Open Access Article
Open Access ArticleSynthesis and biological evaluation of fluorinated 3,4-dihydroquinolin-2(1H)-ones and 2-oxindoles for anti-hepatic fibrosis†
Man Li‡
a,
Fu-Sheng He‡c,
Long-Shan Jia,
Ya-Ting Gaoa,
Xin Zhanga,
Zhuo Yub,
Miao Fanga,
Jie Wu *cde and
Yue-Qiu Gao*ab
*cde and
Yue-Qiu Gao*ab
aLaboratory of Cellular Immunity, Institute of Clinical Immunology, Shanghai Key Laboratory of Traditional Chinese Clinical Medicine, Shuguang Hospital, Affiliated to Shanghai University of Traditional Chinese Medicine, 528 ZhangHeng Road, Shanghai 201203, China. E-mail: gaoyueqiu@hotmail.com
bDepartment of Hepatology, Shuguang Hospital, Affiliated to Shanghai University of Traditional Chinese Medicine, 528 ZhangHeng Road, Shanghai 201203, China
cSchool of Pharmaceutical and Materials Engineering, Taizhou University, 1139 Shifu Avenue, Taizhou 31800, China. E-mail: jie_wu@fudan.edu.cn
dState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Scinences, 354 Fenglin Road, Shanghai 200032, China
eSchool of Chemistry and Chemical Engineering, Henan Normal University, China
First published on 3rd February 2021
Abstract
(Z)-4-(Iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones and fluorinated 3,3-disubstituted 2-oxindoles are synthesized and evaluated for anti-hepatic fibrosis. CCK-8 assay indicates that most of the compounds have no obvious cytotoxicity on the human hepatic stellate cells (HSC) cell line. Collagen I and fibrosin expression levels are tested by ELISA, and the results show that several compounds can inhibit the expression of collagen I and fibrosin. Additionally, results from real time-PCR reveal that only one compound can inhibit the expression level of α-SMA, suggesting that this compound can inhibit the activation of the HSC cell line. These studies demonstrate that this compound may be a potential novel drug candidate for anti-hepatic fibrosis (approximately 5–6 lines).
Hepatic fibrosis is an important pathophysiological process of chronic liver diseases involving excessive deposition of collagen-based extracellular matrix (ECM), which is extremely rich in collagen I and III, since it can lead to cirrhosis and portal hypertension.1 Hepatic stellate cells (HSC) (lipocytes, Ito cells) are identified as the main collagen-producing cells in the liver, which are the main cells involved in liver fibrogenesis.2 Many factors have been shown to affect the activity of HSCs, including interleukin-1, tumor necrosis factor, transforming growth factor (TGF), and basic fibroblast growth factors. TGF-β is confirmed to be the most potent stimulus for the activation of HSC.3 Additionally, fibrosin (FBRS) is one new fibrogenic cytokine that was first identified as a product of inflammatory cells and was cloned as “fibrosin”.4 Our previous studies showed that serum FBRS levels in patients with chronic hepatitis B were positively correlated with the degree of hepatic fibrosis, which could be used to evaluate hepatic fibrosis.5
Many anti-fibrotic treatments have been tried, but none is recognized worldwide. Thus, it is urgent to explore the effective medicine or drug candidate to treat hepatic fibrosis. Meanwhile, nitrogen-containing heterocycles are important in drug-discovery process due to their unique biological activities.6 Therefore, significant efforts have been given to the methodologies development and library construction for the rapid preparation of heterocycles and their derivatives. On the other hand, the incorporation of fluorinated groups into privileged scaffolds has received considerable attention in both synthetic and medicinal chemistry community, with the aim to improve their biological properties.7 Recently, we accomplished the synthesis of (Z)-4-(iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones through a photoinduced radical cyclization of benzene-tethered 1,7-enynes with Togni reagent in the presence of sodium iodide under ultraviolet irradiation.8 Additionally, fluorinated 3,3-disubstituted 2-oxindoles could be generated as well under ultraviolet irradiation through a photoinduced radical cyclization of N-arylacrylamides with fluorinated alkyl iodides.9 Herein, we describe the identification and biological evaluation of (Z)-4-(iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones including 3a–f and fluorinated 3,3-disubstituted 2-oxindoles including 5a–h. The results show that compounds 3b and 5a can inhibit the expression of collagen I and FBRS. Moreover, real-time-PCR shows that only compound 5a can inhibit the expression level of α-SMA, suggesting that compound 5a can inhibit the activation of LX-2 cells. These studies demonstrate that compound 5a may be a potential novel drug candidate for anti-hepatic fibrosis.
Results and discussion
Chemistry: synthesis of (Z)-4-(iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones and fluorinated 3,3-disubstituted 2-oxindoles
The general synthetic route to the target (Z)-4-(iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones and fluorinated 3,3-disubstituted 2-oxindoles is described in Scheme 1. Accordingly, a series of (Z)-4-(iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones were prepared through a three-component cyclization of benzene-tethered 1,7-enynes 1 with Togni reagent 2 in the presence of sodium iodide under ultraviolet irradiation (Scheme 1).8 Additionally, by treatment of N-arylacrylamides 4 and fluorinated alkyl iodides under ultraviolet irradiation at room temperature, the corresponding fluorinated 3,3-disubstituted 2-oxindoles 5 could be generated in moderate to good yields (Scheme 1).9 As a result, a small library of (Z)-4-(iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones and fluorinated 3,3-disubstituted 2-oxindoles was constructed for the subsequent biological evaluation.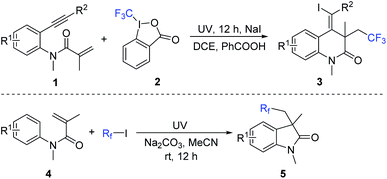 | ||
| Scheme 1 Synthesis of (Z)-4-(iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones and fluorinated 3,3-disubstituted 2-oxindoles. | ||
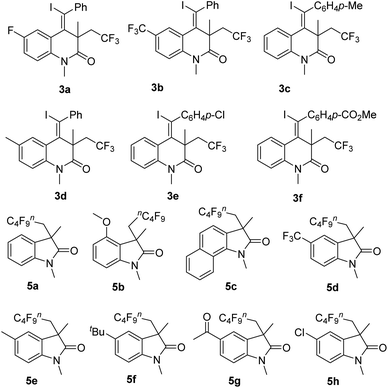
Chemical structure of chemical compounds
(Z)-4-(Iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones 3 included compounds 3a–f and fluorinated 3,3-disubstituted 2-oxindoles included compounds 5a–h. Their chemical structures are presented in Fig. 1. General experimental procedure for the synthesis of (Z)-4-(iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones 3 and fluorinated 3,3-disubstituted 2-oxindoles 5 is presented in the ESI.†Effects of chemical compounds on LX-2 viability
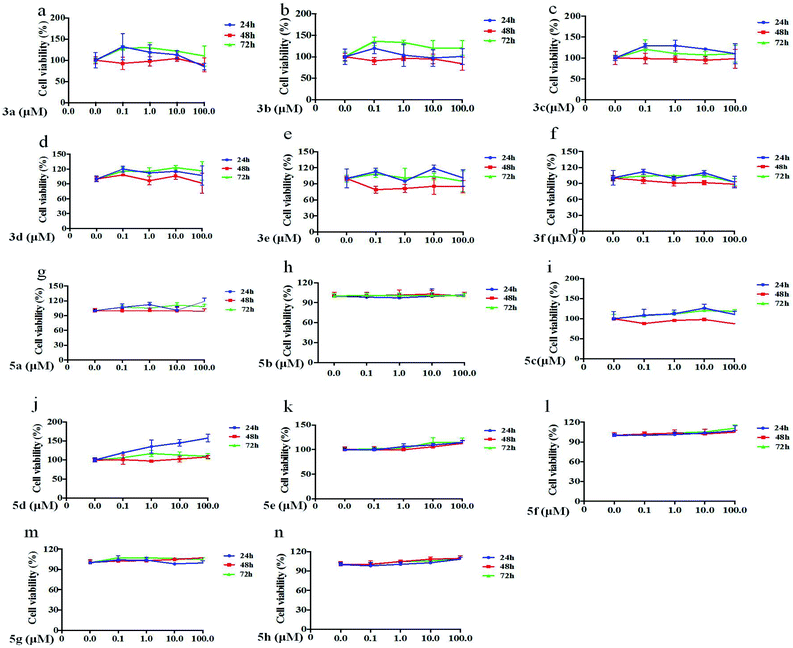
The data showed that all compounds at 0.1 μM, 1 μM, 10 μM and 100 μM had no significant inhibition or cytotoxicity on LX-2 cells after 24 h, 48 h or 72 h treatment, and some compounds contributed to the proliferation of LX-2 cells (Fig. 2).Collagen I expression levels were reduced by some compounds
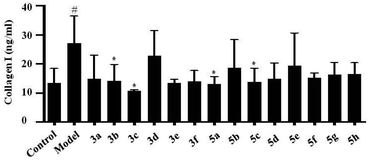
Liver fibrosis is the excessive accumulation of extracellular matrix proteins including collagen that occurs in most types of chronic liver diseases. HSC is responsible for initiating and spreading liver fibrosis, secreting fibrogenic factors that encourage portal fibrocytes, fibroblasts, and bone marrow-derived myofibroblasts.10 Collagen I was markedly expressed by activated HSCs. In this study, LX-2 cells were activated by TGF-β1, and then compounds were individually added into the culture medium. After 72 h, collagen I levels expressed by LX-2 cells were tested by ELISA. The results showed that collagen I level was up-regulated by TGF-β1, and the up-regulation of collagen I was inhibited by compounds 3b, 3c, 5a and 5c, which suggested that the above components could inhibit the expression of collagen I in LX-2 cells (Fig. 3).Elevated expression levels of FBRS on LX-2 were inhibited by compounds
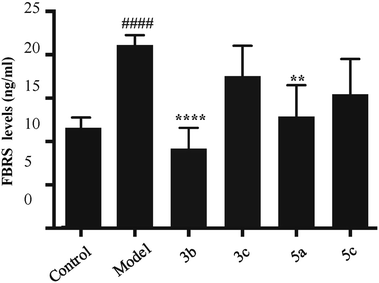
Fibrosin is one fibrogenic cytokine, and our previous studies showed that FBRS levels were closely related to the degree of hepatic fibrosis.5 In this study, some effective components including compounds 3b, 3c, 5a and 5c were further explored in the effects on FBRS expression levels. Our results showed that FBRS levels were up-regulated by TGF-β1, and the up-regulation of FBRS was inhibited by compounds 3b and 5a, which suggested that compounds 3b and 5a might inhibit the progress of fibrosis (Fig. 4).Up-regulation of α-SMA level in LX-2 was decreased by compound 5a
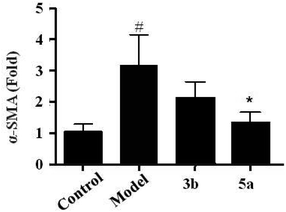
Upon the liver injury, quiescent HSCs become activated and trans-differentiate into myofibroblast-like cells, which are characterized by enhanced cell growth and undergo profound phenotypic changes, including de novo expression of α-smooth muscle actin (α-SMA) and production of large amounts of collagens. We further screened the effects of all compounds on TGF-β1/α-SMA signaling. In this study, LX-2 cells were treated by the compounds including 3b and 5a, and then α-SMA mRNA levels in LX-2 cells induced by TGF-β1 were detected. The result showed that α-SMA mRNA levels was up-regulated by TGF-β1, and the up-regulation of α-SMA was inhibited by compound 5a, which suggested that compound 5a could obviously inhibit the activation of LX-2 cells (Fig. 5).Conclusions
In summary, liver fibrosis is a serious health problem worldwide, which is the final common pathway of chronic or iterative liver damage. The approaches for reversing fibrosis are the major unmet medical need. In this study, (Z)-4-(iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones and fluorinated 3,3-disubstituted 2-oxindoles are synthesized and evaluated for anti-hepatic fibrosis. CCK-8 assay indicates that most of the compounds have no obvious cytotoxicity on human HSC cell line. Collage I and FBRS expression levels are tested by ELISA, and the results show that several compounds can inhibit the expression of collagen I and FBRS. Additionally, results from real-time-PCR reveal that only compound 5a can inhibit the expression level of α-SMA, suggesting that this compound can inhibit the activation of HSC cell line. These studies demonstrate that compound 5a may be a potential novel drug candidate for anti-hepatic fibrosis (approximately 5–6 lines), which decreases the expression levels of collage I and FBRS, and reduced the activation of HSC.Experimental
General experimental procedure for the synthesis of (Z)-4-(iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones
Benzene-tethered 1,7-enynes 1 (0.2 mmol) was added to a mixture of Togni reagent 2 (0.3 mmol), NaI (0.2 mmol) and PhCOOH (0.2 mmol) in DCE (4.0 mL). The mixture, placed around the mercury lamp (purchased from Yuming, Shanghai) with a distance of 10 centimeters, was stirred under UV irradiation (0.67 W cm−1) for 12 hours at room temperature. After completion of reaction as indicated by TLC, the solvent was evaporated, and the residue was purified directly by flash column chromatograph (EtOAc/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to give the desired products 3.
8) to give the desired products 3.
General experimental procedure for the synthesis of fluorinated 3,3-disubstituted 2-oxindoles
Fluorinated alkyl iodide (0.36 mmol) was added to a solution of N-arylacrylamides 4 (0.3 mmol), Na2CO3 (0.3 mmol) in CH3CN (4.0 mL) under N2 atmosphere. The mixture was stirred under ultraviolet irradiation for 12 hours. After completion of reaction as indicated by TLC, the solvent was evaporated, and the residue was purified directly by flash column chromatograph (EtOAc/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to give the desired products 5.
8) to give the desired products 5.
Cell culture and experimental design
The human LX-2 cell line was a well characterized human HSC cell line, that could transdifferentiate in vitro from a quiescent-like phenotype to a more proliferative and activated behavior, and it provided a useful platform to assess anti-fibrotic drugs. LX-2 cells were donated by Institute of Liver Diseases Affiliated to Shanghai University of Traditional Chinese Medicine. LX-2 cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Thermo Fisher Scientific), containing penicillin (100 U mL−1), and streptomycin (100 μg mL−1) and fetal bovine serum at 10% final concentration. Cells were maintained at 37 °C in a humidified atmosphere of 5% CO2, and the medium was changed once per day. When the cell density reached 80–90%, the cells were passaged. To evaluate the effects of chemicals on the activation of LX-2 cells, cells were cultivated in media containing 10% fetal bovine serum in the presence of TGF-β and chemicals.Drug concentration
All chemicals were dissolved in DMSO at 0.05% (v/v). The concentrations of all chemicals were tested for the measurement of cell viability at 0.1 μM, 1 μM, 10 μM and 100 μM.Cytotoxicity assay
The cytotoxicity analyses were performed by a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Gaithersburg, MD, USA). For the assay, 3 × 103 LX-2 cells were cultured in wells of 96-well plates for 12 h, followed by the incubation of different concentrations of chemicals (0.1 μM, 1 μM, 10 μM and 100 μM) for extra 72 h. Next, the cultured cell medium was collected, and CCK-8 solution (10 μL) was added to the culture medium, and the cultures were incubated for 2 h at 37 °C. The absorbance was detested by Microplate Reader (Bio-Rad; Hercules, CA, USA) under the optical density (OD) at 450 nm.Activation of LX-2 cells
To activate LX-2 cells, TGF-β was added to the culture medium at the final concentration of 10 μg mL−1.ELISA
Collagen I and fibrosin levels in culture medium were tested by ELISA. Human collagen I ELISA kit was purchased from Abcam (ab210966). Human fibrosin ELISA kit was purchased from Shanghai Ji Ning Technology Co., Ltd. In brief, 100 μL of sample was added into every well. After incubating 2 h at room temperature, the plate was washed and 100 μL antibody was added to each well. After incubating 1 h at room temperature, the plate was washed and 200 μL substrate solution was added to each well. After incubating for 20 minutes at room temperature, 50 μL of stop solution was added to each well. The absorbance was detested by Microplate Reader (Bio-Rad; Hercules, CA, USA) under the optical density (OD) at 450 nm.RNA extraction and real-time-PCR analyses
Total RNA was isolated from LX-2 cells using Trizol Reagent (Invitrogen) and quantified. RNA was synthesized from 1 μg of total RNA using cDNA Reverse Transcription kit (Invitrogen). Real-time PCR was performed using the Mastercycler ep realplex 4 real-time PCR system (Eppendorf) with an SYBR Green qPCR Master Mix (Fermentas) according to the manufacturer's protocol. The sequences of primers for human α-SMA (NC_000010.11) were 5′-CCACCGCAAATGCTTCTAAGT-3′ (forward) and 5′-GGCAGGAATGATTTGGAAAGG-3′ (reverse). The primers for human GAPDH (NM_002046.3) were 5′-TGCACCACCAACTGCTT AGC-3′ (forward) and 5′-GGCATGGACTGTGGTCATGAG-3′ (reverse). The amplification conditions were as follows: 951C for 10 min, and 40 cycles of 951C for 15 s, 641C for 30 s and 721C 20 s. The amount of α-SMA transcripts of individual samples was normalized to GAPDH.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China (81673767, 81673938, 81874436, 81774240), Science Research Project of Thirteen Five-Year Plan (2018ZX10725-504), “Shuguang Program” by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (18SG39), Program of Shanghai Academic/Technology Research Leader (20XD1423500), Pilot Project of Clinical Cooperation between Chinese and Western Medicine for Major and Difficult Diseases (Hepatic Fibrosis), Si Ming Foundation of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (SGKJ-201903, SGKJ-201904, SGKJ-201905, SGKJ-201912, SGKJ-201904), and the Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University (2020ZD04) is gratefully acknowledged.Notes and references
- (a) T T suchida and SL Friedman, Nat Rev Gastroenterol Hepatol., 2017, 14, 397 CrossRef; (b) T. Tsuchida and SL Friedman, Nat. Rev. Gastroenterol. Hepatol., 2017, 14, 397 CrossRef CAS; (c) M. Giannandrea and W. C. Parks, Dis. Models Mech., 2014, 7, 193 CrossRef CAS.
- (a) R. Lichtinghagen, D. Pietsch, H. Bantel, M. P. Manns, K. Brand and M. J. Bahr, J. Hepatol., 2013, 59, 1365 CrossRef; (b) H. Sekiguchi, N. Hemmi, T. Maki, A. Ozawa, E. Kadowaki, J. Kamiie, M. Yamamoto, K. Arishima and M. Sakaue, J. Vet. Med. Sci., 2013, 75, 553 CrossRef CAS; (c) M. J. Yum, S. Koppula, J. S. Kim, G. M. Shin, Y. J. Chae, T. Yoon, C. S. Chun, J. D. Lee and M. D. Song, Pharm. Biol., 2017, 55, 1577 CrossRef CAS; (d) S. L. Friedman, F. J. Roll, J. Boyles and D. M. Bissell, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 8681 CrossRef CAS; (e) J. J. Maher and R. F. McGuire, J. Clin. Invest., 1990, 86, 1641 CrossRef CAS.
- (a) J. J. Maher, D. M. Bissell, S. L. Friedman and F. J. Roll, J. Clin. Invest., 1988, 82, 450 CrossRef CAS; (b) S. L. Frideman, J. Biol. Chem., 2000, 275, 2247 CrossRef.
- (a) D. J. Wyler, Int. Arch. Allergy Immunol., 1996, 111, 326 CrossRef CAS; (b) S. Prakash, P. W. Robbins and D. J. Wyler, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 2154 CrossRef CAS.
- M. Li, Z. J. Zeng, X. H. Sun, X. J. Zhu, Z. H. Zhou and Y. Q. Gao, J. Clin. Hepatol., 2011, 27, 1312 CAS.
- (a) A. Armstrong and J. C. Collins, Angew. Chem., Int. Ed., 2010, 49, 2282 CrossRef CAS; (b) A. R. Katritzky and S. Rachwal, Chem. Rev., 2010, 110, 1564 CrossRef CAS; (c) M. A. P. Martins, C. P. Frizzo, D. N. Moreira, L. Buriol and P. Machado, Chem. Rev., 2009, 109, 4140 CrossRef CAS; (d) E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234 CrossRef CAS.
- (a) S. Purser, P. R. Moore and S. Swallow, Chem. Soc. Rev., 2008, 37, 320 RSC; (b) D. O'Hagan, Chem. Soc. Rev., 2008, 37, 308 RSC; (c) K. Hagmann, J. Med. Chem., 2008, 51, 4359 CrossRef; (d) Y. Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422 CrossRef CAS.
- Y. An, Y. Kuang and J. Wu, Org. Chem. Front., 2016, 3, 994 RSC.
- Y. An, Y. Li and J. Wu, Org. Chem. Front., 2016, 3, 570 RSC.
- C. Y. Zhang, W. G. Yuan, P. He, J. H. Lei and C. X. Wang, World J. Gastroenterol., 2016, 22, 10512 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra09430g |
| ‡ M. Li and F.-S. He contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |