Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pericardial fluid proteomic label-free quantification of differentially expressed proteins in ischemic heart disease patients with systolic dysfunction by nano-LC-ESI-MS/MS analysis†
Junaid Ullaha,
Satwat Hashmib,
Arslan Ali *c,
Faisal Khanc,
Shahid Ahmed Samid,
Nageeb Basire,
Syeda Saira Bokharie,
Hasanat Sharifd,
Hesham R. El-Seedifg and
Syed Ghulam Musharraf
*c,
Faisal Khanc,
Shahid Ahmed Samid,
Nageeb Basire,
Syeda Saira Bokharie,
Hasanat Sharifd,
Hesham R. El-Seedifg and
Syed Ghulam Musharraf *ac
*ac
aH.E.J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi-75270, Pakistan. E-mail: musharraf1977@yahoo.com; Fax: +92 213 4819018; Fax: +92 213 4819019; Tel: +92 213 4824924 Tel: +92 213 4824925 Tel: +92 213 4819010
bDepartment of Biological and Biomedical Sciences, Agha Khan University, Karachi-74800, Pakistan
cDr Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences, University of Karachi, Karachi-75270, Pakistan. E-mail: arslanali1986@gmail.com
dDepartment of Surgery, The Aga Khan University Hospital, Karachi-74800, Pakistan
eDepartment of Medicine, The Aga Khan University Hospital, Karachi-74800, Pakistan
fPharmacognosy Group, Department of Pharmaceutical Biosciences, BMC, Uppsala University, SE-751 23 Uppsala, Sweden
gInternational Research Center for Food Nutrition and Safety, Jiangsu University, Zhenjiang 212013, China
First published on 23rd December 2020
Abstract
Left ventricular systolic dysfunction (LVSD) is common in patients with pre-existing ischemic heart disease (IHD) and myocardial infarction. An untargeted proteomic approach is used to improve the understanding of the molecular mechanisms associated with LVSD and to find out potential proteomic signatures in pericardial fluid. The pericardial fluid of IHD (n = 45) patients was grouped into two categories according to the left ventricular ejection fraction, LVEF ≥45 (n = 33) and LVEF <45 (n = 12), and analyzed by using nano-liquid chromatography–mass spectrometry (nano-LC-MS/MS) technique. The nano-LC-MS/MS analysis resulted in the identification of 709 pericardial fluid (PF) proteins in both normal and impaired systolic functional groups (LVEF ≥45 vs. LVEF <45). Sixteen proteins were found to be differentially expressed (p < 0.05, fold change >2) including 12 down-regulated and 4 up-regulated in the impaired systolic functional group (LVEF <45) compared to the normal group (LVEF ≥45). Among the differentially expressed proteins the inflammatory marker albumin, atherosclerosis marker apolipoprotein A-IV and hedgehog-interacting protein marker of angiogenesis were predominantly associated with the impaired LVEF <45 group. KEGG pathway analysis revealed that the hedgehog (Hh) signalling pathway is up-regulated in LVSD reflecting the underlying molecular and pathophysiological processes.
Introduction
Cardiovascular disease (CVD) is one of the main causes of mortality and disability worldwide. According to the World Health Organization (WHO), in 2008 approximately 17.3 million people died from cardiovascular disease and this will, expectedly, rise to 23.3 million annually by 2030.1 Ischemic heart disease (IHD) is the most prevalent condition among the CVDs. Left ventricular systolic dysfunction (LVSD) is common in patients with IHD.2 LVSD refers to an impairment of the left ventricular contractility that results in reduced left ventricular ejection fraction (LVEF) and cardiac output. Patients with LVSD were at increased risk of death compared to subjects with a normal LVEF.3 A 10% reduction in LVEF predicts a 39% increase in all-cause mortality, hospitalization and an increased heart failure risk.4 Prevalence of LVSD increases substantially with age and is more prevalent in men (7.6%) as compared to women (2.6%)5The measurement of proteins in biological samples plays a key role in the diagnosis and prognosis of patients that are at high risk of diseases. Pericardial fluid has been used to understand the molecular mechanism of disease in a number of studies.6 Pericardial fluid (PF) is an ultrafiltrate of the plasma and a biochemical window into the heart by which information related to the pathophysiological status of the heart can be obtained.7 Pericardial fluid is present between the two layers; parietal and visceral of the pericardium and is produced by the visceral pericardium of the heart. Under normal conditions, it measures about 15–50 mL in volume. Many diseases including ischemic heart disease, infections, and idiopathic causes can increase the volume and change its composition.8 The proteomic analysis of pericardial fluid may be an important tool to find out the pathophysiological mechanisms underlying cardiac diseases and to find out potential therapeutic targets.9 Several proteomic signatures have been discovered and used in the management of LVSD such as Brain Natriuretic Peptide (BNP), which is released in the pericardial fluid and serum by the ventricles in response to increased wall tension or stretch of cardiac chambers.10 The proteomic analysis of pericardial fluid in patients with lung bullae disease resulted in the identification of 1007 proteins which provided valuable insight into disease mechanism.11 To the best of our knowledge, this is the first study constituting the proteomic profiling of the pericardial fluid in patients with LVSD with established IHD.
The present study aimed to uncover the differentially expressed proteins in the pericardial fluid by a label-free quantitative proteomic approach in IHD patients with impaired (LVEF <45) and normal (LVEF ≥45) systolic function. The study will help to improve the understanding of the molecular mechanisms associated with LVSD and to find out potential proteomic signatures.
Material and methods
Experimental design and statistical analysis
For the present study fourty five patients (43 men and 2 women) were recruited with confirmed ischemic heart disease (IHD) at South city and Aga Khan University Hospital Karachi, Pakistan. A written informed consent was taken from each patient before coronary artery bypass graft (CABG). In this study, patients with renal insufficiency, metabolic bone diseases, valvular disease moderate to severe, constrictive pericarditis and infiltrative, hypertrophic or restrictive cardiomyopathies, established pulmonary disease and malignancy were excluded. Two-dimensional and Doppler echocardiography was used for the assessment of left ventricular systolic function following ASE guideline 2009. Patients were further categorized into two groups according to the LV ejection fraction: group A was 33 patients with normal left ventricular systolic function (LVEF ≥45), and group B was 12 patients with impaired left ventricular systolic function (LVEF <45) as depicted in Table 1. The study was approved by the Ethical Review Board (ERB) of the Aga Khan University Hospital and Institutional Review Board (IRB) of International Centre for Chemical and Biological Sciences (ICCBS), University of Karachi, Pakistan. All procedures followed in this study were in accordance with the ethical principles of the 1964 declaration of Helsinki.| (LVEF ≥45%) | (LVEF <45%) | |
|---|---|---|
| a SD standard deviation, BUN blood urea nitrogen, LVEF left ventricular ejection fraction. | ||
| N | 33 | 12 |
| Age [years; mean (SD)] | 59.7 ± 7.1 | 58.0 ± 9.6 |
| Body weight [kg; mean (SD)] | 72.7 ± 11.3 | 76.6 ± 12.7 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Medical history | ||
| Diabetes/non-diabetes | 18/15 | 9/3 |
| Hypertension/non-hypertension | 23/10 | 7/5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Renal function | ||
| Creatinine [mg dL−1; mean (SD)] | 1.0 ± 0.2 | 1.0 ± 0.3 |
| BUN [mg dL−1; mean (SD)] | 16.0 ± 3.4 | 19.2 ± 11.3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Echocardiographic parameters | ||
| LVEF [%; mean (SD)] | 58.2 ± 4.9 | 36.6 ± 8 |
Sample collection and processing
Pericardial fluid samples were collected from each subject in pre-labeled clean and sterile falcon tubes. Samples were centrifuged at 3500 rpm, for 15 min at 4 °C to remove cellular components. For further analysis aliquoted samples were stored at −80 °C in microcentrifuge tubes. Bicinchoninic acid (BCA) procedure was followed for measurement of total protein concentration according to the Kit protocol.12FASP digestion
Each pericardial fluid sample equivalent to 200 μg protein, were digested using filter-aided sample preparation (FASP) method.13 In short, protein concentrates in 0.5 mL 10 kDa cutoff centrifuge filters were mixed with 500 μL of 6 M urea in 0.1 M tris/HCl and the samples were centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 25 min at 20 °C. After centrifugation, 10 μL of 0.1 M Dithiotreitol (DTT) was added to the filters, and incubated at 56 °C for 30 min. Then, 10 μL of 0.3 M iodoacetamide (IAA) was added to the filters, after which the samples were incubated for 30 min in darkness. Finally, 2 μg of trypsin (MS Grade, Thermo Scientific) in 50 μL of 100 mM NH4HCO3 was added to each filter. The protein to enzyme ratio was 50
000g for 25 min at 20 °C. After centrifugation, 10 μL of 0.1 M Dithiotreitol (DTT) was added to the filters, and incubated at 56 °C for 30 min. Then, 10 μL of 0.3 M iodoacetamide (IAA) was added to the filters, after which the samples were incubated for 30 min in darkness. Finally, 2 μg of trypsin (MS Grade, Thermo Scientific) in 50 μL of 100 mM NH4HCO3 was added to each filter. The protein to enzyme ratio was 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Samples were incubated overnight at 37 °C and peptides were released and collected by centrifugation at 14
1. Samples were incubated overnight at 37 °C and peptides were released and collected by centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g at 20 °C for 25 min.
000g at 20 °C for 25 min.
LC-MS/MS analysis
The protein digest were dissolved in 0.1% TFA and 1 μL (equivalent to 2 μg) was injected on a C18-reverse-phase trapping column (C18, Acclaim™ PepMap™ 100, 75 μm × 2 cm, particle size 3 μm, nanoViper, Thermo Fisher Scientific) and was separated by C18-reverse-phase nano-LC column (75 μm × 15 cm Acclaim™ PepMap™ RSLC C18 column with 2 μm particle size and 100 Å pore size, NanoViper, Thermo Fisher Scientific), using Nano-UHPLC, (Dionex, UltiMate 3000 RSLCnano System, Thermo Scientific, USA) coupled to MaXis II ESI-QTOF Mass spectrometer (Bruker Daltonics, Bremen, Germany). Buffers A and B contained 0.1% formic acid in ultra-pure water and 0.1% formic acid in acetonitrile (ACN), respectively were used for peptide elusion. ACN gradient at 300 nL min−1 was used to separate peptides after 6 min sample load at a flow rate of 5 μL min−1 with 2% B. Elution of the peptides were achieved by applying a linear gradient of 1–35% B over 29 min, with subsequent gradient increase to 95% B over 2 min, hold B for 7 min at 95%, and then decreased to 2% B for 4 min followed by 12 min equilibration at 2% B. Data dependent acquisition (DDA) was carried out in positive ion mode. The parameters were set as follows: end plate offset voltage was 500 V, capillary voltage was 4500 V, dry gas flow rate was 4.0 L min−1 and dry temperature was 180 °C. Scan mode for MS and MS/MS were between 150 to 2200 m/z. The threshold for MS/MS was 2500 and cycle time of 3 s.Label-free quantification
Bruker Daltonics Compass Data Analysis Software (version 4.4) was used to generate the molecular feature list for each LC-MS/MS run. The resulting molecular features were transferred to ProfileAnalysis Software to calculate buckets. A bucket is characterized by its MH+ mass value, retention time, and intensity values. The parameters for bucket calculation were set as, retention time range 6–45 min, mass range 150–2200 m/z, normalization with a quantile, value count of group attribute within bucket were set as 2 and value count of bucket 30. After bucket generation, peptide regulation ratios were calculated using T-test and transferred to ProteinScape software.To link peptide regulation output with the identification results, the LC-MS/MS raw data files of each run were converted to (.xml) using Bruker Daltonics Compass Data Analysis Software (version 4.4). The resulting xml files were imported to ProteinScape software for the Mascot Database search. After the protein database search, peptide identification was linked to their peptide regulation ratios by selecting the retention time correction option and p-value <0.05 as assignment criteria. Finally, protein regulation ratios were calculated from peptide regulation ratios and a list of proteins includes quantification information such as the number of peptides ratios, coefficient of variation (CV) values, and the regulation ratios were generated.14
MS/MS data analysis using ProteinScape
Nano-LC-MS/MS raw data files of each run were converted to (.xml) and were searched using the Mascot search engine (Version 2.6, Matrix Science, London, UK) triggered by ProteinScape against Swiss-Prot database with the following parameters: taxonomy filter was set to homo sapiens, for proteolytic cleavages, trypsin was used with maximum missed cleavages up to 1, oxidation of methionine as variable modifications and carbamidomethyl of cysteine as fixed modifications were allowed. Peptide mass tolerance was set at 0.08 Da and fragment mass tolerance was set at 0.2 Da. Peptide charges were set at +2, +3, and +4. The accepted FDR was set to <1%.Bioinformatic analysis
Label-free quantified proteins were further subjected to bioinformatic analysis, including gene ontology (GO) and KEGG pathway analysis. The 16 differentially expressed proteins sequence information was extracted from the online UniProt knowledgebase database (https://www.uniprot.org/) and retrieved in FASTA format. Functional classification of proteins was done by gene ontology on the basis of biological process, molecular process and cellular component using OmicsBox (version 1.2, https://www.biobam.com/omicsbox/).15 HSP Length cutoff 33 and expect value 1 × 10−3 were set as parameter for blastp-fast. Parameters for the gene ontology (GO) annotations were set as; Hit filter 500, GO weight 5, HSP-Hit coverage cutoff 0, annotation cutoff 55 and E-value-hit-filter 1 × 10−6. The level 2 pie/bar chart configuration was used for showing annotated sequences in GO graphs. The association between differentially expressed quantified proteins with each other and their interaction with other proteins were assessed by the STRING EMBL software (version 11.0, https://string-db.org/). The medium confidence score of 0.400 was used.16Results
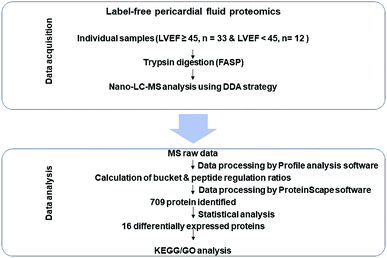
For the present study, workflow is shown in Fig. 1. The data acquisition stage consisted of pericardial fluid sample analysis through nano-LC-ESI-MS/MS by using the data dependent acquisition (DDA) strategy. The statistical analysis stage was used to find out significantly differentially expressed proteins in both groups (LVEF ≥45 vs. LVEF <45) followed by the bioinformatics analysis (GO and KEGG) to understand the biological role of these proteins.Label-free proteomics and identification of differentially expressed proteins
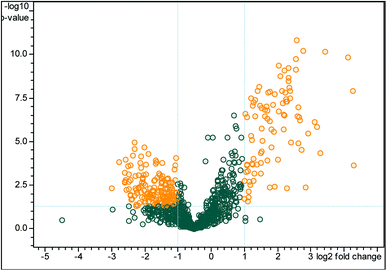
A total of 709 pericardial fluid proteins were identified using the DDA strategy in both groups (LVEF ≥45 vs. LVEF <45) (Table S1†). The annotated spectra of all the identified proteins along with the differentially expressed proteins are given in the ESI file.† For a better look, the double fold change for the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-based values derived from the quotient (LVEF <45/LVEF ≥45) is presented in Fig. 2 as a volcano plot, statistically determined by a P-value <0.05, at 2.0-fold change. The identified proteins in the two groups of the experiment (LVEF ≥45/LVEF <45) were analyzed and compared, 16 proteins were found differentially expressed, including 12 down-regulated and 4 up-regulated proteins in impaired (LVEF <45) systolic function group as compared to normal (LVEF ≥45) systolic function group. Serum albumin, mesothelin, E3 ubiquitin-protein ligase SH3RF3, Rho GTPase-activating protein 44 and serotransferrin were the most down-regulated while AT-rich interactive domain-containing protein 4A, zinc finger protein GLI2, zinc finger protein 831, and hedgehog-interacting protein were the most up-regulated proteins. The results with protein name, accession number, isoelectric point, sequence coverage, molecular weight, fold-change and t-test p-values are presented in Table 2.
2-based values derived from the quotient (LVEF <45/LVEF ≥45) is presented in Fig. 2 as a volcano plot, statistically determined by a P-value <0.05, at 2.0-fold change. The identified proteins in the two groups of the experiment (LVEF ≥45/LVEF <45) were analyzed and compared, 16 proteins were found differentially expressed, including 12 down-regulated and 4 up-regulated proteins in impaired (LVEF <45) systolic function group as compared to normal (LVEF ≥45) systolic function group. Serum albumin, mesothelin, E3 ubiquitin-protein ligase SH3RF3, Rho GTPase-activating protein 44 and serotransferrin were the most down-regulated while AT-rich interactive domain-containing protein 4A, zinc finger protein GLI2, zinc finger protein 831, and hedgehog-interacting protein were the most up-regulated proteins. The results with protein name, accession number, isoelectric point, sequence coverage, molecular weight, fold-change and t-test p-values are presented in Table 2.
| Accession | Protein | MW [kDa] | pI | Mascot score | #Peptides | Sequence coverage [%] | RMS90 [ppm] | Fold change log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (LVEF ≥45): (LVEF<45) 2 (LVEF ≥45): (LVEF<45) |
p-Value | Regulation |
|---|---|---|---|---|---|---|---|---|---|---|
| ALBU_HUMAN | Serum albumin | 69.3 | 5.9 | 3565.8 | 62 | 73.9 | 42.24 | 5.02 | 0.0029 | Down |
| TRFE_HUMAN | Serotransferrin | 77.0 | 6.8 | 2660.8 | 43 | 50.9 | 44.56 | 2.09 | 0.0006 | Down |
| APOA4_HUMAN | Apolipoprotein A-IV | 45.4 | 5.3 | 461.2 | 12 | 32.6 | 44.87 | 1.86 | 0.0029 | Down |
| MSLN_HUMAN | Mesothelin | 68.9 | 6.0 | 76.2 | 3 | 5.4 | 572.57 | 4.60 | 0.0001 | Down |
| RHG44_HUMAN | Rho GTPase-activating protein 44 | 89.2 | 6.1 | 51.1 | 2 | 1.1 | 12.74 | 2.26 | 0.0007 | Down |
| OSBL6_HUMAN | Oxysterol-binding protein-related protein 6 | 106.2 | 6.5 | 38.4 | 2 | 2.1 | 36.23 | 1.89 | 0.0001 | Down |
| GLI2_HUMAN | Zinc finger protein GLI2 | 167.7 | 6.9 | 37.7 | 2 | 1.5 | 664.68 | −4.47 | 0.005 | Up |
| SRBP2_HUMAN | Sterol regulatory element-binding protein 2 | 123.6 | 8.7 | 34.2 | 1 | 1.0 | 1.51 | 1.50 | 0.0147 | Down |
| PHF1_HUMAN | PHD finger protein 1 | 62.1 | 9.3 | 31.2 | 1 | 1.4 | 6.49 | 1.42 | 0.0001 | Down |
| ZN831_HUMAN | Zinc finger protein 831 | 177.8 | 8.7 | 25.7 | 1 | 1.0 | 5.91 | −4.03 | 0.0009 | Up |
| EFC4B_HUMAN | EF-hand calcium-binding domain-containing protein 4B | 45.6 | 4.9 | 20.2 | 1 | 2.5 | 16.97 | 1.50 | 0.0147 | Down |
| NU205_HUMAN | Nuclear pore complex protein Nup205 | 227.8 | 5.8 | 20.0 | 1 | 0.4 | 922.75 | 1.08 | 0.0116 | Down |
| MTUS1_HUMAN | Microtubule-associated tumor suppressor 1 | 141.3 | 7.3 | 19.6 | 1 | 0.8 | 8.45 | 1.50 | 0.0147 | Down |
| HHIP_HUMAN | Hedgehog-interacting protein | 78.8 | 8.2 | 17.6 | 1 | 2.0 | 6.71 | −2.34 | 0.0184 | Up |
| SH3R3_HUMAN | E3 ubiquitin-protein ligase SH3RF3 | 92.7 | 9.1 | 17.0 | 1 | 1.0 | 10.22 | 3.17 | 0.0001 | Down |
| ARI4A_HUMAN | AT-rich interactive domain-containing protein 4A | 142.7 | 5.0 | 15.6 | 1 | 1.0 | 3.54 | −4.49 | 0.0042 | Up |
Functional classification of differentially expressed proteins
A GO classification was performed for the 16 significant differentially expressed proteins in patients with normal (LVEF ≥45) and impaired (LVEF <45) systolic function group as shown in the pie chart at graph level 2 (Fig. 3 and Table S2†). The GO analysis of differentially expressed protein revealed involvement in multiple biological processes in both groups. The highest proportion of (18%, 7 proteins: MTUS1, TRFE, PHF1, MSLN, SH3R3, OSBL6, RHG44) sequences were found to be involved in cellular processes followed by biological regulation (15%, 6 proteins: MTUS1, TRFE, PHF1, SH3R3, OSBL6, RHG44), and metabolic processes (13%, 5 proteins: PHF1, MSLN, SH3R3, OSBL6, APOA4), while the remaining functions such as response to a stimulus; localization, signaling, developmental process, cellular component organization, locomotion, immune system process biological adhesion and multicellular organismal process, were observed in less proportions in both groups (LVEF ≥45/LVEF <45). Differentially expressed, proteins were also classified based on their molecular functions, such as protein involved with binding (81%, 13 proteins: MTUS1, PHF1, MSLN, EFC4B, GLI2, RHG44, TRFE, SRBP2, ARI4A, SH3R3, OSBL6, APOA4, ZN831), transporter activity (12%, 2 proteins: TRFE, OSBL6) and catalytic activity (6%, 1 protein: HHIP).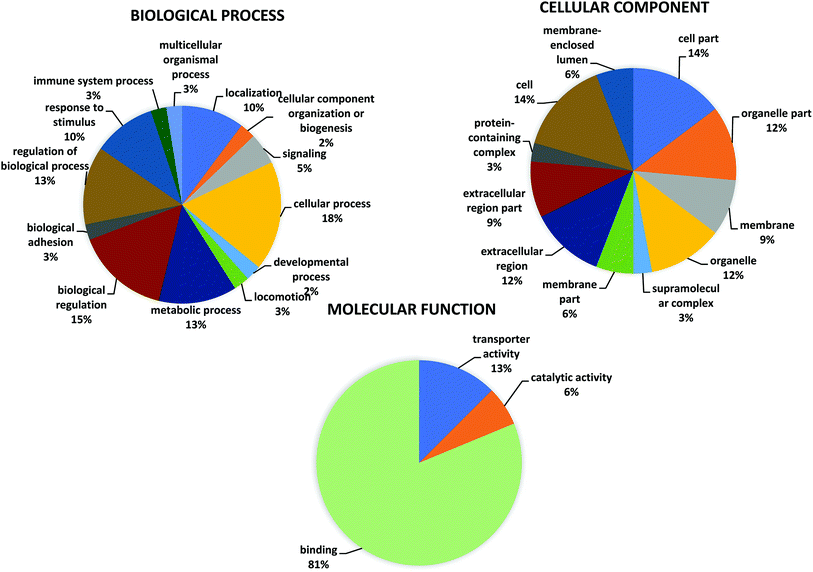 | ||
| Fig. 3 Go annotation of “biological process” “molecular function” and “cellular component” analyze by OmicxBox (version 1.2, https://www.biobam.com/omicsbox/). | ||
OmicsBox analysis, also classified the differentially expressed proteins according to their cellular component. Most of the proteins were assigned to the organelle (12%, 4 proteins MTUS1, MSLN, NU205, OSBL6), followed by extracellular region (12%, 4 proteins: TRFE, MSLN, APOA4, ALBU) and membrane (9%, 3 proteins: MTUS1, MSLN, OSBL6), while the remaining component such as extracellular region part, membrane part, membrane-enclosed lumen, supramolecular complex and protein-containing complex were observed in fewer proportions.
KEGG pathway analysis
STRING was used to gain an insight into the interactive links known to be associated with the 16 proteins expressed differentially in the dataset. Interactive links between the differentially regulated proteins could be traced as shown in Fig. 4. A total of 8 RCTM/KEGG pathways were observed in the present study. The suggested pathways of these differentially regulated proteins are listed in Table 3, along with term ID, term definition, observed gene count, background gene count, FDR and matching proteins within a network.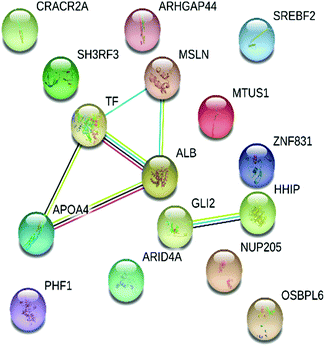 | ||
| Fig. 4 Interactive network analysis and association of 16 differentially expressed pericardial fluid proteins obtained from STRING (version 11.0, https://string-db.org/). | ||
| KEGG/RCTM pathways | |||||
|---|---|---|---|---|---|
| #Term ID | Term description | Observed gene count | Background gene count | False discovery rate | Matching proteins in your network (labels) |
| HSA04340 | Hedgehog signaling pathway | 2 | 46 | 0.009 | GLI2, HHIP |
| HSA05217 | Basal cell carcinoma | 2 | 63 | 0.009 | GLI2, HHIP |
| HSA-381426 | Regulation of insulin-like growth factor (IGF) transport and uptake by insulin-like growth factor binding proteins (IGFBPs) | 3 | 123 | 0.012 | ALB, MSLN, TF |
| HSA-8957275 | Post-translational protein phosphorylation | 3 | 106 | 0.012 | ALB, MSLN, TF |
| HSA-8957322 | Metabolism of steroids | 3 | 146 | 0.012 | ALB, OSBPL6, SREBF2 |
| HSA-8963899 | Plasma lipoprotein remodeling | 2 | 30 | 0.012 | ALB, APOA4 |
| HSA-194068 | Bile acid and bile salt metabolism | 2 | 42 | 0.0157 | ALB, OSBPL6 |
| HSA-5632684 | Hedgehog ‘on’ state | 2 | 82 | 0.0405 | GLI2, HHIP |
Discussion
Proteomics is used to perform quantitative as well as qualitative analyses of a large array of proteins in biological samples.17 Recently, label-free quantitative proteomics (LFQP) has gained more popularity as mass spectrometer's efficiency has significantly improved. The LFQP becomes a widely applied approach for identifying as well as quantifying differentially expressed proteins. It increases the dynamic range of proteins 3 to 4 time as compared to 2DE and is more sensitive and rapid than many other proteomic methods.18In the present study, we have found 16 significantly differentially expressed proteins in LVEF ≥45 vs. LVEF <45. Among the differentially expressed proteins 12 proteins down-regulated in impaired (LVEF <45) systolic function group, including serum albumin, serotransferrin, apolipoprotein A-IV, mesothelin, Rho GTPase-activating protein 44, sterol regulatory element-binding protein 2, oxysterol-binding protein-related protein 6, PHD finger protein 1, nuclear pore complex protein Nup205, EF-hand calcium-binding domain-containing protein 4B, Microtubule-associated tumor suppressor 1, and E3 ubiquitin-protein ligase SH3RF3. While, the remaining 4 proteins were found up-regulated in impaired (LVEF <45) systolic function group including, zinc finger protein 831, zinc finger protein GLI2, AT-rich interactive domain-containing protein 4A and hedgehog-interacting protein. One of the down-regulated protein in patients with impaired (LVEF <45) group is albumin. Albumin is a 66.5 kDa protein which is the most abundant protein in plasma. Albumin plays a significant role in regulating plasma oncotic pressure and fluid distribution throughout the body compartment.19 Serum albumin's physiological properties include anti-inflammatory, antioxidant, anticoagulant activity and regulating the transport of cholesterol.20 Normal pericardial fluid contains between one quarter and one third of the protein of plasma and demonstrates a far higher proportion of albumin to other proteins in the pericardial fluid.21,22 Hypoalbuminemia, with serum albumin levels less than 3.5 g dL−1 (ref. 23) has been linked to several cardiovascular diseases such as coronary artery disease, congenital heart disease, stroke and heart failure.24 Hypoalbuminemia has been reported in one-third of heart failure patients with reduced ejection fraction (LVEF <45).25 In concordance with the findings in the serum, the present findings suggest that the down-regulation of albumin in the pericardial fluid is associated with impaired systolic function of the left ventricle. Hence, it could be used as a potential risk marker in identifying the patients with left ventricular systolic dysfunction.
Another down-regulated protein in impaired (LVEF <45) group is serotransferrin. It is an iron binding protein, responsible for transporting iron from sites of heme degradation and absorption to those of utilization and storage.26 The serum level of serotransferrin decreases in inflammatory conditions such as diabetes mellitus which in turn increases the risk of CVD.27 It has been demonstrated that decrease level of plasma serotransferrin is associated with rheumatic valvular heart disease (RVD),28 and coronary artery dilation (CAD) caused by Kawasaki disease.29 The down-regulation of serotransferrin in impaired (LVEF <45) group may be due to the inflammatory response of the body as its level also decreases in patients with CKD and ESRD.30
Apo A-IV is another protein that is down-regulated in impaired (LVEF <45) group. Apo A-IV, is an oligosaccharide containing protein with a molecular weight of 46 kDa.31 Its concentration in plasma is 15–37 mg dL−1.32 Apo A-IV is synthesized in the intestine and is incorporated into the chylomicrons and secreted into the intestinal lymph during fat absorption.33 Apo A-IV plays a significant role in the lipid and glucose metabolism.34 It also acts as anti-inflammatory agent and potentially exert its anti-atherogenic effect35 by reverse cholesterol pathway, which removes cholesterol from peripheral cells and transports it to the liver.36 Clinical studies have demonstrated an inverse relationship between apo A-IV and coronary artery diseases.37 Apo A-IV deficiency is associated with atherosclerosis, cardiovascular diseases, chronic pancreatitis, malabsorption disorders38 and diabetes mellitus.39 Low Apo A-IV levels in these patients with systolic dysfunction as compared to patients with preserved EF may point towards a disease mechanism underlying the progression from preserved EF to reduced EF.
Among the up-regulated differentially expressed proteins in patients with impaired (LVEF <45) group is AT-rich interactive domain containing protein 4A. It is also known as ARID4A or retinoblastoma – binding protein 1 is encoded by the gene ARID4A.40 Studies shows that ARID4A play a significant role in various types of cancer such as breast cancer and leukemia. ARID4A binds with other proteins and play a key role in cell proliferation and differentiation.41 Zinc finger protein 831 belongs to a large class of zinc finger proteins (ZNFs) is up-regulated in impaired (LVEF <45) group. Zinc finger proteins (ZNFs) have a key role in tissue development and differentiation. Alterations in ZNFs are involved in the development of several diseases such as congenital heart disease, defects of the cardiac outflow tract and diabetes.42
KEGG network analysis with pathway enrichment identified that the hedgehog signaling pathway is up-regulated in impaired (LVEF <45) group as compared to the normal (LVEF ≥45) group. Network analysis revealed that two up-regulated proteins zinc finger protein GLI2 and hedgehog-interacting protein are involved in hedgehog signaling (Hh) pathway. Hh signaling pathway was first identified as a key mediator of organogenesis in invertebrates by Nusslein–Volhard and Wieschaus in 1980.43 Three Hh homologues have been discovered in mammals in the early 1990s, including Indian hedgehog (Ihh), Sonic (Shh) and Desert (Dhh). Sonic is one of the best studied and most widely expressed during embryonic development as compared to Dhh and Ihh.44 Hh signaling pathway is known to regulate cell differentiation and migration during embryonic development but in post-natal life, the Hh signaling can be recapitulated under several pathological conditions, including cardiac ischemia.45 The absence of tissue specific Hh signalling components reduces proangiogenic gene expression and thus loss of coronary vasculature that leads to cardiomyocyte cell death and ventricular failure. The presence of the Hh signalling pathway may protect and limit the extent of myocardial ischemic damage.46 Many theories are postulated to elucidate the regulation mechanism of Hh signaling pathway in ischemic heart tissues. It has been shown that the expression of hypoxia-induced factor-1 (HIF-1) and inflammation in cardiac ischemia, activate the Hh signaling pathway, resulting in elevated expression of angiogenic and proangiogenic factors to facilitate angiogenesis and neovascularization in ischemic tissues.47 In concordance with the previous findings, our results also validate the recapitulation of Hh signaling pathway in ischemic heart disease patients but more prominently in impaired (LVEF <45) group.48 Considering that the Hh signaling pathway is essential for the formation of new coronary vessels, this finding signals an important adaptive mechanism in humans with LVSD.49
KEGG pathway analysis also showed that pathways involved in the Metabolism of steroids (pathway proteins: albumin, oxysterol-binding protein-related protein 6, sterol regulatory element-binding protein 2), plasma lipoprotein remodelling (pathway proteins: albumin, apolipoprotein A-IV) and bile acid and bile salt metabolic pathways (pathway proteins: albumin, oxysterol-binding protein-related protein 6) were down-regulated in impaired (LVEF <45) group as compared to the normal (LVEF ≥45) group.
Conclusion
In conclusion, sixteen proteins were found to be differentially expressed between IHD patients with LVEF <45 and LVEF ≥45. Among the differentially expressed proteins the inflammatory marker albumin, atherosclerosis marker apolipoprotein A-IV and hedgehog-interacting protein marker of angiogenesis were predominantly associated with impaired LVEF <45 group. The KEGG pathway analysis revealed that the hedgehog signaling pathway is up regulated in impaired LVEF <45 group. These findings suggest important associations of heart systolic function with specific proteins reflecting its underlying pathophysiological mechanisms, however, they need to be further validated in a larger number of samples.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work was supported by the Pakistan Academy of Sciences (Grant # 5-9/PAS/745). We are thankful to South City and The Aga Khan University Hospital, Karachi, the cardiothoracic surgeons and the cardiologists for their invaluable work in patient's recruitment, sample aspiration and data collection. We are also thankful to Mr Junaid ul Haq and Mr. Arsalan Tahir, Mass spectrometry section, ICCBS, for their assistance in nano-LC-MS/MS analysis.References
- G. Vilahur, J. J. Badimon, R. Bugiardini and L. Badimon, Eur. Heart J. Suppl., 2014, 16, A7–A11 CrossRef.
- W. E. Chavey, C. S. Blaum, B. E. Bleske, R. Van Harrison, S. Kesterson and J. M. Nicklas, Am. Fam. Physician, 2001, 64, 769 Search PubMed.
- J. D. Sara, T. Toya, R. Taher, A. Lerman, B. Gersh and N. S. Anavekar, European Cardiology Review, 2020, 15, e13 Search PubMed.
- A. G. Japp, S. Moir and P. M. Mottram, Heart, Lung Circ., 2015, 24, 532–535 CrossRef.
- I. Raymond, F. Pedersen, F. Steensgaard-Hansen, A. Green, M. Busch-Sorensen, C. Tuxen, J. Appel, J. Jacobsen, D. Atar and P. Hildebrandt, Heart, 2003, 89, 1422–1429 CrossRef CAS.
- M. Z. Israr, L. M. Heaney and T. Suzuki, Heart failure clinics, 2018, 14, 93–107 CrossRef.
- S. Yavuz, M. Kasap, G. Akpinar, E. Ozbudak, D. Ural and T. Berki, BioMed Res. Int., 2014, 2014, 1–7 Search PubMed.
- T. Ege, M. H. Us, M. Cikirikcioglu, C. Arar and E. Duran, Yonsei Med. J., 2006, 47, 372–376 CrossRef CAS.
- F. Trindade, P. Bastos, A. Leite-Moreira, B. Manadas, R. Ferreira, S. F. Soares, A. L. Daniel-da-Silva, I. Falcão-Pires and R. Vitorino, Arch. Biochem. Biophys., 2017, 634, 1–10 CrossRef CAS.
- S. Amjad, S. A. Sami, M. N. Basir, K. Hameed, M. Fujita and H. R. Ahmad, Asian Cardiovasc. Thorac. Ann., 2013, 21, 160–165 CrossRef CAS.
- F. Xiang, X. Guo, W. Chen, J. Wang, T. Zhou, F. Huang, C. Cao and X. Chen, Proteomics, 2013, 13, 2692–2695 CrossRef CAS.
- A. Bainor, L. Chang, T. J. McQuade, B. Webb and J. E. Gestwicki, Anal. Biochem., 2011, 410, 310–312 CrossRef CAS.
- J. R. Wiśniewski, A. Zougman, N. Nagaraj and M. Mann, Nat. Methods, 2009, 6, 359–362 CrossRef.
- C. O. da Silva Frozza, E. da Silva Brum, A. Alving, S. Moura, J. A. Henriques and M. Roesch-Ely, J. Pharm. Pharmacol., 2016, 68, 1073–1084 CrossRef CAS.
- S. Götz, J. M. García-Gómez, J. Terol, T. D. Williams, S. H. Nagaraj, M. J. Nueda, M. Robles, M. Talón, J. Dopazo and A. Conesa, Nucleic Acids Res., 2008, 36, 3420–3435 CrossRef.
- D. Szklarczyk, A. Franceschini, S. Wyder, K. Forslund, D. Heller, J. Huerta-Cepas, M. Simonovic, A. Roth, A. Santos and K. P. Tsafou, Nucleic Acids Res., 2015, 43, D447–D452 CrossRef CAS.
- R. Favicchio, C. Thepaut, H. Zhang, R. Arends, J. Stebbing and G. Giamas, Cancer Lett., 2016, 382, 86–94 CrossRef CAS.
- M. Wang, J. You, K. G. Bemis, T. J. Tegeler and D. P. Brown, Briefings Funct. Genomics Proteomics, 2008, 7, 329–339 CrossRef CAS.
- S. Suzuki, N. Hashizume, Y. Kanzaki, T. Maruyama, A. Kozuka and K. Yahikozawa, PLoS One, 2019, 14, e0219044 CrossRef CAS.
- S.-C. Chien, C.-Y. Chen, C.-F. Lin and H.-I. Yeh, Biomarker research, 2017, 5, 31 CrossRef.
- K. Vogiatzidis, S. G. Zarogiannis, I. Aidonidis, E. I. Solenov, P.-A. Molyvdas, K. I. Gourgoulianis and C. Hatzoglou, Front. physiol., 2015, 6, 62 CrossRef.
- A. Gibson and M. Segal, J. Physiol., 1978, 277, 367–377 CrossRef CAS.
- D. G. Levitt and M. D. Levitt, Int. J. Gen. Med., 2016, 9, 229 CrossRef CAS.
- S. Arques, Eur. J. Intern. Med., 2018, 52, 8–12 CrossRef CAS.
- M. F. Hussein, Iraqi Academic Scientific Journal, 2012, 11, 411–417 Search PubMed.
- P. Aisen and I. Listowsky, Annu. Rev. Biochem., 1980, 49, 357–393 CrossRef CAS.
- P. T. Gomme, K. B. McCann and J. Bertolini, Drug discovery today, 2005, 10, 267–273 CrossRef CAS.
- G. Gao, C. Xuan, Q. Yang, X.-C. Liu, Z.-G. Liu and G.-W. He, PLoS One, 2013, 8, e72111 CrossRef CAS.
- L. Zhang, W. Wang, J. Bai, Y.-F. Xu, L.-Q. Li, L. Hua, L. Deng and H.-L. Jia, Revista Portuguesa de Cardiologia, 2016, 35, 265–273 CrossRef.
- L. Pupim, C. J. Martin and T. A. Ikizler, in Nutritional Management of Renal Disease, Lippincott Williams & Wilkins, Philadelphia, 2004, pp. 223–240 Search PubMed.
- G. Utermann and U. Beisiegel, Eur. J. Biochem., 1979, 99, 333–344 CrossRef CAS.
- P. H. Weinstock, C. L. Bisgaier, T. Hayek, K. Aalto-Setala, E. Sehayek, L. Wu, P. Sheiffele, M. Merkel, A. Essenburg and J. Breslow, J. Lipid Res., 1997, 38, 1782–1794 CAS.
- P. H. Green, R. M. Glickman, J. W. Riley and E. Quinet, J. Clin. Invest., 1980, 65, 911–919 CrossRef CAS.
- Z. Wang, L. Wang, Z. Zhang, L. Feng, X. Song and J. Wu, Nutr. Metab., 2019, 16, 41 CrossRef.
- N. Duverger, G. Tremp, J.-M. Caillaud, F. Emmanuel, G. Castro, J.-C. Fruchart, A. Steinmetz and P. Denefle, Science, 1996, 273, 966–968 CrossRef CAS.
- A. Steinmetz, R. Barbaras, N. Ghalim, V. Clavey, J. Fruchart and G. Ailhaud, J. Biol. Chem., 1990, 265, 7859–7863 CAS.
- F. Kronenberg, M. Stühlinger, E. Trenkwalder, F. Geethanjali, O. Pachinger, A. von Eckardstein and H. Dieplinger, J. Am. Coll. Cardiol., 2000, 36, 751–757 CrossRef CAS.
- S. Koga, Y. Miyata, A. Funakoshi and H. Ibayashi, Digestion, 1985, 32, 19–24 CAS.
- J. Qu, C.-W. Ko, P. Tso and A. Bhargava, Cells, 2019, 8, 319 CrossRef CAS.
- Y. K. Liang, Z. D. Han, J. M. Lu, Z. Z. Liu, Y. J. Zhuo, X. J. Zhu, J. X. Chen, J. H. Ye, Y. X. Liang, H. C. He and W. D. Zhong, J. Cell. Biochem., 2018, 119, 7245–7255 CrossRef CAS.
- C. Lin, W. Song, X. Bi, J. Zhao, Z. Huang, Z. Li, J. Zhou, J. Cai and H. Zhao, OncoTargets Ther., 2014, 7, 315–324 Search PubMed.
- M. Cassandri, A. Smirnov, F. Novelli, C. Pitolli, M. Agostini, M. Malewicz, G. Melino and G. Raschella, Cell Death Discovery, 2017, 3, 17071 CrossRef.
- C. Nüsslein-Volhard and E. Wieschaus, Nature, 1980, 287, 795–801 CrossRef.
- E. Yao and P.-T. Chuang, J. Formosan Med. Assoc., 2015, 114, 569–576 CrossRef CAS.
- I. Giarretta, E. Gaetani, M. Bigossi, P. Tondi, T. Asahara and R. Pola, Int. J. Mol. Sci., 2019, 20, 5270 CrossRef CAS.
- K. J. Lavine, A. Kovacs and D. M. Ornitz, J. Clin. Invest., 2008, 118, 2404–2414 CAS.
- J. Pan and S. Zhou, Die Pharmazie-An International Journal of Pharmaceutical Sciences, 2012, 67, 475–481 CAS.
- E. G. Lima, F. P. C. d. Carvalho, J. P. P. Linhares Filho, F. G. Pitta and C. V. Serrano Jr, Rev. Assoc. Med. Bras., 2017, 63, 793–800 CrossRef.
- K. J. Lavine, A. C. White, C. Park, C. S. Smith, K. Choi, F. Long, C.-c. Hui and D. M. Ornitz, Genes Dev., 2006, 20, 1651–1666 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08389e |
| This journal is © The Royal Society of Chemistry 2021 |