Photoredox-mediated N-centered radical addition/semipinacol rearrangement for the convenient synthesis of β-amino (spiro)cyclic ketones†
Abstract
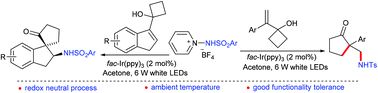
A visible light photoredox-catalyzed N-centered radical addition/semipinacol rearrangement cascade of cycloalkanol-substituted 1H-indenes or styrenes with N-arylsulfonyl protected 1-aminopyridinium salts is presented. This protocol provides an efficient access to β-amino (spiro)cyclic ketones at ambient temperature, and has the advantages of broad substrate scope, good functional group tolerance, simple operation and mild conditions.


 Please wait while we load your content...
Please wait while we load your content...