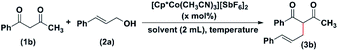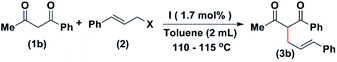Open Access Article
Open Access ArticleMonoallylation and benzylation of dicarbonyl compounds with alcohols catalysed by a cationic cobalt(III) compound†
Mohan Chandra Sau,
Smita Mandal and
Manish Bhattacharjee *
*
Department of Chemistry, Indian Institute of Technology Kharagpur, Kharagpur 721302, India. E-mail: mxb@iitkgp.ac.in
First published on 1st March 2021
Abstract
Monoallylation and monoalkylation of diketones and β-keto esters with allylic and benzylic alcohols catalysed by [Cp*Co(CH3CN)3][SbF6]2 (I) are reported. The method does not require any additive and affords regioselective products. The mechanistic investigations were done by in situ 1H NMR spectroscopy as well as control experiments. It has been shown that reactions proceed via η3-allyl complex formation or ally ether intermediate. The alkylation takes place via only ether intermediate. The resulting allylated and alkylated products have been used for the synthesis of eleven new trisubstituted pyrazoles and one pyrazolone.
Introduction
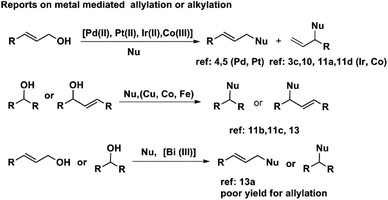
Allylation and alkylation of active methylene compounds are essential in synthetic organic chemistry. Usually, allyl halides and alkyl halides are used for allylation in the presence of a base. However, these reactions are not atom economical or green, as a large amount of salts is generated. Allylation and alkylation using the corresponding alcohol are attractive. The reaction is green as only water is produced as the side product.1 The selective mono-allylated diketones are used as a precursor for synthesising natural and unnatural bioactive molecules.2The hydroxyl group is a weak leaving group. Usually, the alcohols are converted into acetate, carbonate, or halide for smooth allylation and alkylation reactions.3 In addition to the Pd-catalysed Tsuji–Trost reaction,1a,1b which proceeds through palladium π-allyl intermediate, complexes of palladium,4 platinum,5 ruthenium,6 indium,1c molybdenum,7 gold,8 and rhodium9 have been used as catalysts for this kind of allylation reaction. The palladium-based catalysts predominantly afford the linear allylated products, although some diallyl products are also obtained. When complexes of iridium,3c,10 and rhodium, are used as catalysts, branched allylation products were found to be the primary products. Recently, Ohshima et al. reported selective monoallylation of active methylene compound using 2 mol% [Pt(cod)Cl2] 2 mol% xantphos as the ligand along with 10 mol% each, of pyrrolidine, and acetic acid as co-catalysts. However, this catalytic system is useful for only allylic alcohols.5b Cobalt compounds have been used as catalysts for allylation reaction.11 For example, cobalt carbonyl compounds, [(crotyl)Co(CO)3], and Na[Co(CO)4] have been shown to catalyse allylation using ally formate, chloride, and acetate. However, the conversions were found to be low.11a Similarly, cobalt(III) DMG (DMG = dimethylglyoxime) complex was used as a catalyst for the allylation using allyl alcohol.11b But the conversions were low, and the catalyst loading was comparatively higher (5 mol%), and acetic acid was used as the reaction medium. Ackermann and coworkers have reported cobalt(III) catalysed allylative C–H and C–F functionalisation.11e It may be pointed out that most of the reported methods either require additives or are not regioselective. Some of the methods afford a mixture of diallylated and monoallylated products (Scheme 1). Therefore, the development of an additive-free strategy for regioselective allylic substitution is highly desirable.
Recently a high valent cobalt complex, Cp*Co(CO)2I2 has been used as a precatalyst for dehydrogenation of alcohols.12 High valent cationic cobalt complex has been shown to catalyse directed C–H bond activation.13
Lewis acids or Brønsted acids,14 including InCl3,1c Bi(OTf)3,15a FeCl3,15b carbon-supported iron – ionic liquid,15c and PTSA,16 have been used for the benzylation of active methylene compounds by benzylic alcohols. To the best of our knowledge, there is only one report on the benzylation and allylation of β-keto carbonyl compounds using the same catalyst. It has been reported that Bi(OTf)3 can catalyse both allylation and alkylation of active methylene compounds.14 The yields of benzylated products were found to be excellent. Allylation reactions were found to be useful only for cinnamyl alcohol, and the products were obtained in moderate yield. The selective allylation and alkylation reaction with cobalt complexes as catalysts are preferable because its compounds are comparatively inexpensive than those of rhodium, iridium, and palladium.
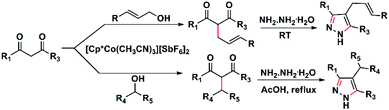
Recently we have shown that [Cp*Co(CH3CN)3][SbF6]2 (I) is an efficient catalyst for the Nakamura reaction.17 In continuation of our studies on the catalytic activity of [Cp*Co(CH3CN)3][SbF6]2 (I), herein, we report the monoallylation and benzylation of diketones and β-keto esters using allylic alcohols and secondary benzyl alcohols, respectively. Eleven new pyrazoles and one pyrazolone have been synthesized from the allylated and alkylated products (Scheme 2).
Results and discussion
The optimization of the allylation reaction was done using 1-phenyl-butane-1,3-dione (1b) and cinnamyl alcohol (2a) as model substrates. In the preliminary stage, we did a blank reaction with 1-benzoyl acetone (1b) and cinnamyl alcohol (2a) at different temperatures (room temperature to 110 °C) in toluene for 24 hours. However, we did not get any desired product (entry 1, Table 1). When we added the catalyst (1 mol%) and heated the reaction solution to 90 °C for 12 hours, the product was isolated in 49% yield (entry 2, Table 1). Next, the reaction solution was heated at 110 °C for 12 hours, and the desired product, (3b), was isolated in 58% yield (entry 3, Table 1). It was anticipated that the addition of base to the reaction mixture would enhance the reaction rate as a base can deprotonate the active methylene –CH2 protons and enolisation of the β-dicarbonyl compounds may happen faster. Thus, we carried out the reaction in the presence of inorganic bases, K2CO3, and Na2CO3, but the yield decreased unexpectedly (entries 4 and 5, Table 1). When we added the base, the yield of the reaction drastically reduced. The reduction in the yield could be due to the catalyst degradation upon addition of the base. When the base was added, the reaction mixture's colour changed, and within a few minutes, the solution became hazy. Then we carried out the reaction in the presence of 1 mol% I in toluene, using 1b and 2a in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 ratio. The yield was found to be 72% (entry 6, Table 1). The reaction carried out in dichloroethane (DCE) afforded the product in moderate yields (entry 7, Table 1). We could not detect any product formation when CH3CN was used as a solvent (entry 8, Table 1). When 1.5 mol% of I was used, maintaining the 1b
1.5 ratio. The yield was found to be 72% (entry 6, Table 1). The reaction carried out in dichloroethane (DCE) afforded the product in moderate yields (entry 7, Table 1). We could not detect any product formation when CH3CN was used as a solvent (entry 8, Table 1). When 1.5 mol% of I was used, maintaining the 1b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a ratio at 1
2a ratio at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, the product was obtained in 76% yield in eight hours (entry 9, Table 1). Similarly, when the catalyst loading was 2 mol%, the product was isolated in 81% yield in eight hours (entry 10, Table 1). When the reactants, 1b and 2a were used in 1
1.5, the product was obtained in 76% yield in eight hours (entry 9, Table 1). Similarly, when the catalyst loading was 2 mol%, the product was isolated in 81% yield in eight hours (entry 10, Table 1). When the reactants, 1b and 2a were used in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 ratio, in the presence of 1.5 mol% of I, the product was isolated in 80% yield (entry 11, Table 1). On increasing the catalyst loading to 1.7 mol%, the product was isolated in 83% yield (entry 12, Table 1).
1.7 ratio, in the presence of 1.5 mol% of I, the product was isolated in 80% yield (entry 11, Table 1). On increasing the catalyst loading to 1.7 mol%, the product was isolated in 83% yield (entry 12, Table 1).
| Entry | I (mol%) | Temperature (°C) | Time (h) | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a 1b (0.2 mmol), 2a (0.2 mmol), I (1 mol%, 0.0015 g), toluene (2 mL).b Isolated yield.c In the presence of K2CO3 (1 equivalent).d In the presence of Na2CO3 (1 equivalent).e 1b (0.2 mmol), 2a (0.3 mmol).f 1b (0.2 mmol), 2a (0.34 mmol). | |||||
| 1 | — | 90/110 | 24 | Toluene | — |
| 2 | 1 | 90 | 12 | Toluene | 49 |
| 3 | 1 | 110 | 12 | Toluene | 58 |
| 4 | 1 | 110 | 12 | Toluene | 19c |
| 5 | 1 | 110 | 12 | Toluene | 13d |
| 6 | 1 | 110 | 8 | Toluene | 72e |
| 7 | 1 | 110 | 8 | DCE | 65e |
| 8 | 1 | 100 | 8 | CH3CN | —e |
| 9 | 1.5 | 110 | 8 | Toluene | 76e |
| 10 | 2 | 110 | 8 | Toluene | 81e |
| 11 | 1.5 | 110 | 8 | Toluene | 80f |
| 12 | 1.7 | 110 | 8 | Toluene | 83f |
After optimizing the time, temperature, solvent, and catalyst loading, we studied the effect of the leaving group. When cinnamyl chloride was used, the product was isolated in a 91% yield in six hours (entry 1, Table 2). The yield decreased drastically to 59% in eight hours, when cinnamyl acetate was used as the reactant (entry 2, Table 2). However, when carbonic acid isobutyl easter-3-phenyl-ally ester was used, the product was isolated in 79% in six hours (entry 3, Table 2). Although the conversion to the product was higher in the case of chloride leaving group, we preferred to use allylic alcohols as the method is green.
| Entry | Leaving group (X) | Time (h) | Yield (%) |
|---|---|---|---|
| a 1b (0.3 mmol), 2a (0.51 mmol), I (0.0040 g, 1.7 mol%), toluene (2 mL), 110–115 °C. | |||
| 1 | Cl | 6 | 91 |
| 2 | OAc | 8 | 59 |
| 3 | OCO2–CH2iPr | 6 | 79 |
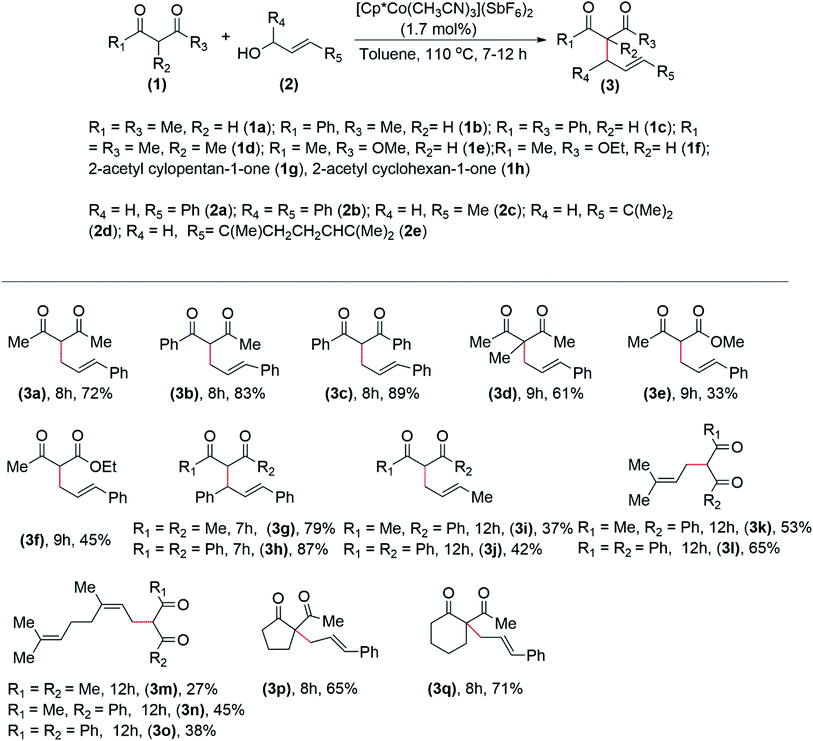
After the optimization of the reaction condition, we proceeded to investigate the scope of the reaction. Reactions were carried out between various diketones and β-keto esters with different allylic alcohols. Pentane-2,4-dione (1a), 1-phenyl-butane-1,3-dione (1b), 1,3-diphenyl propane-1,3-dione (1c), and 3-methyl pentane-2,4-dione (1d) on reaction with cinnamyl alcohol (2a) afforded the monoallylated products 3a, 3b, 3c, and 3d in 72%, 83%, 89% in eight hours, and 61% in nine hours, respectively (Table 3). 3-Oxo-butyric acid-methyl ester (1e) and 3-oxo-butyric acid-ethyl ester (1f) on reaction with cinnamyl alcohol (2a) afford the products, 3e and 3f in 33% and 45% yield, respectively in nine hours (Table 3). The products, 3(1,3-diphenyl-ally)-pentane-2,4-dione (3g) and 2-(1,3-diphenyl-allyl)- 1-phenyl-butane-1,3-dione (3h) were isolated in 79% and 87% yield, respectively in seven hours form the reaction of (E)-1,3-diphenyl-prop-2-en-1-ol (2b) with 1a and 1c (Table 3). Similarly, (E)-but-2-en-1-ol (2c) on reaction with 1b and 1,4-diphenyl-2,3-butanedione (1c) afforded the product 3i and 3j in 37% and 42% yield, respectively, in twelve hours (Table 3). 3-Methyl-2-buten-1-ol (2d), when reacted with 1b, and 1c, the products, 3k and 3l were isolated in 53%, and 65% yield, respectively in 12 hours. 3,6-Dimethyl-2,6-octadiene-1-ol (2e) on reaction with 1a, 1b, and 1c afforded the corresponding products, 3m, 3n, and 3o, in twelve hours, in 27%, 45%, and 38%, respectively.
Thus, it was observed that in the cases of aliphatic allylic alcohols, comparatively lower yields of the products were obtained (Table 3). 2-Acetylcyclopentan-1-one (1g) and 2-acetylcyclohexan-1-one (1h) on reaction with 2a, afforded the products, 3p, and 3q in 65 and 71% yield, respectively.
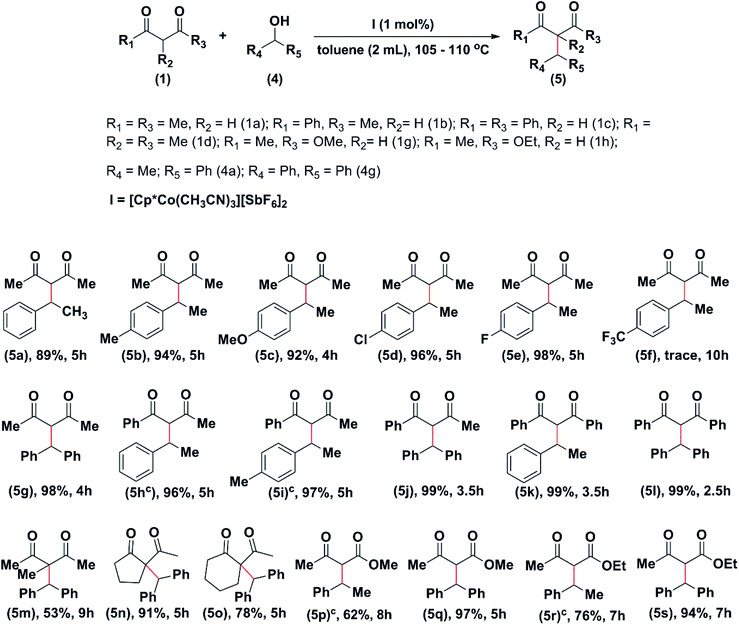
We then proceeded to study the alkylation of diketones using secondary aromatic alcohols. There is only one report on both allylation and alkylation of β-keto carbonyl compounds, catalysed by the same catalyst.15 Thus, 1a on reaction with 1-phenyl-ethan-1-ol (4a) and substituted 1-phenyl-ethan-ols {p-Me (4b), p-OMe (4c), p-Cl (4d), and p-F (4e)} afforded the alkylated products 5a to 5e in excellent yields within four to five hours. However, in the case of 1-(4-triflouromethylphenyl)-ethan-1-ol (4f), the conversion to the product 5f was found to be negligible, even after ten hours of the reaction time. Interestingly, when 1a was reacted with diphenylmethanol (4g) the product, 5g was isolated in almost quantitative yield (98%) in four hours. Similarly, 1b on reaction with 4a, 4b, and 4g afforded the products 5h, 5i, and 5j, respectively, in excellent yields in 3.5 to 5 hours (Table 4). When 1c was reacted with 4a and 4g, the products, 5k, and 5l were obtained in quantitative yields in 3.5 and 2.5 hours, respectively. 3-Methylpentane-2,4-dione (1d) on reaction with 4g afforded the product, 5m in 53% yield in nine hours. Cyclic diketones, 1g, and 1h on reaction with 4g afforded the product, 5n, and 5o in 91% and 78%, respectively, in 5 hours.
The β-ketoester, 1e, was found to react with 4a and 4g to afford the corresponding products in 62% and 97% in 8 and 5 hours, respectively. The β-Ketoester, 1f on reaction with 4a and 4g afforded the products 5r, and 5s in 76% and 94% yield, respectively, in 7 hours (Table 4). It may be noted that no product could be isolated from the reaction of 1c with 2-propanol and 1-(thiophen-2-yl)ethan-1-ol. From the data, it is clear that the highest rate of conversion was found for the reactions of diphenylmethanol (4g) with β-keto compounds. Similarly, the highest conversion rate was observed in the reactions of 1-phenyl-butane-1,3-dione (1b).
There are two possible pathways for allylation of 1,3-diketone by allylic alcohol. One path is through activation of alcohol by the metal centre via the formation of ether, followed by addition. The second pathway is η3-allyl metal complex formation.1c
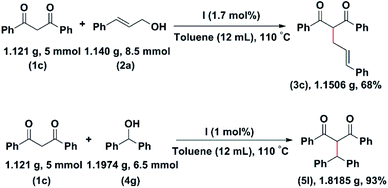
We have carried out reactions between 1,3-diphenyl propane-1,3-dione (1c) with cinnamyl alcohol (2a) and diphenylmethanol (4g) on a gram scale to check the scalability of the reaction. The allylation product 3c has been obtained in 68% yield. The alkylation product 5l has been isolated in 93% yield (Scheme 3).
In a recent publication for this laboratory, we have shown that when acetylacetone is added to the solution of [Cp*Co(CH3CN)3][SbF6]2 (I), it forms [Cp*Co(acac)]+ (m/z = 293.1) in LCMS of the reaction mixture.17
The mechanism was studied by 1H NMR spectroscopy. The solution of cinnamyl alcohol and I (1 mol%) was heated at 100 °C for 18 hours, and the 1H NMR spectrum (Fig. S3, ESI†) was recorded. The NMR spectrum shows a signal at 4.21 ppm (doublet, J = 6 Hz), which can be assigned to the –CH2 protons of the ether (Scheme 3).
Similarly, the 1H NMR spectrum (Fig. S4, ESI†) of the solution of diphenylmethanol and I (1 mol%) was recorded after heating the reaction solution at 110 °C. The NMR spectrum shows a singlet at 5.43 ppm along with the signals of aromatic protons. This signal is due to the –CH proton of the ether.
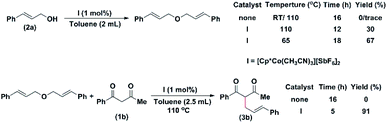
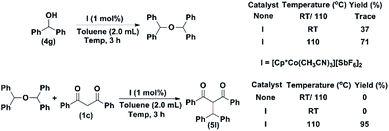
Similarly, when diphenylmethanol (4g) was mixed with I and stirred at room temperature. The corresponding ether was isolated in a 37% yield in three hours. When the reaction was conducted at 110 °C, the ether was isolated in a 71% yield in three hours (Scheme 4). The ether was isolated and was reacted with 1c. In the absence of I, no product formation was detected. Therefore, to investigate the reaction mechanism, we performed two control experiments (Scheme 4). First, cinnamyl alcohol was heated to different temperatures in the absence and in the presence (1 mol%) of the catalyst. In the absence of the catalyst, we could detect the formation of a trace amount of ether (Scheme 4). In the presence of the catalyst, at 65 °C, the ether was isolated in a 67% yield. At 110 °C, the observed yield of the ether decreased to 30%. We then carried out a reaction between the isolated ether and 1b. In the absence of I, no product could be isolated. However, in the presence of the catalyst, the product was isolated in 91% yield in 5 hours (Scheme 4).
However, in the presence I, the product was isolated in 95% yield in 3 hours (Scheme 5).
Yasuda et al. reported InCl3 mediated alkylation of diketones. They suggested that the reaction of alcohols proceed through indium mediated ether formation and subsequent alkylation.1c They have also suggested a direct activation of alcohol for the alkylation. It may be mentioned that Fe(OTf)3 has been shown to catalyse dehydration of alcohols to ether.18
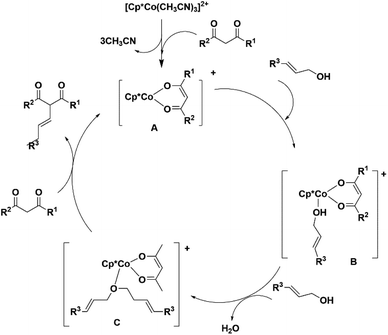
Based on the report from this laboratory17 and other reports, it is proposed that first, the diketone forms the intermediate A. Which reacts with allyl alcohol to produce the intermediate B. The intermediate B on reaction with another molecule of allylic alcohol forms the ether, which is coordinated to the cobalt centre (C). In the next step, the nucleophilic addition to the enolized diketones occurs, followed by the release of the product by proton transfer from another molecule of diketone to produce the intermediate A (Scheme 6). We believe that the alkylation also takes place via the same mechanism.
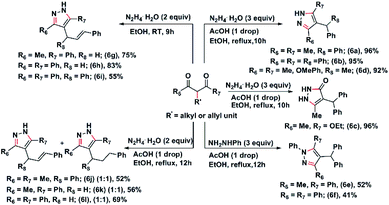
After the synthesis of substituted diketones, we thought of using these for the synthesis of pyrazoles. Pyrazoles and pyrazolones are an important class of compounds having potential medicinal use.19 Thus, we carried out the reaction of the substituted diketones, 5c, 5g, and 5k with hydrazine in ethanol, in the presence of acetic acid. The resulting pyrazoles were isolated in excellent yields (Scheme 7). Similarly, ethyl-2-benzhydryl-3-oxobutanoate (5s) on reaction with hydrazine under the same condition, afforded 4-benzhydryl-5-methyl-1,2-dihydro-pyrazole-3-one (6c) in 96% yield. Corresponding pyrazoles, 6e, and 6f were isolated from the reaction of phenylhydrazine with the diketones, 5j & 5l (Scheme 7). The pyrazoles, 6g, 6h, and 6i, were isolated in excellent yield form the reaction of 3b, 3c, and 3h with hydrazine hydrate in ethanol at room temperature (Scheme 7). Interestingly, when the reaction of diketones, 3g, 3b, and 3c with hydrazine hydrate were carried out in the presence of one drop of acetic acid in ethanol for 12 hours, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 4-(1,3-diphenyl-propyl)-3,5-dimethyl-pyrazole and 4-(1,3-diphenyl-allyl)-3,5-dimethyl-pyrazole (6j) was obtained. The reaction of 3b and 3c with hydrazine hydrate under the same reaction condition afforded the 1
1 mixture of 4-(1,3-diphenyl-propyl)-3,5-dimethyl-pyrazole and 4-(1,3-diphenyl-allyl)-3,5-dimethyl-pyrazole (6j) was obtained. The reaction of 3b and 3c with hydrazine hydrate under the same reaction condition afforded the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the allylated and alkylated pyrazoles 6k and 6l, respectively (Scheme 7).
1 mixture of the allylated and alkylated pyrazoles 6k and 6l, respectively (Scheme 7).
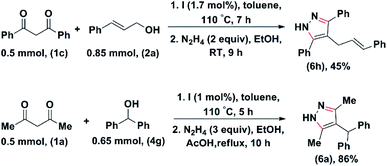
We have carried one-pot sequential reactions for the synthesis of pyrazoles. For example, 1,3-diphenyl propane-1,3-dione (1c) was first reacted with cinnamyl alcohol (2a) and 4g under optimized condition. Upon completion of the reaction a few millilitre EtOH hydrazine hydrate was added, and the corresponding product was isolated (Scheme 8).
Conclusion
In summary, allylation and alkylation of active methylene compounds using the corresponding alcohol are reported. The reaction is regioselective. Also, the method affords only monoallylated and monoalkylated products, unlike most of the reported methods.The reactions have been carried out under aerobic conditions in toluene. These allylated and alkylated diketones and a β-keto ester have been used for the synthesis of eleven new pyrazoles and a new pyrazolone.
Experimental section
General methods and materials
All the reactions are performed in the air without further purification of commercially available reagents and solvents. The catalyst [Cp*Co(CH3CN)3][SbF6]2 was synthesized from the literature procedure.16 The 1H NMR (400 MHz) and 13C NMR (100 MHz) were recorded in CDCl3. The LCMS data of the reaction mixture were recorded with a TOF mass spectrometer in ESI+ mode in MeOH and acetonitrile solvent. The HRMS data of newly synthesized compounds were recorded with a TOF mass spectrometer in ESI+ mode in acetonitrile/water mixture. Flash column chromatography was done in silica gel (60–120 mesh) for purification of product from the crude reaction mixture.General procedure for the reaction of allylic alcohols with diketones and β-keto esters
The catalyst (0.0027 g, 1.7 mol%), toluene (2 mL), the β-keto compound (0.2 mmol), and allylic alcohol (0.34 mmol, 1.7 equivalent) were taken in a screw-cap reaction tube. The reaction tube was placed in an oil-bath at a temperature of 110 °C and continuously stirred for 8–12 hours. The reaction was monitored by TLC. The solvent was removed in vacuo, and the products were purified by silica gel (60–120 mesh) column chromatography using hexane-ethyl acetate (1–5%) as eluent.Synthesis of pyrazoles
The alkylated diketones, 5c, 5g, 5k, and 5s (0.2 mmol) were dissolved in ethanol, and one drop of acetic acid was added to this. The reaction solution was refluxed for 12 hours. The solvent was removed in vacuo, and the products were purified by silica gel (60–120 mesh) column chromatography using hexane-ethyl acetate (1–5%) as eluent.The newly synthesized allylated compounds, 3b, 3c, and 3h (0.2 mmol) and hydrazine hydrate (0.4 mmol) were dissolved in ethanol, and the reaction solution was stirred for 9 hours at room temperature. The solvent was removed in vacuo, and the products were purified by silica gel (60–120 mesh) column chromatography using hexane-ethyl acetate (1–5%) as eluent. In another set of reactions, the reaction solutions were stirred for 12 hours in the presence of one drop of acetic acid.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1). Off white liquid; yield 0.0149 g, 52% w.r.t 6j1; 1H NMR (500 MHz, CDCl3) δ 7.37 (d, J = 7.5 Hz, 1H), 7.32–7.27 (m, 3H), 7.25–7.21 (m, 2H), 7.19 (d, J = 7.0 Hz, 1.4H), 7.17 (d, J = 7.0 Hz, 0.5H), 7.14 (d, J = 7.0 Hz, 1H), 6.62 (dd, J = 15.5, 7.0 Hz, 0.67H), 6.32 (d, J = 16 Hz, 0.64H), 4.84 (d, J = 7.0 Hz, 0.65H), 3.88 (dd, J = 10, 6.3 Hz, 0.53H), 2.65 (ddt, J = 13.8, 9.4, 5.5 Hz, 1H), 2.45–2.39 (m, 0.55H), 2.35–2.27 (m, 0.62H), 2.15 (s, 2.3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 144.4, 142.7, 141.9, 137.3, 131.2, 130.8, 128.5, 128.4, 128.3, 128.3, 128.2, 128.1, 127.5, 127.3, 126.3, 126.2, 125.9, 125.8, 43.6, 39.8, 35.5, 34.2, 11.8, 11.7; DEPT 13C NMR (125 MHz, CDCl3) δ 131.2, 130.8, 128.5, 128.4, 128.4, 128.3, 128.2, 128.1, 127.5, 127.3, 126.3, 126.2, 125.9, 125.8, 43.6, 39.8, 35.5, 34.2, 11.8, 11.7. HRMS (ESI-TOF) m/z: [C20H20N2 + H]+ & [C20H22N2 + H]+ calcd for C20H21N2 & C20H23N2 289.1700 & 291.1856; found 289.1681 & 291.1857.
1). Off white liquid; yield 0.0149 g, 52% w.r.t 6j1; 1H NMR (500 MHz, CDCl3) δ 7.37 (d, J = 7.5 Hz, 1H), 7.32–7.27 (m, 3H), 7.25–7.21 (m, 2H), 7.19 (d, J = 7.0 Hz, 1.4H), 7.17 (d, J = 7.0 Hz, 0.5H), 7.14 (d, J = 7.0 Hz, 1H), 6.62 (dd, J = 15.5, 7.0 Hz, 0.67H), 6.32 (d, J = 16 Hz, 0.64H), 4.84 (d, J = 7.0 Hz, 0.65H), 3.88 (dd, J = 10, 6.3 Hz, 0.53H), 2.65 (ddt, J = 13.8, 9.4, 5.5 Hz, 1H), 2.45–2.39 (m, 0.55H), 2.35–2.27 (m, 0.62H), 2.15 (s, 2.3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 144.4, 142.7, 141.9, 137.3, 131.2, 130.8, 128.5, 128.4, 128.3, 128.3, 128.2, 128.1, 127.5, 127.3, 126.3, 126.2, 125.9, 125.8, 43.6, 39.8, 35.5, 34.2, 11.8, 11.7; DEPT 13C NMR (125 MHz, CDCl3) δ 131.2, 130.8, 128.5, 128.4, 128.4, 128.3, 128.2, 128.1, 127.5, 127.3, 126.3, 126.2, 125.9, 125.8, 43.6, 39.8, 35.5, 34.2, 11.8, 11.7. HRMS (ESI-TOF) m/z: [C20H20N2 + H]+ & [C20H22N2 + H]+ calcd for C20H21N2 & C20H23N2 289.1700 & 291.1856; found 289.1681 & 291.1857.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1). Off white solid; yield 0.0154 g, 56% w.r.t 6k1; 1H NMR (500 MHz, CDCl3) δ 7.54 (d, J = 7.5 Hz, 2H), 7.45 (d, J = 7.0 Hz, 2H), 7.41–7.27 (m, 10H), 7.24 (bs, 1H), 7.21–7.16 (m, 2H), 7.12 (d, J = 7.5 Hz, 2H), 6.39–6.29 (m, 2H), 3.45 (d, J = 4.5 Hz, 2H), 2.62–2.55 (m, 4H), 2.24 (s, 3H), 2.21 (s, 3H), 1.82 (dt, J = 15.4, 7.8 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 142.0, 137.5, 130.2, 128.8, 128.7, 128.6, 128.5, 128.4, 128.3, 127.9, 127.7, 127.6, 127.5, 127.0, 126.1, 125.7, 35.6, 32.0, 26.7, 22.8, 10.9. HRMS (ESI-TOF) m/z: [C19H18N2 + H]+ & [C19H20N2 + H]+ calcd for C19H19N2 & C19H21N2 275.1543 & 277.1700; found 275.1504 & 277.1700.
1). Off white solid; yield 0.0154 g, 56% w.r.t 6k1; 1H NMR (500 MHz, CDCl3) δ 7.54 (d, J = 7.5 Hz, 2H), 7.45 (d, J = 7.0 Hz, 2H), 7.41–7.27 (m, 10H), 7.24 (bs, 1H), 7.21–7.16 (m, 2H), 7.12 (d, J = 7.5 Hz, 2H), 6.39–6.29 (m, 2H), 3.45 (d, J = 4.5 Hz, 2H), 2.62–2.55 (m, 4H), 2.24 (s, 3H), 2.21 (s, 3H), 1.82 (dt, J = 15.4, 7.8 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 142.0, 137.5, 130.2, 128.8, 128.7, 128.6, 128.5, 128.4, 128.3, 127.9, 127.7, 127.6, 127.5, 127.0, 126.1, 125.7, 35.6, 32.0, 26.7, 22.8, 10.9. HRMS (ESI-TOF) m/z: [C19H18N2 + H]+ & [C19H20N2 + H]+ calcd for C19H19N2 & C19H21N2 275.1543 & 277.1700; found 275.1504 & 277.1700.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1). Off white solid; yield 0.0233 g, 69% w.r.t 6l1; 1H NMR (500 MHz, CDCl3) δ 7.64 (d, J = 7.5 Hz, 3H), 7.52 (d, J = 7.5 Hz, 2H), 7.41–7.35 (m, 7H), 7.33–7.28 (m, 3H), 7.23–7.20 (m, 1.6H), 7.17–7.14 (t, J = 7.3 Hz, 0.5H), 7.01 (d, J = 7.0 Hz, 1H), 6.49 (dt, J = 16.0, 5.0 Hz, 1H), 6.36 (d, J = 16.0 Hz, 1H), 3.60 (dd, J = 4.5, 2.0 Hz, 1H), 2.76 (t, J = 8.0 Hz, 1H), 2.52 (t, J = 7.5 Hz, 1H), 1.77 (dt, J = 15.5, 7.5 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 141.8, 137.5, 131.2, 129.7, 128.8, 128.7, 128.5, 128.4, 128.2, 128.1, 127.9, 127.8, 127.7, 127.1, 126.2, 125.7, 115.7, 112.4, 35.5, 31.8, 27.2, 22.9; DEPT 13C NMR (125 MHz, CDCl3) δ 131.2, 129.7, 128.8, 128.7, 128.5, 128.4, 128.2, 128.1, 127.9, 127.8, 127.7, 127.1, 126.2, 125.7, 35.5, 31.8, 27.2, 22.9. HRMS (ESI-TOF) m/z: [C24H20N2 + H]+ & [C24H22N2 + H]+ calcd for C24H21N2 & C24H23N2 337.1700 & 339.1856; found 337.1784 & 339.1857.
1). Off white solid; yield 0.0233 g, 69% w.r.t 6l1; 1H NMR (500 MHz, CDCl3) δ 7.64 (d, J = 7.5 Hz, 3H), 7.52 (d, J = 7.5 Hz, 2H), 7.41–7.35 (m, 7H), 7.33–7.28 (m, 3H), 7.23–7.20 (m, 1.6H), 7.17–7.14 (t, J = 7.3 Hz, 0.5H), 7.01 (d, J = 7.0 Hz, 1H), 6.49 (dt, J = 16.0, 5.0 Hz, 1H), 6.36 (d, J = 16.0 Hz, 1H), 3.60 (dd, J = 4.5, 2.0 Hz, 1H), 2.76 (t, J = 8.0 Hz, 1H), 2.52 (t, J = 7.5 Hz, 1H), 1.77 (dt, J = 15.5, 7.5 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 141.8, 137.5, 131.2, 129.7, 128.8, 128.7, 128.5, 128.4, 128.2, 128.1, 127.9, 127.8, 127.7, 127.1, 126.2, 125.7, 115.7, 112.4, 35.5, 31.8, 27.2, 22.9; DEPT 13C NMR (125 MHz, CDCl3) δ 131.2, 129.7, 128.8, 128.7, 128.5, 128.4, 128.2, 128.1, 127.9, 127.8, 127.7, 127.1, 126.2, 125.7, 35.5, 31.8, 27.2, 22.9. HRMS (ESI-TOF) m/z: [C24H20N2 + H]+ & [C24H22N2 + H]+ calcd for C24H21N2 & C24H23N2 337.1700 & 339.1856; found 337.1784 & 339.1857.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank SERB for the NMR facility (grant number SR/FST/CSII-026/2013).Notes and references
- (a) B. M. Trost, Acc. Chem. Res., 1996, 29, 355 CrossRef CAS; (b) B. M. Trost and D. L. Van Vranken, Chem. Rev., 1996, 96, 395 CrossRef CAS; (c) M. Yasuda, T. Somyo and A. Baba, Angew. Chem., Int. Ed., 2006, 45, 793 CrossRef CAS; (d) Z. Lu and S. Ma, Angew. Chem., Int. Ed., 2008, 47, 258 CrossRef CAS; (e) J. Zhang, J. Liao, Y. Wei, G. Cheng and R. Luo, Mini-Rev. Org. Chem., 2018, 15, 476 CrossRef CAS; (f) N. A. Butta and W. Zhang, Chem. Soc. Rev., 2015, 44, 7929 RSC; (g) G. Brahmachari, RSC Adv., 2016, 6, 64676 RSC.
- B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921 CrossRef CAS.
- (a) A. Lei and X. A. Lu, Org. Lett., 2000, 2, 2357 CrossRef CAS; (b) T. Konno, T. Takehana, T. Ishihara and H. Yamanaka, Org. Biomol. Chem., 2004, 2, 93 RSC; (c) R. Takeuchi and M. Kashio, J. Am. Chem. Soc., 1998, 120, 8647 CrossRef CAS; (d) K. Spielmann, G. Niel, R. M. D. Figueiredo and J. M. Campagne, Chem. Soc. Rev., 2018, 47, 1159 RSC.
- (a) B. C. Ranu, K. Chattopadhyay and L. Adak, Org. Lett., 2007, 9, 4595 CrossRef CAS; (b) H. Kinoshita, H. Shinokubo and K. Oshima, Org. Lett., 2004, 6, 4085 CrossRef CAS; (c) T. Nemoto, T. Matsumoto, T. Masuda, T. Hitomi, K. Hatano and Y. Hamada, J. Am. Chem. Soc., 2004, 126, 3690 CrossRef CAS.
- (a) H. Kurosawa, J. Chem. Soc., Dalton Trans., 1979, 939 RSC; (b) R. Shibuya, L. Lin, Y. Nakahara, K. Mashima and T. Ohshima, Angew. Chem., Int. Ed., 2014, 53, 4377 CrossRef CAS.
- (a) J. L. Renaud, C. Bruneau and B. Demerseman, Synlett, 2003, 3, 408 CrossRef; (b) C. Bruneau, J. L. Renaud and B. Demerseman, Pure Appl. Chem., 2008, 80, 861 CAS; (c) S. Grubera and P. S. Pregosin, Adv. Synth. Catal., 2009, 351, 3235 CrossRef; (d) R. K. G. Siddappa, C. W. Chang and R. J. Chein, Tetrahedron Lett., 2014, 55, 1031 CrossRef CAS.
- H. Yang, L. Fang, M. Zhang and C. Zhu, Eur. J. Org. Chem., 2009, 666 CrossRef CAS.
- P. Kothandaraman, W. Rao, X. Zhang and P. W. H. Chan, Tetrahedron, 2009, 65, 1833 CrossRef CAS.
- (a) S. B. Tang, X. Zhang, H. F. Tu and S. L. You, J. Am. Chem. Soc., 2018, 140, 7737 CrossRef CAS; (b) J. Zheng and B. Breit, Angew. Chem., Int. Ed., 2019, 58, 3392 CrossRef CAS; (c) B. W. H. Turnbul and P. A. Evans, J. Org. Chem., 2018, 83, 11463 CrossRef.
- C. C. Bao, D. S. Zheng, X. Zhang and S. L. You, Organometallics, 2018, 37, 4763 CrossRef CAS.
- (a) J. L. Roustan, J. Y. Merour and F. Houlihan, Tetrahedron Lett., 1979, 20, 3721 CrossRef; (b) M. Mukhopadhyay and J. Iqbal, Tetrahedron Lett., 1995, 36, 6761 CrossRef CAS; (c) J. B. Baruah and A. G. Samuelson, J. Organomet. Chem., 1989, 361, C57 CrossRef CAS; (d) K. Takizawa, T. Sekino, S. Sato, T. Yoshino, M. Kojima and S. Matsunaga, Angew. Chem., Int. Ed., 2019, 58, 9199 CrossRef CAS; (e) D. Zell, V. Müller, U. Dhawa, M. Bursch, R. R. Presa, S. Grimme and L. Ackermann, Chem.–Eur. J., 2017, 23, 12145 CrossRef CAS; (f) Y. Suzuki, B. Sun, K. Sakata, T. Yoshino, S. Matsunaga and M. Kanai, Angew. Chem., Int. Ed., 2015, 54, 9944 CrossRef CAS; (g) H. Wang, M. M. Lorion and L. Ackermann, ACS Catal., 2017, 7, 3430 CrossRef CAS; (h) U. Dhawa, C. Tian, W. Li and L. Ackermann, ACS Catal., 2020, 10, 6457 CrossRef CAS.
- M. K. Gangwar, P. Dahiya, B. Emayavaramban and B. Sundararaju, Chem.–Asian J., 2018, 13, 2445 CrossRef CAS.
- (a) T. Yoshino, H. Ikemoto, S. Matsunga and M. Kanai, Angew. Chem., Int. Ed., 2013, 52, 2207 CrossRef CAS; (b) D. G. Yu, T. Gensch, F. de. Azambuja, S. Vásquez-Céspedes and F. Glorius, J. Am. Chem. Soc., 2014, 136, 17722 CrossRef CAS.
- K. Motokura, N. Fujita, K. Mori, T. Mizugaki, K. Ebitani and K. Kaneda, Angew. Chem., Int. Ed., 2006, 45, 2605 CrossRef CAS.
- (a) M. Rueping, B. J. Nachtsheim and A. Kuenkel, Org. Lett., 2007, 9, 825 CrossRef CAS; (b) Y. Yuan, Z. Shi, X. Feng and X. Liu, Appl. Organomet. Chem., 2007, 21, 958 CrossRef CAS; (c) P. Moriel and A. B. García, Green Chem., 2014, 16, 4306 RSC.
- (a) R. Sanz, A. Martínez, D. Miguel, J. M. Álvarez-Gutiérrez and F. Rodríguez, Adv. Synth. Catal., 2006, 348, 1841 CrossRef CAS; (b) Z. S. Qureshi, K. M. Deshmukh, P. J. Tambade and B. M Bhanage, Tetrahedron Lett., 2010, 51, 724 CrossRef CAS.
- M. C. Sau and M. Bhattacharjee, RSC Adv., 2020, 10, 36014 RSC.
- P. K. Shaoo, S. S. Gawali and C. Gunanathan, ACS Omega, 2018, 3, 124 CrossRef.
- (a) M. J. Naim, O. Alam, F. Nawaz, Md. J. Alam and P. Alam, J. Pharm. BioAllied Sci., 2016, 8, 2 CrossRef CAS; (b) F. Keter and J. Darkwa, BioMetals, 2012, 25, 9 CrossRef CAS; (c) T. M. Beck and B. Breit, Eur. J. Org. Chem., 2016, 5839 CrossRef CAS; (d) P. Chauhan, S. Mahajan and D. Enders, Chem. Commun., 2015, 51, 12890 RSC; (e) M. C. Perez-Aguilar and C. Valdes, Angew. Chem., Int. Ed., 2015, 54, 13729 CrossRef CAS; (f) H. Wang, X. Sun, S. Zhang, G. Liu, C. Wang, L. Zhu and H. Zhang, Synlett, 2018, 29, 2689 CrossRef CAS; (g) S. Fustero, A. Simón-Fuentes and J. F. Sanz-Cervera, Org. Prep. Proced. Int., 2009, 41, 253 CrossRef CAS; (h) S. T. Heller and S. R. Natarajan, Org. Lett., 2006, 8, 2675 CrossRef CAS; (i) D. C. Schmitt, A. P. Taylor, A. C. Flick and R. E. Kyne Jr, Org. Lett., 2015, 17, 1405 CrossRef CAS; (j) N. Panda and A. K. Jena, J. Org. Chem., 2012, 77, 9401 CrossRef CAS.
- C. Cazorla, M. Billamboz, H. Bricout, E. Monflier and C. Len, Eur. J. Org. Chem., 2017, 1078 Search PubMed.
- N. Gürbüz, S. Demir, I. Özdemir, B. Cetinkaya and C. Bruneau, Tetrahedron, 2010, 66, 1346 CrossRef.
- P. N. Liu, Z. Y. Zhou and C. P. Lau, Chem.–Eur. J., 2007, 13, 8610 CrossRef CAS.
- R. B. Watson, A. N. Golonka and C. S. Schindler, Org. Lett., 2016, 18, 1310 CrossRef CAS.
- N. T. Patil and Y. Yamamoto, J. Org. Chem., 2004, 69, 6478 CrossRef CAS.
- (a) Y. Li, Z. Yu and S. Wu, J. Org. Chem., 2008, 73, 5647 CrossRef CAS; (b) X. Zhang, R. Qiu, C. Zhou, J. Yu, N. Li, S. Yin and X. Xu, Tetrahedron, 2015, 71, 1011 CrossRef CAS; (c) M. E. Lloris, N. Galvez, J. Marquet and M. Moreno-Manas, Tetrahedron, 1991, 47, 8031 CrossRef CAS; (d) S. A. Babu, M. Yasuda, Y. Tsukahara, T. Yamauchi, Y. Wada and A. Baba, Synthesis, 2008, 11, 1717 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C NMR spectra and HRMS, and LCMS. See DOI: 10.1039/d0ra09778k |
| This journal is © The Royal Society of Chemistry 2021 |