Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Anti-rheumatic effect of quercetin and recent developments in nano formulation
Feng Guan
*a,
Qi Wang
 *b,
Yongping Bao
*b,
Yongping Bao
 b and
Yimin Chao
b and
Yimin Chao
 c
c
aSchool of Pharmacy, Heilongjiang University of Chinese Medicine, Harbin 150040, P. R. China. E-mail: guanfeng@hljucm.net
bNorwich Medical School, University of East Anglia, Norwich NR4 7UQ, UK. E-mail: q.wang1@uea.ac.uk; y.bao@uea.ac.uk
cSchool of Chemistry, University of East Anglia, Norwich NR4 7TJ, UK. E-mail: y.chao@uea.ac.uk
First published on 11th February 2021
Abstract
Rheumatoid arthritis (RA) is a common worldwide chronic autoimmune disease, characterised by synovial hyperplasia, inflammatory cell infiltration, pannus formation and destruction of articular cartilage and bone matrix. It is one of the most common forms of osteoarthritis bestowing high rates of both disability and death. Increasing attention has been paid to the use of natural medicines and natural products in the treatment of RA and patients' acceptance has increased year by year because of their high efficacy and safety. Flavonoids are a group of important secondary metabolites occurring in many plants which have rich biological activities such as anti-rheumatic, vasodilator, and anti-tumor effects. Many successful medical treatments of RA appear to be attributable to the application of flavonoids. Quercetin, a representative active member of the flavonoid family, is found abundantly in many plants, e.g. apples, berries, cabbages, onions, and ginkgo. In recent years, progress has been made in the research of its anti-rheumatoid effects which indicate that it is potentially a noteworthy prodrug for the treatment of RA. However, the poor solubility of quercetin affects its bioavailability and clinical efficacy. This review aims to provide an up to date summary of the biological effects and mechanism of action of quercetin for the treatment of RA, and the research progress made towards nano formulations of quercetin to improve its solubility and efficacy.
1 Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease, characterised by synovial hyperplasia, inflammatory cell infiltration, pannus formation and destruction of articular cartilage and bone matrix.1 It is one of the most common and disabling forms of osteoarthritis. It is mainly manifested by redness, swelling, a hot sensation, pain, and other symptoms of the small joints of the extremities. The lesions develop symmetrically and destructively, which may eventually lead to joint deformity and loss of function, and can even affect the heart, lungs and nervous system.2,3 In most developed countries, RA affects 0.3–1.0% of the adult population.4,5 At present, steroidal, non-steroidal anti-inflammatory drugs (NSAIDs), disease modifying anti-rheumatic drugs (DMARDs), glucocorticoids, bacterial therapy6 and targeted treatment7 are used to relieve pain and control the disease. Patients with severe joint involvement may suffer disability, and may even require joint repair or replacement.8,9However, long-term administration of these drugs may cause gastrointestinal discomfort, nausea, vomiting, and bleeding, or other adverse reactions such as to the central nervous system or cardiovascular system. Moreover, improper application of hormones may even aggravate the disease. When the disease is difficult to control, biological agents such as tumour necrosis factor inhibitors, abatacept, rituximab, and tocilizumab are often used in clinic. However their use is limited by high cost and adverse events i.e. reactions and infections at infusion and injection sites.4 Increasing attention has been paid to the use of natural medicines or natural products in the treatment of RA, and the patients' acceptance has increased year by year because of their high efficacy and safety.
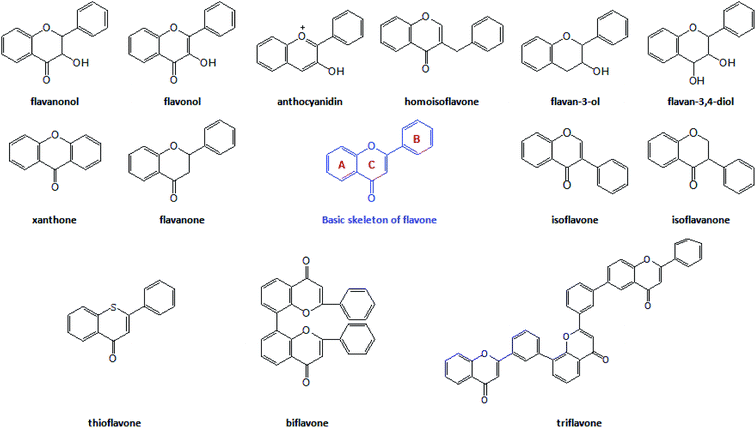
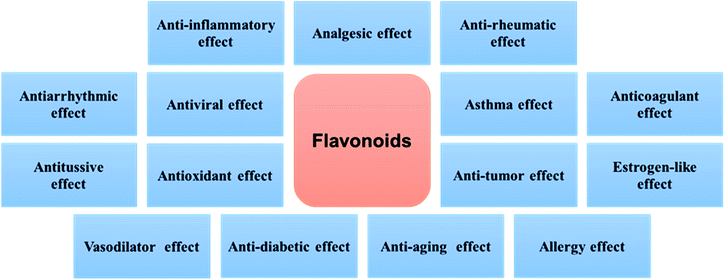
The flavonoids are vital secondary metabolites of many plants with the basic structural skeleton of 2-phenyl chromogenic ketone and consist of C6–C3–C6. It is a polyphenolic compound comprising two aromatic rings (A and B) and a heterocyclic ring (C). There are some flavonoid compounds that have a three-carbon chain but without a ring C. Some flavonoids exist in the form of dimers, trimers and even thioflavones. The main classes of natural flavonoids are flavones, isoflavones, flavanols, dihydroflavones, dihydroflavanols, bioflavonoids, triflavonoids, thioflavones, etc. (Fig. 1). Flavonoids have a number of biological activities (Fig. 2) including anti-inflammatory, analgesic, anti-rheumatic, vasodilator, anti-aging, and anti-tumour effects.10,11 The success of many medical treatments can be attributed to the application of flavonoids and new scientific studies of these compounds and their derivatives have focused on the activities above.12,13
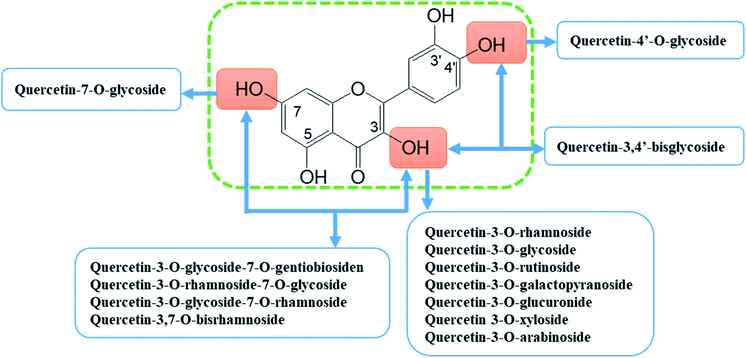
Quercetin (5,7,3′,4′-tetrahydroxyflavonol, C15H10O7) is a representative member of the flavonoid's family. It is found abundantly in a variety of foods including apples, berries, Brassica vegetables, grapes, onions, shallots, as well as many medicinal plants including Ginkgo biloba, Hypericum perforatum, and Sambucus canadensis.14–16 Quercetin frequently occurs as quercetin glycosides where polyhydroxyl substitution appears in its structure.17 The quercetin glycosides derivatives that have been identified include quercetin-3-O-rhamnoside (quercitrin), quercetin-3-O-glycoside (isoquercitrin), quercetin-3-O-rutinoside (rutin), and quercetin-7-O-glycoside (quercimeritrin) (Fig. 3).18–20 Quercetin possesses the typical pharmacological effects of flavonoids, such as anti-inflammatory, analgesic, anti-rheumatic, antioxidant, anti-tumour, etc.12,21–24. In recent years, new progress has been made in the research of its anti-rheumatoid effects which indicate that it is safe to use, with few side effects and thus a noteworthy prodrug for the treatment of RA.
Despite showing promising potential for medicinal use, the real-life application of quercetin has been largely limited due to its poor solubility and bioavailability.13,18 The solubility of quercetin in water is 7 μg mL−1, and it is absorbed and metabolised rapidly after entering the body. Quercetin has strong first pass effect and its bioavailability is very low, less than 3.6%. Quercetin is inactivated by combining with sugar molecules into gluconic acid and so on. Therefore, there is a need to employ modern nanotechnology to improve its solubility and bioavailability, so as to better deploy its anti-rheumatoid effects. This review is intended to provide an insight into the pharmacological function and mechanisms of action of therapeutic use of quercetin for RA. The recent research progress using nano formulation as a strategy to increase/improve quercetin potential in RA treatment have also been summarised. Our review is mainly based on the published journal papers in recent 15 years, excluding patent literature.
2 Anti-rheumatic effects of quercetin
The pathogenesis of RA is complex and has not been fully determined.25 Clinical treatment is mainly based on the purpose of reducing inflammation and alleviating symptoms. There is considerable research interest in the potential health benefits of quercetin. Therefore, the anti-rheumatoid effect of quercetin is summarised from the perspective of anti-inflammatory effects, analgesic effects, and the effect on experimental rheumatoid animal models.2.1 Anti-inflammatory effect and mechanism of action
Inflammation plays a key role in rheumatoid diseases.26 Research results using both in vitro and animal models have shown that quercetin can inhibit the occurrence and development of inflammation, thus having an important potential impact on RA.Liao and Lin studied the pharmacological effects of quercetin on systemic inflammation in septic mice.27 The sepsis mouse model was established by intraperitoneally (i.p.) injecting lipopolysaccharide (LPS). LPS is an important trigger of inflammatory response, which can stimulate a variety of cells in vivo, especially macrophages to synthesize and release many endogenous bioactive factors, leading to inflammatory response. They then administered quercetin to the septic mice in a prophylactic or therapeutic manner. Their results suggested that quercetin administration i.p. at a high dose of 0.15 μmol to each mouse could significantly (P < 0.05) increase Interleukin 10 (IL-10) secretion and had strong anti-inflammatory potential. Huang et al. similarly observed the anti-inflammatory effect of quercetin.28 Their results showed that quercetin at 100, 200, and 400 mg kg−1 could significantly inhibit the auricle swelling of rats caused by xylol, and the degree of swelling and inhibition rate were significantly different from those in the control group (P < 0.01). This suggests that quercetin can have a good inhibitory effect on inflammatory response.
RAW264.7 is a monocyte/macrophage-like cell line that has been frequently used to study immune function. Zhou et al. proved that quercetin significantly inhibited the increase of nitric oxide (NO), Tumour Necrosis Factor alpha (TNF-α), Interleukin 18 (IL-18) and Interleukin 6 (IL-6) in Raw264.7 cells induced by LPS (P < 0.01) showing that it has a good anti-inflammatory effect in vitro.29 These results agreed with the findings from Paul et al.30 and Cessak et al.31 which suggested that quercetin is an effective inhibitor for TNF-α and IL-6. Ren et al. also studied the protective effect of quercetin on LPS induced inflammation in RAW264.7 cells.32 Their results suggested that the protective effect may be related to the regulation of Toll-like receptor 4/nuclear factor kappa-light-chain-enhancer of activated B (TLR4/NF-κB) signalling pathway.
Chronic inflammation, a process linked to increased oxidative stress, may induce many diseases. Yeh et al. investigated the effects of β-carotene on the inflammatory reaction of macrophage model cells (differentiated HL-60 cells and RAW264.7 cells) and their modulation by quercetin or naringenin.33 Their results demonstrated that quercetin partially suppressed the pro-inflammatory effects, synergistically enhanced the inhibitory effects of β-carotene on the secretion of pro-inflammatory mediators and the DNA damaging ability of PMA-stimulated HL-60 cells. The mechanism of action was associated with its antioxidant activity and inhibition of the production of pro-inflammatory cytokines. Avila et al. summarised that quercetin showed a mixed inhibition mechanism towards Adenosine triphosphate (ATP) and that the binding site of quercetin overlaps with both ATP and inhibitor of nuclear factor kappa B (IκBα) binding sites.34
Extracellular High-Mobility Group Box-1 (HMGB-1) is an important late-stage inflammatory transmitter with strong inflammatory activity. It contributes to the pathogenesis of numerous chronic inflammatory and autoimmune diseases including RA. Musumeci et al. indicated that quercetin was a HMGB1 inhibitor and it could limit the activation of mitogen-activated protein kinase.35
2.2 Analgesic effect and mechanism of action
In addition to its significant anti-inflammatory effect, quercetin also shows significant analgesic activity.Liu DN has reported that quercetin had no significant effect on paw withdrawal thermal latency in naïve rats. However, it could significantly increase the threshold of mechanical paw contraction response.36 The study also showed that quercetin has a significant inhibitory effect on bee venom induced spontaneous nociceptive response, pain score, thermal and mechanical pain sensitivity, and also on bee venom induced local inflammatory response.36 This suggests that quercetin's analgesic effect may be related to the blocking of pro-inflammatory factors. In addition, quercetin has a considerable inhibitory effect on the ipsilateral mechanical hyperalgesia and contralateral mechanical hyperalgesia caused by sciatic nerve branch injury model.
In addition to the above studies on the anti-inflammatory and analgesic effects of quercetin monomer, there are also a number related studies of medicinal plant extracts with quercetin as the main component, which further confirm the anti-inflammatory and analgesic effects of quercetin.37–47
2.3 Effects on experimental animal model
There is growing interest in the anti-rheumatoid effects of quercetin. The commonly used animal models of RA mainly include adjuvant induced arthritis (AA), collagen induced arthritis (CIA), oil induced arthritis (OIA), and proteoglycan induced arthritis (PGIA).48–51 In recent years, the research of anti-rheumatoid effect of quercetin has been mainly based on AA and CIA models.Mamani-Matsuda et al. observed that the therapeutic and preventive properties of quercetin in experimental arthritis correlated with decreased macrophage inflammatory mediators.53 Their results indicated that in chronic rat (AA), oral administration of quercetin (30 mg per rat every 2 days, for 10 days) to arthritic rats resulted in a clear decrease of clinical signs compared to untreated controls. The effects of oral administration of quercetin (150 mg kg−1 daily, 28 days) were also investigated in a rat model of adjuvant arthritis by Gardi et al.54. Their results indicated that quercetin lowered levels of Interleukin 1β (IL-1β) (p < 0.003), monocyte chemotactic protein-1 (MCP-1) (p < 0.014) and restored plasma antioxidant capacity.
| Effects | Model inducer | Animal | AM | Dose | SW | PT | WT | PWTL | PWMT | TNF-α | IL-1β | IL-1α | IL-6 | IL-8 | IL-10 | IL-17 | NO | CRP | MCP-1 | PGE2 | MMP-1 | MMP-3 | MMP-13 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Note: ① AM(administration mode), SW (swelling), PT (pain threshold), WT (writhing times), PWTL (paw withdraw thermal latency), PWMT (paw withdrawal mechanical threshold), IFA (incomplete Freund's adjuvant), CRP(C-reactive protein), MCP-1 (monocyte chemotactic protein-1), PMA (phorbol-12-myristate-13-acetate), Ref. (References). ② — not mentioned, ↑ increase, ↓ reduce. | ||||||||||||||||||||||||
| Anti-inflammatory effects | LPS | BALB/c mice | i.p. | 0.15 μmol (each mouse) | — | — | — | — | — | ↑ | ↓ | — | ↑ | — | ↑ | — | — | — | — | — | — | — | — | 27 |
| Anti-inflammatory effects | Sodium urate | SD rats | i.g. | 100, 200, 400 mg kg−1 | ↓ | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 28 |
| Anti-inflammatory effects | Xylene | BALB/c mice | i.g. | 100, 200, 400 mg kg−1 | ↓ | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 28 |
| Anti-inflammatory effects | LPS | In vitro | — | 2.5, 5, 10 μg mL−1 | — | — | — | — | — | ↓ | ↓ | — | ↓ | — | ↓ | — | ↓ | — | — | — | — | — | — | 29 |
| Anti-inflammatory effects | LPS | In vitro | — | 5, 15, 25 μM | — | — | — | — | — | ↓ | ↓ | — | ↓ | — | — | — | ↓ | — | — | — | — | — | — | 32 |
| Anti-inflammatory effects | PMA | In vitro | — | 20 μM | — | — | — | — | — | ↓ | — | — | — | ↓ | — | — | — | — | — | — | — | — | — | 33 |
| Analgesic effects | Hot plate | BALB/c mice | i.g. | 100, 200, 400 mg kg−1 | — | ↑ | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 28 |
| Analgesic effects | Acetic acid | BALB/c mice | i.g. | 100, 200, 400 mg kg−1 | — | — | ↓ | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 28 |
| Analgesic effects | Bee venom | SD rats | i.g. | 40, 80, 120 mg kg−1 | ↓ | — | — | ↑ | ↑ | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 37 |
| Anti-rheumatic effect | Adjuvant-carrageenan | Wistar rats | i.g. | 80 mg kg−1 | ↓ | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 52 |
| Anti-rheumatic effect | LPS | — | — | — | — | — | — | — | — | ↓ | — | — | ↓ | — | — | — | — | — | — | — | — | — | — | 30 |
| Anti-rheumatic effect | AA | Lewis rats | i.g. | 150 mg kg−1 | ↓ | — | — | — | — | — | ↓ | — | — | — | — | — | — | ↓ | ↓ | — | — | — | — | 54 |
| Anti-rheumatic effect | CIA | C57BL/6 mice | i.g. | 30 mg kg−1 | ↓ | — | — | — | — | ↓ | ↓ | ↓ | ↓ | — | — | ↓ | — | — | ↓ | — | — | — | — | 55 |
| Anti-rheumatic effect | CIA | Wistar rats | i.g. | 150 mg kg−1 | ↓ | — | — | — | — | ↓ | ↓ | — | ↓ | — | — | ↓ | — | — | — | ↓ | — | — | — | 56 |
| Anti-rheumatic effect | CIA | Wistar rats | i.p. | 50 mg kg−1 | ↓ | — | — | — | — | — | ↓ | — | — | — | — | — | — | — | — | — | — | — | ↑ | 57 |
| Anti-rheumatic effect | CIA | DBA/1 mice | i.g. | 50 mg kg−1 | ↓ | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 58 |
| Anti-rheumatic effect | TNF-α | In vitro | — | 50, 100 μM | — | — | — | — | — | — | ↓ | — | ↓ | ↓ | — | — | — | — | — | — | ↓ | ↓ | ↓ | 59 |
| Anti-rheumatic effect | Zymosan | Swiss mice | 100 mg kg−1 | ↓ | ↓ | — | — | — | ↓ | ↓ | — | — | — | — | — | — | — | — | — | — | — | — | 108 | |
2.4 Quercetin in vivo metabolism
Metabolism of absorbed flavonoids including quercetin involves their conjugation with glucuronide, sulfate and/or to a limited extent, methylation of the catechol group.72 Glucuronidation requires uridine diphosphate glucuronosyltransferase (UDP-GT) and sulfation is dependent on sulfotransferase activity. In general, the major metabolites of quercetin have less bioactivity in comparison to the aglycone, however, there are exceptions in that metabolites may have greater effects e.g. the inhibitor constant Ki for the inhibition of xanthine oxidase by quercetin glucuronides followed the order 4′- > 3′- > 7- > 3-, with quercetin-4′-glucuronide a particularly potent inhibitor (Ki = 0.25 μM; quercetin Ki = 0.2 μM).72 Quercetin 3-glucuronide, and 3′-methylquercetin 3-glucuronide from 0.1–1 μM inhibited cyclooxygenase-2 (COX-2) expression in lymphocytes ex vivo in a dose-dependent manner. However, a single high dose of quercetin (4 μM) does not change COX-2 mRNA expression in human lymphocytes in vivo.73 To date, a search on flavonoids/quercetin and Arthritis (Rheumatoid) in www.clinicaltrils.gov showed no results.3 Promotion of quercetin pharmaceutical application by nano formulation
Quercetin, as a potential anti rheumatoid drug, is of increasing interest to the pharmaceutical industry. However, its low hydrophilicity and lipophilicity limits its application. Furthermore, it is easily oxidised, and is sensitive to light and temperature. In order to increase its solubility and bioavailability, quite a few researches have been carried out.74–76 Among them, the application of nano technology provides promise for the further development and utilization of quercetin.77 Nanobiotechnology has been recently regarded as a strategy to improve therapy efficacy by promoting the accumulation of hydrophobic bioactive compounds in tissues.78,79 In view of the current research progress, nano formulation of quercetin can improve its solubility, and enhance its bioavailability. At the same time, quercetin nanoparticles can also change the way it is used in medication, control its release rate, and reduce its side effects.3.1 Nano formulation strategies
There has been an increasing focus on the drug delivery potential of nano-formulations in the recent years.80–84 Application of nano formulation strategies on bioactive molecules could increase their solubility, absorption, bioavailability, protect them from degradation, prolong their circulation time in plasma,82,85 and make them selective biodistribution in the inflammatory parts.86 In addition, the nano formulation strategies could also improve intracellular penetration, reduce systemic toxicity and open up the potential for co-delivery of therapeutic agents.87–89 Currently, many nano formulated systems designed for therapeutic use of phytochemicals have reached the clinical trial stage and are increasingly applied in clinical practice.90,91 So far, a wide variety of nano formulation systems have been used for pharmaceutical applications of quercetin. There are organic92,93 and inorganic nanoparticles,94,95 nanomicelles,96,97 nanoliposomes,78 nanoemulsions98 and nanocapsules99 all gathering great interest. Nanoparticles for delivery of quercetin are generally with a diameter of 20–200 nm. They are formulated using different organic and inorganic materials including dietary fibre,92 lactoglobulin,93 zein,100 casein,101 silica,94 and quantum dots.102 Nanomicelles are self-assembling nanosized colloidal dispersions consist of a hydrophobic core and hydrophilic shell.103 Amphiphilic materials are used to synthesise nanomicelles for solubilising hydrophobic biomolecules like quercetin. Nanoemulsions are nanosized emulsions in the range of 20–200 nm, which prepared by either chemical or mechanical methods using mixtures of immiscible liquids, such as water and oil.104 Nanoliposomes represent nanosized self-assembled lipid vesicles with a structure of phospholipid bilayers entrapping one or more therapeutic agents.105 Detailed applications of different nano-formulations of quercetin on RA treatment are described and discussed in the following sections.3.2 Improvement of solubility
Kakran et al. prepared quercetin nanoparticles by evaporation and precipitation nano-suspension (EPN). They studied the type of antisolvent (e.g., water), the effect of concentration and the ratio of solvent to antisolvent of quercetin particles formed in the EPN process. It showed that the solid dispersion significantly improved quercetin solubility.106 According to the experiment, quercetin showed a very low dissolution rate with only 10% dissolved within 120 min. For the quercetin nanoparticles, the dissolution rate improved significantly to about 75% at 120 min. It can also be observed that relative dissolution (RD) for quercetin nanoparticles was 7.69 at 120 min, and the time for 50% dissolution was only 7.9 min compared to more than 120 min for quercetin. Moreover, they reported that the size of quercetin nanoparticles was affected by drug concentration, solvent to anti-solvent (S/AS) ratio, stirring speed and flow rate.107 The results indicated that the dissolution of quercetin nanoparticles was significantly higher compared with quercetin in simulated intestinal fluid.In the studies of Khor et al., quercetin was co-precipitated with dietary fibres into a fast-dissolving nano formulation via antisolvent precipitation. It was found that a high dissolution rate and good storage stability was achieved for quercetin nano formulations with cellulose fibre, resistant starch, or resistant maltodextrin. The nano formulations exhibited higher levels of antioxidant activities in contrast to quercetin alone.92
García-Casas et al. reported that a supercritical antisolvent (SAS) process had been used to precipitate microparticles of quercetin together with nanoparticles of cellulose acetate phthalate (CAP). Release profiles of quercetin were carried out in simulated gastric and intestinal fluids. Higher ratios of quercetin to polymer in the coprecipitates were recommended to achieve faster release and higher solubilities of quercetin.109
Wang et al. reported that amphiphilic chitosan was obtained through grafting of deoxycholic acid modified chitosan and N-acetyl-L-cysteine. Quercetin-loaded nanomicelles (CS-DA-NAC-QNMs) were prepared through a self-assembly method by using amphiphilic chitosan as the wall-material and quercetin as core-material. They demonstrated that there was a bursting release of quercetin from CS-DA-NAC-QNMs for 0 to 8 hours, and then the release rate decreased gradually. After 72 hours, more than 40% of quercetin were released. All the quercetin loaded nano micelles samples showed good hemocompatibility, and their water solubility and biocompatibility was increased significantly.110
Chavoshpour-Natanzi et al. prepared β-Lactoglobulin (BLG) nanoparticles for the encapsulation of quercetin. The nanoparticles had a mean particle size of between 180–300 nm and a loading efficiency (LE) of 13.9%. Protein nanoparticles could be digested at different stages of the gastrointestinal tract, depending on several factors including specificity of proteases e.g. pepsin. This study suggested that nano formulation could overcome BLG resistance to peptic digestion. Thus synthesised BLG-quercetin nanoparticles could achieve controlled release of quercetin under simulated conditions.93
The study performed by Simon et al. used harmless amphiphilic polyoxazolines (POx) to encapsulate quercetin.96 They produced mixed micelles, made of POx and phosphatidylcholine, using a thin film and high-pressure homogeniser process. The obtained nanomicelles that were about 20 nm in diameter with a spherical shape and encapsulation efficiency of 94 ± 4%. They demonstrated improved cell viability and antioxidant activity from these nanomicelles compared to quercetin alone. Subsequently, this group synthesised quercetin encapsulated lipid nanocapsules (LNC) using the same material POx.111 A similar synthesis method has been used as for the production of mixed micelles but implementing an additional short sonication step. The obtained LNC have a well-defined spherical shape and a size of ∼30 nm.
3.3 Enhancement of bioavailability
Jeyadevi et al. investigated the anti-arthritic activity of quercetin with thioglycolic acid capped cadmium telluride quantum dots (TGA-CdTe QDs) as nano carrier on adjuvant induced arthritic Wistar rats.102 Fifteen days after adjuvant induction, arthritic rats received QDs-quercetin complex orally at a dose of 0.2 and 0.4 mg kg−1 daily for 3 weeks. The complex induced a significant reduction in inflammation and improvement in cartilage regeneration.Aditya et al. reported a comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nano emulsions (LNE) of quercetin.112 Encapsulation efficiency (EE) of quercetin in these nanocarriers was above 90%. Maximum bio-accessibility was observed with NLC and LNE (W60%) compared to SLN (W35%) and free quercetin solution (W7%).
Tran et al. developed a quercetin-containing self-nanoemulsifying drug delivery system (Q-SNEDDS). Oil-in-water nanoemulsions were formed to improve quercetin oral bioavailability.98 Following oral administration of Q-SNEDDS in rats (15 mg kg−1), the maximum concentration (Cmax) of plasma quercetin after 24 h was 3.75 ± 0.96 mg L−1, increased by approximately three-fold compared with the native quercetin group (1.20 ± 0.17 mg L−1). The results suggested that Q-SNEDDS can enhance the solubility and oral bioavailability of quercetin. Collectively, Q-SNEDDS increased quercetin Cmax and area under the concentration curve (AUC), from 6.7 ± 1.4 L−1 h−1 to 14.0 ± 2.8 L−1 h−1, without affecting its elimination kinetics, suggesting that Q-SNEDDS improved quercetin bioavailability by enhancing its absorption.
Dinesh Kumar et al. have also studied biodegradable polymeric nanoparticles for the effective delivery of quercetin. The results suggest that optimised formulation of nanoparticles could promote the controlled release and improve the physical stability of quercetin.113
Lee et al. investigated the antioxidative and anti-inflammatory activities of quercetin-loaded silica nanoparticles (QLSNs).94 QLSNs were synthesised using an oil-in-water microemulsion method. The nanoparticles showed comparable cell viability to that of the free quercetin, while the amounts of proinflammatory cytokines produced by macrophages, such as TNF-κB, IL-6, and IL-1β, were significantly reduced.
Caddeo et al. prepared cross-linked chitosan liposomes of quercetin and confirmed that the system had acid resistance and promoted the release under alkaline conditions.114 In addition, Hao et al. proposed a facile electrostatic deposition method to prepare quercetin nanoliposomes coated with chitosan.115 The obtained Q-NPs have high EE (71.14%) and the storage stability and antioxidant activity was improved compared with native quercetin.
Penalva et al. studied the use of zein nanoparticles as a carrier for the oral delivery of quercetin. Quercetin and 2-hydroxypropyl-β-cyclodextrin were encapsulated together in zein nanoparticles. They showed that nanoparticles provided high and sustained levels of quercetin in plasma after oral administration. The Cmax of plasma quercetin was 176 ± 13.4 μg mL−1. The mean values obtained for AUC and the half-life of the terminal phase (t1/2) were 167 ± 8.21 μg h mL−1 and 0.60 ± 0.35 h, respectively. The mean residence time (MRT) was 1.60 ± 0.12 h, whereas the quercetin clearance and its volume of distribution were calculated to be 30 mL h−1 and 26 mL h−1, respectively. The relative oral bioavailability was calculated to be about 60%.116 They further optimised the preparative process of quercetin loaded casein nanoparticles and evaluated the pharmacokinetics of the nanoparticles after oral administration to Wistar rats117 showing that the relative oral bioavailability of quercetin in nanoparticles (close to 37%) was about 9-times higher than the oral solution of quercetin in a mixture of PEG 400 and water. Another study by Li et al. also used zein and soluble soybean polysaccharide (SSPS) nanoparticles. The EE of quercetin was greatly improved to 82.5% and the photochemical stability and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS+) scavenging ability of quercetin in such nanoparticles were significantly enhanced.100
Pivetta et al. produced nanostructured lipid carriers to load quercetin. Their results indicated that the nanoparticles exhibited a low recrystallization index (13.03%) which is important to obtain high entrapment efficiency (97.42%) and avoid drug expulsion during the storage time.118 Furthermore, in a reconstructed human skin model, it was observed that the topical formulation of quercetin-NLC presented no phototoxic potential. Therefore, this developed nanostructure is a vehicle with potential for topical administration of quercetin.
Research by Gokhale et al. reported a quercetin loaded nano emulsion (NE)-based gel for the effective of management RA.119 This study showed that quercetin-NE has no toxic effect on synoviocytes and a strong inhibitory effect on LPS-induced TNF-α production. It has also exhibited adequate rheological behaviour with a good texture profile and improved drug permeation compared to a free quercetin gel. In addition, the gel was found to be non-irritating and inhibited the formation of paw edema in rats induced by Freund's complete adjuvant (CFA) over 24 hours. Another study performed by Ghatak and Iyyaswami used casein particles to encapsulate quercetin to improve its water solubility and bioavailability.101 A maximum encapsulation yield of 97% could be achieved with the addition of 0.5% (w/v) sodium caseinate, 0.1 M of calcium chloride, 0.5 M of di potassium hydrogen phosphate, 0.1 mM CTAB and 1 M of sodium citrate at a pH of 7.
3.4 Regulation of release rate
Liu et al. studied the characterization and biodistribution of quercetin-loaded cationic nanostructured lipid carriers (QR-CNLC) in vivo. QR-CNLC nanoparticles were prepared by emulsification at high temperature and subsequent solidification at low temperature.78 QR-CNLC exhibited an average particle size of 126.6 nm and 89.3% entrapment efficiency. The results demonstrated that QR-CNLC offered slower release compared with quercetin solution in vitro.Mohan et al. reported on TiO2 nanotubes that were loaded initially with quercetin (TNTQ) and then additionally with chitosan coated on the top of the quercetin (TNTQC) to various thicknesses. The drug release of TNTQ and TNTQC were studied in Hanks' solution for 192 hours. The results showed that the release of drug into the local environment during that duration was constant and the local concentration of the drug could be controlled and tuned by controlling the thickness of the chitosan (0.6, 1 and 3 μm).120
Zong et al. studied in vitro release of quercetin-loaded mixed micelles composed of Pluronic P123/Poloxamer 188, and their pharmacokinetics in rat.121 The results indicated that quercetin-loaded mixed micelles have high entrapment efficiency and loading efficiency which could improve the release behaviour in vitro. The nano formulation of quercetin also prolonged the circulation time of quercetin and significantly enhanced the bioavailability of quercetin. Hui et al. prepared and characterised amphiphilic chitosan/quercetin nano micelles (ACS-QNMs) using a novel amphiphilic chitosan (ACS).97 ACS has deoxycholic acid (DA) as the hydrophobic group and n-acetyl-L-cysteine (NAC) as the hydrophilic group. The results showed that quercetin could be released in vivo and was stable when stored at room temperature after being embedded in nano micelles.
Zhao et al. prepared a new nanodrug delivery system (quercetin@mesoporous hydroxyapatite, QUE@MHAs) and investigated its release in vitro.122 The results showed that QUE@MHAs have good stability and a slow drug release rate.
3.5 Transdermal administration
Quercetin is a flavonoid with significant antioxidant and anti-inflammatory activities and can be considered as a potential topical drug for skin. Nevertheless, it suffers from poor water solubility and consequently topical inactivity. To enhance its transdermal absorption, a number of different nano formulations of quercetin have been studied, including liposomes, nanoparticles, micelles, and solid lipid nanoparticles.Tan Qi studied the preparation and evaluation of quercetin-loaded lecithin–chitosan nanoparticles for topical delivery. Quercetin nanoparticles were prepared using organic solvent injection. The results demonstrated that the nanoparticles could clearly increase the amount of drug retention in especially in the epidermis and also in the dermis, and further enhance antioxidation and anti-inflammatory effects.123
Guo et al. evaluated the potential of quercetin-loaded nanostructured lipid carriers (QT-NLCs) as a topical delivery system.124 The nanoparticles were prepared by the method of emulsion evaporation–solidification at low temperature. The results showed that QT-NLCs could promote the permeation of quercetin, increase the amount of quercetin retention in epidermis and dermis, and enhance the effect of anti-oxidation and anti-inflammation exerted by quercetin.
Sapino et al. evaluated the potential of aminopropyl functionalised mesoporous silica nanoparticles (NH2-MSN) as topical carrier system for quercetin delivery. The silica nanoparticle vehicle prevented UV-induced degradation of quercetin over time, which showed positive effect on photostability of quercetin. Epidermal accumulation and transdermal permeation were evaluated ex vivo using porcine skin mounted on Franz diffusion cells. The inclusion complexation with the inorganic nanoparticles increased the penetration of quercetin into the skin after 24 hours post-application without transdermal delivery.95
Hatahet et al. tested three approaches to improve quercetin delivery to skin, including liposomes, lipid nanocapsules (LNC) and smartCrystals®.99 They showed that compared to liposome (0.56 mg mL−1), quercetin smartCrystals® and LNC had a better drug loading with 14.4 mg mL−1 and 10.8 mg mL−1 respectively. SmartCrystals® and LNC demonstrated different skin penetration behaviours. Only LNC allow quercetin to be delivered to viable epidermis that holds potential for treatment of skin inflammatory disorders.
In conclusion, quercetin is not only an important drug source for oral administration but can also be used as a transdermal absorbent by employing nanotechnology to enhance its transdermal absorption capacity. It can be seen from all above reports that different materials and forms of nano encapsulation can have many positive effects on quercetin anti-rheumatoid applications. Nano formulation has significantly improved the solubility and bioavailability of native quercetin, and at the same time avoiding its shortcomings. The anti-rheumatoid related nano formulations of quercetin are summarised in Table 2.
| Type | Nanocarrier | Preparation method | Antisolvent | Size (nm) | Aim | Ref. |
|---|---|---|---|---|---|---|
| a Note: — not mentioned; Ref. (reference). | ||||||
| Nanoparticles | — | Syringe pump | Deionised water | 170 | Improvement of solubility | 107 |
| Nanoparticles | Dietary fiber | Antisolvent precipitation | Water | <100 | Improvement of solubility | 92 |
| Nanoparticles | Cellulose acetate phthalate | Supercritical antisolvent | Supercritical CO2 | 145 | Improvement of solubility | 109 |
| Nanoparticles | β-Lactoglobulin | Antisolvent precipitation | Acetone | 180–300 | Improvement of solubility | 93 |
| Nanoparticles | Thioglycolic acid-capped cadmium telluride quantum dots | Antisolvent precipitation | Acetone | 185 | Enhancement of bioavailability | 102 |
| Nanoparticles | Polycaprolactone | Nano-precipitation | Pluronic F127 | 213–257 | Enhancement of bioavailability | 113 |
| Nanoparticles | Silica | Oil-in-water microemulsion | Water | 70–140 | Enhancement of bioavailability | 94 |
| Nanoparticles | Zein, 2-hydroxypropyl-β-cyclodextrin | Desolvation | Water | 300 | Enhancement of bioavailability | 116 |
| Nanoparticles | Casein, 2-hydroxypropyl-β-cyclodextrin | Simple coacervation | Water | 200 | Enhancement of bioavailability | 117 |
| Nanoparticles | Natural lipids | Emulsion and sonication | Pluronic F68 | 130 | Enhancement of bioavailability | 118 |
| Nanoparticles | Zein, SSPS | Antisolvent precipitation | — | 200 | Enhancement of bioavailability | 100 |
| Nanoparticles | Casein | Emulsion | Ethanol | 114.3–482.1 | Enhancement of bioavailability | 101 |
| Nanoparticles | Mesoporous hydroxyapatite | Magnetic stirring | Deionised water | 169–179 | Regulation of release rate | 122 |
| Nanoparticles | Lecithin, chitosan | Organic solvent injection | Ethanol | 95 | Changes of administration mode | 123 |
| Nanoparticles | Soya lecithin, glyceryl monostearate, stearic acid, media chain triglyceride | Emulsion evaporation–solidification | Water | 215 | Changes of administration mode | 124 |
| Nanoparticles | Mesoporous silica | Impregnation and magnetic stirring | Methanol | 200–300 | Changes of administration mode | 95 |
| Nanomicelles | Amphiphilic chitosan | Grafting deoxycholic acid, N-acetyl-L-cysteine | — | 360–580 | Improvement of solubility | 110 |
| Nanomicelles | Polyoxazolines, phosphatidylcholine | Thin film and high pressure homogeniser | Acetonitrile | 20 | Improvement of solubility | 96 |
| Nanomicelles | Polyoxazolines, Labrafac®, Lipoid® S75 | Thin film and high pressure homogeniser process | Acetonitrile | 30 | Improvement of solubility | 111 |
| Nanomicelles | Pluronic P123/Poloxamer 188 | Film dispersion | Tween80 | — | Regulation of release rate | 121 |
| Nanomicelles | Amphiphilic chitosan | Self assembly | Deionised water | 140–600 | Regulation of release rate | 97 |
| Nanoliposomes | Cross-linked chitosan | Ultrasonication | TPP aqueous solution | 180 | Enhancement of bioavailability | 114 |
| Nanoliposomes | Chitosan | Facile electrostatic deposition | Chloroform, methanol | 350–600 | Enhancement of bioavailability | 115 |
| Nanoliposomes | GMS, MCT, soy lecithin | Emulsifying and solidifying | Transcutol | 118–135 | Regulation of release rate | 78 |
| Nanoliposomes | DPPC, Cremophor® EL | Magnetic stirring | Alcohol | 179 | Changes of administration mode | 99 |
| Nanoemulsions | Lecithin | High pressure homogeniser | — | 73–91 | Enhancement of bioavailability | 112 |
| Nanoemulsions | Self-nanoemulsifying drug delivery system | Gentle stirring | Tween 80, PEG 400 | 204–213 | Enhancement of bioavailability | 98 |
| Nanoemulsion-based gels | Arachis oil, oleic acid | Gentle stirring | Tween 20, PEG 400 | 137 | Enhancement of bioavailability | 119 |
| Nanosuspensions | — | Evaporative precipitation | Hexane | 220 | Improvement of solubility | 106 |
| Nanotubes | TiO2 | Top filling | — | 125 (tubes diameters) | Regulation of release rate | 120 |
| Nanocapsules | Lipid | Magnetic stirring | Milli Q water | 26 | Changes of administration mode | 99 |
4 Conclusions and future perspective
Rheumatoid arthritis is a common worldwide public health problem.125 It is in the top ten of the world's disease spectrum and is also one of the diseases that seriously affect human physical and mental health, with high rates of disability and death. The pathogenesis of RA is very complex and has not been fully elucidated. RA can be managed through conventional treatments, but more attention has recently been paid to the treatment of RA by natural active ingredients from medicinal plants, including food plants, because of their safety and efficacy. In recent years, the consumption of plant-based medicines and other botanicals has increased. According to an estimate of World Health Organization (WHO), nearly 80% of the populations of developing countries rely on traditional medicines.126As a plant derived medicine, quercetin is a striking candidate for use in arthritic therapy.126 As summarised above, there are many reports on the effects of quercetin on RA. It has been confirmed that quercetin has significant anti-inflammatory and analgesic effects in vivo and in vitro and furthermore, quercetin and its derivatives also have significant antioxidant effects, which is one of the possible reasons for their significant anti-rheumatic properties.20 At the same time, it is reported that quercetin is mostly well tolerated and safe to use. Doses up to 1000 mg each day for several months have not produced adverse effects on blood parameters, and liver, and kidney function. As a potential bioavailability enhancer for active pharmaceutical ingredients, quercetin can also be used as one of the options in combination therapy for RA.65 Moreover, it has been shown that with the application of nano formulations, quercetin has not only improved oral bioavailability, but also can be used for external transdermal use, which provides a new reference for the treatment of RA.
Move rover, Susanne Andres et al. reviewed the safety aspects of quercetin as a dietary supplement. It showed that based on animal studies, some possible critical safety aspects of quercetin could be identified such as to enhance nephrotoxic effects in the predamaged kidney or to promote tumour development especially in estrogen-dependent cancer.127 Furthermore, when quercetin interacts with some drugs, the bioavailability of may be altered. Therefore, it suggests that, like any potential drug or active ingredient, a very in-depth study on its safety and applicability should be conducted before clinical application. Future clinical studies are needed to verify the safety and efficacy of nano formulated quercetin as a new RA treatment medicine. Future clinical studies are needed to verify the safety and efficacy of Nano formulated quercetin as a new RA treatment medicine.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is supported by China Scholarships Council for studying abroad and Natural Science Foundation of Heilongjiang Province, China.References
- A. Marino, I. Paterniti, M. Cordaro, R. Morabito, M. Campolo, M. Navarra, E. Esposito and S. Cuzzocrea, PharmaNutrition, 2015, 3, 53–59 CrossRef CAS.
- C. L. Qi Changjie, Med. Recepitula., 2015, 25, 1652–1653 Search PubMed.
- L. S. Zeng Xiaowei, J. Chin. Pract. Diagn. Ther., 2015, 29, 1044–1066 Search PubMed.
- F. Wolfe, D. L. Scott and T. W. J. Huizinga, Lancet, 2010, 1094–1108 Search PubMed.
- A. M. Uttra, M. Alamgeer, M. Shahzad, A. Shabbir, S. Jahan, I. A. Bukhari and A. M. Assiri, J. Ethnopharmacol., 2019, 237, 92–107 CrossRef CAS.
- S. Q. Li, W. Q. Jiang, C. X. Zheng, D. Shao, Y. L. Liu, S. Q. Huang, J. Han, J. X. Ding, Y. Tao and M. Q. Li, J. Controlled Release, 2020, 327, 801–833 CrossRef CAS.
- M. D. Yang, J. X. Ding, X. R. Feng, F. Chang, Y. N. Wang, Z. L. Gao, X. L. Zhuang and X. S. Chen, Theranostics, 2017, 7, 97–105 CrossRef CAS.
- D. Y. Zhao, T. T. Zhu, J. Li, L. G. Cui, Z. Y. Zhang, X. L. Zhuang and J. X. Ding, Bioact. Mater., 2021, 6, 346–360 CrossRef.
- T. T. Zhu, Y. T. Cui, M. R. Zhang, D. Y. Zhao, G. Y. Liu and J. X. Ding, Bioact. Mater., 2020, 5, 584–601 CrossRef.
- X. Hui, M. Jia-ni, L. Jie, H. Chun-yan and J. Qing, China Pharm., 2017, 28, 3882–3885 Search PubMed.
- Y.-h. Zhu, S. Wang, Y. Li, Z.-y. Chen and H.-y. Fan, J. Jilin Med. Univ., 2018, 39, 219–223 Search PubMed.
- G. D'Andrea, Fitoterapia, 2015, 256–271, DOI:10.1016/j.fitote.2015.09.018.
- M. Russo, C. Spagnuolo, I. Tedesco, S. Bilotto and G. L. Russo, Biochem. Pharmacol., 2012, 83, 6–15 CrossRef CAS.
- S. H. Häkkinen, S. O. Kärenlampi, I. M. Heinonen, H. M. Mykkänen and A. R. Törrönen, J. Agric. Food Chem., 1999, 47, 2274–2279 CrossRef.
- Y. Li, J. Yao, C. Han, J. Yang, M. T. Chaudhry, S. Wang, H. Liu and Y. Yin, Nutrients, 2016, 8, 167 CrossRef.
- W. Wiczkowski, J. Romaszko, A. Bucinski, D. Szawara-Nowak, J. Honke, H. Zielinski and M. K. Piskula, J. Nutr., 2008, 138, 885–888 CrossRef CAS.
- J. V. Formica and W. Regelson, Food Chem. Toxicol., 1995, 33, 1061–1080 CrossRef CAS.
- Y. Xiao, M. Li, P. Mao and J. Yuan, J. Henan Univ. Sci. Technol., Nat. Sci., 2019, 40, 123–131 Search PubMed.
- S.-y. Li, Z. Li, J.-l. Li, W.-n. Gao, Z.-q. Zhang and C.-j. Guo, Pharm. J. Chin. People's Liberation Army, 2011, 27, 540–543 CAS.
- M. Lesjak, I. Beara, N. Simin, D. Pintać, T. Majkić, K. Bekvalac, D. Orčić and N. Mimica-Dukić, J. Funct. Foods, 2018, 40, 68–75 CrossRef CAS.
- H. Sun, H. Jin, R.-r. Yang, Z.-g. He, M.-y. Du and J.-l. Shi, Farm Prod. Process, 2019, 83–87 Search PubMed.
- Y.-x. Yu, S.-r. Ge and G.-z. Wang, J. Chin. Pract. Diagn. Ther., 2003, 26, 902–904 Search PubMed.
- A. W. Boots, M. Drent, V. C. de Boer, A. Bast and G. R. Haenen, Clin. Nutr., 2011, 30, 506–512 CrossRef CAS.
- R. K. Davidson, J. Green, S. Gardner, Y. Bao, A. Cassidy and I. M. Clark, Sci. Rep., 2018, 8, 17173 CrossRef.
- L. D. Kumar, R. Karthik, N. Gayathri and T. Sivasudha, Biomed. Pharmacother., 2016, 79, 52–61 CrossRef CAS.
- S. J. Maleki, J. F. Crespo and B. Cabanillas, Food Chem., 2019, 299, 125124 CrossRef CAS.
- Y. R. Liao and J. Y. Lin, Life Science, 2015, 137, 89–97 CrossRef CAS.
- J.-q. Huang, Y. Song, P. Zhao, Y. Feng and Y. Liu, Strait Pharm. J., 2013, 25, 64–67 Search PubMed.
- X.-n. Zhou, C. Han, P.-y. Song, X.-h. Zhao and X.-h. Zhong, Prog. Vet. Med., 2017, 38, 56–61 Search PubMed.
- A. T. Paul, V. M. Gohil and K. K. Bhutani, Drug Discovery Today, 2006, 11, 725–732 CrossRef CAS.
- G. Cessak, O. Kuzawińska, A. Burda, K. Lis, M. Wojnar, D. Mirowska-Guzel and E. Bałkowiec-Iskra, Pharmacol. Rep., 2014, 66, 836–844 CrossRef CAS.
- G.-y. Ren, B.-y. Zhang and J.-l. Huang, Chin. Tradit. Pat. Med., 2019, 41, 1795–1799 Search PubMed.
- S. Yeh, H. M. Wang, P. Y. Chen and T. C. Wu, Chem.-Biol. Interact., 2009, 179, 386–393 CrossRef CAS.
- C. M. Avila, N. C. Romeiro, C. M. Sant'Anna, E. J. Barreiro and C. A. Fraga, Bioorg. Med. Chem. Lett., 2009, 19, 6907–6910 CrossRef CAS.
- D. Musumeci, G. N. Roviello and D. Montesarchio, Pharmacol. Ther., 2014, 141, 347–357 CrossRef CAS.
- D.-N. Liu, Doctoral degree thesis, The Fourth Military Medical University, 2008 Search PubMed.
- Y. Yin, F.-Y. Gong, X.-X. Wu, Y. Sun, Y.-H. Li, T. Chen and Q. Xu, J. Ethnopharmacol., 2008, 120, 1–6 CrossRef CAS.
- M. Mueller, S. Hobiger and A. Jungbauer, Food Chem., 2010, 122, 987–996 CrossRef CAS.
- A. Sowemimo, F. Samuel and M. S. Fageyinbo, J. Ethnopharmacol., 2013, 149, 191–194 CrossRef.
- N. Simin, D. Orcic, D. Cetojevic-Simin, N. Mimica-Dukic, G. Anackov, I. Beara, D. Mitic-Culafic and B. Bozin, LWT--Food Sci. Technol., 2013, 54, 139–146 CrossRef CAS.
- Y.-C. Cho, A. Ju, B. R. Kim and S. Cho, J. Ethnopharmacol., 2015, 162, 140–147 CrossRef.
- D. Huang, Y. Chen, W. Chen, Y. Liu, F. Yao, D. Xue and L. Sun, J. Ethnopharmacol., 2015, 176, 356–364 CrossRef CAS.
- K.-J. Gou, R. Zeng, X.-D. Ren, Q.-L. Dou, Q.-B. Yang, Y. Dong and Y. Qu, Immunol. Lett., 2018, 201, 59–69 CrossRef CAS.
- M. Ondua, E. M. Njoya, M. A. Abdalla and L. J. McGaw, J. Ethnopharmacol., 2019, 234, 27–35 CrossRef.
- T. Macedo, V. Ribeiro, A. P. Oliveira, D. M. Pereira, F. Fernandes, N. G. M. Gomes, L. Araujo, P. Valentao and P. B. Andrade, J. Ethnopharmacol., 2019, 248, 112312 CrossRef.
- J. Y. Lui, J. Jalil, A. Attiq, C. H. Chiew and N. A. Zakaria, J. Ethnopharmacol., 2019, 229, 303–325 CrossRef.
- A. O. d. Silva, A. D. Alves, D. A. T. d. Almeida, S. O. Balogun, R. G. d. Oliveira, A. A. Aguiar, I. M. Soares, P. G. Marson-Ascêncio, S. D. Ascêncio and D. T. d. O. Martins, J. Ethnopharmacol., 2014, 154, 319–329 CrossRef.
- Y. Luan and R. Sun, Chin. J. Pharmacovigil., 2014, 11, 219–221 Search PubMed.
- A. Gong, Y. Ouyang, X. Zhang, X. Zhao and M. Wu, World Lat. Med. Info., 2015, 15, 25–27 Search PubMed.
- K. McNamee, R. Williams and M. Seed, Eur. J. Pharmacol., 2015, 759, 278–286 CrossRef CAS.
- K. Fan, J. Wu, Y. Li and T. Wang, Pract. Pharm. Clin. Rem., 2018, 21, 709–713 Search PubMed.
- T. Guardia, A. E. Rotelli, A. r. O. Juarez and L. E. Pelzer, Farmaco, 2001, 56, 683–687 CrossRef CAS.
- M. Mamani-Matsuda, T. Kauss, A. Al-Kharrat, J. Rambert, F. Fawaz, D. Thiolat, D. Moynet, S. Coves, D. Malvy and M. D. Mossalayi, Biochem. Pharmacol., 2006, 72, 1304–1310 CrossRef CAS.
- C. Gardi, K. Bauerova, B. Stringa, V. Kuncirova, L. Slovak, S. Ponist, F. Drafi, L. Bezakova, I. Tedesco, A. Acquaviva, S. Bilotto and G. L. Russo, Arch. Biochem. Biophys., 2015, 583, 150–157 CrossRef CAS.
- N. Haleagrahara, S. Miranda-Hernandez, M. A. Alim, L. Hayes, G. Bird and N. Ketheesan, Biomed. Pharmacother., 2017, 90, 38–46 CrossRef CAS.
- Y. Yang, X. Zhang, M. Xu, X. Wu, F. Zhao and C. Zhao, Int. Immunopharmacol., 2018, 54, 153–162 CrossRef CAS.
- Y.-q. Wang, S.-f. Chen and Y. Mei, Immunol. J., 2019, 35, 485–491 Search PubMed.
- Q.-y. Jia, Y.-j. Wang, Q.-q. Liang and Q. Shi, Chin. J. Osteoporosis, 2019, 25, 738–741 Search PubMed.
- Q.-y. Jia, Y.-j. Wang, Q.-q. Liang and Q. Shi, J. Osteoporosis, 2018, 24, 1175–1179 Search PubMed.
- C. F. S. Guazelli, L. Staurengo-Ferrari, A. C. Zarpelon, F. A. Pinho-Ribeiro, K. W. Ruiz-Miyazawa, F. Vicentini, J. A. Vignoli, D. Camilios-Neto, S. R. Georgetti, M. M. Baracat, R. Casagrande and W. A. Verri Jr, Biomed. Pharmacother., 2018, 102, 175–184 CrossRef CAS.
- J.-J. Ji, Y. Lin, S.-S. Huang, H.-L. Zhang, Y.-P. Diao and K. Li, Afr. J. Tradit., Complementary Altern. Med., 2013, 10, 418–421 CAS.
- P. Xiao, P. Wang, X. Zhu, J.-P. Sun and X.-j. Wu, Chin. J. Exp. Surg., 2016, 33, 2378–2380 Search PubMed.
- M. K. Pandey, S. C. Gupta, D. Karelia, P. J. Gilhooley, M. Shakibaei and B. B. Aggarwal, Biotechnol. Adv., 2018, 36, 1633–1648 CrossRef CAS.
- S. S. Laev and N. F. Salakhutdinov, Bioorg. Med. Chem., 2015, 23, 3059–3080 CrossRef CAS.
- A. Ajazuddin, A. Alexander, A. Qureshi, L. Kumari, P. Vaishnav, M. Sharma, S. Saraf and S. Saraf, Fitoterapia, 2014, 97, 1–14 CrossRef CAS.
- D. D. Bandawane, S. Beautikumari, S. S. Gate and A. N. Patel, Biomed. Aging Pathol., 2014, 4, 105–115 CrossRef CAS.
- X. Han, D. Su, X. Xian, M. Zhou, X. Li, J. Huang, J. Wang and H. Gao, J. Ethnopharmacol., 2016, 194, 228–235 CrossRef.
- A. Tanwar, R. Chawla, M. M. Ansari, S. A. Neha, P. Thakur, A. S. Chakotiya, R. Goel, H. Ojha, M. Asif, M. Basu, R. Arora and H. A. Khan, Biomed. Pharmacother., 2017, 87, 92–101 CrossRef CAS.
- R. Adhikary, S. Sultana and B. Bishayi, J. Ethnopharmacol., 2018, 210, 209–222 CrossRef CAS.
- G.-S. Zou, S.-J. Li, S.-l. Zheng, X. Pan and Z.-p. Huang, J. Taiwan Inst. Chem. Eng., 2018, 93, 54–62 CrossRef CAS.
- H. Xiong, X. Ding, H. Wang, H. Jiang, X. Wu, C. Tu, C. Wu, Y. Pi, G. Yang, Z. Zhao and Z. Mei, Phytomedicine, 2019, 57, 271–281 CrossRef.
- A. J. Day, Y. Bao, M. R. A. Morgan and G. Williamson, Free Radical Biol. Med., 2000, 29, 1234–1243 CrossRef CAS.
- S. de Pascual-Teresa, K. L. Johnston, M. S. DuPont, K. A. O'Leary, P. W. Needs, L. M. Morgan, M. N. Clifford, Y. Bao and G. Williamson, J. Nutr., 2004, 134, 552–557 CrossRef CAS.
- D.-y. Ning, Q.-y. Zhang, X.-l. Tan and X.-x. Huang, Sci. Technol. Innov., 2019, 41–42 Search PubMed.
- X. Lu and C. Jiang, Her. Med., 2019, 38, 921–926 Search PubMed.
- Y. Guo and R. S. Bruno, J. Nutr. Biochem., 2015, 26, 201–210 CrossRef CAS.
- K. Janakiraman, V. Krishnaswami, V. Rajendran, S. Natesan and R. Kandasamy, Mater. Today Commun., 2018, 17, 200–213 CrossRef CAS.
- L. Liu, Y. Tang, C. Gao, Y. Li, S. Chen, T. Xiong, J. Li, M. Du, Z. Gong, H. Chen, L. Liu and P. Yao, Colloids Surf., B, 2014, 115, 125–131 CrossRef CAS.
- X. R. Feng, W. G. Xu, Z. M. Li, W. T. Song, J. X. Ding and X. S. Chen, Adv. Sci., 2019, 6, 1900101 CrossRef.
- Y. Davatgaran-Taghipour, S. Masoomzadeh, M. H. Farzaei, R. Bahramsoltani, Z. Karimi-Soureh, R. Rahimi and M. Abdollahi, Int. J. Nanomed., 2017, 12, 2689–2702 CrossRef CAS.
- S. Khogta, J. Patel, K. Barve and V. Londhe, J. Herb. Med., 2019, 100300, DOI:10.1016/j.hermed.2019.100300.
- T. Y. Forbes-Hernández, J. Berry Res., 2020, 10, 45–60 Search PubMed.
- S. Z. Moradi, S. Momtaz, Z. Bayrami, M. H. Farzaei and M. Abdollahi, Front. Bioeng. Biotechnol., 2020, 8, 238 CrossRef.
- A. Vaiserman, A. Koliada, A. Zayachkivska and O. Lushchak, Front. Bioeng. Biotechnol., 2020, 7, 447 CrossRef.
- P. Aiello, S. Consalvi, G. Poce, A. Raguzzini, E. Toti, M. Palmery, M. Biava, M. Bernardi, M. A. Kamal, G. Perry and I. Peluso, Semin. Cancer Biol., 2019 DOI:10.1016/j.semcancer.2019.08.029.
- N. B. Feng, M. D. Yang, X. R. Feng, Y. N. Wang, F. Chang and J. X. Ding, ACS Biomater. Sci. Eng., 2018, 4, 4154–4162 CrossRef CAS.
- Q. Wang and Y.-M. Chao, J. Biomed. Res., 2018, 32, 91–106 Search PubMed.
- H. Alshaker, Q. Wang, S. Srivats, Y. Chao, C. Cooper and D. Pchejetski, Breast Cancer Res. Treat., 2017, 165, 531–543 CrossRef CAS.
- Q. Wang, H. Alshaker, T. Böhler, S. Srivats, Y. Chao, C. Cooper and D. Pchejetski, Sci. Rep., 2017, 7, 5901 CrossRef.
- M. Hajialyani, D. Tewari, E. Sobarzo-Sánchez, S. M. Nabavi, M. H. Farzaei and M. Abdollahi, Int. J. Nanomed., 2018, 13, 5023–5043 CrossRef CAS.
- X. R. Feng, J. X. Ding, F. Chang and X. S. Chen, J. Controlled Release, 2017, 252, 108–124 CrossRef.
- C. M. Khor, W. K. Ng, K. P. Chan and Y. Dong, Carbohydr. Polym., 2017, 161, 109–117 CrossRef CAS.
- Z. Chavoshpour-Natanzi and M. Sahihi, Food Hydrocolloids, 2019, 96, 493–502 CrossRef CAS.
- G. H. Lee, S. J. Lee, S. W. Jeong, H. C. Kim, G. Y. Park, S. G. Lee and J. H. Choi, Colloids Surf., B, 2016, 143, 511–517 CrossRef CAS.
- S. Sapino, E. Ugazio, L. Gastaldi, I. Miletto, G. Berlier, D. Zonari and S. Oliaro-Bosso, Eur. J. Pharm. Biopharm., 2015, 89, 116–125 CrossRef CAS.
- L. Simon, M. Vincent, S. Le Saux, V. Lapinte, N. Marcotte, M. Morille, C. Dorandeu, J. M. Devoisselle and S. Bégu, Int. J. Pharm., 2019, 570, 118516 CrossRef CAS.
- W. Hui, Y. Ziming, H. Zuyu, Z. Chuang, W. Chao and L. Puwang, Chin. J. Trop. Crop., 2019, 40, 980–986 Search PubMed.
- T. H. Tran, Y. Guo, D. Song, R. S. Bruno and X. Lu, J. Pharm. Sci., 2014, 103, 840–852 CrossRef CAS.
- T. Hatahet, M. Morille, A. Hommoss, J. M. Devoisselle, R. H. Muller and S. Begu, Int. J. Pharm., 2018, 542, 176–185 CrossRef CAS.
- H. Li, D. Wang, C. Liu, J. Zhu, M. Fan, X. Sun, T. Wang, Y. Xu and Y. Cao, Food Hydrocolloids, 2019, 87, 342–351 CrossRef CAS.
- D. Ghatak and R. Iyyaswami, Food Bioprod. Process., 2019, 115, 100–109 CrossRef CAS.
- R. Jeyadevi, T. Sivasudha, A. Rameshkumar, D. A. Ananth, G. S. Aseervatham, K. Kumaresan, L. D. Kumar, S. Jagadeeswari and R. Renganathan, Colloids Surf., B, 2013, 112, 255–263 CrossRef CAS.
- R. Trivedi and U. B. Kompella, Nanomedicine, 2010, 5, 485–505 CrossRef CAS.
- M. Jaiswal, R. Dudhe and P. K. Sharma, 3 Biotech, 2015, 5, 123–127 CrossRef.
- H. Chan and P. Král, ACS Omega, 2018, 3, 10631–10637 CrossRef CAS.
- M. Kakran, N. G. Sahoo and L. Li, Colloids Surf., B, 2011, 88, 121–130 CrossRef CAS.
- M. Kakran, N. G. Sahoo, L. Li and Z. Judeh, Powder Technol., 2012, 223, 59–64 CrossRef CAS.
- S. M. Borghi, S. S. Mizokami, F. A. Pinho-Ribeiro, V. Fattori, J. Crespigio, J. T. Clemente-Napimoga, M. H. Napimoga, D. L. Pitol, J. P. M. Issa, S. Y. Fukada, R. Casagrande and W. A. Verri Jr, J. Nutr. Biochem., 2018, 53, 81–95 CrossRef CAS.
- I. Garcia-Casas, A. Montes, C. Pereyra and E. J. Martinez de la Ossa, Eur. J. Pharm. Sci., 2017, 100, 79–86 CrossRef CAS.
- H. Wang, Z. Yang, Z. He, C. Zhou, C. Wang, Y. Chen, X. Liu, S. Li and P. Li, Colloids Surf., B, 2019, 179, 519–526 CrossRef CAS.
- L. Simon, V. Lapinte, L. Lionnard, N. Marcotte, M. Morille, A. Aouacheria, K. Kissa, J. M. Devoisselle and S. Begu, Int. J. Pharm., 2020, 579, 8 CrossRef.
- N. P. Aditya, A. S. Macedo, S. Doktorovova, E. B. Souto, S. Kim, P.-S. Chang and S. Ko, LWT--Food Sci. Technol., 2014, 59, 115–121 CrossRef CAS.
- V. Dinesh Kumar, P. R. P. Verma and S. K. Singh, LWT--Food Sci. Technol., 2015, 61, 330–338 CrossRef CAS.
- C. Caddeo, O. Diez-Sales, R. Pons, C. Carbone, G. Ennas, G. Puglisi, A. M. Fadda and M. Manconi, J. Colloid Interface Sci., 2016, 461, 69–78 CrossRef CAS.
- J. Hao, B. Guo, S. Yu, W. Zhang, D. Zhang, J. Wang and Y. Wang, LWT--Food Sci. Technol., 2017, 85, 37–44 CrossRef CAS.
- R. Penalva, C. J. Gonzalez-Navarro, C. Gamazo, I. Esparza and J. M. Irache, Nanomedicine, 2017, 13, 103–110 CrossRef CAS.
- R. Penalva, I. Esparza, J. Morales-Gracia, C. J. Gonzalez-Navarro, E. Larraneta and J. M. Irache, Int. J. Pharm., 2019, 570, 118652 CrossRef CAS.
- T. P. Pivetta, L. B. Silva, C. M. Kawakami, M. M. Araújo, M. P. F. M. Del Lama, R. M. Z. G. Naal, S. S. Maria-Engler, L. R. Gaspar and P. D. Marcato, J. Drug Delivery Sci. Technol., 2019, 53, 101148 CrossRef CAS.
- J. P. Gokhale, H. S. Mahajan and S. J. Surana, Biomed. Pharmacother., 2019, 112, 108622 CrossRef CAS.
- L. Mohan, C. Anandan and N. Rajendran, Int. J. Biol. Macromol., 2016, 93, 1633–1638 CrossRef CAS.
- R. Zong, C. Wang, T.-l. Guo and L.-x. Shen, Chin. Pharmacol. Bull., 2019, 35, 246–250 CrossRef.
- D. Zhao, M. Zheng and Z. Zhang, J. Hebei Univ. Sci. Technol., 2019, 33, 277–280 Search PubMed.
- Q. Tan, Master degree dissertationShandong University, 2012 Search PubMed.
- C. Guo, C. Yang, Q. Li, Q. Tan, Y. Xi, W. Liu and G. Zhai, Int. J. Pharm., 2012, 430, 292–298 CrossRef CAS.
- S. Lü, Q. Wang, G. Li, S. Sun, Y. Guo and H. Kuang, J. Ethnopharmacol., 2015, 176, 177–206 CrossRef.
- D. Prakash, N. S. Katiyar, A. P. Singh and A. K. Gangwar, Eur. J. Pharm. Med. Res., 2016, 3, 305–308 Search PubMed.
- S. Andres, S. Pevny, R. Ziegenhagen, N. Bakhiya, B. Schafer, K. I. Hirsch-Ernst and A. Lampen, Mol. Nutr. Food Res., 2018, 62, 1700447 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |