Open Access Article
Open Access ArticleRecovery of valuable metals from mixed spent lithium-ion batteries by multi-step directional precipitation†
Xuan Yangb,
Yingjie Zhangab,
Qi Meng *a,
Peng Dong*a,
Peichao Ningb and
Qingxiang Lic
*a,
Peng Dong*a,
Peichao Ningb and
Qingxiang Lic
aNational and Local Joint Engineering Laboratory for Lithium-ion Batteries and Materials Preparation Technology, Key Laboratory of Advanced Battery Materials of Yunnan Province, Faculty of Metallurgy and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China. E-mail: mengqi315117@126.com; dongpeng2001@126.com
bFaculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming 650093, China
cShenzhen Zhongjin Lingnan Technology Co., Ltd., Shenzhen 518118, China
First published on 23rd December 2020
Abstract
The novel strategy of multi-step directional precipitation is proposed for recovering valuable metals from the leachate of cathode material obtained by mechanical disassembly from mixed spent lithium-ion batteries. Based on thermodynamics and directional precipitation, Mn2+ is selectively precipitated under conditions of MRNM (molar ratio of (NH4)2S2O8 to Mn2+) = 3, pH = 5.5 and 80 °C for 90 min. Ni2+ was then selectively precipitated using C4H8N2O2 under conditions of pH = 6, MRCN (molar ratio of C4H8N2O2 to Ni2+) = 2, 30 °C and 20 min. Then, the pH was adjusted to 10 to precipitate Co2+ as Co(OH)2. Finally, Li+ was recovered by Na2CO3 at 90 °C. The precipitation rates of Mn, Ni, Co, and Li reached 99.5%, 99.6%, 99.2% and 90%, respectively. The precipitation products with high purity can be used as raw materials for industrial production based on characterization. The economical and efficient recovery process can be applied in industrialized large-scale recycling of spent lithium-ion batteries.
1. Introduction
Lithium-ion batteries (LIBs) have been widely used in portable electronic equipment and new energy vehicles because of their high energy density, low self-discharge rate, and light weight, etc.1,2 With the large consumption and growing demand for LIBs, a large number of spent LIBs will be generated due to their limited average life of 2–4 years.3,4 Spent LIBs contain heavy metals, organic chemicals, plastics, etc., which will cause great harm to the environment.5,6 Meanwhile, spent LIBs contain valuable metals of Ni, Co, Mn, Li, etc., which will cause the squandering of resources without reasonable recycling.7,8 Therefore, the recycling of spent LIBs is necessary for protecting the environment and saving resources.9,10The recycling methods of spent LIBs mainly include pyrometallurgy processes11–13 and hydrometallurgy processes.14–16 Compared with the pyrometallurgy process,17 the hydrometallurgy process is widely used due to its advantages of mild operating conditions, high metal recovery, etc.18,19 Leaching is an important step in hydrometallurgy.20,21 Some researchers have used organic acids as leachates due to its mild and environment friendly conditions, such as tartaric acid,22 citric acid,23 oxalic acid,24 etc. However, the organic acids of leaching process seem low leaching efficiency and unsatisfied for following process.25 Inorganic acid is often used in leaching process because of its cheap price and high leaching efficiency, including HCl,26,27 HNO3,28 H2SO4,29–31 etc. Therefore, the inorganic acid is common reagent for leaching process, which seems be fully developed.
Separation of valuable metals from leachate after leaching has gained more attention, mainly including solvent extraction and chemical precipitation. The selection of extractant is the research focus of solvent extraction. There are a lot of researches on solvent extraction. Wang et al. used D2EHPA to extract copper and manganese.32 Jha et al. used Cyanex 272 to extract Co and extraction rate reaches 99.9%.33 Suzuki et al. used PC-88A/TOA mixed extractant to separate Co from leachate, the extraction rate was more than 98%.34 The solvent extraction methods can obtain high purity products, but it has disadvantages of high toxicity and high cost.
In order to reduce process cost, the chemical precipitation method with simplify process flow and improve process integrity have also been reported. Chen et al. used oxalic acid to separate Co from leach solution and the precipitation rate reached 98%.9 Wang et al. used KMnO4 to separate Mn from leaching solution, the precipitation rate reached 98%.26 Barik et al. used Na2CO3 to separate Li from leachate and the precipitation rate reached 91%.35 The precipitation method has the advantages of simple operation and low cost. However, co-precipitation is easy to occur in the precipitation process, which reduces the purity of the product. Therefore, it is very important to choose a suitable precipitant agent.
Moreover, most recycle research are reported on a single kind of spent LIBs, which was pretreated by manual disassembly with low efficiency and dangerous. Therefore, more effective recycling strategy, such as mechanical disassembly, should be developed for recycling of mixed spent LIBs. However, the related research has been rarely reported.
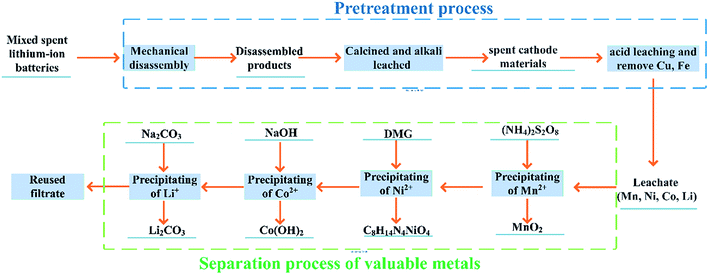
A novel recovery process of multi-step directional precipitation was proposed to recover the valuable metals from mixed spent LIBs. Based on pretreatment process, the leachate was obtained from mixed spent LIBs with mechanical disassembly, leaching and impurities removal step. Then, the selective precipitants were used for step by step separation of Mn, Ni, Co and Li. The effect of variables was investigated to obtain directional separation optimal conditions. To analyze selective precipitation process, the reaction mechanism and precipitation products was characterized by XRD, SEM, FTIR. To further analysis the economy of process, the material balance, energy balance and waste management in the whole process was analyzed. The whole process is green, efficient and economical with high purity precipitation products and without waste liquid, which can provide a new idea for the industrial application of high efficiency and economic recovery of mixed spent LIBs.
2. Experimental section
2.1 Materials and reagents
The mixed spent LIBs used here were collected from BYD Technology Co., Ltd. The disassembled products were obtained by mechanical disassembly. The specific process of mechanical disassembly is shown in Fig. S1.† The disassembled products were analyzed by X-ray Diffraction (XRD) and thermogravimetric analysis-differential scanning calorimetry (TG-DSC). All reagents used here were analytical grade and solutions were prepared with deionized water.2.2 Pretreatment process
10 g of disassembled products were calcined at an optimal temperature based on analysis of TG-DSC to obtain spent residues. The contents of metals in spent residues were measured by inductively coupled plasma optical emission spectrometer (ICP-OES). The results are shown in Table S1.†In order to improve the separation efficiency of Ni, Co, Mn and Li, impurities such as Al, Cu and Fe should be removed before separation step. The spent residues were immersed in 3 mol L−1 NaOH solution at 60 °C for 2 h, and then washed three times with deionized water and filtered to obtain spent cathode materials. The spent cathode materials were leached in 3 mol L−1 H2SO4 under conditions of 3 vol% H2O2, solid–liquid ratio of 20 g L−1, 80 °C for 1 h to obtain leachate. Subsequently, iron was used for remove copper in the leachate under the condition of pH = 1.5 and ultrasound at 30 °C for 20 minutes. After filtration, the pH of leachate was adjusted to 3.5 to remove Fe at 90 °C. Finally, the leachate was obtained and analyzed by ICP-OES. The results are shown in Table S2.†
2.3 Separation process of valuable metals
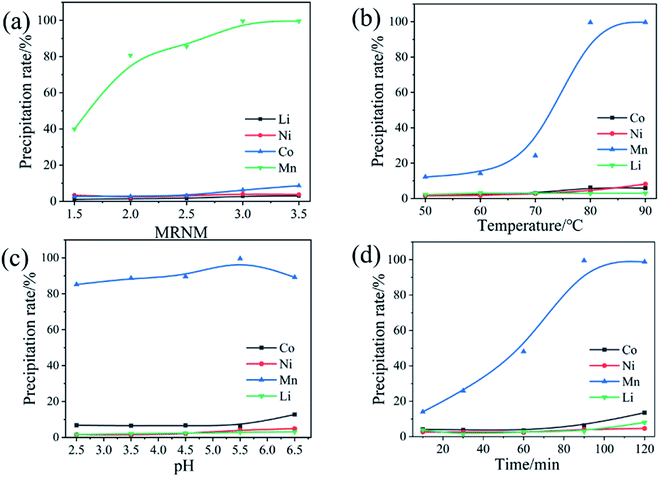
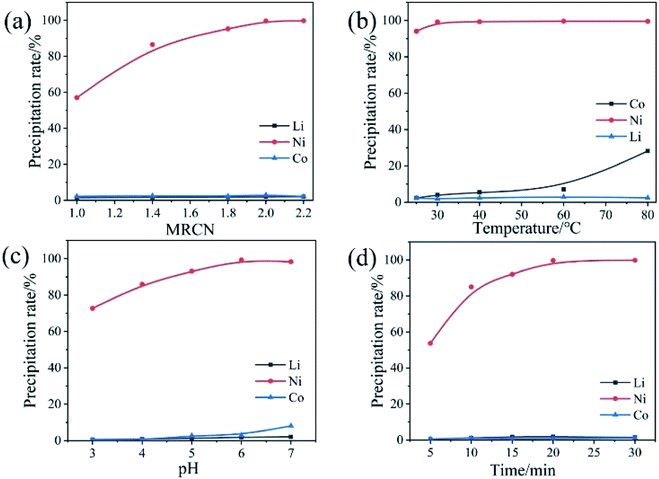
The multi-step directional precipitation method was used to recover valuable metals from the leachate. A certain amount of (NH4)2S2O8 powders were added to a certain volume of leachate in a 250 ml three neck flask and stirring rate was controlled at 300 rpm. The effects of pH value, temperature, molar ratio of (NH4)2S2O8 to Mn2+ (MRNM) and time on the reaction were studied. Under the optimal conditions of Mn recovery, the remaining leachate was treated to obtain the solution enriched with Ni, Co, Li and products of Mn. The same experimental operations were used for recovery of Ni and Co. The Ni from the filtrate was precipitated by C4H8N2O2 (DMG). The effects of pH, molar ratio of C4H8N2O2 to Ni2+(MRCN), temperature and reaction time were studied to obtain optimal conditions. NaOH was added to the filtrate to remove cobalt after the recovery of Ni under the optimum conditions. The pH, temperature and reaction time of the solution are controlled to obtain optimal conditions for cobalt separation. Subsequently, a certain amount of Na2CO3 was added, and the reaction temperature was adjusted. Lithium was recovered as Li2CO3 by high-temperature filtration. The content changes of Mn, Ni, Co, Li in the separation process were shown in Table S3.†The precipitation rates of metals were calculated according to eqn (1):
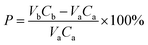 | (1) |
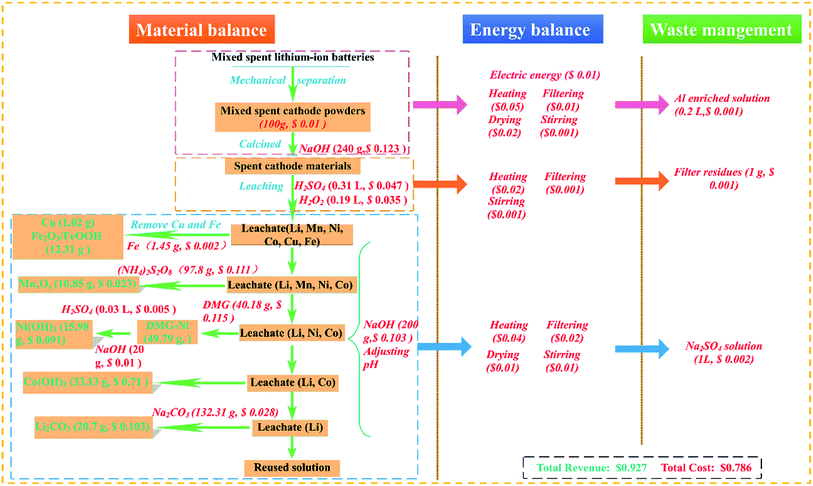
Finally, the recovery process for the recycling of mixed spent LIBs was proposed in Fig. 1. After obtaining the optimal conditions for recovery Mn, Ni, Co, and Li, a scale-up experiment was conducted. 100 g of disassembled products were subjected by recovery process and economic evaluation was carried out.
2.4 Analytical methods
The metal concentration in the solution was measured by ICP-OES (PerkinElmer Optima 8000).A pH meter (PHS-25, 2000) was used for real-time pH monitoring and adjustment. During the experiment, the pH was adjusted using 1 mol L−1 H2SO4 and 1 mol L−1 NaOH solution. A pH meter (PHS-25, 2000) was used for real-time pH monitoring and adjustment. During the experiment, the pH was adjusted using 1 mol L−1 H2SO4 and 1 mol L−1 NaOH solution. The composition of the precipitated product was detected and determined by X-ray diffractometer (XRD, Rigaku Japan). Sample morphology and elemental composition were obtained by scanning electron microscopy (SEM, TESCAN VEGA3, CZE) coupled with energy-dispersive X-ray spectroscopy (EDS). FTIR spectrometer (Nicolet Is10) was used to detect the functional groups of C8H14N4NiO4 to verifying the precipitated product.
3. Results and discussion
3.1 Pretreatment process
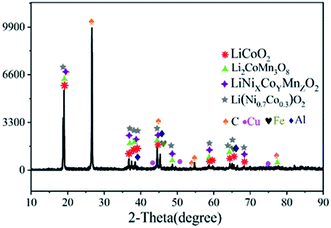
The compositions of the disassembled products were characterized by XRD. As shown in Fig. 2, LiCoO2, LiNixCoyMnzO2, Li(Ni0.7Co0.3)O2, Li2CoMn3O8 and other phases exist in the cathode powders. The phases of LiCoO2, LiNixCoyMnzO2, Li(Ni0.7Co0.3)O2 indicated that the mechanically pulverized spent batteries contain both lithium cobaltite batteries, ternary batteries and possibly other types of batteries. The phase of Li2CoMn3O8 may be generated from the heat of mechanical crushing, which causes various cathode materials to mix and react to form a mixed phase. In addition, the cathode powders contain C, Al, Fe, and Cu. C is mainly derived from the anode materials and acetylene black of the LIBs. Al, Cu and Fe are derived from the positive electrode current collectors, negative electrode current collectors and the case of the spent LIBs, respectively.Fig. 3(a) shows the TG/DSC pattern of the disassembled products. As shown in the Fig. 3(a), the endothermic peak at 0–250 °C corresponds to the loss of crystal water. The exothermic peak at 250–350 °C corresponds to the combustion of the battery separator (PP). The endothermic peak at 350–450 °C corresponds to the PVDF decomposition. The exothermic peak at 550–760 °C corresponds to the combustion of carbon. In order to remove the non-metallic impurities completely, the calcination temperature was selected at 800 °C.
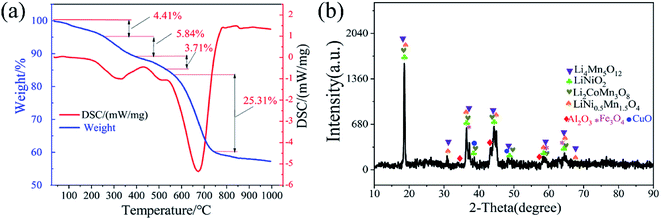 | ||
| Fig. 3 (a) Images of TG/DSC pattern of spent cathode materials; (b) image of XRD pattern of disassembled products after calcined. | ||
Fig. 3(b) shows the XRD diffraction pattern of the disassembled products calcined at 800 °C. As shown in Fig. 3(b), the Al, Fe, and Cu impurities are oxidized to form corresponding oxides while the peak of C disappears. It was attributed to the combustion decomposition of C at a high temperature of 800 °C. Moreover, the phases of LiNiO2 and Li4Mn5O12 appeared in the Fig. 3(b). This is because the structure of the cathode material has been restored to a certain extent, which proves that the spent cathode powders are made up of cathode materials of various spent LIBs. Meanwhile, as shown in Table S1,† the disassembled products contain Al, Fe and Cu, which must be removed before subsequent valuable metal separation process steps. After the impurities of Al, Cu and Fe were removed, the contents of each metal in the leachate were shown in Table S2.† As shown in Table S2,† the contents of Al, Cu and Fe are 0.002 g L−1, 0.002 g L−1 and 0.004 g L−1, respectively. The impurities are basically removed effectively, which provides good conditions for the subsequent separation process. Impurities were effectively removed which indicated that the leachate can be used for subsequent study on the separation of valuable metals.
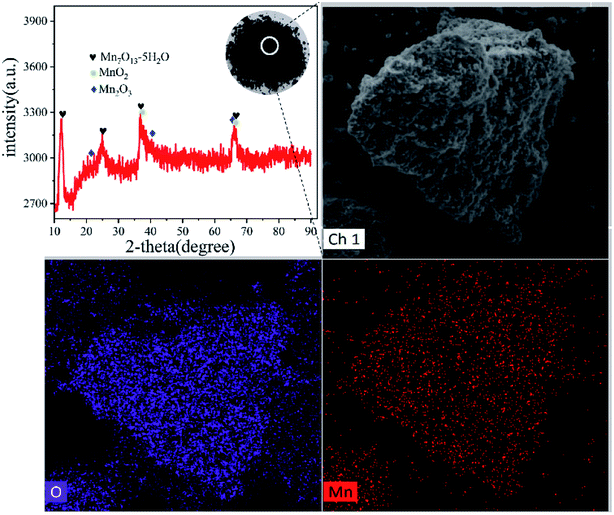
3.2 Analysis of Mn separation process
| Mn2+ + S2O82− + 2H2O = MnO2 + 2SO42− + 4H+ | (2) |
| 2Mn2+ + S2O82− + 3H2O = Mn2O3 + 2SO42− + 6H+ | (3) |
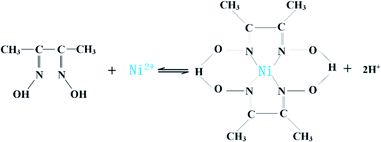
3.3 Ni separation process
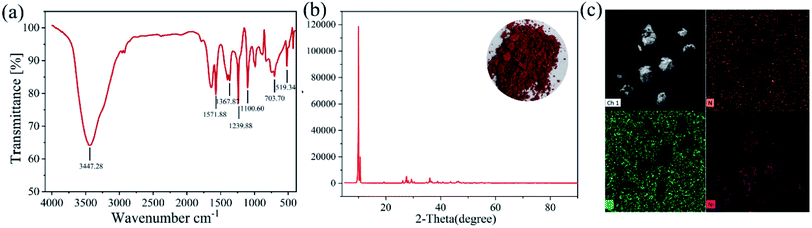
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond; the vibration peak at 1367.87 cm−1 corresponds to N–OH bending vibration peaks. 1239.88 cm−1 and 1100.30 cm−1 correspond to the N–O antisymmetric and symmetric stretching vibration peaks caused by the coordination of N–OH groups with Ni2+, respectively; 730.7 cm−1 and 519.34 cm−1 are all correspond to the absorption peak of N–Ni bond. The XRD diffraction pattern of the precipitated product is shown in Fig. 7(b). XRD pattern indicates that relatively pure precipitation product of DMG–Ni could be obtained. The EDS image of the precipitated product is shown in Fig. 7(c). The main elements on the precipitated surface are O, N and Ni. Besides, the purity of the product is 98.3% obtained by ICP, which indicated that Ni2+ could be effectively recovered from leachate by selective precipitation.
N double bond; the vibration peak at 1367.87 cm−1 corresponds to N–OH bending vibration peaks. 1239.88 cm−1 and 1100.30 cm−1 correspond to the N–O antisymmetric and symmetric stretching vibration peaks caused by the coordination of N–OH groups with Ni2+, respectively; 730.7 cm−1 and 519.34 cm−1 are all correspond to the absorption peak of N–Ni bond. The XRD diffraction pattern of the precipitated product is shown in Fig. 7(b). XRD pattern indicates that relatively pure precipitation product of DMG–Ni could be obtained. The EDS image of the precipitated product is shown in Fig. 7(c). The main elements on the precipitated surface are O, N and Ni. Besides, the purity of the product is 98.3% obtained by ICP, which indicated that Ni2+ could be effectively recovered from leachate by selective precipitation.
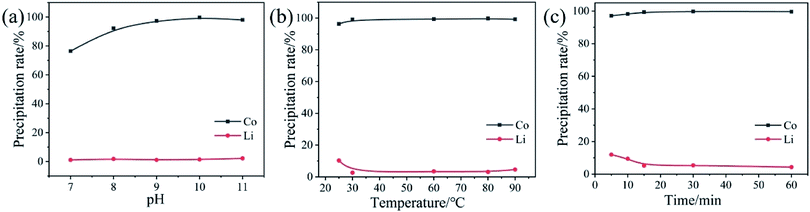
3.4 Analysis of Co separation process
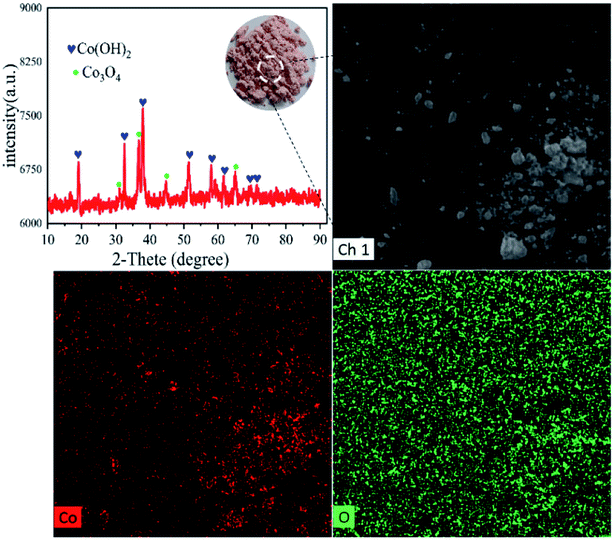
| Co2+ + 2OH− = Co(OH)2 | (4) |
3.5 Recovery of lithium
Li+ was precipitated from the leachate by Na2CO3. The temperature of the leachate was raised to 90 °C, and Na2CO3 was added to the leaching solution in an amount twice that of Li+. About 90% of Li+ was recovered as Li2CO3 with a purity of over 99%. In order to minimize the loss of lithium, the entrained impurity ions must be quickly filtered at high temperature and washed with hot deionized water (close to 100 °C). Fig. S7† shows an XRD pattern of Na2CO3 treatment and precipitation obtained. As shown in Fig. S7,† the precipitated product is Li2CO3 and there are few other peaks, which indicates that the purity of the precipitated product is very high.3.6 Recovery process analysis and economic analysis
The contents of Mn, Ni, Co and Li in the leachate during separation process were shown in Table S3.† As shown in Table S3,† the valuable metals were separated efficiently and the precipitation rates of Mn, Ni, Co, and Li reached 99.5%, 99.6%, 99.2%, and 90% under the optimal conditions. MnxOy, C8H14N4NiO4, Co(OH)2 and Li2CO3 can be used in industrial production or in the resynthesize of cathode materials after simple further purification, such as ion exchange.37 The filtrate (mainly sulfate solution) produced after recovery can be recycled.Fig. 10 shows the material balance, energy balance and waste management in the whole process of recovering 100 g disassembled products. The currency used in this economic analysis is US dollars. The precipitation products of Ni were further treated by sulfuric acid and sodium hydroxide. It can be found that the cost of NaOH ($0.236), DMG (C4H8N2O2, $0.115), (NH4)2S2O8 ($0.111) are the highest. There are four kinds of energy consumption of various experimental operations in the whole process, the total energy consumption is $0.183. Finally, it can be found that the waste generated in the recycling process is very small, and the corresponding total cost of waste treatment is $0.004. About $0.927 can be earned by corresponding products of valuable metals. Therefore, the total profit of recycling 100 g disassembled products should be $0.141.
The recovery process has the following characteristics: (1) the precipitant used in this process has high selectivity, which can avoid the occurrence of co-precipitation to the greatest extent. (2) During the process, the pH changes from low to high throughout the process, which avoids back and forth adjustment of the pH, saves the number of acid–base reagents, and simplifies operations. (3) The mechanical disassembly of the mixed spent LIBs has the characteristics of high efficiency and safety, and the mechanical disassembly device is closed cycle without waste gas releasing.
4. Conclusions
In this paper, a novel recovery process was used to recover valuable metals from the leachate of the mechanically disassembled products. Ni, Co, Mn, Li can be effectively recovered by multi-step directional precipitation. Firstly, Mn2+ was oxidized to form MnxOy by (NH4)2S2O8 on optimal conditions of pH = 5.5, temperature 80 °C, 90 min, MRNM = 3, under which about 99.5% of Mn2+ was recovered. Then, DMG was used to selectively precipitate Ni2+. Under the optimal conditions of pH = 6, 30 °C, MRCN = 2, and 20 min, about 99.6% Ni2+ can be recovered. The relatively pure C8H14N4NiO4 was obtained. NaOH was used to adjust the equilibrium pH of the filtrate to 10, and at 30 °C for 15 min, about 99.2% of Co2+ could be recovered. Finally, Li+ was precipitated with Na2CO3, which about 90% of Li was recovered. The process is efficient, economical and easily for especially suitable for the large-scale processing of mixed spent LIBs, which has great potential for industrial application.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from National Key Research and Development Program of China (2019YFC1907900) the National Natural Science Foundation of China (51764029, 52004116), the National Key Research and Development Program of China (2019YFC1803501), the Science Research Foundation of Yunnan Provincial Department of Education (2020J0070), the Applied Basic Research Plan of Yunnan Province (202001AU070039, 2018FB087), the High-level Talent Introduction Scientific Research Start Project of KUST (20190015) and the Science and Technology Plan of Shenzhen (JSGG20180508154602283).References
- G. Harper, R. Sommerville, E. Kendrick, L. Driscoll, P. Slater, R. Stolkin, A. Walton, P. Christensen, O. Heidrich, S. Lambert, A. Abbott, K. S. Ryder, L. Gaines and P. Anderson, Nature, 2019, 575, 75–86 CrossRef CAS.
- X. Zhang, L. Li, E. Fan, Q. Xue, Y. Bian, F. Wu and R. Chen, Chem. Soc. Rev., 2018, 47, 7239–7302 RSC.
- W. Wang, Y. Zhang, X. Liu and S. Xu, ACS Sustainable Chem. Eng., 2019, 7, 12222–12230 CAS.
- Y. Yang, S. Lei, S. Song, W. Sun and L. Wang, Waste Manage., 2020, 102, 131–138 CrossRef CAS.
- X. Yang, P. Dong, T. Hao, Y. Zhang, Q. Meng, Q. Li and S. Zhou, Jom, 2020, 72, 3843–3852 CrossRef CAS.
- S. Zhou, Y. Zhang, Q. Meng, P. Dong, Z. Fei and Q. Li, J. Environ. Manage., 2021, 277, 111426 CrossRef CAS.
- Q. Li, K. Y. Fung and K. M. Ng, ACS Sustainable Chem. Eng., 2019, 7, 12718–12725 CrossRef CAS.
- Q. Meng, Y. J. Zhang and P. Dong, J. Cleaner Prod., 2018, 180, 64–70 CrossRef CAS.
- X. Chen, D. Kang, L. Cao, J. Li, T. Zhou and H. Ma, Sep. Purif. Technol., 2019, 210, 690–697 CrossRef CAS.
- D. Dutta, A. Kumari, R. Panda, S. Jha, D. Gupta, S. Goel and M. K. Jha, Sep. Purif. Technol., 2018, 200, 327–334 CrossRef CAS.
- C. Hanisch, T. Loellhoeffel, J. Diekmann, K. J. Markley, W. Haselrieder and A. Kwade, J. Cleaner Prod., 2015, 108, 301–311 CrossRef CAS.
- W. S. Fonseca, X. Meng and D. Deng, ACS Sustainable Chem. Eng., 2015, 3, 2153–2159 CrossRef CAS.
- H. Nie, L. Xu, D. Song, J. Song, X. Shi, X. Wang, L. Zhang and Z. Yuan, Green Chem., 2015, 17, 1276–1280 RSC.
- M. Sathiya, A. Prakash, K. Ramesha and A. Shukla, Mater. Res. Bull., 2009, 44, 1990–1994 CrossRef CAS.
- Q. Sa, E. Gratz, M. He, W. Lu, D. Apelian and Y. Wang, J. Power Sources, 2015, 282, 140–145 CrossRef CAS.
- Y. Liu, M. Zhang, Y. Xia, B. Qiu, Z. Liu and X. Li, J. Power Sources, 2014, 256, 66–71 CrossRef CAS.
- T. Georgi-Maschler, B. Friedrich, R. Weyhe, H. Heegn and M. Rutz, J. Power Sources, 2012, 207, 173–182 CrossRef CAS.
- J. Nan, D. Han and X. Zuo, J. Power Sources, 2005, 152, 278–284 CrossRef CAS.
- D. A. Bertuol, C. M. Machado, M. L. Silva, C. O. Calgaro, G. L. Dotto and E. H. Tanabe, Waste Manag., 2016, 51, 245–251 CrossRef CAS.
- R. Zheng, L. Zhao, W. Wang, Y. Liu, Q. Ma, D. Mu, R. Li and C. Dai, RSC Adv., 2016, 6, 43613–43625 RSC.
- P. Meshram, B. Pandey and T. Mankhand, Chem. Eng. J., 2015, 281, 418–427 CrossRef CAS.
- L.-P. He, S.-Y. Sun, Y.-Y. Mu, X.-F. Song and J.-G. Yu, ACS Sustainable Chem. Eng., 2016, 5, 714–721 CrossRef.
- H. Ku, Y. Jung, M. Jo, S. Park, S. Kim, D. Yang, K. Rhee, E.-M. An, J. Sohn and K. Kwon, J. Hazard. Mater., 2016, 313, 138–146 CrossRef CAS.
- X. Zeng, J. Li and B. Shen, J. Hazard. Mater., 2015, 295, 112–118 CrossRef CAS.
- Q. Meng, Y. J. Zhang and P. Dong, Waste Manage., 2017, 64, 214–218 CrossRef CAS.
- R.-C. Wang, Y.-C. Lin and S.-H. Wu, Hydrometallurgy, 2009, 99, 194–201 CrossRef CAS.
- L. Li, J. Lu, Y. Ren, X. X. Zhang, R. J. Chen, F. Wu and K. Amine, J. Power Sources, 2012, 218, 21–27 CrossRef CAS.
- W. Lv, Z. Wang, H. Cao, X. Zheng, W. Jin, Y. Zhang and Z. Sun, Waste Manag., 2018, 79, 545–553 CrossRef CAS.
- D. A. Ferreira, L. M. Z. Prados, D. Majuste and M. B. Mansur, J. Power Sources, 2009, 187, 238–246 CrossRef CAS.
- J. Kang, G. Senanayake, J. Sohn and S. M. Shin, Hydrometallurgy, 2010, 100, 168–171 CrossRef CAS.
- G. Dorella and M. B. Mansur, J. Power Sources, 2007, 170, 210–215 CrossRef CAS.
- F. Wang, R. Sun, J. Xu, Z. Chen and M. Kang, RSC Adv., 2016, 6, 85303–85311 RSC.
- A. K. Jha, M. K. Jha, A. Kumari, S. K. Sahu, V. Kumar and B. D. Pandey, Sep. Purif. Technol., 2013, 104, 160–166 CrossRef CAS.
- T. Suzuki, T. Nakamura, Y. Inoue, M. Niinae and J. Shibata, Sep. Purif. Technol., 2012, 98, 396–401 CrossRef CAS.
- S. Barik, G. Prabaharan and B. Kumar, Waste Manag., 2016, 51, 222–226 CrossRef CAS.
- M. Rath, L. P. Behera, B. Dash, A. R. Sheik and K. Sanjay, Hydrometallurgy, 2018, 176, 229–234 CrossRef CAS.
- E. Pehlivan and T. Altun, J. Hazard. Mater., 2007, 140, 299–307 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra09297e |
| This journal is © The Royal Society of Chemistry 2021 |