 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ionization inhibition in a polyol/water system for boosting H2 generation from NaBH4
Lili Feng a,
Cong Wangb,
Jinrong Xub,
Mingwei Fanga,
Yu Shic,
Lei Xiec,
Jie Zheng*b and
Xingguo Li
a,
Cong Wangb,
Jinrong Xub,
Mingwei Fanga,
Yu Shic,
Lei Xiec,
Jie Zheng*b and
Xingguo Li *b
*b
aSchool of Chemical & Environmental Engineering, China University of Mining and Technology, Beijing, 100083, PR China
bBeijing National Laboratory of Molecular Sciences, State Key Laboratory of Rare Earth Materials Chemistry and Applications, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871, PR China. E-mail: jiezheng@pku.edu.cn; xgli@pku.edu.cn
cSunan Institute for Molecular Engineering, Peking University, Changshu, 215500, PR China
First published on 24th December 2020
Abstract
Alcoholysis and hydrolysis of NaBH4 to produce H2 offer attractive routes to sustainable development with high energy density and environmentally-friendly features. However, the productivity is often limited by the increased alkalinity of the reaction system and the deactivation of catalysts. Here, we present a novel strategy of constructing a polyol/water composite system to promote catalyst free alcoholysis of NaBH4 while inhibiting the ionization of reaction products. The polyol/water system exhibits a NaBH4 conversion of more than 90% in less than 60 min, especially when the erythritol/water system is employed with a conversion of 96% in 80 min. Further study shows that erythritol participates in the reaction and the ionization of reaction products is inhibited by erythritol. Moreover, the analysis of reaction products and control group results reveal that erythritol indeed inhibits the basicity enhancement of the reaction system via reacting with NaB(OH)4. By adjusting the volume of water in the polyol/water system, a quasi-solid phase reaction system is developed for practical applications, which shows an excellent NaBH4 conversion of 94% and high hydrogen storage gravimetric density of 3.9 wt%.
1. Introduction
Hydrogen (H2) is considered to be a promising energy carrier toward sustainable development with high energy density and environmentally-friendly features.1 Due to the gaseous nature and low density of H2 at room temperature, storage and transportation of H2 are still obstacles to the development of hydrogen energy.2 The controllable generation and purity of H2 have led to the hydrolysis of chemical hydrides being widely studied. Among them, sodium borohydride (NaBH4) deserves special attention owing to its high gravimetric hydrogen density (10.5 wt%), environmental friendliness and potential safe operation.3,4 The hydrolysis and alcoholysis of NaBH4 have been widely considered as promising methods for hydrogen generation.Unfortunately, the hydrolysis of NaBH4 is always inhibited by the increased alkalinity of the solution resulting from the ionization of the reaction products. To solve this problem, a variety of catalysts have been developed for efficient hydrolysis of NaBH4, including metals, metal oxides, metal chalcogenides, carbon-based nanomaterials.5–12 The noble metals including Ru, Rh and Pt have been found to be the most efficient catalytic species, but their practical applications are restricted by the expensive price and limited resources.13–15 Moreover, earth abundant materials like Co, Ni, Fe show activities towards hydrolysis of NaBH4, while they suffer from slow kinetics and poor catalytic durability.16–18 For instance, Brown et al. have compared the catalytic activity of different metallic elements and observed that those metallic elements exert a catalytic effect on NaBH4 hydrolysis with an order: Ru, Rh > Pt > Co > Ni > Os > Ir > Fe > Pd.19 Besides, acids such as nitric acid and malic acid are also reported as catalysts to make the reaction more complete.20 The addition of acids can inhibit the ionization of reduction products, but causes environmental pollution.
Since the hydrolysis of NaBH4 is not efficient at low temperature, which may be inconvenient for automotive and portable applications.21 Alcoholysis of NaBH4 has been widely reported as another attractive route with fast reaction kinetics and low activation energy in comparison to hydrolysis of NaBH4. For example, Lo et al. reported that NaBH4 could generate H2 in methanol at low temperatures (253–323 K).22 Wang and co-workers adopted a strategy of constructing bimetallic catalytic system for boosting H2 generation rates from NaBH4 methanolysis. Significantly, the highest H2 generation rate is observed in the case of Ru–Co/C catalyst, achieving 9.36 L min−1 g−1 at 25 °C, which is comparable to the reported pure Ru catalysts.23 Although great progress has been achieved in improving the catalytic activity of catalysts for alcoholysis/hydrolysis of NaBH4, the increasing alkalinity of the solution resulting from the ionization of the reaction products is not fundamentally inhibited. Meanwhile, catalysts still confront great challenges in poor activity, low selectivity, and poor long-term stability. It is still far from satisfaction to achieve practical applications. Moreover, further studies are still necessary to elucidate its underlying mechanisms of alcoholysis/hydrolysis of NaBH4. Thus, rational design of reaction system is important to facilitate the alcoholysis/hydrolysis of NaBH4.
Here, we present a novel strategy of constructing a polyol/water composite system to promote the alcoholysis of NaBH4 without any catalyst while inhibiting the ionization of reaction products. The polyol/water system witnesses a NaBH4 conversion of more than 90% in less than 60 min, especially when the erythritol/water system is employed with a conversion of 96% in 80 min. Further study shows that erythritol participates in the reaction and the ionization of reaction products is inhibited by erythritol. Moreover, the analysis of reaction products and control group results further reveal that erythritol indeed inhibit the basicity enhancement of the reaction system via reacting with NaB(OH)4. By adjusting the volume of water in polyol/water system, a quasi-solid phase reaction system is developed for practical applications, which shows an excellent NaBH4 conversion of 94% and high hydrogen storage gravimetric density of 3.9 wt%.
2. Experimental
2.1 Materials
Sodium borohydride (NaBH4, 98%, Aldrich), methanol (CH3OH, 99%, Aldrich), ethylene glycol (C2H6O2, 99%, Aldrich), glycerol (C3H8O3, 99%, Aldrich), erythritol (C4H10O4, 99%, Adamas), xylitol (C5H12O5, 99%, Adamas), sorbitol (C6H14O6, 99%, Adamas), mannitol (C6H14O6, 99%, Adamas) and sodium metaborate (NaB(OH)4·2H2O, 99%, Adamas) were all analytical grade and used without further purification.2.2 Hydrogen generation
The experiment was inducted in a flask containing NaBH4, polyols and water. Methanol, ethylene glycol, glycerol, erythritol, xylitol, sorbitol and mannitol were adopted as the polyol feedstock and tested individually. The reaction temperature was controlled by a oil bath with magnetic agitation. Upon H2 production from the reaction, the clean/pure H2 can replace the water that was filled in an inverted cylinder to determine the exact volume of the generated H2 from the reaction. To evaluate the reaction performances of different polyol/water composite systems, NaBH4 conversion was adopted as the evaluation index in this work, which was equal to H2 productivity and can be calculated according to the following equation:| NaBH4 conversion (%) = (Vr/Vt) × 100% | (1) |
| Vt = (WNaBH4/MNaBH4) × (4/1) × 24.5 (L mol−1 at 298 K) | (2) |
2.3 Characterization
The chemical of reaction products were confirmed by the Fourier transform infrared (FTIR) spectra at 4 cm−1 resolution in the spectral range of 4000–500 cm−1 by FTIR spectrometer (Bruker, Tensor 27). The crystallization phase of reaction products was probed by X-ray powder diffraction (XRD) obtained using a Rigaku RX III powder diffractometer with a Cu Kα radiation. Moreover, the chemistry of reaction products was measured with a nuclear magnetic resonance (NMR) which was recorded in D2O at 500 MHz on a Bruker AV-400 NMR spectrometer.3. Results and discussion
3.1 Hydrogen generation in different polyol/water system
The conversions of NaBH4 in different polyol/water system are shown in Fig. 1. It is clearly that the NaBH4 conversions in all polyol/water system increase rapidly within 20 min and then stabilize. The NaBH4 conversion in methanol/water system is merely 43% after 140 min, which is much lower than that in ethylene glycol/water (88% after 135 min), glycerol/water (92% after 135 min), erythritol/water (96% after 80 min), xylitol/water (90% after 55 min), sorbitol/water (92% after 115 min) and mannitol/water (96% after 105 min) systems. It suggests that polyol can improve the reactivity and H2 productivity of alcoholysis of NaBH4. In general, the conversion of NaBH4 is high in erythritol/water, xylitol/water, sorbitol/water and mannitol/water systems, resulting in a H2 productivity of over 90% within 60 min. Considering the relatively high NaBH4 conversion and low cost, the erythritol/water system is a promising candidate for hydrogen generation of NaBH4 in comparision of xylitol, sorbitol and mannitol. Thus, the further study focused on hydrogen generation from NaBH4 in the erythritol/water system. | ||
Fig. 1 The conversion of NaBH4 in different polyol/water system. Mole ratio of NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alcohol hydroxyl alcohol hydroxyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. Reaction temperature is 50 °C. 8. Reaction temperature is 50 °C. | ||
In order to prove that polyols participate in the reaction, the relationship between conversion and mole ratio of erythritol/NaBH4 was systematically studied. As shown in Fig. 2(A), the positive linear relationship between conversion and mole ratio of erythritol/NaBH4 indicates that erythritol participates in the reaction rather than acts as catalysts. In order to further compare the difference between methanol/water system and erythritol/water system, the pH values of reaction products in methanol/water system and erythritol/water system were compared in Fig. 2(B). Obviously, the pH value of products in erythritol/water system is about 9, which is significantly lower than that in methanol/water system (pH = 11). It means that the ionization of reaction products in erythritol/water system is inhibited by erythritol, allowing the reaction more complete.
3.2 Reaction product characterization
A detailed analysis was carried out to further clarify the chemical structure of the reaction products. Gas-phase products were analyzed using a gas chromatograph (GC). As can be seen from Fig. 3(a), only H2 is detected from the gas product. Furthermore, non gaseous products were analyzed by XRD, FTIR and NMR techniques. Prior to all the measurements, the solution after reaction was dried under vacuum and the solid samples were obtained. XRD patterns in Fig. 3(b) show that there is no obvious crystal peak, indicating that the product is amorphous. Thus, it can be inferred that the solid products might be a complex amorphous mixture. The FTIR spectra in Fig. 3(c) show absorption bands corresponding to the vibration absorption of O–H at 3430 cm−1 and 1600 cm−1. The bands at 2900–3000 cm−1, 1100 cm−1 and 1300–1500 cm−1 can be assigned to stretching vibrations absorption of C–H, C–O and B–O groups, respectively. And the vibration absorption peaks in the range of 1100–1500 cm−1 point to typical borate structures, in which boron atoms are coordinated with multiple oxyalkyl groups. Interestingly, the vibration absorption peak of B–O bond is wider than that of standard absorption peak, which might be caused by the different coordination environment of B and O in solid products. In order to further study the coordination environment of B atoms, 11B-NMR spectroscopy was employed. As can be seen from Fig. 3(d), 6 peaks could be observed on 11B-NMR spectrum, indicating that there are 6 chemical environments of B atoms.In previous studies, Yoshinobu Miyazaki et al. investigated the products from the reaction between erythritol, boric acid (H3BO3) and sodium tetrahydroxyborate (NaB(OH)4).24 The specific structures corresponding to different chemical shifts in NMR boron spectra were determined by 11B-NMR and DFT calculations, shown in Fig. 3(e). The coordination environment of B atoms represented by the 6 peaks in Fig. 3(d) could be identified using the knowledge from the previous literature report. Apparently, peak-2, 3 are much higher than the others, indicating that structure-2, 3 (δ = 5.6–5.9) have a high proportion in the products. In the corresponding structure, a B atom binds to one or two erythritol groups to form a five-membered ring, while the remaining coordination oxygen may come from additional erythritol or hydroxyl groups. It is suggested that water not only acts as solvent but also part of the reaction, and the hydroxyl group of H2O goes into the final products.
3.3 Reaction mechanism
Over the past decades, the hydrolysis mechanism of NaBH4 has been widely studied and a common reaction procedure has been obtained as the following steps:Step 1: BH4− + H+ ⇌ H2BH3
Step 2: BH4− + H2O ⇌ H2BH3 + OH−
Step 3: H2BH3 ⇌ H2 + BH3
Step 4: BH3 + 3H2O + OH− → B(OH)4− + 3H2
Based on the analysis of previous experimental results in this work, it is speculated that there might be another subsequent reaction step in the erythritol/water system:
Step 5: B(OH)4− + CH2OH(CHOH)2CH2OH → solid products
That is, erythritol might further react with NaB(OH)4 to produce the final products. To confirm this conjecture, the reaction of NaB(OH)4 with erythritol/water system was carried out under the same conditions and the reaction products were comprehensively characterized. As described in Fig. 4, the XRD patterns, FTIR spectra and 11B-NMR spectra of the products from the reaction of NaB(OH)4 with erythritol/water system are very similar with those between NaBH4 and erythritol/water system. It means that erythritol can indeed further react with NaB(OH)4 and inhibit the ionization of B(OH)4−, thus increasing the productivity of reaction.
3.4 Optimization of reaction system
In light of above findings, it can be found that the volume of water in the actual reaction system is much larger than the theoretical demand, thus decreasing the gravimetric density of hydrogen generation. Seeking to meet the practical applications of hydrogen energy requirements, the erythritol/water system was further optimized to a quasi-solid phase reaction system via adjusting the volume of water. We first optimized the reaction system by turning the mole ratio of the reactants to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Using this configuration, excellent NaBH4 conversion (85%) and gravimetric density of hydrogen storage (3.7 wt%) are observed.
1. Using this configuration, excellent NaBH4 conversion (85%) and gravimetric density of hydrogen storage (3.7 wt%) are observed.
Meanwhile, the temperature of reaction system as the reaction proceeded was tracked (Fig. 5). The temperature curve reveals the presence of a critical temperature point, i.e., 48 °C. Obviously, it takes only about 95 seconds to reach the critical temperature point due to the reduced volume of water. The NaBH4 conversion increases rapidly after the critical temperature point. As we adjusted the mole ratio by increasing water volume slowly from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 to 1
0.6 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3, monotonic increment in the conversion of NaBH4 and the gravimetric density of hydrogen generation were observed. As can be clearly seen from Table 1, when the mole ratio of the reactants is 1
1.3, monotonic increment in the conversion of NaBH4 and the gravimetric density of hydrogen generation were observed. As can be clearly seen from Table 1, when the mole ratio of the reactants is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3, the NaBH4 conversion was as high as 94%, and the gravimetric density of hydrogen storage was 3.9 wt%, close to the requirements for practical applications.
1.3, the NaBH4 conversion was as high as 94%, and the gravimetric density of hydrogen storage was 3.9 wt%, close to the requirements for practical applications.
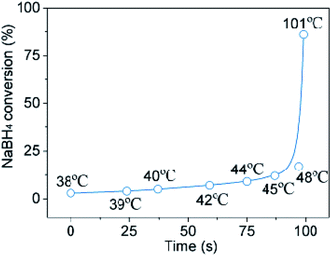 | ||
Fig. 5 The temperature effect on NaBH4 conversion during the reaction. Mole ratio of NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) erythritol erythritol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
Mole ratio of NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) erythritol erythritol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
NaBH4 Conversion (%) | Gravimetric H2 storage capacity (wt%) |
|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 |
73 | 3.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
88 | 3.8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
89 | 3.8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
94 | 3.9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 1.7 |
80 | 3.2 |
4. Conclusions
In summary, a novel strategy of constructing a polyol/water composite system to induce the alcoholysis of NaBH4 while inhibiting the ionization of reaction products was developed. The polyol/water composite systems can be fully reacted (NaBH4 conversion of more than 90%) in a relatively short time (within 60 min), especially the erythritol/water system. Erythritol participates in the reaction rather than acts as a catalyst, and the pH value of erythritol/water system is lower than that of methanol/water system. The ionization of reaction products is inhibited by erythritol, thus promoting the conversion of NaBH4. Moreover, the chemical structural analysis of reaction products and control group further prove that erythritol can indeed react with NaB(OH)4 and inhibit the ionization of B(OH)4−. On the basis of erythritol/water system, a quasi-solid phase reaction system was developed via adjusting the volume of water, which shows an excellent NaBH4 conversion (94%) and high gravimetric density of hydrogen storage (3.9 wt%). Briefly, this work provides a new perspective for the design of catalyst free alcoholysis/hydrolysis of NaBH4 and further deepens the understanding of mechanism of H2 generation from NaBH4.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the Ministry of Science and Technology of China (2018YFB1502104) and the Fundamental Research Funds for the Central Universities (2017QH02).References
- K. Müller and W. Arlt, Status and development in hydrogen transport and storage for energy applications, Energy Technology, 2013, 1, 501–511 CrossRef.
- U. B. Demirci and P. Miele, Sodium borohydride versus ammonia borane, in hydrogen storage and direct fuel cell applications, Energy Environ. Sci., 2009, 2, 627–637 RSC.
- Y. Wang, Y. S. Lu, D. Wang, S. W. Wu, Z. Q. Cao, K. Zhang, H. X. Liu and S. G. Xin, Hydrogen generation from hydrolysis of sodium borohydride using nanostructured Ni-B catalysts, Int. J. Hydrogen Energy, 2016, 41, 16077–16086 CrossRef CAS.
- H. Imamura, T. Takada and S. Tsuchiya, Hydrogen storage in metal hydrides by the use of methanol: A new way of hydriding by chemical hydrogen carriers, Int. J. Hydrogen Energy, 1988, 13, 11–13 CrossRef CAS.
- M. Zahmakiran and S. Özkar, Intrazeolite ruthenium(0) nanoclusters: a superb catalyst for the hydrogenation of benzene and the hydrolysis of sodium borohydride, Langmuir, 2008, 24, 7065–7067 CrossRef CAS.
- U. B. Demirci and F. Garin, Kinetics of Ru-promoted sulphated zirconia catalysed hydrogen generation by hydrolysis of sodium tetrahydroborate, J. Mol. Catal. A: Chem., 2008, 279, 57–62 CrossRef CAS.
- X. J. Li, G. Y. Fan and C. M. Zeng, Synthesis of ruthenium nanoparticles deposited on graphene-like transition metal carbide as an effective catalyst for the hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2014, 39, 14927–14934 CrossRef CAS.
- Y. H. Li, Q. Zhang, N. W. Zhang, L. H. Zhu, J. B. Zheng and B. H. Chen, Ru–RuO2/C as an efficient catalyst for the sodium borohydride hydrolysis to hydrogen, Int. J. Hydrogen Energy, 2013, 38, 13360–13367 CrossRef CAS.
- Y. H. Li, X. Zhang, Q. Zhang, J. B. Zheng, N. W. Zhang, B. H. Chen and K. J. Smith, Activity and kinetics of ruthenium supported catalysts for sodium borohydride hydrolysis to hydrogen, RSC Adv., 2016, 6, 29371–29377 RSC.
- Y. Liang, H. B. Dai, L. P. Ma, P. Wang and H. M. Cheng, Hydrogen generation from sodium borohydride solution using a ruthenium supported on graphite catalyst, Int. J. Hydrogen Energy, 2010, 35, 3023–3028 CrossRef CAS.
- D. Bhattacharjee and S. Dasgupta, Trimetallic NiFePd nanoalloy catalysed hydrogen generation from alkaline hydrous hydrazine and sodium borohydride at room temperature, J. Mater. Chem. A, 2015, 3, 24371–24378 RSC.
- D. Kılınç, Ö. Şahin and C. Saka, Investigation on salisylaldimine-Ni complex catalyst as an alternative to increasing the performance of catalytic hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2017, 42, 20625–20637 CrossRef.
- C. C. Su, M. C. Lu, S. L. Wang and Y. H. Huang, Ruthenium immobilized on Al2O3 pellets as a catalyst for hydrogen generation from hydrolysis and methanolysis of sodium borohydride, RSC Adv., 2012, 2, 2073–2079 RSC.
- H. A. Bandal, A. R. Jadhav and H. Kim, Cobalt impregnated magnetite-multiwalled carbon nanotube nanocomposite as magnetically separable effificient catalyst for hydrogen generation by NaBH4 hydrolysis, J. Alloys Compd., 2017, 699, 1057–1067 CrossRef CAS.
- Y. S. Wei, M. S. Wang, W. Y. Fu, L. Wei, X. S. Zhao, X. Y. Zhou, M. Ni and H. J. Wang, Highly active and durable catalyst for hydrogen generation by the NaBH4 hydrolysis reaction: CoWB/NF nanodendrite with an acicular array structure, J. Alloys Compd., 2020, 836, 155429 CrossRef CAS.
- D. W. Zhuang, H. B. Dai, Y. J. Zhong, L. X. Sun and P. Wang, A new reactivation method towards deactivation of honeycomb ceramic monolith supported cobalt–molybdenum–boron catalyst in hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2015, 40, 9373–9381 CrossRef CAS.
- H. B. Dai, Y. Liang and P. Wang, Effect of trapped hydrogen on the induction period of cobalt–tungsten–boron/nickel foam catalyst in catalytic hydrolysis reaction of sodium borohydride, Catal. Today, 2011, 170, 27–32 CrossRef CAS.
- Y. Liang, P. Wang and H. B. Dai, Hydrogen bubbles dynamic template preparation of a porous Fe–Co–B/Ni foam catalyst for hydrogen generation from hydrolysis of alkaline sodium borohydride solution, J. Alloys Compd., 2010, 491, 359–365 CrossRef CAS.
- H. C. Brown and C. A. Brown, New, Highly active metal catalysts for the hydrolysis of borohydride, J. Am. Chem. Soc., 1962, 84, 1493–1494 CrossRef CAS.
- A. Balbay and C. Saka, The effect of the concentration of hydrochloric acid and acetic acid aqueous solution for fast hydrogen production from methanol solution of NaBH4, Int. J. Hydrogen Energy, 2018, 43, 14265–14272 CrossRef CAS.
- V. G. Minkina, S. I. Shabunya, V. I. Kalinin and A. Smirnova, Hydrogen generation from sodium borohydride solutions for stationary applications, Int. J. Hydrogen Energy, 2016, 41, 9227–9233 CrossRef CAS.
- C. F. Lo, K. Karan and B. R. Davis, Kinetic studies of reaction between sodium borohydride and methanol, water, and their mixtures, Ind. Eng. Chem. Res., 2007, 46, 478–5484 CrossRef.
- F. H. Wang, Y. N. Wang, Y. J. Zhang, Y. M. Luo and H. Zhu, Highly dispersed RuCo bimetallic nanoparticles supported on carbon black: enhanced catalytic activity for hydrogen generation from NaBH4 methanolysis, J. Mater. Sci., 2018, 53, 6831–6841 CrossRef CAS.
- Y. Miyazaki, T. Fujimori, H. Okita, T. Hirano and K. Yoshimura, Thermodynamics of complexation reactions of borate and phenylboronate with diol, triol and tetritol, Dalton Trans., 2013, 42, 10473–10486 RSC.
| This journal is © The Royal Society of Chemistry 2021 |



