Open Access Article
Open Access ArticleVinyl sulfonyl chemistry-driven unidirectional transport of a macrocycle through a [2]rotaxane†
Arthur H. G.
David
 ,
Pablo
García–Cerezo
,
Pablo
García–Cerezo
 ,
Araceli G.
Campaña
,
Araceli G.
Campaña
 ,
Francisco
Santoyo–González
,
Francisco
Santoyo–González
 * and
Victor
Blanco
* and
Victor
Blanco
 *
*
Departamento de Química Orgánica, Facultad de Ciencias, Unidad de Excelencia de Química Aplicada a Biomedicina y Medioambiente (UEQ), Universidad de Granada (UGR), Avda. Fuente Nueva S/N, 18071 Granada, Spain. E-mail: fsantoyo@ugr.es; victorblancos@ugr.es
First published on 26th November 2021
Abstract
By applying a combination of the coupling-and-decoupling (CAD) chemistry of the vinyl sulfonate group with the click thia-Michael addition to the vinyl sulfone group (MAVS) we performed the irreversible unidirectional transportation of the ring through the linear component in a [2]rotaxane by a chemically and pH-driven flashing energy ratchet mechanism. The design is based on a monostoppered thread precursor bearing a sulfonate stopper, a vinyl sulfone group on the unstoppered end and a dibenzylammonium unit as recognition site for the dibenzo-24-crown-8 macrocycle. First, the ring enters from the vinyl sulfone side and the rotaxane is capped through a thia-Michael addition reaction. Then, the cleavage of the sulfonate group of the opposite stopper using MgBr2 as chemical stimulus and subsequent addition of base (Et3N) promoted the controlled and directional release of the macrocycle into the bulk under mild conditions. The efficiency of the system allowed the in situ operation as demonstrated by NMR and HRMS techniques.
Introduction
The main reason for the importance and relevance of mechanically interlocked molecules (MIMs),1 such as rotaxanes2 and catenanes,3 is probably their use to design, prepare and operate artificial molecular machines in an attempt to mimic nature or to perform a certain task.4 Typically, molecular machines built on the basis of MIMs rely on molecular switches in which the position of the macrocycle can be controlled through the use of external stimuli. For rotaxanes, the translational motion of the macrocycle along its axle induced by these stimuli gave rise to the preparation of nanovalves,5 the production of macroscopic work,6 the modulation of photophysical properties,7 and to applications in switchable catalysis8 or molecular electronics.9 Moreover, the rotaxane architecture also enabled the generation of synthesizers,10 elevators11 or muscles.12The development of directional transport systems using interlocked structures is one of the most significant progress in the field of molecular machines since it allows an operation away from the equilibrium, a crucial element for the development of molecular motors or pumps.13 Indeed, the key feature of these synthetic devices is the unidirectional movement of a component of the system respect to another. This net transport is produced following Brownian ratchet mechanisms14 and is generally controlled by photo-switches,15 redox processes,16 or chemical fuels.17
Recently, cleavable rotaxanes18 started to attract attention and arose as an interesting alternative to induce a net transport of the macrocycle along the axle. Thus, Chen and co-workers reported the unidirectional transportation of a ring in a rotaxane architecture by performing a CuAAC reaction on one side of a mono-stoppered thread in a pseudorotaxane as the capping step of the process, followed by a selective DBU-catalyzed elimination on the vicinity of a triazolium group on the other side of the rotaxane as a stopper cleavage reaction (decoupling step) that enabled the release of the macrocycle.19 Later on, they proposed another design based on the use of fluoride to release the wheel by cleavage of a tert-butyldiphenylsilyl group.20 More recently, Stoddart and co-workers have applied the same concept of stopper cleavage, in this case light-triggered, to release the macrocycle from a molecular pump.21
On the other hand, the click Michael-type addition reaction of nucleophiles to vinyl sulfonyl groups (MAVS),22 such as vinyl sulfone or vinyl sulfonate, is an efficient and versatile tool for the synthesis of rotaxanes, as we recently demonstrated.23 Furthermore, the vinyl sulfonate moiety is an appropriate group for coupling–and–decoupling chemistry (CAD),24 since after the MAVS, it can undergo substitution by nucleophiles under relatively mild conditions.25 Thus, we demonstrated the utility of this CAD chemistry both to build a rotaxane and to subsequently chemically cleave it in a controlled manner to disassemble it into its components, thus, allowing the development of a cleavable rotaxane.23
Therefore, we envisaged that the chemistry of vinyl sulfone and vinyl sulfonate groups could be an efficient tool for the development of rotaxanes that could enable the pH- and chemically-controlled unidirectional transport of the ring through the linear component. In this context, here we report an irreversible unidirectional transportation system of a macrocycle based on a [2]rotaxane architecture that relies on both the MAVS and the vinyl sulfonate CAD chemistry.
Results and discussion
Strategy and system design
The proposed operation for the unidirectional macrocycle transport is based on a flashing energy ratchet mechanism employing chemical and acid/base stimuli. For this purpose we designed a monostoppered axle precursor bearing a vinyl sulfone group on one end while on the other a sulfonate stopper was previously introduced through a thia-Michael addition to a vinyl sulfonate moiety. The system also incorporates a dibenzylammonium unit as binding site for the dibenzo-24-crown-8 (DB24C8) macrocycle (Ka = 2.7 × 104 M−1 in CDCl3).26 The operation would start with the pseudorotaxane assembly under thermodyamic control driven by the establishment of hydrogen bond interactions between the ammonium unit and the macrocycle followed by the rotaxane formation via a capping approach through a MAVS reaction to the vinyl sulfone unit. Nucleophilic displacement of the sulfonate group to cleave the corresponding sulfonate-functionalized stopper group and addition of base to deprotonate the amine and remove the hydrogen bond interactions with the macrocycle would lead to the release of the macrocycle through the opposite side of its entrance, thus, achieving the unidirectional transport of the macrocycle (Fig. 1). | ||
| Fig. 1 Proposed strategy for the unidirectional and irreversible transportation of a macrocycle through the linear element of a [2]rotaxane following a flashing energy ratchet mechanism and exploiting the reactivity of the vinyl sulfone and vinyl sulfonate groups. The binding constant between DB24C8 and the dibenzylammonium motif in CDCl3 has been taken from ref. 26. | ||
During this operation, the establishment of a pseudorotaxane would represent a lower energy situation than the free axle and macrocycle and, therefore, it would be energetically favoured. Although the complexation is an equilibrium process, the relatively high binding constant along with the appropriate experimental conditions would allow a high degree of complexation. In this process, the bulky stopper would constitute an unsurmountable energy barrier, thus, the ring would be threaded through the vinyl sulfone side. After the subsequent capping and formation of the [2]rotaxane, the potential energy barrier of the vinyl sulfone side would increase, avoiding the dissassembly of the system. The subsequent decoupling of the sulfonate moiety would cause a decrease of the potential energy barrier on that side, while the addition of base would imply a destabilization of the resulting pseudorotaxane as the hydrogen bonding interactions are no longer present and complexation cannot occur. As result, the macrocycle would be released into the bulk towards a lower energy status, completing its unidirectional path (Fig. 1).
Synthesis, operation and characterization
The synthetic route towards our system started with the preparation of aldehyde 6 (Scheme 1). Williamson reaction between 4-hydroxybenzaldehyde (1) and 3-bromo-1-propanol, followed by a protection of the aldehyde group as an acetal employing 2,2-dimethyl-1,3-propanediol afforded intermediate 2 in good yield (85% and 73% for the first and second step, respectively). Treatment with 2-chloroethanesulfonyl chloride in basic medium gave vinyl sulfonate 3 in 80% yield. Subsequent MAVS reaction with thiol stopper 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 yielded compound 5 in excellent yield (quant). Finally, cleavage of the acetal moiety with trifluoroacetic acid afforded aldehyde 6 in 95% yield.
23 yielded compound 5 in excellent yield (quant). Finally, cleavage of the acetal moiety with trifluoroacetic acid afforded aldehyde 6 in 95% yield.
To obtain the target thread precursor we performed a reductive amination between aldehyde 6 and amine 7 (see the ESI† for its synthesis) followed by a Boc-protection of the resulting dibenzylamine derivative giving intermediate 8 in very good yield (80%). Finally, treatment with CF3CO2H and Et3SiH cleaved both trityl and Boc protecting groups. Subsequent reprotection of the amine followed by a MAVS reaction between the resulting thiol and divinyl sulfone afforded 9. A final deprotection of the amine, followed by protonation and counterion exchange, afforded the thread precursor 10·PF6− in an overall 44% yield (from 8) (Scheme 2).
Having synthesized the thread precursor 10·PF6−, we carried out the synthesis of free thread S12·PF6− (see ESI†) as reference for the 1H NMR analysis and to test the thia-Michael addition reaction, which afforded good results. Then, we started the stepwise transportation of macrocycle 12 through the linear component, tackling the synthesis of rotaxane 13·PF6−. Therefore, we carried out the supramolecular assembly of the pseudorotaxane composed by 10·PF6− and DB24C8 (12). This threading is an equilibrium process, being the complexation between macrocycle and dibenzylammonium derivate favoured by the moderately high binding constant in CDCl3, which ensures a good degree of association at NMR concentrations. However, to further displace the equilibrium towards the pseudorotaxane and maximize the extent of complexation, higher concentrations (0.021 M or higher for 10·PF6−) and an excess of DB24C8 (5 equiv.) were used (see Experimental section in the ESI†).271H NMR analysis showed, once equilibrium was reached, two set of signals, one for the pseudorotaxane and a second one for the excess of macrocycle. In this sense, the right integration of the diagnostic NMR signals (see below) and the fact that no free 10·PF6− was detected (e.g. no signals for uncomplexed Hd and He were observed), supported a high degree of association (Fig. S1 and S2†). Subsequently we performed a DMAP-catalyzed MAVS reaction23,28 of thiol stopper 11 (see ESI† for the synthesis) to the terminal vinyl sulfone group in 10·PF6− at 0 °C, yielding the target [2]rotaxane 13·PF6− in 66% yield (Scheme 3a).
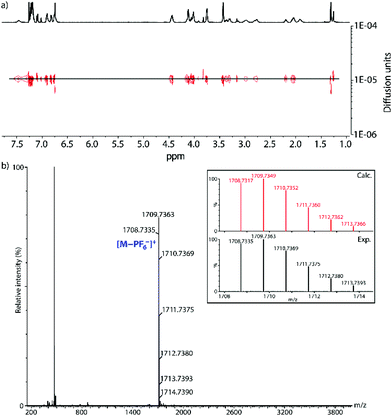
[2]rotaxane 13·PF6− was characterized by means of 1D and 2D NMR techniques. As it can be seen on the comparison of its 1H NMR spectrum with those of the free thread and macrocycle, the aromatic hydrogen nuclei on both rotaxane components suffered some changes (Fig. 2). Thus, the signals of the crown ether aromatic H atoms (H4 and H5) no longer showed the same chemical shift observed in the spectrum of the free macrocycle in CDCl3 as a result of a more pronounced difference in the chemical environment of those nuclei, while those from the axle suffer an upfield shift. Regarding the CH2 signals, the benzylic protons from the thread show the typical downfield shift (ΔδHb,Hc = 0.53 ppm) associated with the establishment of hydrogen bonds between the ammonium unit and the macrocycle, meanwhile those from the ring are shielded (ΔδH1 = −0.40 ppm; ΔδH2 = −0.17 ppm; ΔδH3 = −0.03 ppm). These variations in the 1H NMR spectrum are typical for this type of recognition motif based on hydrogen bonding, supporting the formation of the interlocked rotaxane architecture. Furthermore, the ammonium H atoms also exhibit an upfield shift (ΔδHa = −1.03 ppm), because of their interaction with the crown ether macrocycle (Fig. 2). Moreover, the DOSY NMR spectrum of 13·PF6− exhibits the signals from the both the ring and the axle diffusing at the same diffusion coefficient (Fig. 3a).
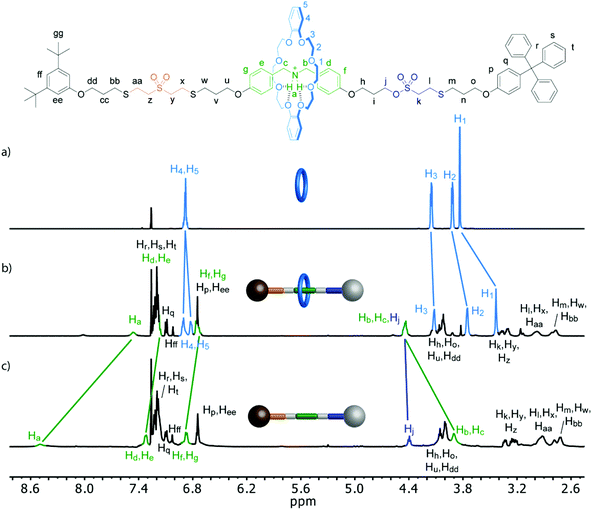 | ||
| Fig. 2 1H NMR (500 MHz, CDCl3) spectra of: (a) Macrocycle 12. (b) Rotaxane 13·PF6−. (c) Thread S12·PF6−. | ||
To further confirm the interlocked nature of 13·PF6 we also recorded and analyzed the NMR spectrum of the isolated compound in DMSO-d6. DMSO is a hydrogen bond acceptor, so its strong competitive character in hydrogen bonding interactions precludes any binding between macrocycle and dibenzylammonium motifs in non-interlocked species.26 In this case, we could observe changes in the chemical shift of the signals of the dibenzylammonium unit and that of macrocycle H1 that follow the same trends already seen and discussed in CDCl3 (Fig. S3†). Moreover, the DOSY experiment in DMSO-d6 also shows that the structure diffuses as a whole, with the signals corresponding to the macrocycle and the thread showing the same diffusion coefficient (Fig. S4†).
Lastly, analysis by ESI-TOF high-resolution mass spectrometry confirmed the identity of [2]rotaxane 13·PF6−. Indeed, the spectrum shows a signal corresponding to an ion resulting from the loss of the PF6− counterion with an exact mass (m/z = 1708.7335) and isotopic distribution that nicely match the theoretical ones (Fig. 3b).
The next step was the cleavage of [2]rotaxane 13·PF6− exploiting the CAD chemistry of the vinyl sulfonate group. For this purpose, we ideally wanted to find a nucleophilic substitution proceeding without the need of a base, at room temperature and in a chlorinated solvent. After a few unsuccessful attempts, we rediscovered a reaction described by Gore and co-workers in 1976,29 which was scarcely employed since then. Thus, MgBr2 was capable of displacing sulfonate groups in CH2Cl2 or CHCl3 at room temperature in a near quantitative fashion, creating bromoalkanes. Therefore, after successfully testing this reaction with thread S11 (see ESI†) we applied it to our system. [2]rotaxane 13·PF6− was treated with MgBr2, resulting in the controlled cleavage of its sulfonate unit. As no hydrogen bonding interactions are established between DB24C8 and non-protonated secondary amines, the addition of a base to deprotonate the dibenzylamine moiety led to a full release of the macrocycle from the cleaved thread (Fig. S5b in the ESI†). After treatment with Et3N, the compounds in the reaction mixture were separated by column chromatography. In this fashion we could isolate macrocycle 12 and a mixture of thread fragments 14/15 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in very good isolated yields (92% and 70% respectively) (Scheme 3b). Their identity was confirmed by NMR and MS techniques (see experimental procedure and Fig. S5, S61–S65 and S82–S85 in the ESI† for further details). The isolation of these compounds confirms the cleavage in a selective and controlled manner of the sulfonate moiety, supporting the release of the macrocycle through the opposite side of its entrance and the proposed operation mechanism.
1) in very good isolated yields (92% and 70% respectively) (Scheme 3b). Their identity was confirmed by NMR and MS techniques (see experimental procedure and Fig. S5, S61–S65 and S82–S85 in the ESI† for further details). The isolation of these compounds confirms the cleavage in a selective and controlled manner of the sulfonate moiety, supporting the release of the macrocycle through the opposite side of its entrance and the proposed operation mechanism.
In situ operation
Having demonstrated that the transportation can be accomplished stepwise, our final goal was the in situ unidirectional transport of DB24C8 (12). For this purpose, we performed the sequence of reactions of the previous stepwise operation in CDCl3 and monitored them by HRMS and 1H NMR spectroscopy.The first step was the assembly of a pseudorotaxane between DB24C8 (12) and thread precursor 10·PF6−, with an unstoppered vinyl sulfone group on one end and a bulky sulfonate stopper on the opposite one. Therefore, by stirring both compounds in CDCl3 under the concentration and stoichiometry conditions discussed for the rotaxane synthesis, the generation of the pseudorotaxane was achieved. This was confirmed by the analysis of the chemical shift and integration of the diagnostic signals (e.g. those of Ha, Hb, Hc or H1) in the 1H NMR spectrum. These signals displayed chemical shifts and integration similar to those already discussed for the stepwise operation that can be associated with the establishment of hydrogen bond interactions between the crown ether and the ammonium unit (Fig. 4a–c). As previously observed, only one set of signals was oberved for 10·PF6−, supporting a high degree of complexation with the concentration of free 10·PF6− below the NMR detection limit. The pseudorotaxane was also detected by HRMS. Thus, the spectra of the mixture of 12 and 10·PF6− showed a peak (m/z = 1428.5527), which matched the threaded species (Fig. S10 and S11†).30
Subsequent addition of thiol stopper 11 and a catalytic amount of DMAP as catalyst for the MAVS reaction between the thiol and the vinyl sulfone moieties afforded rotaxane 13·PF6−. As shown in Fig. 4d, the chemical shifts of the key signals remained essentially unaltered respect to the pseudorotaxane and were comparable to those of pure rotaxane 13·PF6− (Fig. 4d).31 Moreover, the existence of rotaxane 13·PF6− was confirmed by HRMS since we detected the peak corresponding to its exact mass (m/z = 1708.7354 for [M–PF6−]+) (Fig. S13 and S14†), which displayed an isotopic distribution that nicely matched the calculated one. In this way, the threading of the macrocycle through the unstoppered side of the thread and its capture by capping was achieved, completing the first part of the directional transport.
To finish the operation and release the macrocycle through the opposite side of the thread we performed the cleavage of the bulky sulfonate group of rotaxane 13·PF6− with MgBr2. Positive-mode HRMS allowed the detection of a new signal belonging to the pseudorotaxane formed by macrocycle 12 and cleaved axle 14-H+·PF6− (m/z: 1270.5002 [M–PF6−]+) (Fig. S16 and S17†).30 Moreover, in negative mode, a signal corresponding to the sulfonate cleaved stopper 15 (m/z: 517.1504 [M]−) was also present (Fig. S18 and S19†). Lastly, after the addition of Et3N, the 1H NMR spectrum showed the appearance of a triplet at δ = 3.60 ppm, which matches a CH2–Br bond newly created, thus, corresponding to Hj. In addition, those of DB24C8 (12) shifted back to their original position (Fig. 4e). These results confirm the second part of the operation, in which the negigible interaction between crown ethers and non-protonated secondary amines promotes the macrocycle to be relased from the thread through the opposite side of its entrance by cleavage of the sulfonate group, initially stoppered.
Therefore, the in situ unidirectional transport of DB24C8 in a [2]rotaxane by a combination of click MAVS reactions and the CAD chemistry of the vinyl sulfonate group is demonstrated.
Conclusions
In summary, we have described the irreversible unidirectional transportation of a DB24C8 macrocycle along a dibenzylamine/ammonium axle, through a [2]rotaxane, exploiting the chemistry of the vinyl sulfone and the vinyl sulfonate groups. This system is based on a pH- and chemically-driven flashing ratchet mechanism. Indeed, initially, the macrocycle enters by the non-stoppered vinyl sulfone side of the molecule and via a thia-Michael addition of a thiol stopper to the latter, the rotaxane is capped. Subsequently, the MIM is cleaved through a nucleophilic attack on the sulfonate moiety derived from a vinyl sulfonate group on the opposite side and, finally, after addition of base, the macrocycle is liberated through the opposite side to its entrance, achieving the unidirectional transportation. During the operation, the efficiency of the click MAVS chemistry and the CAD chemistry of the vinyl sulfonate group has again been demonstrated. As result, the operation of the system has also been demonstrated in situ.Thus, the CAD chemistry of the vinyl sulfonate group is an efficient tool for the development of cleavable rotaxanes. Moreover, since it is possible to control the unidirectional translational movement of a ring through a rotaxane using a combination of the MAVS and CAD chemistries, the latter could be interesting potential tools for the development of future advanced delivery systems.
Author contributions
A.H.G.D.: Investigation, formal analysis, validation, writing-original draft. P.G.-C.: Investigation, formal analysis, validation. A.G.C.: Conceptualization, funding acquisition, supervision. F.S.-G.: Funding acquisition, methodology, supervision. V.B.: Conceptualization, funding acquisition, formal analysis, methodology, supervision, project administration, writing-original draft.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been financially supported by FEDER(EDRF)/Junta de Andalucía-Consejería de Transformación Económica, Industria, Conocimiento y Universidades (P18-FR-2877), grant PID2020-112906GA-I00 funded by MCIN/AIE (/10.13039/501100011033) and Ministerio de Economía y Competitividad (MINECO, Spain) (CTQ2014-55474-C2-2-R and CTQ2017-86125-P, co-financed by FEDER funds). Funding for open access APCs provided by Universidad de Granada through a Paid by Read & Publish agreement with RSC.Notes and references
- C. J. Bruns and J. F. Stoddart, The Fundamentals of Making Mechanical Bonds, John Wiley & Sons, Hoboken, 2016 Search PubMed.
- M. Xue, Y. Yang, X. Chi, X. Yan and F. Huang, Development of Pseudorotaxanes and Rotaxanes: From Synthesis to Stimuli-Responsive Motions to Applications, Chem. Rev., 2015, 115, 7398–7501 CrossRef CAS PubMed.
- (a) N. H. Evans and P. D. Beer, Progress in the synthesis and exploitation of catenanes since the Millennium, Chem. Soc. Rev., 2014, 43, 4658–4683 RSC; (b) G. Gil-Ramírez, D. A. Leigh and A. J. Stephens, Catenanes: Fifty Years of Molecular Links, Angew. Chem., Int. Ed., 2015, 54, 6110–6150 CrossRef.
- (a) S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Artificial Molecular Machines, Chem. Rev., 2015, 115, 10081–10206 CrossRef CAS; (b) L. Zhang, V. Marcos and D. A. Leigh, Molecular machines with bio-inspired mechanisms, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9397–9404 CrossRef CAS PubMed; (c) A. W. Heard and S. M. Goldup, Simplicity in the Design, Operation, and Applications of Mechanically Interlocked Molecular Machines, ACS Cent. Sci., 2020, 6, 117–128 CrossRef CAS PubMed; (d) I. Aprahamian, The Future of Molecular Machines, ACS Cent. Sci., 2020, 6, 347–358 CrossRef CAS PubMed.
- (a) T. D. Nguyen, H.-R. Tseng, P. C. Celestre, A. H. Flood, Y. Liu, J. F. Stoddart and J. I. Zink, A reversible molecular valve, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10029–10034 CrossRef CAS PubMed; (b) K. Patel, S. Angelos, W. R. Dichtel, A. Coskun, Y.-W. Yang, J. I. Zink and J. F. Stoddart, Enzyme-Responsive Snap-Top Covered Silica Nanocontainers, J. Am. Chem. Soc., 2008, 130, 2382–2383 CrossRef CAS; (c) A. Martinez-Cuezva, S. Valero-Moya, M. Alajarin and J. Berná, Light-responsive peptide [2]rotaxanes as gatekeepers of mechanised nanocontainers, Chem. Commun., 2015, 51, 14501–14504 RSC.
- (a) Y. Liu, A. H. Flood, P. A. Bonvallet, S. A. Vignon, B. H. Northrop, H.-R. Tseng, J. O. Jeppesen, T. J. Huang, B. Brough, M. Baller, S. Magonov, S. D. Solares, W. A. Goddard, C.-M. Ho and J. F. Stoddart, Linear Artificial Molecular Muscles, J. Am. Chem. Soc., 2005, 127, 9745–9759 CrossRef CAS PubMed; (b) J. Berná, D. A. Leigh, M. Lubomska, S. M. Mendoza, E. M. Pérez, P. Rudolf, G. Teobaldi and F. Zerbetto, Macroscopic transport by synthetic molecular machines, Nat. Mater., 2005, 4, 704–710 CrossRef; (c) A. Goujon, T. Lang, G. Mariani, E. Moulin, G. Fuks, J. Raya, E. Buhler and N. Giuseppone, Bistable [c2] Daisy Chain Rotaxanes as Reversible Muscle-like Actuators in Mechanically Active Gels, J. Am. Chem. Soc., 2017, 139, 14825–14828 CrossRef CAS PubMed; (d) S. Ikejiri, Y. Takashima, M. Osaki, H. Yamaguchi and A. Harada, Solvent-Free Photoresponsive Artificial Muscles Rapidly Driven by Molecular Machines, J. Am. Chem. Soc., 2018, 140, 17308–17315 CrossRef CAS.
- (a) G. Bottari, D. A. Leigh and E. M. Pérez, Chiroptical Switching in a Bistable Molecular Shuttle, J. Am. Chem. Soc., 2003, 125, 13360–13361 CrossRef CAS PubMed; (b) Q.–C. Wang, D.–H. Qu, J. Ren, K. Chen and H. Tian, A Lockable Light-Driven Molecular Shuttle with a Fluorescent Signal, Angew. Chem., Int. Ed., 2004, 43, 2661–2665 CrossRef CAS; (c) D. A. Leigh, M. Á. F. Morales, E. M. Pérez, J. K. Y. Wong, C. G. Saiz, A. M. Z. Slawin, A. J. Carmichael, D. M. Haddleton, A. M. Brouwer, W. J. Buma, G. W. H. Wurpel, S. León and F. Zerbetto, Patterning through Controlled Submolecular Motion: Rotaxane-Based Switches and Logic Gates that Function in Solution and Polymer Films, Angew. Chem., Int. Ed., 2005, 44, 3062–3067 CrossRef CAS; (d) H. Onagi and J. Rebek, Jr., Fluorescence resonance energy transfer across a mechanical bond of a rotaxane, Chem. Commun., 2005, 4604–4606 RSC; (e) Z.-Q. Cao, Z.-L. Luan, Q. Zhang, R.-R. Gu, J. Ren and D.-H. Qu, An acid/base responsive side-chain polyrotaxane system with a fluorescent signal, Polym. Chem., 2016, 7, 1866–1870 RSC; (f) Y. Sagara, M. Karman, A. Seki, M. Pannipara, N. Tamaoki and C. Weder, Rotaxane-Based Mechanophores Enable Polymers with Mechanically Switchable White Photoluminescence, ACS Cent. Sci., 2019, 5, 874–881 CrossRef CAS PubMed; (g) A. H. G. David, R. Casares, J. M. Cuerva, A. G. Campaña and V. Blanco, A [2]Rotaxane-Based Circularly Polarized Luminescence Switch, J. Am. Chem. Soc., 2019, 141, 18064–18074 CrossRef CAS PubMed; (h) W.-J. Li, Q. Gu, X.-Q. Wang, D.-Y. Zhang, Y.-T. Wang, X. He, W. Wang and H.-B. Yang, AIE-Active Chiral [3]Rotaxanes with Switchable Circularly Polarized Luminescence, Angew. Chem., Int. Ed., 2021, 60, 9507–9515 CrossRef CAS.
- (a) V. Blanco, A. Carlone, K. D. Hänni, D. A. Leigh and B. Lewandowski, A Rotaxane-Based Switchable Organocatalyst, Angew. Chem., Int. Ed., 2012, 51, 5166–5169 CrossRef CAS; (b) V. Blanco, D. A. Leigh, V. Marcos, J. A. Morales-Serna and A. L. Nussbaumer, A Switchable [2]Rotaxane Asymmetric Organocatalyst That Utilizes an Acyclic Chiral Secondary Amine, J. Am. Chem. Soc., 2014, 136, 4905–4908 CrossRef CAS; (c) A. Martinez-Cuezva, A. Saura-Sanmartin, T. Nicolas-Garcia, C. Navarro, R.-A. Orenes, M. Alajarin and J. Berna, Photoswitchable interlocked thiodiglycolamide as a cocatalyst of a chalcogeno-Baylis–Hillman reaction, Chem. Sci., 2017, 8, 3775–3780 RSC; (d) C. Biagini, S. D. P. Fielden, D. A. Leigh, F. Schaufelberger, S. Di Stefano and D. Thomas, Dissipative Catalysis with a Molecular Machine, Angew. Chem., Int. Ed., 2019, 58, 9876–9880 CrossRef CAS PubMed; (e) M. Calles, J. Puigcerver, D. A. Alonso, M. Alajarin, A. Martinez-Cuezva and J. Berna, Enhancing the selectivity of prolinamide organocatalysts using the mechanical bond in [2]rotaxanes, Chem. Sci., 2020, 11, 3629–3635 RSC.
- (a) C. P. Collier, E. W. Wong, M. Belohradsky, F. M. Raymo, J. F. Stoddart, P. J. Kuekes, R. S. Williams and J. R. Heath, Electronically Configurable Molecular-Based Logic Gates, Science, 1999, 285, 391–394 CrossRef CAS; (b) J. E. Green, J. W. Choi, A. Boukai, Y. Bunimovich, E. Johnston-Halperin, E. DeIonno, Y. Luo, B. A. Sheriff, K. Xu, Y. S. Shin, H.-R. Tseng, J. F. Stoddart and J. R. Heath, A 160-kilobit molecular electronic memory patterned at 1011 bits per square centimetre, Nature, 2007, 445, 414–417 CrossRef CAS.
- (a) B. Lewandowski, G. De Bo, J. W. Ward, M. Papmeyer, S. Kuschel, M. J. Aldegunde, P. M. E. Gramlich, D. Heckmann, S. M. Goldup, D. M. D'Souza, A. E. Fernandes and D. A. Leigh, Sequence-Specific Peptide Synthesis by an Artificial Small-Molecule Machine, Science, 2013, 339, 189–193 CrossRef CAS; (b) G. De Bo, M. A. Y. Gall, M. O. Kitching, S. Kuschel, D. A. Leigh, D. J. Tetlow and J. W. Ward, Sequence-Specific β-Peptide Synthesis by a Rotaxane-Based Molecular Machine, J. Am. Chem. Soc., 2017, 139, 10875–10879 CrossRef CAS; (c) C. T. McTernan, G. De Bo and D. A. Leigh, A Track-Based Molecular Synthesizer that Builds a Single-Sequence Oligomer through Iterative Carbon-Carbon Bond Formation, Chem, 2020, 6, 2964–2973 CrossRef CAS; (d) J. Echavarren, M. A. Y. Gall, A. Haertsch, D. A. Leigh, J. T. J. Spence, D. J. Tetlow and C. Tian, Sequence-selective decapeptide synthesis by the parallel operation of two artificial molecular machines, J. Am. Chem. Soc., 2021, 143, 5158–5165 CrossRef CAS PubMed.
- J. D. Badjić, V. Balzani, A. Credi, S. Silvi and J. F. Stoddart, A Molecular Elevator, Science, 2004, 303, 1845–1849 CrossRef PubMed.
- (a) C. J. Bruns and J. F. Stoddart, Rotaxane-Based Molecular Muscles, Acc. Chem. Res., 2014, 47, 2186–2199 CrossRef CAS PubMed; (b) A. Goujon, E. Moulin, G. Fuks and N. Giuseppone, [c2]Daisy Chain Rotaxanes as Molecular Muscles, CCS Chem., 2019, 1, 83–96 CAS.
- (a) S. Kassem, T. van Leeuwen, A. S. Lubbe, M. R. Wilson, B. L. Feringa and D. A. Leigh, Artificial molecular motors, Chem. Soc. Rev., 2017, 46, 2592–2621 RSC; (b) M. Baroncini, S. Silvi and A. Credi, Photo- and Redox-Driven Artificial Molecular Motors, Chem. Rev., 2020, 120, 200–268 CrossRef CAS PubMed; (c) Y. Qiu, Y. Feng, Q.-H. Guo, R. D. Astumian and J. F. Stoddart, Pumps through the Ages, Chem, 2020, 6, 1954–1979 Search PubMed; (d) Y. Feng, M. Ovalle, J. S. W. Seale, C. K. Lee, D. J. Kim, R. D. Astumian and J. F. Stoddart, Molecular Pumps and Motors, J. Am. Chem. Soc., 2021, 143, 5569–5591 CrossRef CAS; (e) S. Corra, L. Casimiro, M. Baroncini, J. Groppi, M. La Rosa, M. Tranfić Bakić, S. Silvi and A. Credi, Artificial Supramolecular Pumps Powered by Light, Chem. – Eur. J., 2021, 27, 11076–11083 CrossRef CAS.
- (a) M. N. Chatterjee, E. R. Kay and D. A. Leigh, Beyond Switches: Ratcheting a Particle Energetically Uphill with a Compartmentalized Molecular Machine, J. Am. Chem. Soc., 2006, 128, 4058–4073 CrossRef CAS PubMed; (b) V. Serreli, C.-F. Lee, E. R. Kay and D. A. Leigh, A molecular information ratchet, Nature, 2007, 445, 523–527 CrossRef CAS; (c) M. Alvarez-Pérez, S. M. Goldup, D. A. Leigh and A. M. Z. Slawin, A Chemically-Driven Molecular Information Ratchet, J. Am. Chem. Soc., 2008, 130, 1836–1838 CrossRef.
- (a) D. A. Leigh, J. K. Y. Wong, F. Dehez and F. Zerbetto, Unidirectional rotation in a mechanically interlocked molecular rotor, Nature, 2003, 424, 174–179 CrossRef CAS PubMed; (b) J. V. Hernández, E. R. Kay and D. A. Leigh, A Reversible Synthetic Rotary Molecular Motor, Science, 2004, 306, 1532–1537 CrossRef; (c) M. Baroncini, S. Silvi, M. Venturi and A. Credi, Photoactivated Directionally Controlled Transit of a Non-Symmetric Molecular Axle Through a Macrocycle, Angew. Chem., Int. Ed., 2012, 51, 4223–4226 CrossRef CAS; (d) G. Ragazzon, M. Baroncini, S. Silvi, M. Venturi and A. Credi, Light-powered autonomous and directional molecular motion of a dissipative self-assembling system, Nat. Nanotechnol., 2015, 10, 70–75 CrossRef CAS; (e) M. Canton, J. Groppi, L. Casimiro, S. Corra, M. Baroncini, S. Silvi and A. Credi, Second-Generation Light-Fueled Supramolecular Pump, J. Am. Chem. Soc., 2021, 143, 10890–10894 CrossRef CAS PubMed.
- (a) H. Li, C. Cheng, P. R. McGonigal, A. C. Fahrenbach, M. Frasconi, W.-G. Liu, Z. Zhu, Y. Zhao, C. Ke, J. Lei, R. M. Young, S. M. Dyar, D. T. Co, Y.-W. Yang, Y. Y. Botros, W. A. Goddard III, M. R. Wasielewski, R. D. Astumian and J. F. Stoddart, Relative Unidirectional Translation in an Artificial Molecular Assembly Fueled by Light, J. Am. Chem. Soc., 2013, 135, 18609–18620 CrossRef CAS PubMed; (b) C. Cheng, P. R. McGonigal, W.-G. Liu, H. Li, N. A. Vermeulen, C. Ke, M. Frasconi, C. L. Stern, W. A. Goddard III and J. F. Stoddart, Energetically Demanding Transport in a Supramolecular Assembly, J. Am. Chem. Soc., 2014, 136, 14702–14705 CrossRef CAS; (c) C. Cheng, P. R. McGonigal, S. T. Schneebeli, H. Li, N. A. Vermeulen, C. Ke and J. F. Stoddart, An artificial molecular pump, Nat. Nanotechnol., 2015, 10, 547–553 CrossRef CAS PubMed; (d) Y. Qiu, B. Song, C. Pezzato, D. Shen, W. Liu, L. Zhang, Y. Feng, Q.-H. Guo, K. Cai, W. Li, H. Chen, M. T. Nguyen, Y. Shi, C. Cheng, R. D. Astumian, X. Li and J. F. Stoddart, A precise polyrotaxane synthesizer, Science, 2020, 368, 1247–1253 CrossRef CAS PubMed; (e) L. Feng, Y. Qiu, Q.-H. Guo, Z. Chen, J. S. W. Seale, K. He, H. Wu, Y. Feng, O. K. Farha, R. D. Astumian and J. F. Stoddart, Active mechanisorption driven by pumping cassettes, Science, 2021, 374, 1215–1221 CrossRef PubMed.
- (a) M. R. Wilson, J. Solá, A. Carlone, S. M. Goldup, N. Lebrasseur and D. A. Leigh, An autonomous chemically fuelled small-molecule motor, Nature, 2016, 534, 235–240 CrossRef CAS PubMed; (b) S. Erbas-Cakmak, S. D. P. Fielden, U. Karaca, D. A. Leigh, C. T. McTernan, D. J. Tetlow and M. R. Wilson, Rotary and linear molecular motors driven by pulses of a chemical fuel, Science, 2017, 358, 340–343 CrossRef CAS PubMed; (c) S. Amano, S. D. P. Fielden and D. A. Leigh, A catalysis-driven artificial molecular pump, Nature, 2021, 594, 529–534 CrossRef CAS.
- (a) C. Reuter, W. Wienand, G. M. Hübner, C. Seel and F. Vögtle, High-Yield Synthesis of Ester, Carbonate, and Acetal Rotaxanes by Anion Template Assistance and their Hydrolytic Dethreading, Chem. – Eur. J., 1999, 5, 2692–2697 CrossRef CAS; (b) A. Fernandes, A. Viterisi, F. Coutrot, S. Potok, D. A. Leigh, V. Aucagne and S. Papot, Rotaxane-Based Propeptides: Protection and Enzymatic Release of a Bioactive Pentapeptide, Angew. Chem., Int. Ed., 2009, 48, 6443–6447 CrossRef CAS PubMed; (c) R. Barat, T. Legigan, I. Tranoy-Opalinski, B. Renoux, E. Péraudeau, J. Clarhaut, P. Poinot, A. E. Fernandes, V. Aucagne, D. A. Leigh and S. Papot, A mechanically interlocked molecular system programmed for the delivery of an anticancer drug, Chem. Sci., 2015, 6, 2608–2613 RSC; (d) C. C. Slack, J. A. Finbloom, K. Jeong, C. J. Bruns, D. E. Wemmer, A. Pines and M. B. Francis, Rotaxane probes for protease detection by 129Xe hyperCEST NMR, Chem. Commun., 2017, 53, 1076–1079 RSC; (e) S.–T. Tung, H.–T. Cheng, A. Inthasot, F.–C. Hsueh, T.–J. Gu, P.–C. Yan, C.–C. Lai and S.–H. Chiu, Interlocked Photo-degradable Macrocycles Allow One-Off Photo-triggerable Gelation of Organo- and Hydrogelators, Chem. – Eur. J., 2018, 24, 1522–1527 CrossRef CAS PubMed; (f) M. Zhang and G. De Bo, Mechanical Susceptibility of a Rotaxane, J. Am. Chem. Soc., 2019, 141, 15879–15883 CrossRef CAS.
- Z. Meng, J.-F. Xiang and C.-F. Chen, Directional Molecular Transportation Based on a Catalytic Stopper-Leaving Rotaxane System, J. Am. Chem. Soc., 2016, 138, 5652–5658 CrossRef CAS.
- H.-Y. Zhou, Y. Han, Q. Shi and C.-F. Chen, Directional Transportation of a Helic[6]arene along a Nonsymmetric Molecular Axle, J. Org. Chem., 2019, 84, 5872–5876 CrossRef CAS.
- Q.-H. Guo, Y. Qiu, X. Kuang, J. Liang, Y. Feng, L. Zhang, Y. Jiao, D. Shen, R. D. Astumian and J. F. Stoddart, Artificial Molecular Pump Operating in Response to Electricity and Light, J. Am. Chem. Soc., 2020, 142, 14443–14449 CrossRef CAS PubMed.
- (a) B. A. Frankel, M. Bentley, R. G. Kruger and D. G. McCafferty, Vinyl Sulfones: Inhibitors of SrtA, a Transpeptidase Required for Cell Wall Protein Anchoring and Virulence in Staphylococcus aureus, J. Am. Chem. Soc., 2004, 126, 3404–3405 CrossRef CAS PubMed; (b) S. Liu, B. Zhou, H. Yang, Y. He, Z.-X. Jiang, S. Kumar, L. Wu and Z.-Y. Zhang, Aryl Vinyl Sulfonates and Sulfones as Active Site-Directed and Mechanism-Based Probes for Protein Tyrosine Phosphatases, J. Am. Chem. Soc., 2008, 130, 8251–8260 CrossRef CAS PubMed; (c) J. Morales-Sanfrutos, F. J. Lopez-Jaramillo, F. Hernandez-Mateo and F. Santoyo-Gonzalez, Vinyl Sulfone Bifunctional Tag Reagents for Single-Point Modification of Proteins, J. Org. Chem., 2010, 75, 4039–4047 CrossRef CAS PubMed; (d) T. del Castillo, J. Marales-Sanfrutos, F. Santoyo-González, S. Magez, F. J. Lopez-Jaramillo and J. A. Garcia-Salcedo, Monovinyl Sulfone β-Cyclodextrin. A Flexible Drug Carrier System, ChemMedChem, 2014, 9, 383–389 CrossRef CAS; (e) S. Chatani, C. Wang, M. Podgórski and C. N. Bowman, Triple Shape Memory Materials Incorporating Two Distinct Polymer Networks Formed by Selective Thiol–Michael Addition Reactions, Macromolecules, 2014, 47, 4949–4954 CrossRef CAS; (f) F. M. Tomlin, U. I. M. Gerling-Driessen, Y.-C. Liu, R. A. Flynn, J. R. Vangala, C. S. Lentz, S. Clauder-Muenster, P. Jakob, W. F. Mueller, D. Ordoñez-Rueda, M. Paulsen, N. Matsui, D. Foley, A. Rafalko, T. Suzuki, M. Bogyo, L. M. Steinmetz, S. K. Radhakrishnan and C. R. Bertozzi, Inhibition of NGLY1 Inactivates the Transcription Factor Nrf1 and Potentiates Proteasome Inhibitor Cytotoxicity, ACS Cent. Sci., 2017, 3, 1143–1155 CrossRef CAS PubMed; (g) J. Sinha, M. Podgórski, S. Huang and C. N. Bowman, Multifunctional monomers based on vinyl sulfonates and vinyl sulfonamides for crosslinking thiol-Michael polymerizations: monomer reactivity and mechanical behavior, Chem. Commun., 2018, 54, 3034–3037 RSC.
- A. H. G. David, P. García–Cerezo, A. G. Campaña, F. Santoyo–González and V. Blanco, [2]Rotaxane End-Capping Synthesis by Click Michael-Type Addition to the Vinyl Sulfonyl Group, Chem. – Eur. J., 2019, 25, 6170–6179 CrossRef CAS.
- R. Bielski and Z. Witczak, Strategies for Coupling Molecular Units if Subsequent Decoupling Is Required, Chem. Rev., 2013, 113, 2205–2243 CrossRef CAS.
- C. M. Cruz, M. Ortega–Muñoz, F. J. López–Jaramillo, F. Hernández–Mateo, V. Blanco and F. Santoyo–González, Vinyl Sulfonates: A Click Function for Coupling-and-Decoupling Chemistry and their Applications, Adv. Synth. Catal., 2016, 358, 3394–3413 CrossRef CAS.
- P. R. Ashton, P. J. Campbell, E. J. T. Chrystal, P. T. Glink, S. Menzer, D. Philp, N. Spencer, J. F. Stoddart, P. A. Tasker and D. J. Williams, Dialkylammonium Ion/Crown Ether Complexes: The Forerunners of a New Family of Interlocked Molecules, Angew. Chem., Int. Ed. Engl., 1995, 34, 1865–1869 CrossRef CAS.
- It should be noted that the complexation process between DB24C8 and dibenzylammonium derivatives is slow on the NMR timescale. Therefore, by performing the operation with an excess of macrocycle, we also simplify the monitoring by NMR, especially during the in situ operation, avoiding the presence of an additional set of signals corresponding to the unbound 10·PF6−. In addition, the excess of 12 also helps to improve the solubility of 10·PF6− in CDCl3, usually quite poor.
- K. Nakazono, T. Oku and T. Takata, Synthesis of rotaxanes consisting of crown ether wheel and sec-ammonium axle under basic condition, Tetrahedron Lett., 2007, 48, 3409–3411 CrossRef CAS.
- P. Place, M. L. Roumestant and J. Gore, Reaction of magnesium halides with sulfonates, Bull. Soc. Chim. Fr., 1976, 169–176 CAS.
- During the analysis of the pseudorotaxanes assembled from DB24C8 (12) and 10·PF6− or 14-H+·PF6−, peaks corresponding to the free ammonium salts (calculated m/z: 980.3358 [M−PF6−]+ for 10·PF6−; 822.2895 [M−PF6−]+ for 14-H+·PF6−) can also be detected in some cases (e. g. Fig. S10†) depending on the experimental conditions, such as concentration or ionization potential. In fact, the dissociation of the pseudorotaxane is not surprising at low concentrations and in the presence of a hydrogen bonding competitive solvent as MeOH, conditions used in ESI mass spectrometry measurements.
- Minor traces of an unknown by-product that seems to possess an alkene group with peaks in the region between 6.2 and 6.4 ppm can be in some cases detected by NMR spectroscopy.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data, NMR and HRMS spectra and additional supporting figures. See DOI: 10.1039/d1qo01491a |
| This journal is © the Partner Organisations 2022 |