Open Access Article
Open Access ArticleRapid growth of the CH3NH3PbCl3 single crystal by microwave irradiation
Moch Wisnu Arif Sektiono ab,
Fitri Aulia Permatasari
ab,
Fitri Aulia Permatasari a,
Akfiny Hasdi Aimona and
Ferry Iskandar
a,
Akfiny Hasdi Aimona and
Ferry Iskandar *ac
*ac
aDepartment of Physics, Faculty of Mathematics and Natural Science, Institut Teknologi Bandung, Bandung, 40132, Indonesia
bPSDKU Kediri-Politeknik Negeri Malang, Kediri, 64114, Indonesia
cResearch Center for Nanosciences and Nanotechnology, Bandung, 40132, Indonesia. E-mail: ferry@fi.itb.ac.id
First published on 5th January 2021
Abstract
We report a rapid growth of the CH3NH3PbCl3 single crystal through microwave irradiation. A systematic evaluation of the structural and optical properties of the obtained single crystal was also conducted. 1 minute is the optimum microwave irradiation time that generated a large single crystal of dimension (5 × 5 × 2.5) mm3. The obtained crystal exhibits broad absorption in UV range and near-visible light luminescence under UV excitation with an optical bandgap around 2.8 eV. A fast and simple synthesis method of CH3NH3PbCl3 single crystal with these outstanding properties could be potentially applied for any optoelectronic application with scale-up production.
Introduction
For over a decade, lots of research on the organometal trihalide perovskites (OTPs) has been conducted that focus on both the low-cost production and various superior properties. OTPs have a chemical structure of ABX3, where A is occupied by a monovalent organic cation, B is occupied by divalent metal cations (e.g., Mn2+, Cu2+, Pb2+, Sn2+), and the X site is occupied by halide elements such as I−, Br− or Cl−.1–4Owing to these outstanding properties, OTPs have demonstrated excellent performance in optoelectronic devices like photodetectors and solar cells.5–7 Superior properties of OTPs including visible light absorption coefficients,8 direct bandgap,9,10 long carrier lifetime, and balanced exciton mobility,11,12 strongly depend on high purity, especially crystallinity. Polycrystalline OTPs is relatively easier to be prepared by various method than the single crystal OTPs.13 However, it suffers more limitations such as grain boundaries, voids, and defects on the surface that lead to a lower charge mobility compared to the single crystal OTPs.14–17 A single crystal OTPs contains only one quantum well with a high order crystallization resulted in a superior optical and electrical properties compare to the polycrystalline structure.18,19 However, in practically, the synthesis of single crystal OTPs is still challenging in various ways.
Recently, a solution growth method has been reported successfully for synthesizing the single crystal OTPs.20,21 Dang et al., successfully prepared a monocrystalline NH(CH3)3SnX3 (X = Cl, Br) with the dimensions 13 × 8 × 6 mm3 and 8 × 6 × 4 mm3.20 The crystal growth process started from immerse the crystal seed through a bottom seed solution growth (BSSG) method under atmospheric environmental condition. The morphology of the formed crystals strongly depending on the speed of tool's rotation, the orientation of the crystal seed used, and the temperature. Maculan et al., prepared a CH3NH3PbCl3 single crystal through an inverse temperature crystallization (ITC) method in oil bath resulted in CH3NH3PbCl3 single crystals in sized 2 × 4 × 4 mm3.21 The ITC method requires the well-controlled of the high and continuous temperature conditions of the solution in range of 100 °C to 120 °C. Despite a large crystal size, these methods used a conduction or convection route from an external heat source then spread into a centre of sample, consuming a relatively long time crystallization process.22
On the other hand, microwave-assisted preparation has been reported successful to synthesize the polycrystalline OTPs films.23,24 Contrast to conventional heating, microwave irradiation can exploit the multiple molecules in compounds (liquid, gaseous, or solid) to convert energy directly from electromagnetic to heat result in an abruptly heating process.25 Moreover, the interaction between microwave irradiation and precursors facilitates the chemical reaction that results in a short time reaction. A microwave irradiation could then be a potential method to accelerate the crystallization process of single crystal OTPs as long a well-controlled heating rate. A microwave irradiation has also been reported successfully to promote the growth of the single crystal OTPs i.e., MAPbI3 and MAPbBr3.26 The low power of microwave irradiation that used led to the well-controlled temperature result in the similar crystal growth as the conventional ITC. Although the crystal growth process represents a low energy consumption, it still consumes a relatively long-time crystallization process in several hours with a relatively smaller bulk dimension compared to the single crystal prepared from the conventional ITC method. Therefore, an exploration of an effective and efficient way for preparing the single crystal OTPs is still needed.
In this research, a rapid growth process of CH3NH3PbCl3 single crystal through a commercial microwave preparation was conducted. The crystallization process was undergoing without the presence of the crystal seed that reduces the consuming time. A large CH3NH3PbCl3 single crystal in a dimension of 5 × 5 × 2.5 mm3 was obtained by microwave irradiation for 1 minute time. To the best of our knowledge, a minute is the fastest for synthesizing a relatively large CH3NH3PbCl3 single crystal. The effect of irradiation time to its crystallinity and optical properties were also investigated systematically. Thus, a commercial microwave used could reduce the crystallization time and the production cost of the single crystal OTPs' preparation.
Materials and methods
Materials
Perovskite CH3NH3PbCl3 solution is synthesized using the wet chemical routes as reported elsewhere.14,21 In brief, the material used as CH3NH3Cl salt is synthesized by reacting the hydrochloride acid solution (Merck, 37 wt% in a water solvent) and methylamine (Sigma Aldrich, 33 wt% in absolute ethanol solvent), in the absolute ethanol solvent (Merck, ≥99.5%) and under nitrogen atmospheric. 2 M solution of PbCl2 powder (Sigma Aldrich, 99%) and CH3NH3Cl salt (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) in dimethyl sulfoxide or DMSO (Sigma Aldrich, anhydrous, ≥99.9%) was dissolved at 100 °C on a hotplate result in a precursor solution.
1 molar ratio) in dimethyl sulfoxide or DMSO (Sigma Aldrich, anhydrous, ≥99.9%) was dissolved at 100 °C on a hotplate result in a precursor solution.
Synthesis of CH3NH3PbCl3 single crystal
The prepared CH3NH3PbCl3 solution was divided by 2 ml into 10 ml vials, then irradiated by a commercial microwave (Panasonic type NN-ST342M). Under a low microwave irradiation mode, the irradiation time varied from one to four minutes, resulting in single crystal samples in various dimensions. The temperature of the solution was monitored through an irradiation process by the infrared thermometer (Infrared Thermometer type GM320).Characterizations
The crystal structure of samples was characterized by X-ray diffraction (Philips PW 1710) with Cu anode, Kα![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = 1.542 Å. The diffraction angle (2θ) was monitored in range of 10° into 60°. The absorption features of samples were observed by UV-Vis spectrophotometer (Ocean Optic UV-Vis spectrophotometer). The resulted absorption feature was evaluated further by using the Tauc plot to estimate the bandgap of samples. Last, the photoluminescence properties of samples were also observed by PL spectrofluorometer (RF 5300PC, Shimadzu Corp.) using Xe lamps as a light source.
= 1.542 Å. The diffraction angle (2θ) was monitored in range of 10° into 60°. The absorption features of samples were observed by UV-Vis spectrophotometer (Ocean Optic UV-Vis spectrophotometer). The resulted absorption feature was evaluated further by using the Tauc plot to estimate the bandgap of samples. Last, the photoluminescence properties of samples were also observed by PL spectrofluorometer (RF 5300PC, Shimadzu Corp.) using Xe lamps as a light source.
Results and discussion
The samples were prepared from the precursors that consist of CH3NH3Cl and PbCl2 as the starting materials. Microwave heating was used to accelerate the precursor's dissolution process due to the difference dielectric characteristics to the solvent in precursors. Dimethyl sulfoxide (DMSO) was selected as a solvent due to its excellent performance for dissolving CH3NH3Cl and PbCl2.14,21 In addition, as an aprotic polar solvent, DMSO also plays an important role in kinetic reaction owing to its high microwave absorbing feature with permittivity (ε) ≈ 47 and loss factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) = 0.825 at the frequency of 2.45 GHz in 20 °C.22,27,28 The commercial microwave that used can be adjusted based on two parameters, i.e., duty cycle and irradiation time. The low duty cycle mode is the most reliable mode to prepare the crystal bulk since excessive evaporation has occurred when medium or high duty cycle mode was used for a minute. Then, the microwave irradiation time was varying from 1 to 4 minutes. Notably, less than 1 minute irradiation time does not generate the crystal growth and irradiation for more than 4 minutes suffer excessive evaporation that hinders the crystallization process.
δ) = 0.825 at the frequency of 2.45 GHz in 20 °C.22,27,28 The commercial microwave that used can be adjusted based on two parameters, i.e., duty cycle and irradiation time. The low duty cycle mode is the most reliable mode to prepare the crystal bulk since excessive evaporation has occurred when medium or high duty cycle mode was used for a minute. Then, the microwave irradiation time was varying from 1 to 4 minutes. Notably, less than 1 minute irradiation time does not generate the crystal growth and irradiation for more than 4 minutes suffer excessive evaporation that hinders the crystallization process.
Photograph of the obtained crystal samples were shown in Fig. 1. The sample was rapidly growing under a low duty cycle mode of the 800 W microwave irradiation in several minutes. It is worth pointing out that 1 minute is the fastest time to grow the crystal sample reported. Despite the other method, such as the conventional ITC method, this method does not need a crystal seed to initiate the crystal growth. The largest sample has dimension size of 5 × 5 × 2.5 mm3 obtained from 1 minute microwave irradiation. As a longer microwave irradiation time up to 4 minutes, the smaller dimension sample size was obtained. Then, the microwave irradiation time longer than 4 minutes provokes the fast evaporation of precursors completely due to its high temperature.
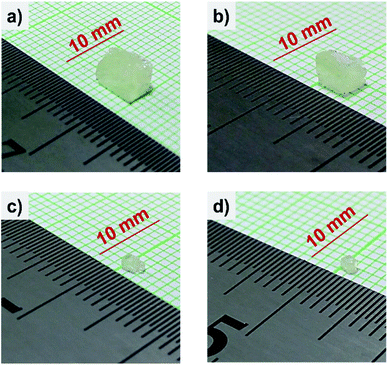 | ||
| Fig. 1 Photograph taken from the as-grown CH3NH3PbCl3 single crystal that prepared by microwave irradiation in varying irradiation time. (a) 1 minute; (b) 2 minutes; (c) 3 minutes; (d) 4 minutes. | ||
Fig. 2a shows XRD pattern of the obtained crystal samples particulates ground from a piece of corresponding large sample. Comparing to the starting materials (CH3NH3Cl and PbCl2), the XRD pattern of samples indicated that all samples are pure CH3NH3PbCl3 without any residual or contaminant. All samples are pure in phase and the crystalline phase of the CH3NH3PbCl3 perovskite, indicated by (100) and (110) planes.31 A set of preferred orientations at 15.5°, 31.4°, and 35.2° was observed, with assigned to (100), (200) and (210) planes of the CH3NH3PbCl3 perovskite cubic structure,14 respectively as shown in Fig. 2b. Through a Rietveld refined data using Pm3m space group superimposed on the CH3NH3PbCl3 as shown in Table 1, the crystal lattice constant of sample is in a reasonable value of a = 5.6 Å which indicated a good quality fitting and consistent with the other reports.9,14,21,29,30 This value is also in good agreement with the estimated lattice constant value calculated based on the Bragg equation. The CH3NH3PbCl3 that was prepared in 1 minute up to 3 minutes has the strongest peaks located at 2θ = 15.5° and 2θ = 31.4° that correspond to (100) and (200) planes in CH3NH3PbCl3 crystal structure, respectively.18 These indicated that for the samples that were prepared in 1 up to 3 minutes microwave irradiation might be dominated by the crystal in orientation of (100) and (200) planes. While for 4 minutes microwave irradiation, the crystal orientation was dominated by (110), (111), (210), (211), and (220) planes.
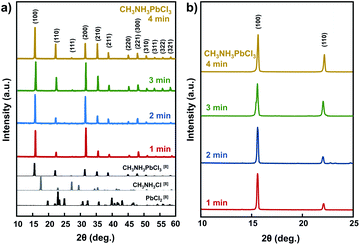 | ||
| Fig. 2 X-ray diffraction patterns of the obtained single crystal samples prepared in varying microwave irradiation time. (a) Full XRD pattern; (b) zoom-in XRD pattern for 2θ = 10–25 degree. | ||
| Sample | Space group = Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, α = β = γ = 90°, a = b = c (Å) m, α = β = γ = 90°, a = b = c (Å) |
Volume (Å3) |
|---|---|---|
| Liu, Y. et al., (2015)14 | 5.6855 | 183.783(3) |
| Maculan, G. et al., (2015)21 | 5.67 | 182.284(3) |
| Baikie, T. et a., (2015)9 | 5.68415(6) | 183.653(4) |
| Cheng, X et al., (2018)29 | 5.6768 | 182.9408(9) |
| Nandi, P. et al., (2019)30 | 5.6834 | 183.5797 |
| This work (1 min) | 5.6904(3) | 184.2551(8) |
| This work (2 min) | 5.6854(8) | 183.77(5) |
| This work (3 min) | 5.6827(2) | 183.513(4) |
| This work (4 min) | 5.6803(7) | 183.286(5) |
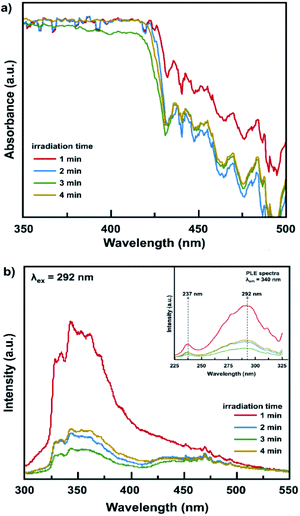
The optical properties of the obtained CH3NH3PbCl3 single crystal were characterized by UV-Vis and photoluminescence spectroscopy. Fig. 3a shows the identical absorption spectrum of all CH3NH3PbCl3 single crystal samples that were prepared in varying irradiation times. It indicates that all samples are pure CH3NH3PbCl3 corresponds to the XRD results, as discussed in Fig. 2. The CH3NH3PbCl3 single crystal has a clear colour showing a strong absorption a noticeably short wavelength region (<500 nm). All crystal samples also do not exhibit an absorption tail or excitonic peak indicating high quality crystal with low defect concentration.14
Surprisingly, the obtained single crystals exhibit a strong UV luminescence with peak ∼340 nm under 292 nm excitation wavelength as shown in Fig. 3b. The UV luminescence was also observed on the single crystal OTPs that synthesized by other methods.14,32,33 In varying microwave irradiation time, the PL intensity of single crystal was varying with the identical emission spectra. The single crystal prepared under 1 minute irradiation exhibits the most intense emission intensity that might be ascribed to the anisotropic crystal structure. The crystal bulk consists of the repeating atoms and the arrangements vary in different crystal orientations, which leads to an anisotropy of the crystal structure. The crystal anisotropy plays a crucial role in the optoelectronic properties in perovskite crystals. As discussed in Fig. 2b, the 1 minute sample was dominated by the (100) plane that was reported has a higher charge density than others. This charge density is related to the charge density distribution for [CH3NH3]+ and [PbCl6]4− in the crystal plane.29 While, other samples that were dominated not only by (100) plane but also (110) plane might be suffering a great defect migration path, since the CH3NH3PbCl3 has a pseudo-cubic structure.34 The lattice defect because of a strong ion migration was also possibly present even though in a single crystal structure.
Fig. 4 shows a Tauc-plots that derived from the absorption spectra of CH3NH3PbCl3 single crystal. The optical bandgap (Eg) is obtained by fitting the linear region between the axis of photon energy (hν) and the (αhν)1/n with n = 1/2, owing to its direct bandgap properties.14,20,35,36 Based on this approximation, the optical bandgap of the CH3NH3PbCl3 single crystal was estimated at ∼2.8 eV. This value is closed to the thin film polycrystalline perovskite (∼3.1 eV) that is in the preferable range for optoelectronic application, including solar cells and field effect transistor.33 It indicates that the obtained CH3NH3PbCl3 single crystal could be potentially applied in the optoelectronic application.
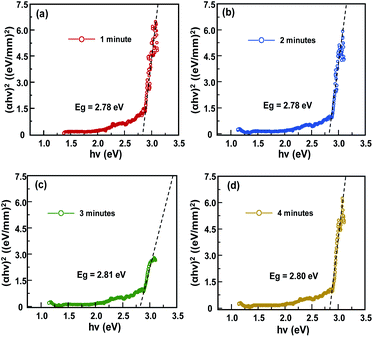 | ||
| Fig. 4 Tauc-plots obtained from the absorbance spectra of samples. The intercept dash line with the horizontal axis estimate the optical band gap energy of samples. | ||
Varying the microwave irradiation of precursor results in various dimensions of single crystal. The correlation between the sample dimension that was evaluate in volumetric crystal value with the microwave irradiation was shown in Fig. 5. The final temperature of sample right after the microwave irradiation was also presented in that figure in right y-axis. When the microwave irradiation time is prolonged, the single crystal size is getting smaller. Longer interaction between microwave with the precursor gives an elevated final temperature of sample. The microwave irradiation from 1 to 2 minutes gives a slightly elevated temperature of 2 °C, while the crystal size decrease significantly. It was an intriguing phenomenon since in conventional heating methods, the crystal size strongly depends on the synthesis temperature.14,21 Thus, the possible mechanism of crystallization process might be also differing from the conventional heating.
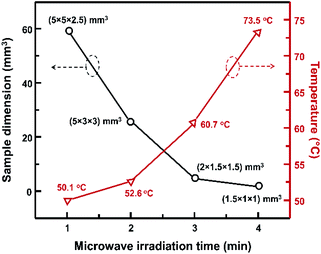 | ||
| Fig. 5 The dependence of the sample dimension to the microwave irradiation time and the sample temperature right after microwave irradiation. | ||
As a comparison, several CH3NH3PbCl3 single crystal that have been prepared by other methods were summarized in Table 2. In conventional heating, a crystal seed was required to preserve the crystal growth process via a first-order mechanism to the crystal seed surface.14,29 The crystal seed was prepared from a precursor solution through conventional heating for several hours at high temperatures (i.e., 80 and 100 °C). The nucleation process of crystal seed was controlled by the temperature and precursor concentration. Since the perovskite has inversely dissolution temperature characteristic, the crystal seed was limited only to the small size seed. Then, the obtained single crystal was strongly depending on the crystal seed's surface area itself. While the inverse temperature crystallization method utilizes the room temperature as a growth crystal environment condition from a prepared single crystal seed.21,41 Even though the crystal seed process took place under a lower temperature than the conventional heating, the crystal growth process took a set of repeated crystallization processes for a long time, up to several weeks. It might correspond to the slow kinetic crystal growth process under room temperature. Despite of both methods, the solvent evaporation method that was proposed by Nandi et al., did not required a crystal seed.30 The nucleation was initiated along the evaporation of precursors solution under room temperature. Thus, several weeks was required to obtain the CH3NH3PbCl3 single crystal with the dimension of 8 × 5 × 1 mm. Regardless of the single crystal dimension, this microwave method successfully obtained a single crystal in noticeably short time without involving the crystal seed. It suggested that this method could meet the large-scale production requirement for commercial application.
| Preparation method | CH3NH3PbCl3 single crystal dimension (in mm) | Required seed crystal | Crystal growth duration | Ref. |
|---|---|---|---|---|
| a The written of crystal growth duration of ITC methods was referred to the crystal seed preparation time.b The written of crystal growth duration of M-ITC methods was referred to one cycle of crystal seed preparation up to room temperature cooling process. The crystal growth took place under room temperature for a couple of days through repeated cycles. | ||||
| Conventional heating | (13.5 × 13.5 × 4.5) | Yes | 100 °C, 72 h | Liu et al.14 |
| Conventional heating | (5 × 5 × 2) | Yes | 80 °C, 6–7 days | Cheng et al.29 |
| Inverse temperature crystallization (ITC)a | (2 × 4 × 4) | Yes | 50 °C, 6h | Maculan et al.21 |
| Modified inverse temperature crystallization (M-ITC)b | (6 × 6 × 2) | Yes | 20–60 °C, 5h | Chen. et al.41 |
| Solvent evaporation | (8 × 5 × 1) | No | Room temperature, 2 weeks | Nandi et al.30 |
| Microwave heating | (5 × 5 × 2.5) | No | 50 °C, 1 minute | This work |
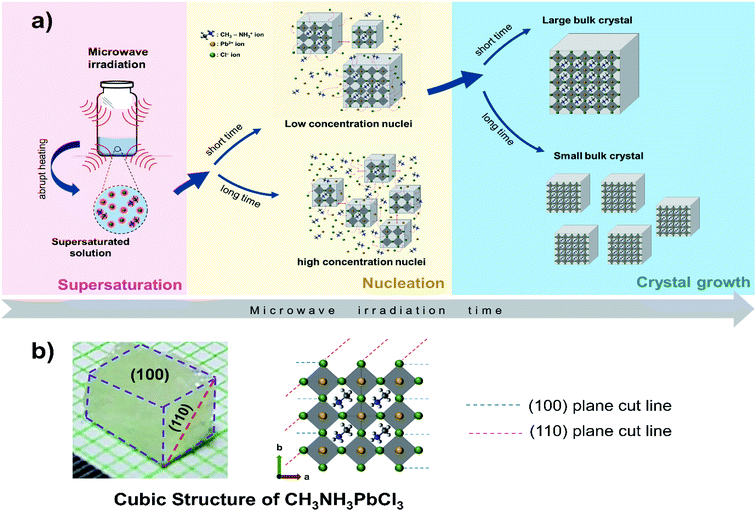
Possible mechanism of crystallization process of the CH3NH3PbCl3 crystal bulk could be considered based on the classical nucleation theory, which explains three consecutive steps of the crystallization process, namely supersaturation, nucleation, and growth of the solid phase.37–39 In the initial phase, the precursors solution is converted to the supersaturation of ionic solution. A systematic experiment revealed that the optimum precursor is found to be ≈ 2 M in DMSO that was also observed in other repots.14,21 When microwave irradiation started, an elevated temperature of solution led to the dissolution of complex compounds increase of ion concentrations (CH3NH3+, Pb2+, and Cl−) and acid content of the ionic solution.40 This resulted ionic solution perform an efficient microwave absorption owing to its high ionic conductivity and polarizability and subsequently, the nucleation process was initiated.22,25
The possible mechanism of nucleation and growth process of CH3NH3PbCl3 single crystal by microwave irradiation was proposed in Fig. 6a shows. The nucleation stage of the crystal was generated by an abrupt supersaturation through a microwave irradiation. Short time irradiation (1 minute) gives an elevated solution temperature only up to ∼50 °C that might be drift the low resulted nuclei concentration. In contrast, the longer evaporation was occurred when medium or high duty cycle mode was used for a minute. Then, the microwave irradiation time was varying from 1 to 4 minutes. Notably, less than 1 minute irradiation time do not generate the crystal growth and irradiation for more than 4 minutes suffer excessive evaporation that hinder the crystallization process irradiation time (up to 4 minutes) give an elevated solution temperature up to ∼70 °C that could possibly generate more nuclei. Subsequent growth of nuclei could depend on its nuclei diffusion that correspond to the provide energy from microwave irradiation. Naturally, the low concentration of resulted nuclei could harvest a larger crystal than a high concentration of nuclei. The nucleation and growth process could possibly inhomogeneous correspond to the abrupt supersaturation during microwave irradiation.
Based on the XRD results, the resulted CH3NH3PbCl3 single crystal could be anisotropically growing in two major directions, i.e. (100) and (110).31 Fig. 6b shows the possible crystal orientation of the CH3NH3PbCl3 single crystal that was prepared by 1 minute microwave irradiation time. This crystal was dominated by (100) plane that might be originated from strong interaction between [CH3NH3]+ and [PbCl6]4− ions. The [CH3NH3]+ ions rotate disorderly in the ab plane and over four similar orientations in the form of the orthorhombic phase and subsequently directed towards the distorted cubic faces in the direction of (100), (010) and (001) plane.42
The CH3NH3PbCl3 single crystal has highly potential application in various applications including photovoltaics, LEDs, lasers, photodetectors and other optoelectronic devices.5,14,26 It is worth to note that the CH3NH3PbCl3 single crystal with the (100) and (110) planes orientations exhibits superior opto-electronical properties compare to other crystal orientation, especially for the photodetector application. Owing to its anisotropic properties, the (100) planes was reported to possesses stronger charge transportation capacity than other orientation.29 It was led by the high charge density of (100) plane that is attributed to the higher distribution density of [CH3NH3]+ ions and [PbCl]− ions. In addition, the electron excitation energy in (100) plane lies on wide range and resulted high photocurrents. Thus, it was implied that the obtained CH3NH3PbCl3 single crystal by microwave heating method was highly potential applied as a photodetector with excellent performances.
Conclusions
In summary, the CH3NH3PbCl3 perovskite single crystal has been successfully synthesized through microwave irradiation in a short time. The crystal size could be well controlled by adjusting the microwave irradiation time. Short microwave irradiation time could possibly generate a low nuclei concentration result in a large crystal size. The XRD characterization of the resulted crystal indicates the as-grown crystal are in cubic phase of CH3NH3PbCl3 with a lattice constant of a = 5.6 Å. The obtained crystal also exhibits broad absorption and a strong luminescence in near-visible light range under UV excitation. The Tauc-plots of the absorption spectra estimate the optical bandgap of the resulted crystal is around 2.8 eV. These outstanding properties of the CH3NH3PbCl3 single crystal generating by short time microwave irradiation could be potentially applied in any optoelectronic application. Moreover, a simple apparatus, commercially microwave that used open the development of an efficient perovskite single crystal preparation method for scale-up production.Conflicts of interest
There are no conflicts to declare.Acknowledgements
F. A. P would like to thank Ministry of Finance Secretary General Indonesia Endowment Fund for Education (LPDP) for the doctoral scholarship. This work was supported by World Class University Institut Teknologi Bandung (WCU ITB) through Program Penelitian Luar Negeri Fiscal Year 2020.References
- G. Kieslich, S. Sun and A. K. Cheetham, Chem. Sci., 2014, 5, 4712–4715 RSC.
- B. Saparov and D. B. Mitzi, Chem. Rev., 2016, 116, 4558–4596 CrossRef CAS.
- G. F. Needham, R. D. Willett and H. F. Franzen, J. Phys. Chem., 1984, 88, 674–680 CrossRef CAS.
- D. Weber, Z. Naturforsch., B: J. Chem. Sci., 1978, 33, 1443–1445 Search PubMed.
- M. I. Saidaminov, V. Adinolfi, R. Comin, A. L. Abdelhady, W. Peng, I. Dursun, M. Yuan, S. Hoogland, E. H. Sargent and O. M. Bakr, Nat. Commun., 2015, 6, 8724 CrossRef CAS.
- M. D. Birowosuto, D. Cortecchia, W. Drozdowski, K. Brylew, W. Lachmanski, A. Bruno and C. Soci, Sci. Rep., 2016, 6, 37254 CrossRef CAS.
- M. A. Green and T. Bein, Nat. Mater., 2015, 14, 559–561 CrossRef CAS.
- S. De Wolf, J. Holovsky, S.-J. Moon, P. Löper, B. Niesen, M. Ledinsky, F.-J. Haug, J.-H. Yum and C. Ballif, J. Phys. Chem. Lett., 2014, 5, 1035–1039 CrossRef CAS.
- T. Baikie, Y. Fang, J. M. Kadro, M. Schreyer, F. Wei, S. G. Mhaisalkar, M. Graetzel and T. J. White, J. Mater. Chem. A, 2013, 1, 5628 RSC.
- T. Wang, B. Daiber, J. M. Frost, S. A. Mann, E. C. Garnett, A. Walsh and B. Ehrler, Energy Environ. Sci., 2017, 10, 509–515 RSC.
- S. D. Stranks, G. E. Eperon, G. Grancini, C. Menelaou, M. J. P. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Science, 2013, 342, 341–344 CrossRef CAS.
- G. Xing, N. Mathews, S. Sun, S. S. Lim, Y. M. Lam, M. Gratzel, S. Mhaisalkar and T. C. Sum, Science, 2013, 342, 344–347 CrossRef CAS.
- J. Chen, S. Zhou, S. Jin, H. Li and T. Zhai, J. Mater. Chem. C, 2016, 4, 11–27 RSC.
- Y. Liu, Z. Yang, D. Cui, X. Ren, J. Sun, X. Liu, J. Zhang, Q. Wei, H. Fan, F. Yu, X. Zhang, C. Zhao and S. F. Liu, Adv. Mater., 2015, 27, 5176–5183 CrossRef CAS.
- G.-J. A. H. Wetzelaer, M. Scheepers, A. M. Sempere, C. Momblona, J. Ávila and H. J. Bolink, Adv. Mater., 2015, 27, 1837–1841 CrossRef CAS.
- A. Mei, X. Li, L. Liu, Z. Ku, T. Liu, Y. Rong, M. Xu, M. Hu, J. Chen, Y. Yang, M. Gratzel and H. Han, Science, 2014, 345, 295–298 CrossRef CAS.
- Y. Shao, Z. Xiao, C. Bi, Y. Yuan and J. Huang, Nat. Commun., 2014, 5, 1–7 Search PubMed.
- X. Chi, K. Leng, B. Wu, D. Shi, Y. Choy, Z. Chen, Z. Chen, X. Yu, P. Yang, Q.-H. Xu, T. C. Sum, A. Rusydi and K. P. Loh, Adv. Opt. Mater., 2018, 6, 1800470 CrossRef.
- K. Leng, W. Fu, Y. Liu, M. Chhowalla and K. P. Loh, Nat. Rev. Mater., 2020, 5, 482–500 CrossRef CAS.
- Y. Dang, C. Zhong, G. Zhang, D. Ju, L. Wang, S. Xia, H. Xia and X. Tao, Chem. Mater., 2016, 28, 6968–6974 CrossRef CAS.
- G. Maculan, A. D. Sheikh, A. L. Abdelhady, M. I. Saidaminov, M. A. Haque, B. Murali, E. Alarousu, O. F. Mohammed, T. Wu and O. M. Bakr, J. Phys. Chem. Lett., 2015, 6, 3781–3786 CrossRef CAS.
- C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 CrossRef CAS.
- Q. Cao, S. Yang, Q. Gao, L. Lei, Y. Yu, J. Shao and Y. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 7854–7861 CrossRef CAS.
- T. Kollek, C. Fischer, I. Göttker-Schnetmann and S. Polarz, Chem. Mater., 2016, 28, 4134–4138 CrossRef CAS.
- M. Bhattacharya and T. Basak, Energy, 2016, 97, 306–338 CrossRef.
- J. Jancik, A. Jancik Prochazkova, M. C. Scharber, A. Kovalenko, J. Másilko, N. S. Sariciftci, M. Weiter and J. Krajcovic, Cryst. Growth Des., 2020, 20, 1388–1393 CrossRef CAS.
- Z. Lu, E. Manias, D. D. Macdonald and M. Lanagan, J. Phys. Chem. A, 2009, 113, 12207–12214 CrossRef CAS.
- Q. Jie and J. Guo-Zhu, J. Phys. Chem. A, 2013, 117, 12983–12989 CrossRef CAS.
- X. Cheng, L. Jing, Y. Zhao, S. Du, J. Ding and T. Zhou, J. Mater. Chem. C, 2018, 6, 1579–1586 RSC.
- P. Nandi, C. Giri, D. Swain, U. Manju and D. Topwal, CrystEngComm, 2019, 21, 656–661 RSC.
- S. Sun, Y. Fang, G. Kieslich, T. J. White and A. K. Cheetham, J. Mater. Chem. A, 2015, 3, 18450–18455 RSC.
- L. Dimesso, M. Dimamay, M. Hamburger and W. Jaegermann, Chem. Mater., 2014, 26, 6762–6770 CrossRef CAS.
- N. Kitazawa, Y. Watanabe and Y. Nakamura, J. Mater. Sci., 2002, 37, 3585–3587 CrossRef CAS.
- Z. Zuo, J. Ding, Y. Zhao, S. Du, Y. Li, X. Zhan and H. Cui, J. Phys. Chem. Lett., 2017, 8, 684–689 CrossRef CAS.
- S. Tsunekawa, T. Fukuda and A. Kasuya, J. Appl. Phys., 2000, 87, 1318–1321 CrossRef CAS.
- J. Tauc, Mater. Res. Bull., 1968, 3, 37–46 CrossRef CAS.
- L. L. Regel and W. R. Wilcox, Cryst. Growth Des., 2005, 5, 1657 CrossRef CAS.
- K. J. Laidler and M. C. King, J. Phys. Chem., 1983, 87, 2657–2664 CrossRef CAS.
- A. A. Zhumekenov, V. M. Burlakov, M. I. Saidaminov, A. Alofi, M. A. Haque, B. Turedi, B. Davaasuren, I. Dursun, N. Cho, A. M. El-Zohry, M. De Bastiani, A. Giugni, B. Torre, E. Di Fabrizio, O. F. Mohammed, A. Rothenberger, T. Wu, A. Goriely and O. M. Bakr, ACS Energy Lett., 2017, 2, 1782–1788 CrossRef CAS.
- P. K. Nayak, D. T. Moore, B. Wenger, S. Nayak, A. A. Haghighirad, A. Fineberg, N. K. Noel, O. G. Reid, G. Rumbles, P. Kukura, K. A. Vincent and H. J. Snaith, Nat. Commun., 2016, 7, 13303 CrossRef CAS.
- Y. Chen, X. Hou, S. Tao, X. Fu, H. Zhou, J. Yin, M. Wu and X. Zhang, Chem. Commun., 2020, 56, 6404–6407 RSC.
- M. T. Weller, O. J. Weber, P. F. Henry, A. M. Di Pumpo and T. C. Hansen, Chem. Commun., 2015, 51, 4180–4183 RSC.
| This journal is © The Royal Society of Chemistry 2021 |