Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Simultaneous determination of 114 pesticides in complex Chinese herbal medicine Fritillaria using ordered mesoporous carbon CMK-3 as a reversed-dispersive solid phase extraction sorbent†
Tong Wua,
Peipei Qi‡
bcd,
Jiao Wangbcd,
Zhiwei Wangbcd,
Shanshan Dibcd,
Hao Xubcd,
Huiyu Zhaobcd,
Changshan Zhao*a and
Xinquan Wang *bcd
*bcd
aCollege of Agriculture, Northeast Agricultural University, No. 600 Changjiang Road, Harbin, 150030, P. R. China. E-mail: csz-hlj@sohu.com; Tel: +86 451 55191775
bState Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products, Institute of Quality and Standard of Agro-products, Zhejiang Academy of Agricultural Sciences, Hangzhou 310021, P. R. China. E-mail: wangxinquan212@163.com; Tel: +86 571 86419051
cAgricultural Ministry Key Laboratory for Pesticide Residue Detection, Hangzhou 310021, P. R. China
dKey Laboratory of Detection for Pesticide Residue and Control of Zhejiang, Hangzhou 310021, P. R. China
First published on 20th January 2021
Abstract
Fritillaria, a traditional Chinese herbal medicine, is classified into many medicinal species and contains numerous complex components. It is thus difficult to simultaneously detect multiple pesticide residues in different Fritillaria species. An easy, reliable, and widely applicable analytical method based on a modified Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) method coupled with LC-MS/MS was developed to solve these problems encountered during pesticide residue analysis in complex Fritillaria matrices. Ordered mesoporous carbon CMK-3 and a primary secondary amine (PSA) were used as efficient purification sorbents by optimization of the QuEChERS process. Systematic method validation was performed for four species of Fritillaria. The matrix effect of pesticides varied among different Fritillaria species, and matrix-matched standard solutions were thus employed for quantitative analysis. The mean recoveries of all pesticides ranged from 88.6% to 95.5%, with mean relative standard deviations (RSD) lower than 6% at spiked concentrations of 30, 120, and 240 μg kg−1. The limits of quantification (LOQ) for the developed method were in the range of 30–120 μg kg−1. This method was further used to analyze 47 Fritillaria samples from Zhejiang province, China, and seven pesticides were detected in 22 Fritillaria samples. These results demonstrate that the developed method is suitable for an accurate analysis of multiple pesticide residues in various Fritillaria.
1. Introduction
Fritillaria, an important traditional Chinese herbal medicine with antitussive and expectorant properties, is widely used as an ingredient in patented medicines (herbal products) and health foods. Currently, there are more than 200 different Chinese patented medicines containing Fritillaria for the treatment of cough and phlegm.1 The expanding demand for herbal products has led to an increase in the cultivation area and export volume of Fritillaria.2 To improve the yield and quality of Fritillaria, pesticides are inevitably used to prevent pests (e.g., grub, cutworm), diseases (e.g., gray mold, black spot), and weeds during cultivation. However, only eight pesticides are currently registered for use on Fritillaria in China. These eight pesticides are not enough to prevent all of the diseases and pests on Fritillaria. Therefore, some pesticides that are not registered for Fritillaria cultivation are used,3 the scientific dosage standards of which are not set for use on Fritillaria. The abuse of pesticides often occurs when unregistered pesticides are used by growers, and the abuse of these pesticides in China has led to large amounts of multiple pesticide residues in Fritillaria, which have been confirmed by previous reports.3,4Considering that pesticide residues in herbal medicines may pose potential health risks to consumers, some countries and organizations have established maximum residue limit (MRL) regulations for these medicinal plants. While China has issued MRLs of 21 pesticides in several herbs such as ginseng,5 the European Union (EU) has set more than 400 MRLs for herbal infusions.6 However, there are no detailed regulations regarding the MRLs for Fritillaria, and the risk of pesticide residues in Fritillaria can only be assessed on the basis of existing MRLs for similar herbs.7 There are differences in the MRL standards established by different countries or organizations, which poses an obstacle to the assessment of pesticide residue risk in Fritillaria and the export of its products. Therefore, it is necessary to develop an efficient and reliable method for the simultaneous detection of multiple pesticide residues in Fritillaria, and monitor and assess the associated risks.
Fritillaria contains various active components, such as steroidal alkaloids, fatty acids, terpenoids, saponins, amino acids, polysaccharides,8,9 which can interfere with the quantification of target analytes. According to the records of the Chinese Pharmacopeia, there are many Fritillaria species with pharmacological effects, and the types and contents of the active components vary with the species. For example, Fritillaria cirrhosa D.Don (FC) and F. ussuriensis Maxim. (FU) contain imperialine (an isosteroidal alkaloids), but F. thunbergii Miq. (FT) does not contain imperialine. Meanwhile, FC contains more imperialine than FU.10 Thus, it is a great challenge to determine the residue levels of multiple pesticides simultaneously in the different Fritillaria species. Previous studies have focused only on several kinds of pesticides and the individual specie of Fritillaria,11,12 as such, there is no comprehensive method to determine multiple pesticides in different species of Fritillaria. The development of a high-throughput detection method for various Fritillaria is essential for monitoring the quality of these Fritillaria and protecting consumer safety.
Sample preparation is essential to ensure accurate quantification of target analytes in pesticide residue analysis. In previous reports, accelerated solvent extraction,13 solid phase extraction,14,15 and Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) method4,16,17 have been used for the preparation of Fritillaria samples. Compared to the other methods, the QuEChERS method has advantages such as simplified operation steps and reduced solvent consumption. Moreover, the QuEChERS method has demonstrated wide application during multiple pesticide residues analysis in complex matrices.18,19 Therefore, the aim of the present study is to establish a rapid, widely applicable detection method based on QuEChERS for multiple pesticides, and solve the practical problem of pesticide residue determination in different Fritillaria species.
The typical QuEChERS method includes acetonitrile extraction and purification by dispersive solid phase extraction (dSPE).20 The dSPE purification procedure removes interferences from the matrix by cleanup sorbents, and the retained target analytes can be accurately quantified.21,22 Thus, the application of a suitable sorbent plays a key role in effectively removing the matrix interferences during dSPE. Ordered mesoporous carbon is a new type of carbonaceous material with a high surface area, large pore volume, and ordered structure. Ordered mesoporous carbon and its functionalized materials have been successfully used as sorbents for the removal of phenol,23 antibiotics,24 proteins,25 dyes26 and heavy metals,27 and for the adsorption and enrichment of some pesticides from water samples.28,29 Moreover, the excellent adsorption capacity of ordered mesoporous carbons for alkaloids has also been reported.30 Based on these studies, we were inspired to consider that ordered mesoporous carbon could be used as a sorbent to remove interferences, such as steroidal alkaloids, from the Fritillaria matrix. Hence, ordered mesoporous carbon CMK-3 was selected as the purification sorbent, because it is commercially available and has a high adsorption rate and adsorption capacity.23,31
The overall aim of this study was to develop an easy, rapid, and reliable QuEChERS method using CMK-3 as a sorbent for simultaneous determination of multiple pesticide residues in different Fritillaria species by LC-MS/MS. A total of 114 common pesticides that may be used in Fritillaria cultivation were selected as target analytes, and the recovery and matrix effect of all pesticides were used as a guide to select the optimal extraction and purification conditions. The optimized method was used to detect pesticide residue levels in 47 Fritillaria samples from Zhejiang province, China.
2. Experimental
2.1. Materials and chemicals
The four medicinal species of Fritillaria from various areas of China were used in the experiment, including FT from Zhejiang province, FU from Liaoning province, FC from Sichuan province, Fritillaria delavayi Franch. (FD) from Tibet autonomous region. These samples were analyzed and proved to be uncontaminated. The FT samples were used during the method optimization. CMK-3 was obtained from Nanjing XFNANO Materials Tech Co. Ltd. (Nanjing, China), and the primary secondary amine (PSA) was obtained from Agilent (DE, USA). Anhydrous magnesium sulfate (MgSO4) and sodium chloride (NaCl) were obtained from Bonna-Agela Technologies Co. Ltd. (Tianjin, China).A total of 114 pesticide standards were purchased from the Ministry of Agriculture (Tianjin, China), Agro-Environmental Protection Institute, and Shanghai Pesticide Research Institute (Shanghai, China). A mixed standard solution of 114 pesticides at a concentration of 5.0 mg L−1 was prepared in methanol and stored at 4 °C. HPLC-grade acetonitrile (ACN) and methanol were supplied by Merck (New Jersey, USA). HPLC-grade ammonium formate was provided by Tedia (Fairfield, USA). Purified water was prepared by a Millipore Milli-Q system. Acetic acid (HAC) was an analytical grade reagent.
2.2. Sample preparation
The Fritillaria samples were ground and stored at room temperature. Fritillaria powder (2 g) was weighed into a 50 mL centrifuge tube, and 5 mL water was added. After mixing for 1 min, 6 mL acetonitrile was added and the mixture was vortexed for 1 min. Next, NaCl (1.5 g) and anhydrous MgSO4 (6 g) were added and after vortexing for 1 min, the mixtures were centrifuged at 7000 rpm for 5 min.After centrifugation, 1 mL of the supernatant acetonitrile phase were collected and transferred into a 2 mL centrifuge tube containing 5 mg of CMK-3, 30 mg of PSA and 150 mg of anhydrous MgSO4. The mixtures were vortexed for 1 min and then centrifuged at 7000 rpm for 5 min. Next, 0.5 mL of the supernatants were mixed with 0.5 mL of water, and filtered through 0.22 μm filters for analysis by LC-MS/MS.
In addition, an appropriate volume of the mixed standard solution was evenly dropped into Fritillaria powder (2 g) for method optimization and recovery experiments. After standing for 1 h, the spiked samples were extracted and purified according to the above procedures.
2.3. LC-MS/MS analysis
The LC-MS/MS system consisted of Nexera X2 LC-30AD (Shimadzu, Kyoto, Japan) and a Shimadzu 8050 triple-quadrupole mass spectrometer (Shimadzu, Kyoto, Japan). A chromatographic column ACE Excel 2 C18 (100 mm × 2.1 mm, 2 μm) was used for the separation of the target pesticides and maintained at 35 °C. The mobile phases were 5 mmol L−1 ammonium formate aqueous solution (A) and pure methanol (B) at a flow rate of 0.3 mL min−1. The gradient elution program was presented as follows: 0–1.00 min, 40% B; 1.00–3.00 min, 40–80% B; 3.00–5.00 min, 80–95% B; 5.00–8.00 min, 95% B; 8.00–8.01 min, 95–5% B; 8.01–10.00 min, 5% B. The total run time was 10 min and the injection volume was 2 μL.The MS/MS detection was performed using electrospray ionization and multiple reaction monitoring mode. The operation parameters were as follows: capillary voltage, 4000 V and −3000 V for the positive and negative ion modes, respectively; capillary temperature, 300 °C; flow rate of the nebulizing gas (nitrogen, 99.9% purity), 3 L min−1; flow rate of the drying gas (nitrogen, 99.9% purity), 10 L min−1; collision gas, argon. Table S1† lists the precursor ions, product ions, collision energies, and deviations for all the target pesticides.
2.4. Validation study
The developed method was validated for four Fritillaria species, namely, FT, FU, FC, FD. The linearity, matrix effect, recovery, precision, limit of detection (LOD) and limit of quantification (LOQ) of the developed method were evaluated.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and solvent standard solutions were prepared in ACN/water (1
1, v/v), and solvent standard solutions were prepared in ACN/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The linear regression equations and correlation coefficients (R) of the calibration curves were calculated by plotting the pesticide peak area versus concentration. The R was higher than 0.99, indicating good linearity of pesticides.
1, v/v). The linear regression equations and correlation coefficients (R) of the calibration curves were calculated by plotting the pesticide peak area versus concentration. The R was higher than 0.99, indicating good linearity of pesticides.
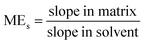 | (1) |
For the investigation of the adsorbent purification effect in Section 3.2, the peak area ratio of pesticides in matrix-matched standard solution versus those in solvent standard solution at the same concentration was used to evaluate the matrix effect (MEp),33 because it was conducive to more visually display the improvement degree of the matrix effect after using the purification adsorbents, and the calculation equation is as follows:
 | (2) |
The values of MEs and MEp closer to 1 represent the weaker matrix effect. The values of MEs and MEp less than 1 or greater than 1 indicate matrix suppression or matrix enhancement, respectively.
3. Results and discussion
3.1. Optimization of the extraction solvent
The selection of extraction solvent is the first step to be overcome in multi-residue analysis. ACN was widely used as the extraction solvent because of its satisfactory extraction effect for most pesticides.36 It has been reported that adding acid to the extraction solvent has a positive influence on the extraction efficiency and stability of base-sensitive pesticides in multi-residue analysis.37,38 Thus, the extraction effects of ACN, 0.5% HAC in ACN, and 1.0% HAC in ACN were evaluated.All 114 pesticides were spiked to the FT samples at the concentration of 120 μg kg−1, and extracted with 6 mL of different extraction solutions (ACN, 0.5% HAC in ACN, or 1.0% HAC in ACN). The extracted supernatants were directly injected into LC-MS/MS for analysis without a purification step. The recoveries for each pesticide were calculated, and the overall recovery distributions of the pesticides were evaluated. As shown in Fig. 1, it was observed that there were no significant differences in the recoveries using different extraction solvents, with over 98.2% of the pesticide recoveries in the range of 70–120%. This indicated that all the extraction solvents achieved satisfactory extraction effects. However, further observation of the pesticide recovery distributions revealed some differences. When ACN was used as the extraction solvent, all pesticide recoveries were larger than 70%, and 71.1% of the pesticide recoveries were in the range of 90–110%. However, only 53.5% and 61.4% of the pesticide recoveries were between 90–110% using 0.5% HAC in ACN and 1.0% HAC in ACN, respectively. Taking both convenient extraction process and extraction efficiency into consideration, ACN was chosen as the optimum extraction solvent for further experiments.
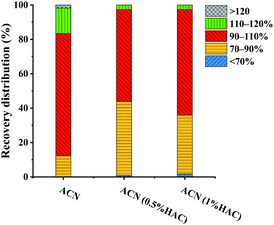 | ||
| Fig. 1 Influence of extraction solvent (ACN, ACN containing 0.5% HAC or ACN containing 1.0%) on the recovery distribution of the pesticides (extracted from the FT sample spiked at 120 μg kg−1). | ||
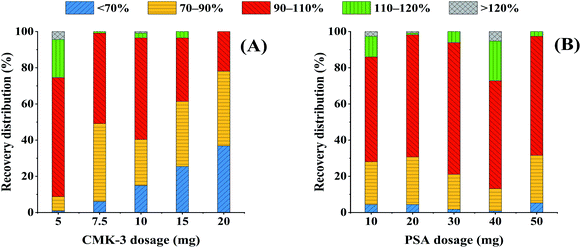
3.2. Optimization of the sorbent amount
Fritillaria mainly contains steroidal alkaloids, fatty acids, and other active components, yet these active components are the main interference factors in multi-pesticide residue analysis, which makes the sample matrix complicated. Therefore, the focus of this experiment was to select efficient purification sorbents and remove the interferences in the sample matrix based on the QuEChERS method. CMK-3 was selected as a sorbent to remove alkaloids, and PSA was selected to remove polar substances such as fatty acids in the sample matrix.39,40 The amounts of CMK-3 and PSA were optimized to obtain the most efficient purification effect and pesticide recoveries. In the following optimization experiments, 6 mL of ACN was used to extract the analytes in the spiked FT samples at a concentration of 120 μg kg−1, and 1 mL of the obtained extraction solutions was drawn for purification analysis.In summary, the modified QuEChERS method for the analysis of multi-pesticide residues in Fritillaria samples utilized 6 mL of ACN as the extraction solvent, and 5 mg of CMK-3 and 30 mg of PSA as the purification sorbents.
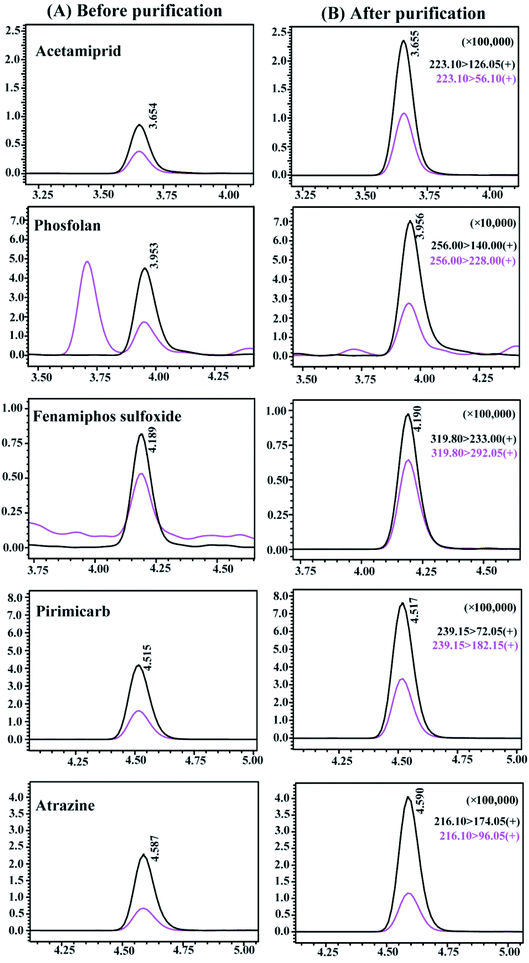
The MEP values of all pesticides with different purification methods are summarized in Fig. 3. The MEP values of all pesticides were classified into four groups according to the interference degree of the matrix compounds on the target analytes, where MEP values of 0–0.2 indicated strong signal suppression, 0.2–0.5 indicated relatively lower signal suppression than those in the range of 0–0.2, 0.5–0.8 indicated medium signal suppression, and 0.8–1.2 indicated low signal suppression or enhancement. Comparing with the unpurified treatment, the percentage of pesticides with MEP values in the range of 0–0.5 decreased from 61.4% to 33.3%, and the percentage of pesticides with MEP of 0.5–1.2 increased from 38.6% to 66.7% by using CMK-3. In particular, the percentages of pesticides within the range of 0–0.2 and 0.8–1.2 were significantly changed, with decrease of 17.5% of pesticides and increase of 19.3% of pesticides, respectively. It is confirmed that even 5 mg of CMK-3 made a significant contribution to improving matrix effects. PSA was additionally coupled with CMK-3 as purification sorbents to reduce the percentages of pesticides with MEP values in the range of 0–0.8, and again increased by 9.65% of pesticides in the range of 0.8–1.2. Compared to the unpurified treatment, the optimal purification treatment (CMK-3 + PSA) gave rise to an increase of the percentage of pesticides with MEP of 0.8–1.2 from 7.02% to 36.0%. It can be concluded that the use of CMK-3 and PSA played a good cleanup role in the purification process of Fritillaria matrix. In addition, the chromatographic peaks of pesticides under different treatments were compared and the chromatograms of some representative pesticides are presented in Fig. 4. It can be clearly observed that the responses of some pesticides were enhanced and the impurity peaks of the sample matrix were reduced after purification. The above results indicated that the combination of CMK-3 and PSA effectively reduced the interference of the Fritillaria matrix components on the target analytes such that the quantitative results of the target analytes were closer to the actual levels in a pure solvent. Moreover, the purified sample matrix could reduce the contamination of the instrument and protect the instrument. Thus, it was acceptable to effectively improve the matrix effect of samples and obtain less quantitative error at the cost of a slight decrease in the recoveries of a few pesticides. Furthermore, the matrix effects of the purified extracts obtained by the peak area ratio of the standard solution in this experiment were similar to those of the FT sample obtained by the slope ratio of the method validation calibration curve.
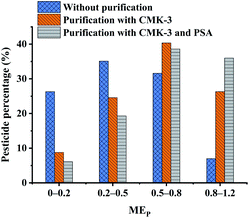 | ||
| Fig. 3 Comparison of the matrix effects between the unpurified, CMK-3 purified and CMK-3 (5 mg) + PSA (30 mg) purified matrix-matched solutions at 20 μg L−1. | ||
3.3. Method validation
The applicability of the developed method was validated in all Fritillaria samples (FT, FU, FC and FD) by evaluating its linearity, matrix effect, recovery, precision, LOD and LOQ.| Samples | Mean recoveries (%) ± mean RSD (%) of pesticides at 30, 120 and 240 μg kg−1 in each Fritillaria sample | The percentages of pesticides with recoveries between 70–120% at 30, 120 and 240 μg kg−1 in each Fritillaria sample | |||||
|---|---|---|---|---|---|---|---|
| 30 μg kg−1 | 120 μg kg−1 | 240 μg kg−1 | Mean ± RSD | 30 μg kg−1 (%) | 120 μg kg−1 (%) | 240 μg kg−1 (%) | |
| FT | 94.1 ± 6 | 94.2 ± 4 | 89.4 ± 4 | 92.6 ± 4 | 98.2 | 100 | 98.2 |
| FU | 88.6 ± 5 | 95.5 ± 3 | 93.2 ± 4 | 92.5 ± 4 | 84.2 | 89.5 | 89.5 |
| FC | 89.3 ± 5 | 90.9 ± 4 | 93.4 ± 4 | 91.2 ± 4 | 86.0 | 91.2 | 93.0 |
| FD | 94.8 ± 6 | 89.0 ± 3 | 88.8 ± 4 | 90.9 ± 4 | 92.1 | 90.4 | 91.2 |
| Minimum | 88.6 ± 5 | 89.0 ± 3 | 88.8 ± 4 | ||||
| Maximum | 94.8 ± 6 | 95.5 ± 3 | 93.4 ± 4 | ||||
Overall, these validation results indicate that the developed method for multi-residue analysis of pesticides has satisfactory linearity, matrix effect, recovery, precision and sensitivity, and is suitable for the detection of pesticide residues in various Fritillaria species.
3.4. Application to real samples
Forty-seven F. thunbergii samples from Zhejiang province, China, were analyzed using the optimized method. The analysis revealed that seven pesticides were present in twenty-two samples in contaminated concentrations of 0.030–0.203 mg kg−1. Among all the detected samples, seven samples contained two pesticides, and one sample contained four pesticides. The sample analysis results were evaluated based on the MRL of other herbal medicines, such as ginseng, set by China and the EU, owing to the lack of MRLs of Fritillaria. Details of the detected pesticides and the corresponding MRLs are presented in Table 2. It was found that the detected frequency of carbendazim was 38.3%, which was the highest among all the detected pesticides, followed by fluopyram (10.6%), difenoconazole (6.38%), thiophanate-methyl (4.26%), dimethomorph (4.26%), tebuconazole (2.13%), and propiconazole (2.13%). Referring to the MRL regulations of other herbs issued by China and the EU, it was observed that the residue levels of detected carbendazim in two samples exceeded the EU MRLs (Fig. S1†), but none of the detected pesticides exceeded the Chinese MRLs. Multiple pesticides were detected simultaneously in Fritillaria samples, which revealed the severity of pesticide abuse in the cultivation of Fritillaria, confirming the necessity and urgency for monitoring the multi-pesticide residues in the quality and safety supervision of Fritillaria products.| Detected pesticides | CN MRLa (mg kg−1) | EU MRL (mg kg−1) | Frequency and concentration range of detected pesticides | No. | |
|---|---|---|---|---|---|
| Frequency (%) | Range (mg kg−1) | ||||
| a The “CN MRL” represents the “Chinese MRL”.b The “—” represents the MRL for herbs is not set by China. | |||||
| Carbendazim | 1.00 | 0.10 | 38.3 | 0.030–0.203 | 2 |
| Thiophanate-methyl | —b | 0.10 | 4.26 | 0.034, 0.094 | |
| Dimethomorph | — | 0.05 | 4.26 | 0.034, 0.042 | |
| Fluopyram | — | 2.50 | 10.6 | 0.036–0.084 | |
| Tebuconazole | 3.00 | 0.15 | 2.13 | 0.048 | |
| Propiconazole | 0.10 | 0.05 | 2.13 | 0.037 | |
| Difenoconazole | 0.50 | 20.00 | 6.38 | 0.034–0.057 | |
4. Conclusions
In this study, a modified QuEChERS coupled with LC-MS/MS method was developed for the simultaneous determination of 114 pesticides in Fritillaria. In view of the complexity of Fritillaria matrix and abundant medicinal species, the process of the QuEChERS method was systematically optimized, and the combination of CMK-3 and PSA was selected to effectively reduce the matrix effect. To the best of our knowledge, the present work is the first to apply CMK-3 as cleanup sorbent in pesticide residue analysis to remove matrix interference in Fritillaria. The established method was fully validated in four Fritillaria samples, and good linearity, weak matrix effect, satisfactory recovery, good precision and high sensitivity were obtained, demonstrating its applicability for the analysis of various Fritillaria species. Moreover, the method was successfully applied to 47 real Fritillaria samples from Zhejiang province, China, of which 22 samples were found to have residues of one or more pesticides. Among all of the seven detected pesticides, carbendazim exceeded EU MRLs in two samples but did not exceed the Chinese MRLs. These results demonstrated that this method is suitable for the analysis of multiple pesticide residues in Fritillaria and can be used as a helpful tool in routine monitoring of the quality of medicinal Fritillaria.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported the National Key Research and Development Program of China [Grant No. 2017YFC1601600]; the Key Research and Development Program of Zhejiang Province [Grant No. 2020C02023]; the fund of State Key Laboratory for Quality and Safety of Agro-products [Grant No. 2010DS700124-KF1905; 2010DS700124-ZZ2009]; and Special Fund for Public Projects of Zhejiang Province [Grant No. LGN19B070001].References
- S. Li, J. Liu, X. Gong, X. Yang, Y. Zhu and Z. Cheng, Biochem. Syst. Ecol., 2013, 46, 130–136 CrossRef CAS.
- A. B. Cunningham, J. A. Brinckmann, S.-J. Pei, P. Luo, U. Schippmann, X. Long and Y.-F. Bi, J. Ethnopharmacol., 2018, 223, 142–151 CrossRef CAS.
- L. Luo, L. Dong, Q. Huang, S. Ma, P. Fantke, J. Li, J. Jiang, M. Fitzgerald, J. Yang, Z. Jia, J. Zhang, H. Wang, Y. Dai, G. Zhu, Z. Xing, Y. Liang, M. Li, G. Wei, J. Song, J. Wei, C. Peng, H. Zhang, W. Zhang, S. Wang, K. Mizuno, A. A. G. Marco, L. Wu, J. Xu, C. Xiong and S. Chen, Chemosphere, 2021, 262, 127477 CrossRef CAS.
- R. Dai, X. Ren, X. He and Y. Huo, Bull. Environ. Contam. Toxicol., 2011, 86, 559–564 CrossRef CAS.
- National Standard of the People's Republic of China, National food safety standard—Maximum residue limits for pesticides in food, GB2763-2019.
- European Commission, EU Pesticides Database, https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/mrls/?event=search.pr, accessed 5 December, 2020 Search PubMed.
- X. Duan, L. Tong, D. Li, Z. Yu and Y. Zhao, Chromatographia, 2018, 81, 545–556 CrossRef CAS.
- D. Hao, X. Gu, P. Xiao and Y. Peng, Chin. J. Nat. Med., 2013, 11, 330–344 CrossRef CAS.
- W. Shan and J. Wu, China Mod. Med., 2016, 23, 135–136+139 Search PubMed.
- X. Wu, S. W. Chan, J. Ma, P. Li, P. C. Shaw and G. Lin, J. Ethnopharmacol., 2018, 210, 39–46 CrossRef CAS.
- L. Ma, W. Li and W. Zhao, Chin. Tradit. Herb. Drugs, 2014, 45, 849–853 CAS.
- J. Wang, L. Fan and J. Wu, Chin. J. Pestic. Sci., 2016, 18, 664–668 Search PubMed.
- H. Lv, L. Shou, L. Hang and X. Hang, Chin. J. Pharm. Anal., 2010, 30, 491–494 Search PubMed.
- X. Li, C. Fang, L. Ma and M. Zhu, Chin. J. Pharm. Anal., 2012, 32, 419–423 CAS.
- L. Wang, Z. Wang, W. Gao, J. Chen, M. Yang, Y. Kuang, L. Huang and S. Chen, Food Chem., 2013, 138, 1048–1054 CrossRef CAS.
- L. Chen, F. Song, Z. Liu, Z. Zheng, J. Xing and S. Liu, Anal. Bioanal. Chem., 2014, 406, 1481–1491 CrossRef CAS.
- J. Yu, T. Cang, F. Dai and X. Zhao, Chin. J. Pestic. Sci., 2018, 20, 370–376 Search PubMed.
- B. Reichert, A. de Kok, I. R. Pizzutti, J. Scholten, C. D. Cardoso and M. Spanjer, Anal. Chim. Acta, 2018, 1004, 40–50 CrossRef CAS.
- N. S. Sulaiman, K. Rovina and V. M. Joseph, J. Consum. Prot. Food Saf., 2019, 14, 209–221 CrossRef.
- M. Anastassiades, S. J. Lehotay, D. Stajnbaher and F. J. Schenck, J. AOAC Int., 2003, 86, 412–431 CrossRef CAS.
- W. Yao, Z. Zhang, S. Song, X. Hao, Y. Xu and L. Han, Food Anal. Meth., 2018, 12, 176–189 CrossRef.
- A. Lawal, R. C. S. Wong, G. H. Tan, L. B. Abdulra'uf and A. M. A. Alsharif, J. Chromatogr. Sci., 2018, 56, 656–669 CAS.
- Z. Guo and Y. Yuan, Chem. J. Chin. Univ., 2007, 28, 289–292 CAS.
- L. Ji, F. Liu, Z. Xu, S. Zheng and D. Zhu, Environ. Sci. Technol., 2010, 44, 3116–3122 CrossRef CAS.
- A. Vinu, K. Z. Hossian, P. Srinivasu, M. Miyahara, S. Anandan, N. Gokulakrishnan, T. Mori, K. Ariga and V. V. Balasubramanian, J. Mater. Chem., 2007, 17, 1819–1825 RSC.
- G. Hao, W. Li, S. Wang, S. Zhang and A. Lu, Carbon, 2010, 48, 3330–3339 CrossRef CAS.
- Y. Zhou, F. Zhang, L. Tang, J. Zhang, G. Zeng, L. Luo, Y. Liu, P. Wang, B. Peng and X. Liu, Sci. Rep., 2017, 7, 43831 CrossRef.
- V. O. Njoku, M. Azharul Islam, M. Asif and B. H. Hameed, J. Anal. Appl. Pyrolysis, 2014, 110, 172–180 CrossRef CAS.
- Q. Wu, Y. Zhao, C. Wang, M. Sun, X. Ma and Z. Wang, Anal. Methods, 2015, 7, 901–908 RSC.
- Y. Li, B. Yuan, J. Fu, S. Deng and X. Lu, J. Colloid Interface Sci., 2013, 408, 181–190 CrossRef CAS.
- R. Wu, Q. Ye, K. Wu, S. Cheng, T. Kang and H. Dai, J. Environ. Sci., 2020, 87, 289–298 CrossRef.
- B. Zhu, X. Xu, J. Luo, S. Jin, W. Chen, Z. Liu and C. Tian, Food Chem., 2019, 276, 202–208 CrossRef CAS.
- S. Wang, P. Qi, S. Di, J. Wang, S. Wu, X. Wang, Z. Wang, Q. Wang, X. Wang, C. Zhao and Q. Li, Anal. Chim. Acta, 2019, 1074, 108–116 CrossRef CAS.
- European Commission, Analytical quality control and method validation procedures for pesticide residues and analysis in food and feed, SANTE/12682/2019, 2019 Search PubMed.
- A. Shrivastava and V. B. Gupta, Chron. Young Sci., 2011, 2, 21–25 CrossRef.
- A. Goon, Z. Khan, D. Oulkar, R. Shinde, S. Gaikwad and K. Banerjee, J. Chromatogr. A, 2018, 1532, 105–111 CrossRef CAS.
- I. Hrynko, B. Łozowicka and P. Kaczyński, Food Anal. Meth., 2018, 11, 2307–2319 CrossRef.
- Y. Ni, H. Yang, H. Zhang, Q. He, S. Huang, M. Qin, S. Chai, H. Gao and Y. Ma, J. Chromatogr. A, 2018, 1537, 27–34 CrossRef CAS.
- E. Rutkowska, B. Łozowicka and P. Kaczyński, Food Anal. Meth., 2018, 11, 709–724 CrossRef.
- M. Saint-Hilaire, C. Inthavong, T. Bertin, G. Lavison-Bompard, T. Guérin, A. Fournier, C. Feidt, G. Rychen and J. Parinet, Food Chem., 2018, 252, 147–153 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra07229j |
| ‡ Peipei Qi is the co-first author owing to the equal contribution. |
| This journal is © The Royal Society of Chemistry 2021 |