu, G. Mao, W. Yu, Z. Ding, Y. Cao, J. Zheng, D. Chu and H. Tong, J. Alloys Compd., 2020, 841, 155670 CrossRef CAS.
- H. Zhang and S. Liu, J. Alloys Compd., 2020, 842, 155873 CrossRef CAS.
- K. Wang and Z. Li, J. Nanosci. Nanotechnol., 2020, 20, 7673–7679 CrossRef CAS.
- D. Liu, Z. Wei, B. Zhong, L. Liu, T. Zhang, X. Duan, M. Chen, H. Wang and X. Huang, Appl. Surf. Sci., 2020, 519, 146190 CrossRef CAS.
- X. Zuo, B. Li, K. Chang, H. Tang and Z. Chang, RSC Adv., 2017, 7, 53126–53134 RSC.
- D. Usachov, O. Vilkov, A. Gruneis, D. Haberer, A. Fedorov, V. K. Adamchuk, A. B. Preobrajenski, P. Dudin, A. Barinov, M. Oehzelt, C. Laubschat and D. V. Vyalikh, Nano Lett., 2011, 11, 5401–5407 CrossRef CAS.
- H. Song, N. Li, H. Cui and C. Wang, Nano Energy, 2014, 4, 81–87 CrossRef CAS.
- X. Zhou, L. J. Wan and Y. G. Guo, Adv. Mater., 2013, 25, 2152–2157 CrossRef CAS.
- N. Wu, W. Du, X. Gao, L. Zhao, G. Liu, X. Liu, H. Wu and Y. B. He, Nanoscale, 2018, 10, 11460–11466 RSC.
- J. P. Paraknowitsch and A. Thomas, Energy Environ. Sci., 2013, 6, 2839–2855 RSC.
- K. Wu, B. Shi, L. Qi, Y. Mi, B. Zhao, C. Yang, Q. Wang, H. Tang, J. Lu, W. Liu and H. Zhou, Electrochim. Acta, 2018, 291, 24–30 CrossRef CAS.
- U. N. Maiti, W. J. Lee, J. M. Lee, Y. Oh, J. Y. Kim, J. E. Kim, J. Shim, T. H. Han and S. O. Kim, Adv. Mater., 2014, 26, 40–67 CrossRef CAS.
- A. Verchere, S. Mishra, E. Jeanneau, H. Guillon, J.-M. Decams and S. Daniele, Inorg. Chem., 2020, 59, 7167–7180 CrossRef CAS.
- Y. Sun, S. Cheng, Z. Yu, L. Li, C. Li and J. Yang, J. Alloys Compd., 2020, 834, 155184 CrossRef CAS.
- M. Maleki, Acta Phys. Pol., A, 2020, 137, 272–275 CrossRef CAS.
- W. Wang, Y. Liu and S. Liu, Cryst. Growth Des., 2020, 20, 2742–2752 CrossRef CAS.
- C. R. Ma, W. M. Zhang, Y. S. He, Q. Gong, H. Y. Che and Z. F. Ma, Nanoscale, 2016, 8, 4121–4126 RSC.
- L. Wen, F. Li and H.-M. Cheng, Adv. Mater., 2016, 28, 4306–4337 CrossRef CAS.
- B. A. Zhang, Q. B. Zheng, Z. D. Huang, S. W. Oh and J. K. Kim, Carbon, 2011, 49, 4524–4534 CrossRef CAS.
- J. Wang, F. Fang, T. Yuan, J. Yang, L. Chen, C. Yao, S. Zheng and D. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 3544–3553 CrossRef CAS.
- S. K. Kulshreshtha, M. M. Gadgil and R. Sasikala, Catal. Lett., 1996, 37, 181–185 CrossRef CAS.
- A. Jayalakshmi, N. Venugopal, K. P. Raja and M. M. Rao, J. Power Sources, 2006, 158, 1538–1543 CrossRef.
- S.-H. Shin, J.-M. Song, S.-W. Kim, J.-K. Shin, S.-M. Lee, J.-C. Ro and S.-J. Suh, Phys. Status Solidi A, 2020, 217, 1900996 CrossRef CAS.
- D. Shao, X. Zhang, Z. Wang, Y. Zhang, G. Tan and W. Yan, Appl. Surf. Sci., 2020, 515, 146003 CrossRef CAS.
- D. Shao, Y. Zhang, W. Lyu, X. Zhang, G. Tan, H. Xu and W. Yan, J. Hazard. Mater., 2020, 390, 122174 CrossRef CAS.
- M. Liu, H. Fan, O. Zhuo, J. Chen, Q. Wu, L. Yang, L. Peng, X. Wang, R. Che and Z. Hu, Nano Energy, 2020, 68, 104368 CrossRef CAS.
- G. Luo, W. Liu, S. Zeng, C. Zhang, X. Yu, Y. Fang and L. Sun, Electrochim. Acta, 2015, 184, 219–225 CrossRef CAS.
- Y. Liu, Y. Jiao, B. Yin, S. Zhang, F. Qu and X. Wu, J. Mater. Chem. A, 2015, 3, 3676–3682 RSC.
- Q. Tian, J. Yan, L. Yang and J. Chen, Electrochim. Acta, 2018, 282, 38–47 CrossRef CAS.
- H. Xie, M. Chen and L. Wu, Small, 2017, 13, 1604283 CrossRef.
- H. Yang, T. Song, S. Lee, H. Han, F. Xia, A. Devadoss, W. Sigmund and U. Paik, Electrochim. Acta, 2013, 91, 275–281 CrossRef CAS.
- J. S. Chen, C. M. Li, W. W. Zhou, Q. Y. Yan, L. A. Archer and X. W. Lou, Nanoscale, 2009, 1, 280–285 RSC.
- Y. Wang, J. Xu, H. Wu, M. Xu, Z. Peng and G. Zheng, J. Mater. Chem., 2012, 22, 21923–21927 RSC.
- D.-F. Zhang, L.-D. Sun, C.-J. Jia, Z.-G. Yan, L.-P. You and C.-H. Yan, J. Am. Chem. Soc., 2005, 127, 13492–13493 CrossRef CAS.
- W.-W. Wang, Mater. Res. Bull., 2008, 43, 2055–2059 CrossRef CAS.
- W. Zhou, C. Cheng, J. Liu, Y. Y. Tay, J. Jiang, X. Jia, J. Zhang, H. Gong, H. H. Hng, T. Yu and H. J. Fan, Adv. Funct. Mater., 2011, 21, 2439–2445 CrossRef CAS.
- Z. X. Chen, M. Zhou, Y. L. Cao, X. P. Ai, H. X. Yang and J. Liu, Adv. Energy Mater., 2012, 2, 95–102 CrossRef CAS.
- T. Yuan, Y. Jiang, Y. Li, D. Zhang and M. Yan, Electrochim. Acta, 2014, 136, 27–32 CrossRef CAS.
- W. Wu, Y. Zhao, J. Li, C. Wu and L. Guan, J. Energy Chem., 2014, 23, 376–382 CrossRef CAS.
- J. Guo, H. Zhu, Y. Sun, L. Tang and X. Zhang, J. Mater. Chem. A, 2016, 4, 16101–16107 RSC.
- G. D. Park, J. K. Lee and Y. C. Kang, J. Mater. Chem. A, 2017, 5, 25319–25327 RSC.
- B.-R. Koo, D.-H. Lee, H.-J. Ahn and Y.-E. Sung, J. Nanosci. Nanotechnol., 2016, 16, 10558–10562 CrossRef CAS.
- J. Ma, J. Li, S. Yang, H. Lu, L. Liu and R. Wang, J. Power Sources, 2020, 453, 227866 CrossRef CAS.
- Y. Qi, H. Zhang, N. Du, C. Zhai and D. Yang, RSC Adv., 2012, 2, 9511–9516 RSC.
- J. Hou, C. Cao, F. Idrees and X. Ma, ACS Nano, 2015, 9, 2556–2564 CrossRef CAS.
- Y. N. Sun, M. Goktas, L. Zhao, P. Adelhelm and B. H. Han, J. Colloid Interface Sci., 2020, 572, 122–132 CrossRef CAS.
- X. Li, Y. Zhang, T. Li, Q. Zhong, H. Li and J. Huang, Electrochim. Acta, 2014, 147, 40–46 CrossRef CAS.
- W. Tao, M. Wang, B. Zhu, W. Huo, R. Yang, H. Xiong, H. Tang, Z. Wei and Y. Wang, Electrochim. Acta, 2020, 334, 135569 CrossRef CAS.
- B. Huang, Z. Pan, X. Su and L. An, J. Power Sources, 2018, 395, 41–59 CrossRef CAS.
- H. Zhou, Y. Zhong, Z. He, L. zhang, J. Wang, J. Zhang and C.-n. Cao, Appl. Surf. Sci., 2014, 314, 1–6 CrossRef CAS.
- J. Ding, X. Gao, L. Cha, M.-Q. Cai and J. Ma, RSC Adv., 2016, 6, 108310–108314 RSC.
- Q. Tian, Z. Zhang, L. Yang and S.-i. Hirano, RSC Adv., 2015, 5, 40303–40309 RSC.
- F. Yang, Y. Zhu, X. Li, C. Lai, W. Guo and J. Ma, RSC Adv., 2015, 5, 98717–98720 RSC.
- Y. Xing, S. Wang, B. Fang, G. Song, D. P. Wilkinson and S. Zhang, J. Power Sources, 2018, 385, 10–17 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- L. Zhou, H. Guo, T. Li, W. Chen, L. Liu, J. Qiao and J. Zhang, Sci. Rep., 2015, 5, 15252 CrossRef CAS.
- Z. Yang, G. Du, Z. Guo, X. Yu, Z. Chen, T. Guo and R. Zeng, Nanoscale, 2011, 3, 4440–4447 RSC.
- Z. Yi, Q. Han, P. Zan, Y. Cheng, Y. Wu and L. Wang, J. Mater. Chem. A, 2016, 4, 12850–12857 RSC.
- J. Y. Cheong, C. Kim, J. S. Jang and I.-D. Kim, RSC Adv., 2016, 6, 2920–2925 RSC.
- A. Xu, X. Dai, K. Wei, W. Han, J. Li, X. Sun, J. Shen and L. Wang, RSC Adv., 2017, 7, 37806–37814 RSC.
- L. Xu, Y. Wang and W. Zhang, RSC Adv., 2019, 9, 39242–39251 RSC.
- Z. Yang, G. Du, Q. Meng, Z. Guo, X. Yu, Z. Chen, T. Guo and R. Zeng, RSC Adv., 2011, 1, 1834–1840 RSC.
- H. Yoo, G. Lee and J. Choi, RSC Adv., 2019, 9, 6589–6595 RSC.
- Z. Yang, Q. Meng, Z. Guo, X. Yu, T. Guo and R. Zeng, J. Mater. Chem. A, 2013, 1, 10395–10402 RSC.
- L. He, C. Wang, X. Yao, R. Ma, H. Wang, P. Chen and K. Zhang, Carbon, 2014, 75, 345–352 CrossRef CAS.
- Z. Lu and H. Wang, CrystEngComm, 2014, 16, 550–555 RSC.
- Y. Tang, D. Wu, S. Chen, F. Zhang, J. Jia and X. Feng, Energy Environ. Sci., 2013, 6, 2447–2451 RSC.
- S. Bai and X. Shen, RSC Adv., 2012, 2, 64–98 RSC.
- R. K. Singh, R. Kumar and D. P. Singh, RSC Adv., 2016, 6, 64993–65011 RSC.
- H. F. Xiang, Z. D. Li, K. Xie, J. Z. Jiang, J. J. Chen, P. C. Lian, J. S. Wu, Y. Yu and H. H. Wang, RSC Adv., 2012, 2, 6792–6799 RSC.
- G. Zhao, T. Wen, C. Chen and X. Wang, RSC Adv., 2012, 2, 9286–9303 RSC.
- A. Abbasnezhad, H. Asgharzadeh, A. Ansari Hamedani and S. Hayat Soytas, Dalton Trans., 2020, 49, 5890–5897 RSC.
- S. Bai, S. Chen, X. Shen, G. Zhu and G. Wang, RSC Adv., 2012, 2, 10977–10984 RSC.
- L. Tao, J. Zai, K. Wang, Y. Wan, H. Zhang, C. Yu, Y. Xiao and X. Qian, RSC Adv., 2012, 2, 3410–3415 RSC.
- C. Y. Du, M. Chen, X. Y. Cao, G. P. Yin and P. F. Shi, Electrochem. Commun., 2009, 11, 496–498 CrossRef CAS.
- J. Zhao, W. Shan, X. Xia, Q. Wang and L. Xing, Sci. China: Technol. Sci., 2014, 57, 1081–1084 CrossRef CAS.
- J. Sun, Q. Wang, Q. Wang, D. A. Zhang, L. L. Xing and X. Y. Xue, Sci. Adv. Mater., 2016, 8, 1280–1285 CrossRef CAS.
- R. Cai, X. Yu, X. Liu and Z. Shao, J. Power Sources, 2010, 195, 8244–8250 CrossRef CAS.
- Y. Hui, L. Cao, Z. Xu, J. Huang, H. Ouyang and J. Li, J. Electroanal. Chem., 2016, 763, 45–50 CrossRef CAS.
- J. Gao, C. Jiang, J. Ying and C. Wan, J. Power Sources, 2006, 155, 364–367 CrossRef CAS.
- K. Song, D.-H. Seo, M. R. Jo, Y.-I. Kim, K. Kang and Y.-M. Kang, J. Phys. Chem. Lett., 2014, 5, 1368–1373 CrossRef CAS.
- F. Mueller, D. Bresser, V. S. K. Chakravadhanula and S. Passerini, J. Power Sources, 2015, 299, 398–402 CrossRef CAS.
- A. Chen, S. J. Xia, H. Y. Pan, J. H. Xi, H. Y. Qin, H. W. Lu and Z. G. Ji, J. Electroanal. Chem., 2018, 824, 169–174 CrossRef CAS.
- Y. H. Cui and Y. J. Feng, J. Mater. Sci., 2005, 40, 4695–4697 CrossRef CAS.
- V. Subramanian, K. I. Gnanasekar and B. Rambabu, Solid State Ionics, 2004, 175, 181–184 CrossRef CAS.
- T. Jia, J. Chen, Z. Deng, F. Fu, J. Zhao, X. Wang and F. Long, Mater. Sci. Eng., B, 2014, 189, 32–37 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |