Open Access Article
Open Access ArticleStep-saving synthesis of star-shaped hole-transporting materials with carbazole or phenothiazine cores via optimized C–H/C–Br coupling reactions†
Jui-Heng Chena,
Kun-Mu Lee*b,
Chang-Chieh Tinga and
Ching-Yuan Liu *a
*a
aDepartment of Chemical and Materials Engineering, National Central University, Jhongli District, Taoyuan 320, Taiwan, Republic of China. E-mail: cyliu0312@ncu.edu.tw
bDepartment of Chemical and Materials Engineering, Chang Gung University, Department of Pediatrics, Chang Gung Memorial Hospital, Linkou, Taoyuan 333, Taiwan, Republic of China. E-mail: kmlee@mail.cgu.edu.tw
First published on 26th February 2021
Abstract
In most research papers, synthesis of organic hole-transporting materials relies on a key-reaction: Stille cross-couplings. This requires tedious prefunctionalizations including the preparation and treatment of unstable organolithium and toxicity-concern organotin reagents. In contrast to traditional multistep synthesis, this work describes that a series of star-shaped small molecules with a carbazole or phenothiazine core can be efficiently synthesized through a shortcut using optimized direct C–H/C–Br cross-couplings as the key step, thus avoiding dealing with the highly reactive organolithium or the toxic organotin species. Device fabrication of perovskite solar cells employing these molecules (6–13) as hole-transporting layers exhibit promising power conversion efficiencies of up to 17.57%.
Introduction
Perovskite solar cells (PSCs) using organic molecules as hole-transporting materials (HTMs) have attracted significant research interest because of their promising power conversion efficiencies (PCE).1–13 Compared to π-conjugated polymers, oligomers possess several advantages including well-defined conjugation lengths, flexible molecular design and reproducible chemical synthesis with higher compound purities.14–21 By far, 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamino)-9,9′-spirobifluorene (spiro-OMeTAD) is the most used organic HTM in perovskite-based solar cells.22 However, it was also stated that spiro-OMeTAD owns a number of drawbacks such as low conductivity, low hole-mobility, and high commercial price. Besides, synthetically speaking, accessing spiro-OMeTAD requires costly spirobifluorene as starting material. Therefore, present research attention has been switched to the development of non-spirolinked small molecules.23 Among various nonspiro-type building blocks, triphenylamine (TPA), carbazole (Cbz), and phenothiazine (PTZ) were very attractive to material scientists since they are inexpensive, readily available and usually exhibit remarkable PCEs while incorporated in PSCs as hole-transport layers.24 Recently, we also demonstrated a series of small-molecule HTMs incorporating TPA- or Cbz-derivatives as end-groups for efficient PSCs.25 However, we have noticed that the employment of Cbz or PTZ as core-moiety still remained less explored. Even though few examples were reported, synthetic approaches for these HTMs were neither step-economical nor optimized.24,26 Moreover, we envisaged that introduction of a carbazole or phenothiazine group as core for HTMs might further improve their hole-mobilities and the performance of PSCs. Therefore, we report herein, an efficient access to eight star-shaped new molecules with Cbz- or PTZ-cores (Cbz: carbazole for 6–9; PTZ: phenothiazine for 10–13) through a step-saving synthetic strategy under pre-optimized direct C–H arylation reaction conditions (Fig. 1). Property and photovoltaic performance of perovskite solar cells using these compounds as HTMs were also investigated.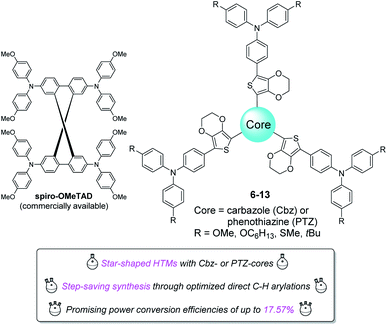 | ||
| Fig. 1 Eight star-shaped HTMs with carbazole or phenothiazine cores: step-saving synthesis and application for perovskite solar cells. | ||
Results and discussion
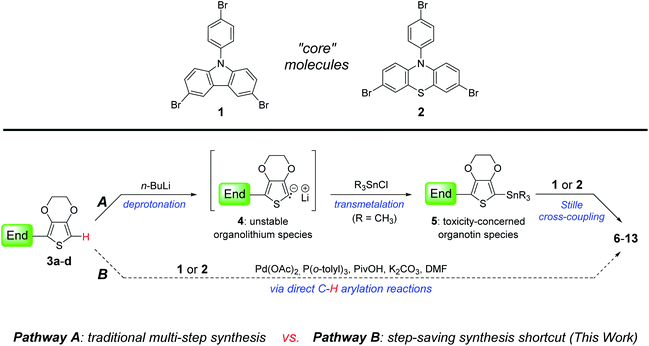
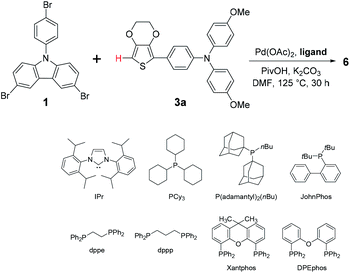
In Scheme 1, a comparison of two synthetic pathways to JHC01–08 was demonstrated. Traditionally, as shown in pathway A, the end-group molecules 3a–d 11 underwent deprotonation with n-butyl lithium at low temperature (usually −78 °C) to give unstable organolithium intermediates 4 which were subsequently transmetalated with trialkyltin chloride to afford organotin reagents 5. The final products can be readily reached by performing Stille cross-coupling reactions of 5 with the core molecules (1 or 2), even though the preparation and treatment of organotin species is toxicity-concerned and time-consuming. On the other hand, in pathway B, we proposed a step-saving shortcut to 6–13 via direct C–H bond activation/arylation of 3a–d with core 1 or 2, by which the generation of organolithium and the contact of organotin compounds can be entirely avoided.Based on pathway B, we carried out a facile synthesis of 6–13. In order to find optimum reaction conditions, the direct C–H/C–Br cross-coupling was investigated using core 1 and end 3a as model study focusing on ligand screening. First, as shown in Table 1, reaction of 3a with tribromocarbazole was conducted under general C–H activation/arylation conditions: Pd(OAc)2, PPh3, PivOH, and K2CO3 in DMF (125 °C, 30 h), giving desired 6 in 38% (isolated yield, entry 1). Other triarylphosphine monodentate ligands were also examined to produce 6 in 29–57% yields (entries 2–4). N-Heterocyclic carbene (NHC) ligand such as IPr gave product in 38% (entry 5). The C–H arylation was then performed with three phosphine ligands bearing alkyl groups (entries 6–8), furnishing 6 of up to 62% isolated yield. From entry 9 to 12, the bidentate phosphine ligands had also been tested. However, they were shown to be less efficient toward C–H/C–Br coupling reactions, providing 6 in only 18–23% yields.
| Entry | Ligand | Yieldb (%) |
|---|---|---|
| a Direct C–H arylation of 3a (0.96 mmol) with 1 (0.30 mmol) was conducted under N2 in the presence of Pd(OAc)2 (15 mol%), ligand (30 mol%), pivalic acid (60 mol%), and K2CO3 (1.08 mmol) in DMF (3 mL) at 125 °C for 30 h.b Isolated yields.c 1 mL DMF was used. | ||
| 1 | PPh3 | 38 |
| 2 | P(o-tolyl)3 | 57 |
| 3 | P(m-tolyl)3 | 29 |
| 4 | Tris(o-methoxyphenyl)phosphine | 53 |
| 5 | IPr | 38 |
| 6 | P(Cy)3 | 51 |
| 7 | P(adamantyl)2(nBu) | 62 |
| 8 | JohnPhos | 35 |
| 9 | dppe | 20 |
| 10 | dppp | 23 |
| 11 | Xantphos | 21 |
| 12 | DPEphos | 18 |
| 13c | P(o-tolyl)3 | 70 |
| 14c | P(adamantyl)2(nBu) | 67 |
According to above results, we selected P(o-tolyl)3 (entry 2) and P(adamantyl)2(nBu) (entry 7) as the optimum ligands for additional examinations. In last two entries (13 and 14), the direct C–H arylations proceeded under a more concentrated condition (solvent reduced to 1 mL) and we were pleased to find the yield of 6 could be further improved (67–70%).
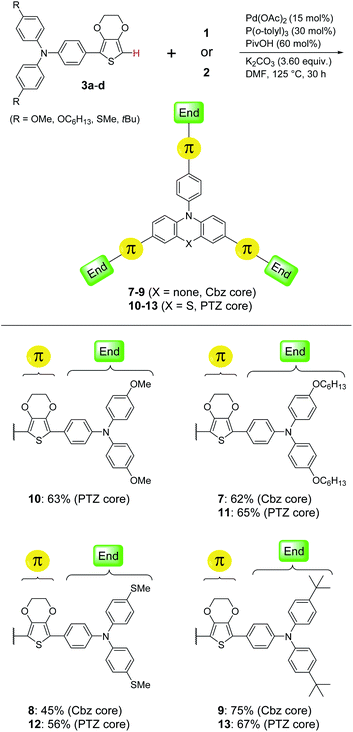
Therefore, in Scheme 2, we decided to use P(o-tolyl)3 for the efficient synthesis of other HTM molecules (7–9 with a Cbz core; 10–13 with a PTZ core) because of its lower commercial price. In addition to the methoxy group (–OMe) that was often equipped with small-molecule HTMs, we also explored other potential and previously used end-substituents11 including n-hexyloxy group (–OC6H13), methyl sulfide group (–SMe) and tertiary-butyl group (–tBu). Reaction of 3b (R = OC6H13) with either core-1 (Cbz) or -2 (PTZ) under optimum reaction conditions produced 7 or 11 in moderate isolated yields (62%; 65%). These two compounds exhibited fairly good solubility in common organic solvents owing to their longer alkyl chains, which might be beneficial to follow-up solution-based device fabrication and film formation through spin-coating techniques.
Next, we investigated the optical, electrochemical, electrical and thermal properties of synthesized 6–13 and the results were summarized in Table 2 (relevant spectra were provided in ESI†). In general, the PTZ core-based 10–13 possess smaller optical bandgaps (ΔEg = 2.56–2.57 eV) than Cbz core-based 6–9 (ΔEg = 2.73–2.81 eV). Based on the electrochemical measurements and calculations, we obtained each EHOMO (the highest occupied molecular orbital) of eight HTM molecules, all of which were located between the EHOMO of perovskite layer (MAPbI3, EHOMO = −5.43 eV) and Ag electrode (EHOMO = −4.20 eV), revealing that 6–13 had appropriate energy levels to serve as potential hole-transport materials. In addition, the hole-mobility (μh) of 6–13 was evaluated by measuring their current density–voltage (J − V) in the region of space-charge limited current (SCLC). It was found that, HTMs bearing –tBu groups (9 and 13) exhibited outstanding hole mobilities (μh = 6.46 × 10−4 and 7.53 × 10−4 cm2 V−1 s−1) that are close to the μh of spiro-OMeTAD (8.38 × 10−4 cm2 V−1 s−1). Among all HTMs, the molecules with –SMe groups (8 and 12) showed the lowest hole mobilities (μh = 1.35 × 10−4 and 6.68 × 10−5 cm2 V−1 s−1), which implied that –SMe might not be a suitable substituent for the design of small-molecule HTMs. We also obtained the thermal analysis data of all HTMs. It is interesting to note that, except for hole mobilities, 9 and 13 (with tBu groups) also showed superior decomposition temperature (Td = 426 °C; 415 °C) and glass transition temperature (Tg = 223 °C; 228 °C) than those of other HTM molecules. Among eight HTMs, 7 and 11 carrying long alkoxyl groups (–OC6H13) demonstrated obviously lower Tg (88–91 °C), presumably resulting from the thermally caused motion of alkyl chains.27,28 Furthermore, we studied the hole-extraction capability of eight molecules by conducting the experiments of steady-state photoluminescence (PL). The required devices were fabricated as glass/MAPbI3/6–13, respectively. The spectra provided in Fig. 2 revealed that 9 & 13 (tBu groups) had remarkable PL quenching abilities. This implied they could extract holes efficiently from the interface between perovskite- and hole-transport layers, whereas the 8 & 12 (–SMe groups) showed poor hole-extraction capabilities.29 We speculated this might be attributed to the inferior coordination of methyl sulfide groups to the perovskite layer.
| HTMs | ΔEga [eV] | EHOMOb [eV] | ELUMOc [eV] | Hole mobility (cm2 V−1 s−1) | Td [°C] | Tg [°C] |
|---|---|---|---|---|---|---|
| a ΔEg was calculated based on the spectra of UV/vis absorption and photoluminescence (measurements performed in CH2Cl2).b EHOMO = −[E1/2 (vs. Ag/AgClsat'd) + 0.197 (vs. NHE) + 4.500] eV (the electrochemical experiments were performed in CH2Cl2).c ELUMO = EHOMO + ΔEg. | ||||||
| 6 | 2.76 | −5.21 | −2.45 | 1.71 × 10−4 | 370 | 177 |
| 7 | 2.73 | −5.20 | −2.47 | 1.59 × 10−4 | 396 | 88 |
| 8 | 2.81 | −5.25 | −2.46 | 1.35 × 10−4 | 406 | 192 |
| 9 | 2.78 | −5.21 | −2.43 | 6.46 × 10−4 | 426 | 223 |
| 10 | 2.57 | −5.16 | −2.59 | 2.34 × 10−4 | 406 | 184 |
| 11 | 2.56 | −5.20 | −2.64 | 4.10 × 10−4 | 382 | 91 |
| 12 | 2.57 | −5.22 | −2.65 | 6.68 × 10−5 | 387 | 192 |
| 13 | 2.56 | −5.20 | −2.64 | 7.53 × 10−4 | 415 | 228 |
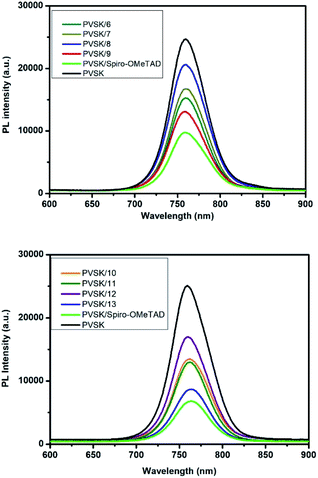 | ||
| Fig. 2 The steady-state photoluminescence spectra of devices fabricated as: glass/MAPbI3 (perovskite layer)/6–13 (hole-transport layer). | ||
Device fabrication of perovskite solar cells using 6–13 as hole-transporting layers was detailed in ESI.† In general, 6–13 was each dissolved in chlorobenzene and the solutions were heated to 90–100 °C to ensure the HTM molecule was completely dissolved. Each solution was then doped with lithium bis(trifluoromethane)sulfonimide (Li-TFSI) and 4-tert-butylpyridine (TBP). After the PSC devices were obtained via spin-coating techniques, we evaluated their photovoltaic performances and the data were summarized in Table 3. It is interesting to note that PSCs based on 9 and 13 (with tBu groups) showed exceptional power conversion efficiencies (PCEs = 17.51%, 17.57%) regardless of the variation of their core molecules (Cbz or PTZ), which is comparable to the PCE of reference cells (spiro-OMeTAD, PCE = 17.65%). Based on the high Tg of 9 and 13 shown in last section (Table 2), we speculated that PSC devices incorporating 9- or 13 as hole-transporting layer might possess more stable film morphology at high operational temperature, thus leading to higher PCEs. In addition, we found that there was no significant difference in PCEs of the solar cells fabricated with HTM molecules bearing either –OMe groups (6 & 10) or the longer –OC6H13 chains (7 & 11), all of which demonstrated excellent PCEs of 15.28–17.10%. PSCs with 8 & 12 (–SMe groups), as expected, displayed relatively poor PCEs (3.95%, 7.78%), which is consistent with the hole-mobility data acquired in Table 2.
| HTMs | Voc [V] | Jsc [mA cm−2] | FF [%] | PCEs [%] | |
|---|---|---|---|---|---|
| a Reverse scans.b Statistical data were calculated based on 6–8 cells. | |||||
| 6 | Best | 1.05 | 21.41 | 75.50 | 16.96 |
| Average | 1.02 ± 0.02 | 21.25 ± 0.42 | 73.40 ± 2.50 | 15.96 ± 0.92 | |
| 7 | Best | 1.04 | 19.85 | 74.30 | 15.28 |
| Average | 0.98 ± 0.04 | 19.73 ± 1.34 | 70.40 ± 5.50 | 13.61 ± 1.21 | |
| 8 | Best | 0.87 | 19.99 | 22.80 | 3.95 |
| Average | 0.60 ± 0.22 | 16.86 ± 3.36 | 30.90 ± 7.30 | 2.98 ± 0.93 | |
| 9 | Best | 1.02 | 22.18 | 77.40 | 17.51 |
| Average | 1.03 ± 0.01 | 22.00 ± 0.25 | 74.30 ± 2.20 | 16.82 ± 0.56 | |
| 10 | Best | 1.06 | 21.37 | 73.30 | 16.61 |
| Average | 1.02 ± 0.03 | 19.84 ± 1.39 | 74.30 ± 2.50 | 15.04 ± 1.04 | |
| 11 | Best | 1.03 | 22.57 | 73.60 | 17.10 |
| Average | 1.01 ± 0.01 | 22.07 ± 0.40 | 70.60 ± 6.80 | 15.82 ± 1.77 | |
| 12 | Best | 0.87 | 14.68 | 61.00 | 7.78 |
| Average | 0.81 ± 0.14 | 12.14 ± 1.63 | 66.00 ± 7.00 | 6.38 ± 1.24 | |
| 13 | Best | 1.09 | 23.44 | 68.80 | 17.57 |
| Average | 1.05 ± 0.02 | 22.20 ± 1.26 | 70.10 ± 1.00 | 16.42 ± 1.08 | |
| spiro-OMeTAD | Best | 1.05 | 22.50 | 74.50 | 17.65 |
| Average | 1.04 ± 0.01 | 21.59 ± 0.82 | 74.40 ± 1.60 | 16.77 ± 0.78 | |
Conclusions
Based on the optimized direct C–H arylation approach, we have efficiently synthesized eight new molecules bearing carbazole (6–9) or phenothiazine (10–13) cores in moderate to good isolated yields. Compared to traditional multistep synthesis, this step-saving synthetic methodology omitted previously required prefunctionalization steps such as deprotonative lithiation and transmetalation/stannylation. Perovskite solar cells fabrication using 6–13 as hole-transporting materials demonstrated power conversion efficiencies of 3.95–17.57%. Most noteworthy was that t-butyl substituted HTMs (9 and 13) exhibited exceptional hole-mobilities, thermal properties (high Td and Tg), and the devices based on 9 or 13 displayed very promising power conversion efficiencies of 17.51–17.57%. Development of succinct synthetic routes combining direct C–H activation/arylation reactions30 for the facile preparation of new small-molecule HTMs is currently underway in our laboratory.Experimental
General procedure for the synthesis of 6–13
To a solution of Pd(OAc)2 (15 mol%), P(o-tolyl)3 (30 mol%), PivOH (60 mol%) and K2CO3 (3.60 equiv.) in DMF (3 mL) in a flame-dried Schlenk flask were added 3,6-dibromo-9-(4-bromophenyl)-9H-carbazole (1) or 3,7-dibromo-10-(4-bromophenyl)-10H-phenothiazine (2) (0.30 mmol) and the corresponding end-groups (3a–d) (0.96 mmol) under N2. The reaction mixture was then heated at 125 °C under N2 for 30 h. After the reaction mixture had cooled to room temperature, water (10 mL) was added. The mixture was extracted with dichloromethane (2 × 20 mL), and the combined organic layers were washed with brine (50 mL), dried (Na2SO4) and concentrated in vacuo. Purification by flash chromatography afforded the desired products 6–13.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support provided by the Ministry of Science and Technology (MOST), Taiwan (MOST 108-2113-M-008-013 and 108-2628-E-182-003-MY3), National Central University (NCU), Chang Gung University (QZRPD181) and Chang Gung Memorial Hospital, Linkou, Taiwan (CMRPD2G0302 and CMRPD2J0041) are gratefully acknowledged. We also thank the instrument center (R & D office, NCU) for the technical support of NMR and mass analysis.Notes and references
- H. D. Pham, T. C.-J. Yang, S. M. Jain, G. J. Wilson and P. Sonar, Development of dopant-free organic hole transporting materials for perovskite solar cells, Adv. Energy Mater., 2020, 1903326 CrossRef CAS.
- C. Rodriguez-Seco, L. Cabau, A. Vidal-Ferran and E. Palomares, Advances in the synthesis of small molecules as hole transport materials for lead halide perovskite solar cells, Acc. Chem. Res., 2018, 51, 869–880 CrossRef CAS.
- S. Ameen, M. A. Rub, S. A. Kosa, K. A. Alamry, M. S. Akhtar, H.-S. Shin, H.-K. Seo, A. M. Asiri and M. K. Nazeeruddin, Perovskite solar cells: influence of hole transporting materials on power conversion efficiency, ChemSusChem, 2016, 9, 10–27 CrossRef CAS.
- L. Calió, S. Kazim, M. Grätzel and S. Ahmad, Hole-transport materials for perovskite solar cells, Angew. Chem., Int. Ed., 2016, 55, 14522–14545 CrossRef.
- M. L. Petrus, K. Schutt, M. T. Sirtl, E. M. Hutter, A. C. Closs, J. M. Ball, J. C. Bijleveld, A. Petrozza, T. Bein, T. J. Dingemans, T. J. Savenije, H. Snaith and P. Docampo, New generation hole transporting materials for perovskite solar cells: amide-based small-molecules with nonconjugated backbones, Adv. Energy Mater., 2018, 1801605 CrossRef.
- D. E. M. Rojas, K. T. Cho, Y. Zhang, M. Urbani, N. Tabet, G. la Torre, M. K. Nazeeruddin and T. Torres, Tetrathienoanthracene and tetrathienylbenzene derivatives as hole-transporting materials for perovskite solar cell, Adv. Energy Mater., 2018, 1800681 CrossRef.
- C. Steck, M. Franckevicius, S. M. Zakeeruddin, A. Mishra, P. Bäuerle and M. Grätzel, A–D–A-type S,N-heteropentacene-based hole transport materials for dopant-free perovskite solar cells, J. Mater. Chem. A, 2015, 3, 17738–17746 RSC.
- A. Abate, S. Paek, F. Giordano, J. P. C. Baena, M. Saliba, P. Gao, T. Matsui, J. Ko, S. M. Zakeeruddin, K. H. Dahmen, A. Hagfeldt, M. Grätzel and M. K. Nazeeruddin, Silolothiophene-linked triphenylamines as stable hole transporting materials for high efficiency perovskite solar cells, Energy Environ. Sci., 2015, 8, 2946–2953 RSC.
- C. Huang, W. Fu, C.-Z. Li, Z. Zhang, W. Qiu, M. Shi, P. Heremans, A. K.-Y. Jen and H. Chen, Dopant-free hole-transporting material with a C3h symmetrical truxene core for highly efficient perovskite solar cells, J. Am. Chem. Soc., 2016, 138, 2528–2531 CrossRef CAS.
- Y. Liu, Z. Hong, Q. Chen, H. Chen, W.-H. Chang, M. Yang, T.-B. Song and Y. Yang, Perovskite solar cells employing dopant-free organic hole transport materials with tunable energy levels, Adv. Mater., 2016, 28, 440–446 CrossRef CAS.
- Y.-C. Chang, K.-M. Lee, C.-H. Lai and C.-Y. Liu, Direct C–H arylation meets perovskite solar cells: tin-free synthesis shortcut to high-performance hole-transporting materials, Chem.–Asian J., 2018, 13, 1510–1515 CrossRef CAS; P.-H. Lin, K.-M. Lee, C.-C. Ting and C.-Y. Liu, Spiro-tBuBED: a new derivative of a spirobifluorene-based hole-transporting material for efficient perovskite solar cells, J. Mater. Chem. A, 2019, 7, 5934–5937 RSC; Y.-K. Peng, K.-M. Lee, C.-C. Ting, M.-W. Hsu and C.-Y. Liu, Making benzotrithiophene derivatives dopant-free for perovskite solar cells: step-saving installation of π-spacers by a direct C–H arylation strategy, J. Mater. Chem. A, 2019, 7, 24765–24770 RSC.
- A. Abudulimu, R. S. Torrientes, I. Zimmermann, J. Santos, M. K. Nazeeruddin and N. Martín, Hole transporting materials for perovskite solar cells and a simple approach for determining the performance limiting factors, J. Mater. Chem. A, 2020, 8, 1386–1393 RSC.
- K. Rakstys, S. Paek, A. Drevilkauskaite, H. Kanda, S. Daskeviciute, N. Shibayama, M. Daskeviciene, A. Gruodis, E. Kamarauskas, V. Jankauskas, V. Getautis and M. K. Nazeeruddin, Carbazole-terminated isomeric hole-transporting materials for perovskite solar cells, ACS Appl. Mater. Interfaces, 2020, 12, 19710–19717 CrossRef CAS.
- C. H. Teh, R. Daik, E. L. Lim, C. C. Yap, M. A. Ibrahim, N. A. Ludin, K. Sopian and M. A. M. Teridi, A review of organic small molecule-based hole transporting materials for meso-structured organic–inorganic perovskite solar cells, J. Mater. Chem. A, 2016, 4, 15788–15822 RSC.
- S. F. Völker, S. Collavini and J. L. Delgado, Organic charge carriers for perovskite solar cells, ChemSusChem, 2015, 8, 3012–3028 CrossRef.
- J. Wang, K. Liu, L. Ma and X. Zhan, Triarylamine: versatile platform for organic, dye-sensitized, and perovskite solar cells, Chem. Rev., 2016, 116, 14675–14725 CrossRef CAS.
- W. Zhou, Z. Wen and P. Gao, Less is more: dopant-free hole transporting materials for high-efficiency perovskite solar cells, Adv. Energy Mater., 2018, 1702512 CrossRef.
- J. U. Mora, I. G. Benito, A. M. Ontoria and N. Martín, Hole transporting materials for perovskite solar cells: a chemical approach, Chem. Soc. Rev., 2018, 47, 8541–8571 RSC.
- Z. Yu and L. Sun, Recent progress on hole-transporting materials for emerging organometal halide perovskite solar cells, Adv. Energy Mater., 2015, 5, 1500213 CrossRef.
- S. J. Park, S. Jeon, I. K. Lee, J. Zhang, H. Jeong, J.-Y. Park, J. Bang, T. K. Ahn, H.-W. Shin, B.-G. Kim and H. J. Park, Inverted planar perovskite solar cells with dopant free hole transporting material: Lewis base-assisted passivation and reduced charge recombination, J. Mater. Chem. A, 2017, 5, 13220–13227 RSC.
- D. R. Kil, C. Lu, J.-M. Ji, C. H. Kim and H. K. Kim, Dopant-free triazatruxene-based hole transporting materials with three different end-capped acceptor units for perovskite solar cells, Nanomaterials, 2020, 10, 936 CrossRef CAS.
- U. Bach, D. Lupo, P. Comte, J. E. Moser, F. Weissortel, J. Salbeck, H. Spreitzer and M. Grätzel, Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies, Nature, 1998, 395, 583–585 CrossRef CAS; U. Bach, Y. Tachibana, J. E. Moser, S. A. Haque, J. R. Durrant, M. Grätzel and D. R. Klug, Charge separation in solid-state dye-sensitized heterojunction solar cells, J. Am. Chem. Soc., 1999, 121, 7445–7446 CrossRef; M. Liu, M. B. Johnston and H. J. Snaith, Efficient planar heterojunction perovskite solar cells by vapour deposition, Nature, 2013, 501, 395–398 CrossRef; H. Zhou, Q. Chen, G. Li, S. Luo, T. B. Song, H. S. Duan, Z. Hong, J. You, Y. Liu and Y. Yang, Interface engineering of highly efficient perovskite solar cells, Science, 2014, 345, 542–546 CrossRef.
- Y. Wu, Z. Wang, M. Liang, H. Cheng, M. Li, L. Liu, B. Wang, J. Wu, R. P. Ghimire, X. Wang, Z. Sun, S. Xue and Q. Qiao, Influence of nonfused cores on the photovoltaic performance of linear triphenylamine-based hole-transporting materials for perovskite solar cells, ACS Appl. Mater. Interfaces, 2018, 10, 17883–17895 CrossRef CAS; Z. Li, Z. Zhu, C.-C. Chueh, S. B. Jo, J. Luo, S.-H. Jang and A. K.-Y. Jen, Rational design of dipolar chromophore as an efficient dopant-free hole-transporting material for perovskite solar cells, J. Am. Chem. Soc., 2016, 138, 11833–11839 CrossRef; B. Pashaei, S. Bellani, H. Shahroosvand and F. Bonaccorso, Molecularly engineered hole-transport material for low-cost perovskite solar cells, Chem. Sci., 2020, 11, 2429–2439 RSC.
- K. Rakstys, A. Abate, M. I. Dar, P. Gao, V. Jankauskas, G. Jacopin, E. Kamarauskas, S. Kazim, S. Ahmad, M. Grätzel and M. K. Nazeeruddin, Triazatruxene-based hole transporting materials for highly efficient perovskite solar cells, J. Am. Chem. Soc., 2015, 137, 16172–16178 CrossRef CAS; H. Choi, J. W. Cho, M. S. Kang and J. Ko, Stable and efficient hole transporting materials with a dimethylfluorenylamino moiety for perovskite solar cells, Chem. Commun., 2015, 51, 9305–9308 RSC; H. Choi, S. Park, S. Paek, P. Ekanayake, M. K. Nazeeruddin and J. Ko, Efficient star-shaped hole transporting materials with diphenylethenyl side arms for an efficient perovskite solar cell, J. Mater. Chem. A, 2014, 2, 19136–19140 RSC; H. Choi, S. Paek, N. Lim, Y. H. Lee, M. K. Nazeeruddin and J. Ko, Efficient perovskite solar cells with 13.63% efficiency based on planar triphenylamine hole conductors, Chem.–Eur. J., 2014, 20, 10894–10899 CrossRef; K. Do, H. Choi, K. Lim, H. Jo, J. W. Cho, M. K. Nazeeruddin and J. Ko, Star-shaped hole transporting materials with a triazine unit for efficient perovskite solar cells, Chem. Commun., 2014, 50, 10971–10974 RSC; B. Xu, E. Sheibani, P. Liu, J. Zhang, H. Tian, N. Vlachopoulos, G. Boschloo, L. Kloo, A. Hagfeldt and L. Sun, Carbazole-based hole-transport materials for efficient solid-state dye-sensitized solar cells and perovskite solar cells, Adv. Mater., 2014, 26, 6629–6634 CrossRef; M. S. Kang, S. D. Sung, I. T. Choi, H. Kim, M. P. Hong, J. Kim, W. I. Lee and H. K. Kim, Novel carbazole-based hole-transporting materials with star-shaped chemical structures for perovskite-sensitized solar cells, ACS Appl. Mater. Interfaces, 2015, 7, 22213–22217 CrossRef; C. Lu, I. T. Choi, J. Kim and H. K. Kim, Simple synthesis and molecular engineering of low-cost and star-shaped carbazole-based hole transporting materials for highly efficient perovskite solar cells, J. Mater. Chem. A, 2017, 5, 20263–20276 RSC; S. Thokala and S. P. Singh, Phenothiazine-based hole transport materials for perovskite solar cells, ACS Omega, 2020, 5, 5608–5619 CrossRef; J. Salunke, X. Guo, Z. Lin, J. R. Vale, N. R. Candeias, M. Nyman, S. Dahlström, R. Österbacka, A. Priimagi, J. Chang and P. Vivo, Phenothiazine-based hole-transporting materials toward ecofriendly perovskite solar cells, ACS Appl. Energy Mater., 2019, 2, 3021–3027 CrossRef; J. Salunke, X. Guo, M. Liu, Z. Lin, N. R. Candeias, A. Priimagi, J. Chang and P. Vivo, N-substituted phenothiazines as environmentally friendly hole transporting materials for low-cost and highly stable halide perovskite solar cells, ACS Omega, 2020, 5, 23334–23342 CrossRef; M. R. Maciejczyk, R. Chen, A. Brown, N. Zheng and N. Robertson, Beyond efficiency: phenothiazine, a new commercially viable substituent for hole transport materials in perovskite solar cells, J. Mater. Chem. C, 2019, 7, 8593–8598 RSC; X. Liu, X. Tan, Q. Chen, H. Shan, C. Liu, J. Xu, Z.-K. Chen, W. Huang and Z.-X. Xu, Facile synthesis of a dopant-free hole transporting material with a phenothiazine core for planar perovskite solar cells, RSC Adv., 2017, 7, 53604–53610 RSC.
- K.-M. Lu, K.-M. Lee, C.-H. Lai, C.-C. Ting and C.-Y. Liu, One-pot synthesis of D–π–D–π–D type hole-transporting materials for perovskite solar cells by sequential C–H (hetero)arylations, Chem. Commun., 2018, 54, 11495–11498 RSC.
- M. Li, Z. Wang, M. Liang, L. Liu, X. Wang, Z. Sun and S. Xue, Low-cost carbazole-based hole-transporting materials for perovskite solar cells: influence of S,N-heterocycle, J. Phys. Chem. C, 2018, 122, 24014–24024 CrossRef CAS; J. Zhang, B. Xu, M. B. Johansson, N. Vlachopoulos, G. Boschloo, L. Sun, E. M. J. Johansson and A. Hagfeldt, Strategy to boost the efficiency of mixed-ion perovskite solar cells: changing geometry of the hole transporting material, ACS Nano, 2016, 10, 6816–6825 CrossRef; J. Wu, C. Liu, B. Li, F. Gu, L. Zhang, M. Hu, X. Deng, Y. Qiao, Y. Mao, W. Tan, Y. Tian and B. Xu, Side-chain polymers as dopant-free hole-transporting materials for perovskite solar cells—the impact of substituents' positions in carbazole on device performance, ACS Appl. Mater. Interfaces, 2019, 11, 26928–26937 CrossRef; H. Wang, A. D. Sheikh, Q. Feng, F. Li, Y. Chen, W. Yu, E. Alarousu, C. Ma, M. A. Haque, D. Shi, Z.-S. Wang, O. F. Mohammed, O. M. Bakr and T. Wu, Facile synthesis and high performance of a new carbazole-based hole-transporting material for hybrid perovskite solar cells, ACS Photonics, 2015, 2, 849–855 CrossRef; J. A. Christians, P. Schulz, J. S. Tinkham, T. H. Schloemer, S. P. Harvey, B. J. T. Villers, A. Sellinger, J. J. Berry
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) and and J. M. Luther, Tailored interfaces of unencapsulated perovskite solar cells for >1000 hour operational stability, Nat. Energy, 2018, 3, 68–74 CrossRef; L. Gao, T. H. Schloemer, F. Zhang, X. Chen, C. Xiao, K. Zhu and A. Sellinger, Carbazole-based hole-transport materials for high-efficiency and stable perovskite solar cells, ACS Appl. Energy Mater., 2020, 3, 4492–4498 CrossRef; T. H. Schloemer, T. S. Gehan, J. A. Christians, D. G. Mitchell, A. Dixon, Z. Li, K. Zhu, J. J. Berry, J. M. Luther and A. Sellinger, Thermally stable perovskite solar cells by systematic molecular design of the hole-transport layer, ACS Energy Lett., 2019, 4, 473–482 CrossRef.
and and J. M. Luther, Tailored interfaces of unencapsulated perovskite solar cells for >1000 hour operational stability, Nat. Energy, 2018, 3, 68–74 CrossRef; L. Gao, T. H. Schloemer, F. Zhang, X. Chen, C. Xiao, K. Zhu and A. Sellinger, Carbazole-based hole-transport materials for high-efficiency and stable perovskite solar cells, ACS Appl. Energy Mater., 2020, 3, 4492–4498 CrossRef; T. H. Schloemer, T. S. Gehan, J. A. Christians, D. G. Mitchell, A. Dixon, Z. Li, K. Zhu, J. J. Berry, J. M. Luther and A. Sellinger, Thermally stable perovskite solar cells by systematic molecular design of the hole-transport layer, ACS Energy Lett., 2019, 4, 473–482 CrossRef. - K. He, W. Li, H. Tian, J. Zhang, D. Yan, Y. Geng and F. Wang, Asymmetric conjugated molecules based on [1]benzothieno[3,2-b][1]benzothiophene for high-mobility organic thin-film transistors: influence of alkyl chain length, ACS Appl. Mater. Interfaces, 2017, 9, 35427–35436 CrossRef CAS; S. Inoue, H. Minemawari, J. Tsutsumi, M. Chikamatsu, T. Yamada, S. Horiuchi, M. Tanaka, R. Kumai, M. Yoneya and T. Hasegawa, Effects of substituted alkyl chain length on solution-processable layered organic semiconductor crystals, Chem. Mater., 2015, 27, 3809–3812 CrossRef.
- I. Zimmermann, J. U. Mora, P. Gratia, J. Aragó, G. Grancini, A. M. Ontoria, E. Orti, N. Martin and M. K. Nazeeruddin, High-efficiency perovskite solar cells using molecularly engineered, thiophene-rich, hole-transporting materials: influence of alkyl chain length on power conversion efficiency, Adv. Energy Mater., 2016, 1601674 Search PubMed.
- Q. Wang, E. Mosconi, C. Wolff, J. Li, D. Neher, F. Angelis, G. P. Suranna, R. Grisorio and A. Abate, Rationalizing the molecular design of hole-selective contacts to improve charge extraction in perovskite solar cells, Adv. Energy Mater., 2019, 9, 1900990 CrossRef CAS; S. Lee, J. Lee, H. Park, J. Choi, H. W. Baac, S. Park and H. J. Park, Defect-passivating organic/inorganic bicomponent hole-transport layer for high efficiency metal-halide perovskite device, ACS Appl. Mater. Interfaces, 2020, 12, 40310–40317 CrossRef.
- L. Ackermann, Metalla-electrocatalyzed C–H activation by earth-abundant 3d metals and beyond, Acc. Chem. Res., 2020, 53, 84–104 CrossRef CAS; P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz and L. Ackermann, 3d Transition metals for C–H activation, Chem. Rev., 2019, 119, 2192–2452 CrossRef; L. Ackermann, R. Vicente and A. R. Kapdi, Transition-metal-catalyzed direct arylation of (hetero)arenes by C–H bond cleavage, Angew. Chem., Int. Ed., 2009, 48, 9792–9826 CrossRef; T. Yanagi, S. Otsuka, Y. Kasuga, K. Fujimoto, K. Murakami, K. Nogi, H. Yorimitsu and A. Osuka, Metal-free approach to biaryls from phenols and aryl sulfoxides by temporarily sulfur-tethered regioselective C–H/C–H coupling, J. Am. Chem. Soc., 2016, 138, 14582–14585 CrossRef; C. Lu, M. Paramasivam, K. Park, C. H. Kim and H. K. Kim, Phenothiazine functionalized multifunctional A–π–D–π–D–π–A-type hole-transporting materials via sequential C–H arylation approach for efficient and stable perovskite solar cells, ACS Appl. Mater. Interfaces, 2019, 11, 14011–14022 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available: NMR spectra (1H and 13C) of compounds 6–13. See DOI: 10.1039/d0ra10190g |
| This journal is © The Royal Society of Chemistry 2021 |