Open Access Article
Open Access ArticleDemonstration of the photothermal catalysis of the Sabatier reaction using nickel nanoparticles and solar spectrum light†
Timothy McCormick Steeves and
Aaron P. Esser-Kahn *
*
Pritzker School of Molecular Engineering, University of Chicago, USA. E-mail: aesserkahn@uchicago.edu
First published on 23rd February 2021
Abstract
The promise of light-to-chemical energy conversion has led researchers to explore photo-thermal processes for chemical reactivity of nanoparticles. Previous work has examined particles as an element of a supported catalyst, but not as an unsupported nanopowder. We present a preliminary examination of a photo-thermal Sabatier reaction performed on suspended Ni nanoparticles. This new system performs a catalytic Sabatier reaction at lower bulk temperatures than reported for a standard reaction, driven by light. This result occurs only when the particles are suspended freely in a gaseous mixture; implying that particle isolation enhances photo-thermal catalysis.
Introduction
Heterogeneous catalysis is a critical step in many industrial processes, from the formation of ammonia to the conversion of CO2 into methane.1,2 In academic research, such catalytic reactions use a variety of energy sources, including photocatalysts that use light through electron injection, as well as through traditional heating, via an oven or other external heat source.3,4 Both photocatalytic and thermal catalysts can perform CO2 methanation. Many of these academically tested methods have limitations of efficiency, heat loss/transfer and cost that hinder industrial implementation. Moreover, innovation in these processes comes both from improvements in the catalysts and improvements in reactor conditions. In this report, we focus on a finding which shows potential has implications for reactor design. In thermal processes, energy is expended to maintain the reactor at an appropriate temperature, which can be mitigated by reducing mass of catalyst. In photocatalytic processes, thermal energy loss impacts both efficiency and reactor design.5,6 Recent research into photothermal heating of nanomaterials could have unique benefits for heterogeneous catalysis as it relates to reactor design. Photothermal heating offers high efficiency conversion from light to heat and high local temperatures at the catalytic surface, but low bulk temperatures of the system.7–13 In this communication we demonstrate that the photothermal heating of simple, ball-milled nickel nanoparticles perform the Sabatier reaction via photothermal heating using broad wavelength solar spectrum light. The Sabatier process is the conversion of CO2 and H2 on the surface of a metal catalyst to form CH4 and H2O.3,14 The reaction occurs while the reactor temperature remains below those previously reported for this type of catalysis (see Table 2). This reduction in bulk heating is achieved by altering the reaction conditions – dispersing the nanoparticles throughout the reaction mixture via strong mechanical agitation. The particles become active while surrounded by reactant rather than resting on a support layer. We selected the Sabatier reaction as model reaction for the examination of this process due to (1) its well-studied kinetics and (2) its need for controlled heat in a heterogeneous reactor, and finally (3) its potential use in sustainable energy production. Other pioneering studies with supported particles have demonstrated the potential of a photothermal Sabatier process using Ru nanoparticles or supported Ni catalysts, but heat a catalytic bed.15,16 Another study constructed selectively absorbent nanosheets coated in single-Ni atoms, that prevented thermal energy from radiating out of the system.17 In contrast, the photo-thermal reactor performs reactions at reduced temperatures and the reaction only occurs when nanoparticles are dispersed throughout the reactant gas mixture. We report on the unique aspects and potential benefits of this photothermal catalysis. These include, (1) distinct product distributions of the Sabatier process which deviate other reports of heterogeneous Ni catalysts, (2) the contribution of heating in the particle bed vs. dispersed particles. From these observations, we hypothesize that particle isolation and the subsequent high heat gradients lead to a distinct reactivity profile. In addition, we report a rate of reaction for a batch process of 1.3 mmol h−1 production of methane, from total conversion of CO2 to CH4 in a small batch reaction illuminated by light with intensity of 12 kW m−2, well within the range of current solar concentration technology.18 A simple approach to catalyzing this reaction at low temperatures with only sunlight has been successfully demonstrated and could provide the basis for further investigation of heterogenous catalysis with unsupported nanoparticles (Table 1).| Time (min) | Nickel (g) | Light intensity | mL methane | mmol methane | Unadjusted efficiency |
|---|---|---|---|---|---|
| 20 | 10.0 | 12 kW m−2 | 7.6 | 0.31 | 0.09 mmol h−1 g−1 |
| 180 | 10.0 | 2 kW m−2 | 0.85 | 0.034 | 0.001 mmol h−1 g−1 |
| Source | Catalyst temperature (°C) | Light intensity (kW m−2) | Catalyst | Selectivity | Catalyst efficiency mmol (g h)−1 |
|---|---|---|---|---|---|
| This publication | 170 | 20 | Nickel nanoparticles | At least 99.9% | 0.09 |
| This publication | 100 | 2 | Nickel nanoparticles | At least 99.9% | 0.001 |
| Sastre et al. 2019 (ref. 21) | 230 | 10.1 | Ruthenium | 52 | |
| O'Brien et al. 2018 (ref. 15) | 150 | 2.47 | Ruthenium film on inverted silicon opal crystal | 2.8 | |
| Jantarang et al. 2018 (ref. 22) | 285 | 300 W Xe lamp | Ni on CexTiyO2 supports | Not reported | 17 |
| Jelle et al. 2018 (ref. 23) | 170 | 22 | Ruthenium on TiO2 nanoparticles | 4.4 | |
| Meng et al. 2014 (ref. 24) | 365 | 300 W Xe lamp | Ruthenium on alumina support | 99.22 | 18.16 |
Results and discussion
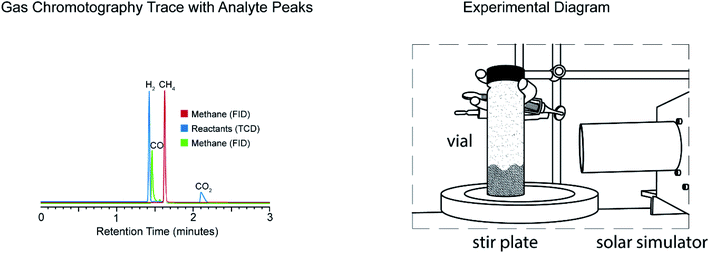
In previous work, we determined that photothermal heating of CO2 capture fluids with carbon black nanoparticles was sufficient to release CO2, achieving reactivity normally observed at 120 °C at bulk temperatures of 60 °C.19 In this work, we extended this idea to heterogeneous catalysis. To simplify our test system, we selected a gaseous reaction to minimize thermal transfer to the medium. We selected the Sabatier reaction for its utility – conversion of CO2 into methane and its well documented thermal reaction profile – the production of CO and CH4 as a function of bulk temperature. We wanted to examine if the local heating we observed in CO2 capture solutions, could be translated to the activation of CO2 for catalytic conversion and how the lower bulk-temperatures might alter the product distribution.In our initial experiments, we designed a simplified reactor which consisted of (1) a light source with variable intensity from 1 to 20 kW m−2, (2) a stoichiometric mixture of Sabatier reactants (H2/CO2 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and (3) nickel nanoparticles dispersed within the sealed, stirred vessel (Fig. 1). In our initial test system, 10.0 g of nickel nanoparticles (99.5%, 300 nm, SkySpring Nanomaterials) were placed in a 40 mL gas sample vial. Absorbance and BET analysis of particles was performed, data available in ESI.† The vial was purged three times with a stoichiometric reactant mixture (H2
1), and (3) nickel nanoparticles dispersed within the sealed, stirred vessel (Fig. 1). In our initial test system, 10.0 g of nickel nanoparticles (99.5%, 300 nm, SkySpring Nanomaterials) were placed in a 40 mL gas sample vial. Absorbance and BET analysis of particles was performed, data available in ESI.† The vial was purged three times with a stoichiometric reactant mixture (H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 4
CO2 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), with the end result being a vial filled with 0.7 mmol CO2 and 2.8 mmol H2. During the reaction, a positive pressure of either hydrogen or additional reactant mixture was applied via balloon. No CO was detected in these experiments, with an instrumental detection limit of 0.25% (see ESI† for details).
1), with the end result being a vial filled with 0.7 mmol CO2 and 2.8 mmol H2. During the reaction, a positive pressure of either hydrogen or additional reactant mixture was applied via balloon. No CO was detected in these experiments, with an instrumental detection limit of 0.25% (see ESI† for details).
After screening different illumination levels, we observed that at an intensity of 4 kW m−2 nanoscale heating achieved the highest CH4 production. At these conditions, the reactor vial temperature as measured by thermocouple never rose above 90 °C (see ESI† for details). This temperature is more than 100 °C below temperatures observed for Sabatier reactions with Ni.16,20 While the conversion and efficiency are not optimized in this system, this result demonstrates the possibility of running this reaction with low bulk-temperatures. Further optimization and reactor design would be required to generate an efficient system using this approach if it is even possible at all.
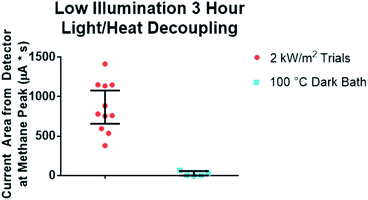
This surprising result led us to examine if photothermal heating of the particles was responsible. To do this, we performed several control experiments. We first tested if the bulk temperature of the reaction could be responsible on its own by heating a particle/gas mixture via an oil-bath without light. An oil bath was set at a temperature of 105 °C and a vial was covered in aluminum foil to prevent light from entering. The system was then held in equilibrium at the max temperature for the equivalent time as the test reaction. Methane production was compared to identify the difference in reactivity. We observed that the bulk temperature alone was insufficient to account for the conversion we observed (Fig. 2, -light control). Next, we sought to test if the particle isolation was responsible for the conversion. In this control, the reaction vial was setup in an identical fashion, but not stirred. In this control, at intensities of 4–12 kW m−2, the unstirred particle control did not produce methane at measurable levels. At intensities above 12 kW m−2, results were inconsistent. The reactor occasionally produced comparable amounts of methane to the light-free thermal controls and other times none. These two controls leave the most reasonable explanation for the high conversion and unique product distribution as the result of the agitated particles being heated beyond the temperature of the reaction vial during their agitation. From these experiments we conclude that photo-thermal heating of the particles can perform a Sabatier reaction without bulk heating of the reaction.
While the low bulk temperature provides an interesting example for a new design of the catalytic process, for a Sabatier process, high catalytic efficiency is often considered a critical factor when comparing between systems. This system is only an experimental demonstration. It remains far from optimized and only a small portion of the particles are even being used for catalysis at any given time. We've done rudimentary analysis of the effective dispersed density of the particles when stirred, by comparing the reduction in illumination in the vial to a derived Beer's law curve (see ESI†). It does show how a reactor employing photo-thermal heating might operate and thus explored an a lower-limit of what might be possible with these processes. In most photo-catalytic processes, high efficiency results from high intensity and subsequent high bulk-temperatures of the catalyst bed. To determine the efficiency of photothermal particle dispersions, we examined the reactivity by increasing the illumination to 12, then 20 kW m−2. These experiments show how photothermal heating of particles might be applied in a large-scale catalytic process, where bulk temperatures already rise to above 200 °C from the exothermic nature of the reaction and increased intensity. A summary of these results, as well as a comparison to our decoupled lower intensity systems, is shown in Table 2.
While this process remains unoptimized, to provide context for the reader we compared our process with reported efficiency values for photocatalytic systems. Table 2 includes a summary of our experimental efficiencies from our systems along with reported values from recent literature.
Conclusions
In conclusion, we identified photothermal heating of nanoparticles as a method for heterogeneous catalysis processes that require high temperatures. Our test system uses the Sabatier reaction on nickel nanoparticles. By tuning the illumination level, we controlled the bulk temperature and reactivity of our system. In low irradiance, reactivity derives predominantly from nanoscale heating. At higher irradiances closer to focused light applications, high overall efficiency was achieved over a thermal only control – indicating improved yield from photo-thermal catalysis.The study represents the first example of using suspended nanoparticles to achieve high temperature reactions while maintaining low bulk temperatures – suggesting that photo-thermal heating of nanoparticles might hold promise as a method for heterogeneous catalysis. In addition, the specific Sabatier reaction shown here compares well with other published methods indicating its potential use in CO2 reduction or methane production. This study is preliminary, and further examination of catalyst stability and reusability is necessary to quantify industrial viability. Regardless, we are excited by the potential that this new approach to photo-thermal catalysis might offer for reactor design and new process design. Reactions such as ammonia synthesis, ethylene epoxidation, or any number of other carbon fixing reactions are all excellent candidates for localized high-temperature reactions.
Finally, there is tremendous opportunity for further optimization of this system. The need for the particles to be suspended means that only a small fraction of particles is active at any given time. Advances in reactor design could allow for constant use of almost all particles, increasing efficiency and potentially allowing photothermally-driven heterogeneous catalysis to be competitive or improve on other modern methanation processes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the Pritzker School of Molecular Engineering for their financial and institutional support. Thank you to the Yang and the University of Chicago Mass Spectrometry Facility for use of their respective Gas Chromatography setups. Thank you to Adarsh Suresh for running the BET experiments for this project and to Mason Dearborn for assisting with final experiments.References
- P. Sabatier – Nobel Lecture, The Method of Direct Hydrogenation by Catalysis, https://www.nobelprize.org/nobel_prizes/chemistry/laureates/1912/sabatier-lecture.html, (accessed 1 February 2018) Search PubMed.
- G. Ertl, Angew. Chem., Int. Ed., 2008, 47, 3524–3535 CrossRef CAS.
- K. R. Thampi, J. Kiwi and M. Grätzel, Nature, 1987, 327, 506–508 CrossRef CAS.
- C. Kim, S. Hyeon, J. Lee, W. D. Kim, D. C. Lee, J. Kim and H. Lee, Nat. Commun., 2018, 9, 3027 CrossRef.
- K. P. Brooks, J. Hu, H. Zhu and R. J. Kee, Chem. Eng. Sci., 2007, 62, 1161–1170 CrossRef CAS.
- C. Junaedi, Compact and Lightweight Sabatier Reactor for Carbon Dioxide Reduction, 41st International Conference on Envionmental Systems, Portland, OR, United States, 2011 Search PubMed.
- O. Neumann, A. D. Neumann, S. Tian, C. Thibodeaux, S. Shubhankar, J. Müller, E. Silva, A. Alabastri, S. W. Bishnoi, P. Nordlander and N. J. Halas, ACS Energy Lett., 2017, 2, 8–13 CrossRef CAS.
- S. Ishii, R. P. Sugavaneshwar and T. Nagao, J. Phys. Chem. C, 2016, 120, 2343–2348 CrossRef CAS.
- X. Wang, Y. He, X. Liu, L. Shi and J. Zhu, Sol. Energy, 2017, 157, 35–46 CrossRef CAS.
- O. Neumann, A. S. Urban, J. Day, S. Lal, P. Nordlander and N. J. Halas, ACS Nano, 2013, 7, 42–49 CrossRef CAS.
- R. Joseph Fortenbaugh and B. J. Lear, Nanoscale, 2017, 9, 8555–8559 RSC.
- K. M. Haas and B. J. Lear, Chem. Sci., 2015, 6, 6462–6467 RSC.
- O. Neumann, C. Feronti, A. D. Neumann, A. Dong, K. Schell, B. Lu, E. Kim, M. Quinn, S. Thompson, N. Grady, P. Nordlander, M. Oden and N. J. Halas, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 11677–11681 CrossRef CAS.
- S. Rönsch, J. Schneider, S. Matthischke, M. Schlüter, M. Götz, J. Lefebvre, P. Prabhakaran and S. Bajohr, Fuel, 2016, 166, 276–296 CrossRef.
- P. G. O'Brien, K. K. Ghuman, A. A. Jelle, A. Sandhel, T. E. Wood, J. Y. Y. Loh, J. Jia, D. Perovic, C. V. Singh, N. P. Kherani, C. A. Mims and G. A. Ozin, Energy Environ. Sci., 2018, 11, 3443–3451 RSC.
- E. T. Kho, S. Jantarang, Z. Zheng, J. Scott and R. Amal, Engineering, 2017, 3, 393–401 CrossRef.
- Y. Li, J. Hao, H. Song, F. Zhang, X. Bai, X. Meng, H. Zhang, S. Wang, Y. Hu and J. Ye, Nat. Commun., 2019, 10, 2359 CrossRef.
- M. Khamooshi, H. Salati, F. Egelioglu, A. Hooshyar Faghiri, J. Tarabishi and S. Babadi, Int. J. Photoenergy, 2014, 2014, 1–17 CrossRef.
- D. T. Nguyen, R. Truong, R. Lee, S. A. Goetz and A. P. Esser-Kahn, Energy Environ. Sci., 2014, 7, 2603–2607 RSC.
- X. Su, J. Xu, B. Liang, H. Duan, B. Hou and Y. Huang, J. Energy Chem., 2016, 25, 553–565 CrossRef.
- F. Sastre, C. Versluis, N. Meulendijks, J. Rodríguez-Fernández, J. Sweelssen, K. Elen, M. K. Van Bael, T. den Hartog, M. A. Verheijen and P. Buskens, ACS Omega, 2019, 4, 7369–7377 CrossRef CAS.
- S. Jantarang, E. C. Lovell, T. H. Tan, J. Scott and R. Amal, Prog. Nat. Sci.: Mater. Int., 2018, 28, 168–177 CrossRef CAS.
- A. A. Jelle, K. K. Ghuman, P. G. O'Brien, M. Hmadeh, A. Sandhel, D. D. Perovic, C. V. Singh, C. A. Mims and G. A. Ozin, Adv. Energy Mater., 2018, 8, 1702277 CrossRef.
- X. Meng, T. Wang, L. Liu, S. Ouyang, P. Li, H. Hu, T. Kako, H. Iwai, A. Tanaka and J. Ye, Angew. Chem., 2014, 126, 11662–11666 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed descriptions of experimental procedures and gas chromatography analysis, methane calibration curve and procedure, characterization of the particles by XPS and SEM, details of our estimation of active catalyst by way of a Beer's law curve for suspended Ni nanoparticles and BET surface adsorption data. See DOI: 10.1039/d0ra09939b |
| This journal is © The Royal Society of Chemistry 2021 |