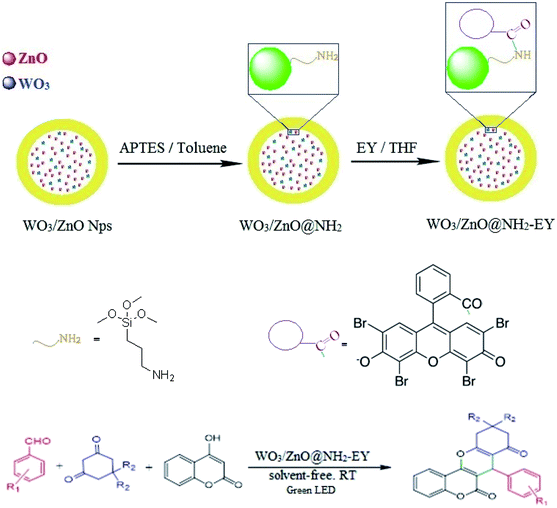
Open Access Article
Open Access ArticleEco-friendly synthesis of chromeno[4,3-b]chromenes with a new photosensitized WO3/ZnO@NH2-EY nanocatalyst†
Zahra Jalili ,
Reza Tayebee
,
Reza Tayebee * and
Farrokhzad M. Zonoz
* and
Farrokhzad M. Zonoz
Department of Chemistry, School of Sciences, Hakim Sabzevari University, Sabzevar, 96179-76487, Iran. E-mail: rtayebee@hsu.ac.ir
First published on 18th May 2021
Abstract
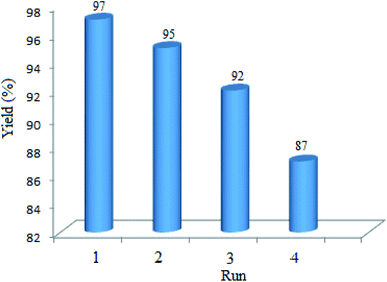
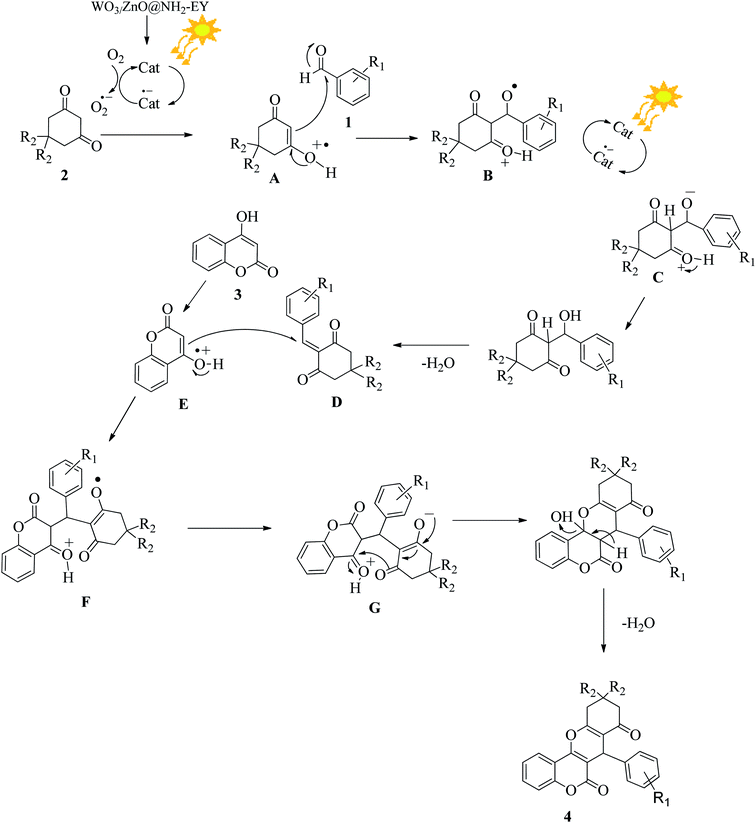
A new heterogeneous photoredox nanocatalyst WO3/ZnO@NH2-EY (EY: eosin Y) was fabricated and characterized employing some instrumental techniques such as XRD, FT-IR, ICP, TGA, and SEM. The photocatalytic efficiency of the prepared material was investigated in the preparation of various chromeno[4,3-b]chromenes via a simple and practical method. The chromene derivatives were prepared through the condensation of aromatic aldehydes, dimedone, and coumarin under an open-air atmosphere in the presence of a green LED under solventless conditions. The significant advantages of this new method include low reaction time, easy work-up, cost-effective, wide substrate scope, excellent yield, and complete atom economy of the final products. Moreover, the prepared photocatalyst could be frequently recovered up to four times with only a little decrease in the catalytic activity. Furthermore, the progress of the condensation reaction is demonstrated to occur via a radical mechanism, which shows that reactive species such as ˙O2− and OH˙ together with h+ would be involved in the photocatalytic process. Stability and reusability studies also warranty good reproducibility of the nanocatalyst for at least 4 runs. Eventually, a hot filtration test ensured that the nanohybrid catalyst is stable in the reaction medium and its catalytic activity originates from the whole undecomposed conjugated composite.
1 Introduction
Multi-component reactions have been known to be powerful tools over conventional multi-step reactions and have emerged as a novel promotion in organic synthesis.1,2 These reactions offer a direct fast route and enable the assembly of highly complicated and diversified molecules in a one-pot single-step process with enhanced atom economy.3–7 Chromenes are crucial oxygenated compounds with different biological activities such as antihypertensive,8 antioxidative,9 antitumor,10 antiviral,11 antibacterial,12 antileishmanial,13 anti-tubulin,14 and anticoagulant,15 and enable to turn on potassium channels to inhibit dihydrofolate reductase and phosphodiesterase.16 In the area of anti-cancer chemotherapy, 4H-chromenes have been known as a new class of molecules that bind to Bcl-2 anti-apoptotic proteins and promote the apoptotic processes in the cancerous cells.17–20 Therefore, novel chromenes should be further progressed as potential therapeutic species against cancer cell lines such as glioma, liver, melanoma, and prostate.19 In addition, the utilization of chromenes is well known in industrial areas such as pigments, biodegradable agrochemicals, cosmetics, optical brightener, fluorescence markers, and laser dyes.21–23Considering the emerging properties of chromene derivatives, the progress of advanced, clean, and uncomplicated methodologies for the efficient catalytic synthesis of these compounds with accessible reagents is of great importance. Some routes have been reported for the synthesis of chromeno[4,3-b]chromenes by the reaction of cyclic-1,3-dicarbonyl compounds, arylaldehydes, and 4-hydroxycoumarine in the presence of SnCl2·2H2O,24 ZnO nanoparticles,25 Mg(ClO4)2,26 molybdic acid-magnetic nanoparticles,27 p-toluene sulfonic acid,28 nano-CuFe2O4@SO3H,29 I2/HOAc,30 heteropolyacids,31 and Zn (L-proline)2.32
Although most of the reported routes have some benefits, however, some disadvantages are also combined with many of them using environmentally toxic organic solvents, expensive mediators, long reaction time, corrosive nature, tedious work-up, non-recyclable catalysts, limited substrate scope, high temperature, and low yields. Therefore, the development of effective and environmental benign methods is desirable for the synthesis of chromene compounds.
Sunlight is an accessible, plentiful, and clean energy source for chemical reactions. Photochemical reactions using visible light are attractive areas in organic synthesis, which provide ubiquitous, non-toxic, eco-friendly, sustainable, inexpensive, and a universally available energy source.33 However, due to the increasing attention on the usage of green platforms in organic synthesis, scholars are trying to redesign the synthetic methods by means of visible light.34–37 Visible light involves a major part of the arriving solar radiation, which can be used to drive various photochemical transformations. Since most organic compounds do not absorb visible light, the application of photochemical reactions has been restricted. Therefore, the development of new and efficient visible light photocatalysts towards chemical transformations is a necessity. Photoredox catalysts absorb visible light to provide the required energy to initiate a photochemical reaction. Among metal-free organic dyes, EY has been used as an economic and environment-friendly alternative to many transition metal photoredox catalysts.
The photochemistry of EY has been well examined via excitation by visible light and this molecule goes through a fast intersystem crossing to the lowest energy states.38 EY can easily absorb green light; therefore, various applications have emerged for this organic photosensitizer in visible light-promoted organic synthesis.39–41 However, because EY has harmful effects on the environment, a solution is required. One of the most efficient ways to solve this problem is to prepare a heterogeneous catalyst from this material using suitable catalytic substrates. There is an example of the heterogenization of EY on TiO2 and its application in the degradation of diclofenac via the solar activation of titanium dioxide.42 In addition, a new metal–organic framework photocatalyst modified by EY “EY@UiO-66-NH2” has been synthesized and used in the C–H activation of tertiary amines.43 Also, the reduction of 4-nitrophenol has been achieved by handling solar irradiation under green conditions by utilizing an effective polymer-supported EY photocatalyst.44 Further transformations involving oxidation of thioethers and phenylboronic acids45 as well as C–C and C–P coupling reactions46 were also performed by EY-supported photocatalysts.
As far as we know, semiconducting metal oxides have a very crucial impact in a wide domain of chemical industries such as lithium ion batteries,47 sensors,48 optoelectronics,49 and photocatalysts.50 Among various types of these semiconductors, zinc oxide and tungsten(VI) oxide are very appropriate to be applied as photocatalysts because of their proper bandgap (3.2–3.35 eV).51 However, semiconductor composites can improve the electron transitions between the (e−)/(h+) pairs in the large energy gaps and can be regarded as effectual photocatalysts.52 To date, ZnO-WO3,53 CuO/WO3/TiO2,54 ZnO–TiO2,55 and ZnO/CeO2 (ref. 56) are prepared and used as effective photocatalysts in a number of routine transformations under visible light irradiation.
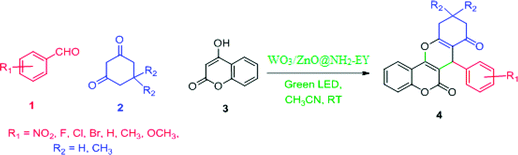
In the extension of our previous studies on the development of green synthetic protocols to achieve important heterocyclic compounds57–59 and to recognize the extensive applications of chromene derivatives, herein, we report a simple and convenient new method to synthesize different substituted chromeno[4,3-b]chromene derivatives by means of a green LED through the multicomponent condensation of aryl aldehydes, 1,3-cyclohexanedione, and 4-hydroxylcoumarin, using an EY-supported nanocomposite (WO3/ZnO) as a green heterogeneous nanocatalyst under solventless conditions (Scheme 1).
The interaction of EY with the solid support material can occur via three routes including hydrophobic, hydrogen bond, and Lewis acid–base interactions. The hydrophobic interaction refers to the adsorption affinity of EY via its non-hydrophobic functional groups. This interaction is not too strong, however, it should be considered. Hydrogen bonding also widely happens in the adsorption of this polar compound to the support material through the lone-pair hydrogen bonding donors on EY with the acceptor sites on the support. Finally, Lewis acid–base interaction occurs via the carboxyl group of EY with the basic sites such as the pendant amino group of the alkyl chain. Therefore, all of the above interactions could be regarded as electronic interactions between EY and WO3/ZnO@NH2.60–63 However, precise adsorption isotherm experiments are necessary to elucidate the exact mechanism of adsorption.
2 Experimental section
2.1 Materials and methods
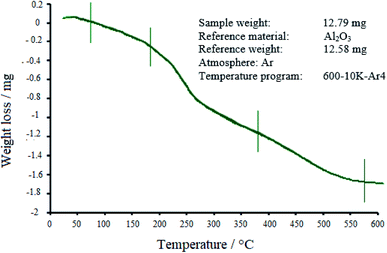
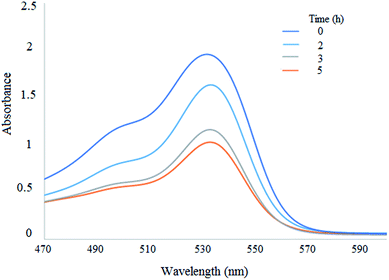
All chemicals including zinc chloride, sodium hydroxide, zinc acetate, sodium tungstate, EY, benzaldehyde, dimedone, 4-hydroxycoumarin, (3-aminopropyl)triethoxysilane (APTES), and solvents were purchased from Sigma-Aldrich, Merck, and Fluka, and utilized as received without further purification. The morphology of the synthesized nanophotocatalyst was studied by a Mira 3-XMU field emission scanning electron microscope (FE-SEM). 1H- and 13C-NMR spectra were recorded on a 300 MHz Bruker AVANCEC III spectrometer using TMS as the internal reference. Melting points were attained on a Stuart BI Branstead Electrothermal IA9200 apparatus. Fourier transform infrared (FT-IR) spectra were recorded on a Shimadzu 8700 Fourier transform spectrometer in the range of 400–4000 cm−1 with KBr pellets. UV-visible spectra were obtained using a Photonix UV-visible array spectrophotometer. X-ray diffraction patterns (XRD) were attained on an Xpert MPD diffractometer with Cu Kα radiation at 30 mA and 40 keV and a scanning rate of 3° min−1 in the 2θ domain from 5° to 80°. Thermogravimetric analysis was carried out with a TGA 92 Setaram at 10 °C min−1. The chemical composition of the catalyst was determined by an inductively coupled plasma spectrometer (ICP-MS; model VARIAN VISTA-PRO).2.2 Preparation of WO3/ZnO nanoparticles
About 30 mL NaOH (4 M) was added drop-wise into a 20 mL mixture of zinc acetate (1 M) and 2 mL of Na2WO4·2H2O (0.5 M). Then, the produced suspension remained at room temperature under mild stirring for 3 h to generate the desired precursor. Thereafter, the obtained suspension was subsequently transferred to a 250 mL ground-glass stopped conical flask and aged at 95 °C for 10 h. Finally, the deposited precipitate at the bottom of the conical flask was gathered, completely washed with water, and air-dried under ambient conditions.2.3 Surface modification of WO3/ZnO via the anchoring of APTES
800 mg WO3/ZnO was dispersed in an anhydrous solution of toluene (60 mL). To this suspension, 1.6 mL APTES was added and the obtained mixture was refluxed for 24 h. Finally, the attained precipitate was filtered and dried in an oven at 60 °C for 12 h.2.4 Surface modification of WO3/ZnO@NH2 with EY
WO3/ZnO@NH2-EY was supplied through a well-known wet-impregnation method. According to this procedure, 20 × 10−3 mL EY was dissolved in THF. Then, 0.5 g of WO3/ZnO@NH2 was gradually added to this solution for 12 h in a dark atmosphere. Eventually, the resulting precipitate was dried at 80 °C under air for 24 h.2.5 A general procedure for the synthesis of substituted chromeno[4,3-b]chromene
Benzaldehyde (100 mg, 1 mmol), dimedone (140 mg, 1 mmol), 4-hydroxycoumarin (162 mg, 1 mmol), and the desired amount of WO3/ZnO@NH2-EY (30 mg) were successively added to a test tube equipped with a magnet. Then, the reaction mixture was irradiated with a green light-emitting diode (λmax = 535 nm) at room temperature in an open-air atmosphere and completion of the reaction was monitored by TLC (n-hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate, 3
ethyl acetate, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After completion of the reaction, the mixture was quenched with H2O (3 mL) and the desired chromene product was dissolved in EtOAc. Then, the EtOAc layer was removed and the aqueous fraction was again extracted with EtOAc (3 × 3 mL). Finally, the attained organic layer was dried over sodium sulfate and concentrated in vacuo. The crude accomplished product was purified through column chromatography (100–200 mesh silica gel; EtOAc/hexane) to afford the pure chromene.
1). After completion of the reaction, the mixture was quenched with H2O (3 mL) and the desired chromene product was dissolved in EtOAc. Then, the EtOAc layer was removed and the aqueous fraction was again extracted with EtOAc (3 × 3 mL). Finally, the attained organic layer was dried over sodium sulfate and concentrated in vacuo. The crude accomplished product was purified through column chromatography (100–200 mesh silica gel; EtOAc/hexane) to afford the pure chromene.
2.6 Spectral data
3 Results and discussion
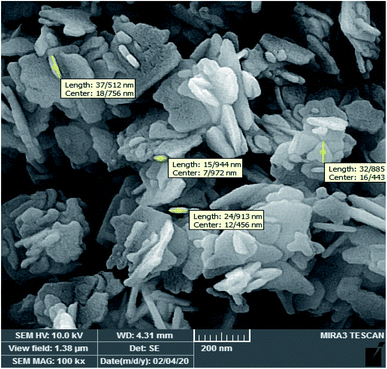
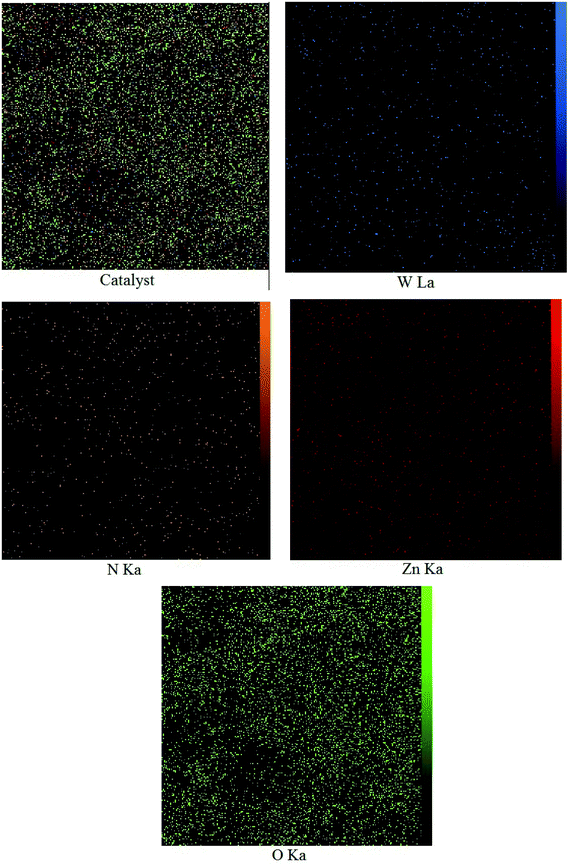
3.1 Characterization and physicochemical properties of WO3/ZnO@NH2-EY nanoparticles
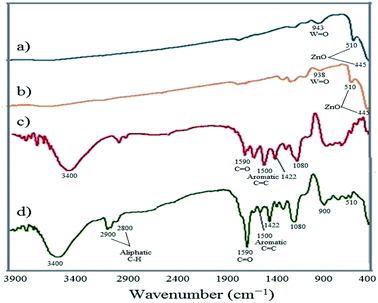
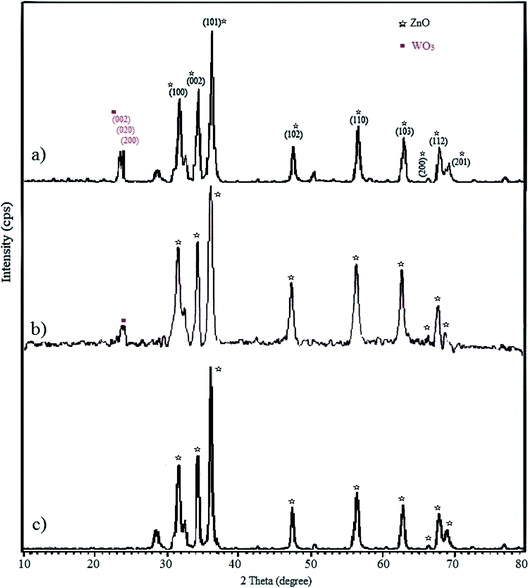
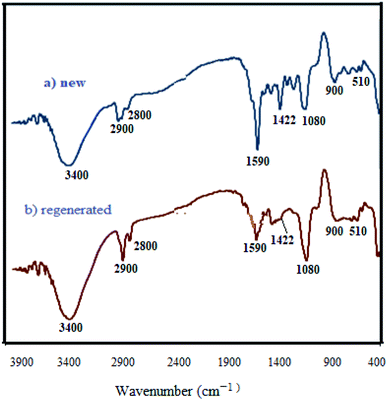
The morphology and physicochemical properties of the prepared WO3/ZnO@NH2-EY nanocatalyst were investigated by means of FT-IR, SEM, ICP, UV-Vis, and XRD. FT-IR and UV-Vis techniques are the most informative techniques to examine the surface interaction of EY with the surface functional groups of WO3/ZnO@NH2.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in the octahedral WO3.64,65 The grafting of the amine group on the surface of WO3/ZnO can be approved by the observation of the corresponding aliphatic bands in 2900 and 3400 cm−1.66 The appearance of the EY characteristic bands in the FT-IR of WO3/ZnO@NH2-EY proved that the structure of EY is unchanged after immobilization. The FT-IR spectrum of WO3/ZnO@NH2-EY was also compared to that of the parent EY. The observation of the absorption bands at 1590 and 1500 cm−1 due to the carboxyl functional group and the C
O bonds in the octahedral WO3.64,65 The grafting of the amine group on the surface of WO3/ZnO can be approved by the observation of the corresponding aliphatic bands in 2900 and 3400 cm−1.66 The appearance of the EY characteristic bands in the FT-IR of WO3/ZnO@NH2-EY proved that the structure of EY is unchanged after immobilization. The FT-IR spectrum of WO3/ZnO@NH2-EY was also compared to that of the parent EY. The observation of the absorption bands at 1590 and 1500 cm−1 due to the carboxyl functional group and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of aromatic phenyl rings, respectively, unambiguously confirmed the loading of EY onto the surface of the WO3/ZnO@NH2 framework.67,68 However, there were no detectable peaks due to the WO3 species and it seems that a little surface segregated WO3 had no apparent effects on the infrared spectrum of ZnO nanoparticles.
C of aromatic phenyl rings, respectively, unambiguously confirmed the loading of EY onto the surface of the WO3/ZnO@NH2 framework.67,68 However, there were no detectable peaks due to the WO3 species and it seems that a little surface segregated WO3 had no apparent effects on the infrared spectrum of ZnO nanoparticles.
3.2 Catalytic tests
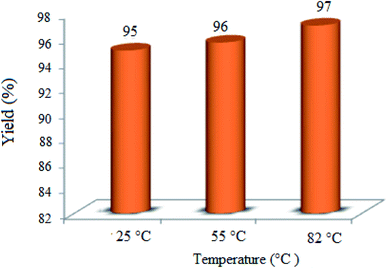
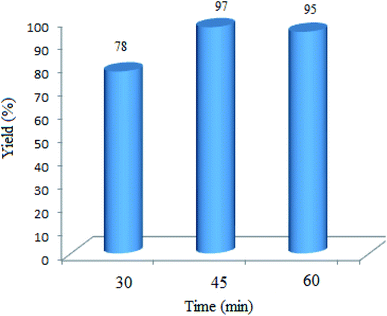
To achieve the best reaction conditions, we performed a model condensation reaction using benzaldehyde (1 mmol), dimedone (1 mmol), and 4-hydroxycoumarin (1 mmol) in the presence of WO3/ZnO@NH2-EY (0.03 g) under solventless conditions. Then, the reaction mixture was irradiated with a green light-emitting diode (λmax, 535 nm) at room temperature in an open-air container. Thereafter, the desired chromeno[4,3-b]chromene was formed in good yield after 45 min (Table 1). This study demonstrated that if we remove any of the aforementioned requirements such as EY, WO3/ZnO@NH2-EY, and LED, the reaction yield will be significantly diminished.| Entry | Light | Light source | WO3/ZnO@NH2-EY (g) | Time (min) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: dimedone (1.0 mmol), benzaldehyde (1.0 mmol), and 4-hydroxycoumarin (1.0 mmol) under solvent-free conditions in an open-air atmosphere at room temperature. | |||||
| 1 | — | — | — | 45 | 4 |
| 2 | Green LED | 25 W, λ = 535 nm | — | 45 | 9 |
| 3 | Dark | — | 0.03 | 45 | 43 |
| 4 | White LED | 20 × 1 W | 0.03 | 45 | 55 |
| 5 | Green LED | 25 W, λ = 535 nm | 0.03 | 45 | 97 |
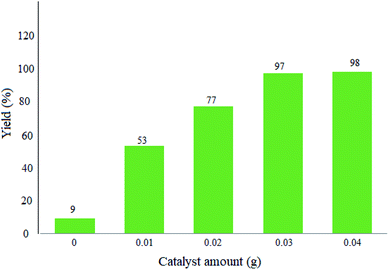 | ||
| Fig. 7 Effect of the catalyst amount on the condensation of dimedone, benzaldehyde, and 4-hydroxycoumarin under the selected reaction conditions below Table 1 in the presence of a green LED (25 W, λ = 535 nm) after 45 min. | ||
| Entry | Green LED (25 W, λ = 535 nm) | Catalyst | Air | Time (min) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions are described below Table 1. 0.03 g of the catalyst was used under solvent-free conditions. | |||||
| 1 | + | — | + | 45 | 9 |
| 2 | + | EY | + | 45 | 72 |
| 3 | + | WO3/ZnO@NH2 | + | 45 | 42 |
| 4 | + | WO3/ZnO@NH2-EY | + | 45 | 97 |
| 5 | − | WO3/ZnO@NH2-EY | + | 45 | 43 |
| 6 | + | WO3/ZnO@NH2-EY | N2 | 45 | 18 |
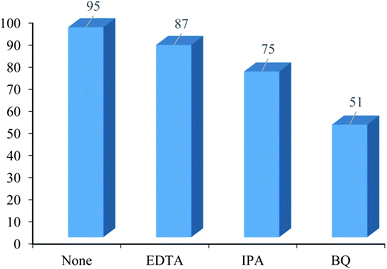 | ||
| Fig. 10 Effect of some familiar scavengers on the reaction progress under the standard reaction conditions described below Table 1. | ||
| Aldehyde | Dimedone | Product | Yield (%), select. (%) | TONb | TOFc | M.P. |
|---|---|---|---|---|---|---|
| a Reaction conditions are described below Table 1. Green LED (2.5 W, λ = 535 nm) under solvent-free conditions in air at room temperature. Reaction time was 45 min and 0.03 g of WO3/ZnO@NH2-EY was used in all the cases.b TON is calculated based on the moles of the converted substrate/mol of the catalyst.c TOF = (yield/time (h))/catalyst amount (g). | ||||||
 |
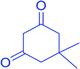 |
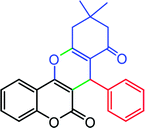 |
97, (98) | 29.66 | 39.5 | 220–222 (ref. 78) |
 |
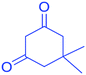 |
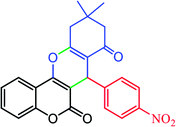 |
91, (85) | 30.33 | 40.44 | 208–210 (ref. 28) |
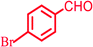 |
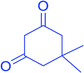 |
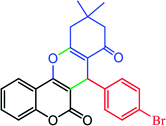 |
73, (94) | 24.33 | 32.44 | 228–230 (ref. 28) |
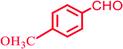 |
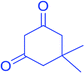 |
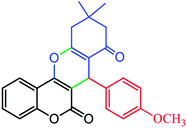 |
72, (96) | 24 | 32 | 187–189 (ref. 78) |
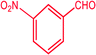 |
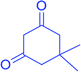 |
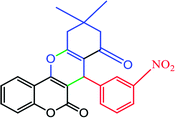 |
73, (88) | 24.33 | 32.44 | 240–245 (ref. 79) |
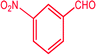 |
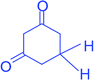 |
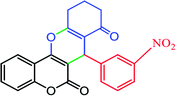 |
76, (92) | 25.33 | 33.78 | 218–220 (ref. 80) |
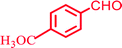 |
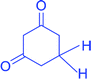 |
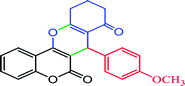 |
78, (97) | 26 | 34.67 | 196–197 (ref. 81) |
| Catalyst | Catalyst amount | Time (h) | Temp (°C) | Solvent | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions are described below Table 1. | |||||
| Fe(DS)3 | 10 mol% | 1.5 | 70 | H2O (2.5 mL) | 90 (ref. 76) |
| Fe3O4@SiO2@(CH2)3OMoO3H | 0.002 g | 0.75 | 80 | — | 90 (ref. 27) |
| Nano-CuFe2O4@SO3H | (0.05 g) | 2.5 | 70 | EtOH (5 mL) | 90 (ref. 29) |
| t-ZrO2 np | 10 mol%, 12.3 mg | 0.5 | 80 | H2O (5 mL) | 91 (ref. 82) |
| Eozin Y | 2 mol% | 3.5 | r.t green LED | CH3CN (3 mL) | 77 (ref. 83) |
| WO3/ZnO@NH2-EY | 30 mg (∼0.21 mmol EY per g of photocatalyst) | 0.75 | r.t. green LED | — | 97 (this work) |
4 Conclusion
In conclusion, we have reported an ecofriendly and efficient method to achieve visible light-promoted synthesis of chromeno[4,3-b]chromenes under mild conditions. The photoredox catalyst WO3/ZnO@NH2-EY is used as a superior green alternative catalyst for the desired heterocyclic condensation reaction. The heterogeneous photoredox nanocatalyst was characterized by means of XRD, FT-IR, ICP, TGA, and SEM. The mild reaction conditions, wide substrate tolerance, solventless reactor, easy workup, high atom economy, cost-effectiveness, and good to excellent yields are the main features of this new photocatalytic system. The WO3/ZnO@NH2-EY nanocomposite exhibits its photocatalytic functions via a series of electron transfer reactions in the presence of visible light through a radical mechanism, as proven using various scavengers. This investigation showed that the reactive species such as ˙O2− and OH˙ along with h+ are the main reactive species involved in the photochemical synthesis of chromenes. The WO3/ZnO@NH2-EY photocatalyst could be frequently recovered over four times without the loss of the catalytic activity. Eventually, a hot filtration test ensured that the nanohybrid catalyst is stable in the reaction mixture and its catalytic activity originates from the whole undecomposed nanocomposite.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors/creators would like to acknowledge the financial support of Ministry of Science, Research and Technology for this project under grant number 12-99-02-000029. Also, the work has been supported by Hakim Sabzevari University.References
- R. C. Cioc, E. Ruijter and R. V. Orru, Green Chem., 2014, 16, 2958–2975 RSC.
- M. B. Gawande, V. D. Bonifácio, R. Luque, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522–5551 RSC.
- X. Guo and W. Hu, Acc. Chem. Res., 2013, 46, 2427–2440 CrossRef CAS PubMed.
- A. Dömling, Chem. Rev., 2006, 106, 17–89 CrossRef PubMed.
- F. Wang, Y. Shen, H. Hu, X. Wang, H. Wu and Y. Liu, J. Org. Chem., 2014, 79, 9556–9566 CrossRef CAS PubMed.
- C. Simon, T. Constantieux and J. Rodriguez, Eur. J. Org. Chem., 2004, 2004, 4957–4980 CrossRef.
- E. Ruijter, R. Scheffelaar and R. V. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234–6246 CrossRef CAS PubMed.
- A. Amr, M. Abdalla, S. Essaouy, M. Areef, M. Elgamal, T. Nassear and A. Haschich, Russ. J. Gen. Chem., 2017, 87, 1826–1833 CrossRef CAS.
- S. W. Ng, L. H. Chung, C. F. Yeung, H. S. Lo, H. L. Shek, T. S. Kang, C. H. Leung, D. L. Ma and C. Y. Wong, Chem.–Eur. J., 2018, 24, 1779–1783 CrossRef CAS PubMed.
- W. H. Zhang, S. Chen, X. L. Liu, X. W. Liu and Y. Zhou, Bioorg. Med. Chem. Lett., 2020, 30, 127410 CrossRef CAS PubMed.
- I. V. Ilyina, O. S. Patrusheva, V. V. Zarubaev, M. A. Misiurina, A. V. Slita, I. L. Esaulkova, D. V. Korchagina, Y. V. Gatilov, S. S. Borisevich and K. P. Volcho, Bioorg. Med. Chem. Lett., 2020, 31, 127677 CrossRef PubMed.
- N. Baral, D. R. Mishra, N. P. Mishra, S. Mohapatra, B. P. Raiguru, P. Panda, S. Nayak, M. Nayak and P. S. Kumar, J. Heterocycl. Chem., 2020, 57, 575–589 CrossRef CAS.
- A. Neghra, M. Lecsö, M. J. Butel, L. S. Espindola, B. Deguin and E. Seguin, Nat. Prod. Commun., 2017, 12, 1459–1463 CrossRef.
- K. Haider, S. Rahaman, M. S. Yar and A. Kamal, Expert Opin. Ther. Pat., 2019, 29, 623–641 CrossRef CAS PubMed.
- I. Zghab, B. Trimeche, M. B. Mansour, M. Hassine, D. Touboul and H. B. Jannet, Arabian J. Chem., 2017, 10, S2651–S2658 CrossRef CAS.
- F. Borges, F. Roleira, N. Milhazes, L. Santana and E. Uriarte, Curr. Med. Chem., 2005, 12, 887–916 CrossRef CAS PubMed.
- M. Mahdavi, J. Davoodi, M. R. Zali and A. Foroumadi, Biomed. Pharmacother., 2011, 65, 175–182 CrossRef CAS PubMed.
- J.-L. Wang, D. Liu, Z.-J. Zhang, S. Shan, X. Han, S. M. Srinivasula, C. M. Croce, E. S. Alnemri and Z. Huang, Proc. Natl. Acad. Sci., India, 2000, 97, 7124–7129 CAS.
- S. A. Patil, J. Wang, X. S. Li, J. Chen, T. S. Jones, A. Hosni-Ahmed, R. Patil, W. L. Seibel, W. Li and D. D. Miller, Bioorg. Med. Chem. Lett., 2012, 22, 4458–4461 CrossRef CAS PubMed.
- A. M. Shestopalov, Y. M. Litvinov, L. A. Rodinovskaya, O. R. Malyshev, M. N. Semenova and V. V. Semenov, ACS Comb. Sci., 2012, 14, 484–490 CrossRef CAS PubMed.
- G. A. Kraus and I. Kim, J. Org. Chem., 2003, 68, 4517–4518 CrossRef CAS PubMed.
- J. G. Tangmouo, A. L. Meli, J. Komguem, V. Kuete, F. N. Ngounou, D. Lontsi, V. P. Beng, M. I. Choudhary and B. L. Sondengam, Tetrahedron Lett., 2006, 47, 3067–3070 CrossRef CAS.
- R. O. S. Kitamura, P. Romoff, M. C. M. Young, M. J. Kato and J. H. G. Lago, Phytochemistry, 2006, 67, 2398–2402 CrossRef PubMed.
- M. Kamali, Synth. Commun., 2020, 51, 1–9 CrossRef.
- H. Anaraki-Ardakani, Russ. J. Gen. Chem., 2017, 87, 1820–1825 CrossRef CAS.
- H. Emtiazi and M. A. Amrollahi, S. Afr. J. Chem., 2014, 67, 175–179 Search PubMed.
- F. Khosravian, B. Karami and M. Farahi, New J. Chem., 2017, 41, 11584–11590 RSC.
- H. Anaraki-Ardakani, R. Ghanavatian and M. Akbari, World Appl. Sci. J., 2013, 22, 802–808 CAS.
- S. Vajar and M. Mokhtary, Polycyclic Aromat. Compd., 2019, 39, 111–123 CrossRef CAS.
- X.-J. Sun, J.-F. Zhou and S.-J. Zhi, Synth. Commun., 2012, 42, 1987–1994 CrossRef CAS.
- R. Tayebee, A. Pejhan, H. Ramshini, B. Maleki, N. Erfaninia, Z. Tabatabaie and E. Esmaeili, Appl. Organomet. Chem., 2018, 32, e3924 CrossRef.
- B. Maleki, S. Babaee and R. Tayebee, Appl. Organomet. Chem., 2015, 29, 408–411 CrossRef CAS.
- S. K. Sahoo, Renewable Sustainable Energy Rev., 2016, 59, 927–939 CrossRef.
- A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. L. Belser and A. V. von Zelewsky, Coord. Chem. Rev., 1998, 84, 85–277 CrossRef.
- P. Melchiorre, Angew. Chem., Int. Ed., 2009, 48, 1360–1363 CrossRef CAS PubMed.
- D. A. Nagib, M. E. Scott and D. W. MacMillan, J. Am. Chem. Soc., 2009, 131, 10875–10877 CrossRef CAS PubMed.
- M. A. Ischay, Z. Lu and T. P. Yoon, J. Am. Chem. Soc., 2010, 132, 8572–8574 CrossRef CAS PubMed.
- Y. Zhang, C. Ye, S. Li, A. Ding, G. Gu and H. Guo, RSC Adv., 2017, 7, 13240–13243 RSC.
- V. Srivastava and P. P. Singh, RSC Adv., 2017, 7, 31377–31392 RSC.
- D. P. Hari and B. König, Chem. Commun., 2014, 50, 6688–6699 RSC.
- S. Yadav, M. Srivastava, P. Rai, B. P. Tripathi, A. Mishra, J. Singh and J. Singh, New J. Chem., 2016, 40, 9694–9701 RSC.
- N. Hashim, S. Thakur, M. Patang, F. Crapulli and A. K. Ray, Environ. Technol., 2017, 38, 933–944 CrossRef CAS PubMed.
- G. Kumar, P. Solanki, M. Nazish, S. Neogi, R. I. Kureshy and H. K. Noor-ul, J. Catal., 2019, 371, 298–304 CrossRef CAS.
- S. Gazi and R. Ananthakrishnan, Appl. Catal., B, 2011, 105, 317–325 CrossRef CAS.
- A. Sridhar, R. Rangasamy and M. Selvaraj, New J. Chem., 2019, 43, 17974–17979 RSC.
- P. Li, G.-W. Wang, X. Zhu and L. Wang, Tetrahedron, 2019, 75, 3448–3455 CrossRef CAS.
- L. Wang, S. Yue, Q. Zhang, Y. Zhang, Y. R. Li, C. S. Lewis, K. J. Takeuchi, A. C. Marschilok, E. S. Takeuchi and S. S. Wong, ACS Energy Lett., 2017, 2, 1465–1478 CrossRef CAS.
- R. Celiesiute, A. Ramanaviciene, M. Gicevicius and A. Ramanavicius, Crit. Rev. Anal. Chem., 2019, 49, 195–208 CrossRef CAS PubMed.
- X. Yu, T. J. Marks and A. Facchetti, Nat. Mater., 2016, 15, 383–396 CrossRef CAS PubMed.
- A. K. Arora, V. S. Jaswal, K. Singh and R. Singh, Orient. J. Chem., 2016, 32, 2035 CrossRef CAS.
- M. J. Limo, A. Sola-Rabada, E. Boix, V. Thota, Z. C. Westcott, V. Puddu and C. C. Perry, Chem. Rev., 2018, 118, 11118–11193 CrossRef CAS PubMed.
- M. B. Tahir, G. Nabi and N. Khalid, Mater. Sci. Semicond. Process., 2018, 84, 36–41 CrossRef CAS.
- E. Mugunthan, M. Saidutta and P. Jagadeeshbabu, J. Photochem. Photobiol., A, 2019, 383, 111993 CrossRef CAS.
- F. Akhlaghian and A. Najafi, Sci. Iran., 2018, 25, 3345–3353 Search PubMed.
- N. Geetha, S. Sivaranjani, A. Ayeshamariam, M. K. Micheal, D. Saravankkumar, S. Fowziya, A. Mohideen and M. Jayachandran, J. Adv. Microsc. Res., 2018, 13, 3–11 CrossRef.
- Z. Xiong, Z. Lei, Z. Xu, X. Chen, B. Gong, Y. Zhao, H. Zhao, J. Zhang and C. Zheng, J. CO2 Util., 2017, 18, 53–61 CrossRef CAS.
- B. Maleki, H. Natheghi, R. Tayebee, H. Alinezhad, A. Amiri, S. A. Hossieni and S. M. M. Nouri, Polycyclic Aromat. Compd., 2020, 40, 633–643 CrossRef CAS.
- B. Maleki, M. Chahkandi, R. Tayebee, S. Kahrobaei, H. Alinezhad and S. Hemmati, Appl. Organomet. Chem., 2019, 33, e5118 CrossRef.
- R. Tayebee, M. Fattahi Abdizadeh, N. Erfaninia, A. Amiri, M. Baghayeri, R. M. Kakhki, B. Maleki and E. Esmaili, Appl. Organomet. Chem., 2019, 33, e4959 CrossRef.
- H. Zhao, X. Liu, Z. Cao, Y. Zhan, X. Shi, Y. Yang, J. Zhou and J. Xu, J. Hazard. Mater., 2016, 310, 235–245 CrossRef CAS PubMed.
- Y. Liu, J. Xu, Z. Cao, R. Fu, C. Zhou, Z. Wang and X. Xu, J. Colloid Interface Sci., 2020, 559, 215–225 CrossRef CAS PubMed.
- X. Liu, J. Xu, Y. Zhao, H. Shi and C.-H. Huang, Chemosphere, 2019, 226, 726–735 CrossRef CAS PubMed.
- J. Xu, Z. Cao, Y. Zhang, Z. Yuan, Z. Lou, X. Xu and X. Wang, Chemosphere, 2018, 195, 351–364 CrossRef CAS PubMed.
- K. S. Babu, A. R. Reddy, C. Sujatha, K. V. Reddy and A. Mallika, J. Adv. Ceram., 2013, 2, 260–265 CrossRef.
- B. Li, R. Tayebee, E. Esmaeili, M. S. Namaghi and B. Maleki, RSC Adv., 2020, 10, 40725–40738 RSC.
- X. Z. Yajun Wang, L. Duan, F. Wang, H. Niu, W. Guo and A. Ali, Mater. Sci. Semicond. Process., 2015, 29, 372–379 CrossRef.
- D. Jaiswal, A. Mishra, P. Rai, M. Srivastava, B. P. Tripathi, S. Yadav, J. Singh and J. Singh, Res. Chem. Intermed., 2018, 44, 231–246 CrossRef CAS.
- D. A. Young, T. B. Freedman, E. D. Lipp and L. A. Nafie, J. Am. Chem. Soc., 1986, 108, 7255–7263 CrossRef CAS.
- A. Chiolerio, A. Chiodoni and P. Allia, Thin Solid Films, 2008, 516, 8453–8461 CrossRef CAS.
- F. Liu, X. Chen, Q. Xia, L. Tian and X. Chen, RSC Adv., 2015, 5, 77423–77428 RSC.
- H. Alinezhad, M. Tarahomi, B. Maleki and A. Amiri, Appl. Organomet. Chem., 2019, 33, e4661 CrossRef.
- V. Kandathil, B. D. Fahlman, B. Sasidhar, S. A. Patil and S. A. Patil, New J. Chem., 2017, 41, 9531–9545 RSC.
- R. K. Sharma, B. Arora, S. Sharma, S. Dutta, A. Sharma, S. Yadav and K. Solanki, Mater. Chem. Front., 2020, 4, 605–620 RSC.
- Z. Yin, M. Han, Z. Hu, L. Feng, Y. Liu, Z. Du and L. Zhang, Chem. Eng. J., 2020, 390, 124532 CrossRef CAS.
- X. Zheng, J. Yuan, J. Shen, J. Liang, J. Che, B. Tang, G. He and H. Chen, J. Mater. Sci.: Mater. Electron., 2019, 30, 5986–5994 CrossRef CAS.
- K. Pradhan, S. Paul and A. R. Das, Tetrahedron Lett., 2013, 54, 3105–3110 CrossRef CAS.
- F. Adibian, A. R. Pourali, B. Maleki, M. Baghayeri and A. Amiri, Polyhedron, 2020, 175, 114179 CrossRef.
- Z. Chen, Q. Zhu and W. Su, Tetrahedron Lett., 2011, 52, 2601–2604 CrossRef CAS.
- K. T. Patil, L. Walekar, S. Undare, G. Kolekar, M. B. Deshmukh, P. Choudhari and P. V. Anbhule, Indian J. Chem., 2016, 55, 1151–1159 Search PubMed.
- A. Shafiee, R. Motamedi, O. Firuzi, S. Meili, A. R. Mehdipour and R. Miri, Med. Chem. Res., 2011, 20, 466–474 CrossRef CAS.
- R. Motamedi, S. Baghbani and F. F. Bamoharram, Synth. Commun., 2012, 42, 1604–1612 CrossRef CAS.
- A. Saha, S. Payra and S. Banerjee, RSC Adv., 2015, 5, 101664–101671 RSC.
- A. K. Sharma, J. Tiwari, D. Jaiswal, S. Singh, J. Singh and J. Singh, Curr. Organocatal., 2019, 6, 222–230 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra09737c |
| This journal is © The Royal Society of Chemistry 2021 |