Controllable chemoselectivity in the reaction of 2H-indazoles with alcohols under visible-light irradiation: synthesis of C3-alkoxylated 2H-indazoles and ortho-alkoxycarbonylated azobenzenes†
Abstract
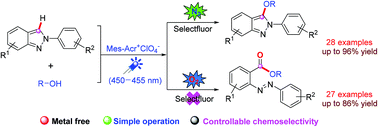
A high chemoselectivity in the visible-light-induced reaction of 2H-indazoles with alcohols controlled by the reaction atmosphere (N2 or O2) was achieved. Under a N2 atmosphere, an alkoxylation of 2H-indazoles with alcohols in the presence of Mes-Acr+ClO4− as a photosensitizer and Selectfluor as an oxidant generated C3-alkoxylated 2H-indazoles in high yields. Under an O2 atmosphere in the absence of Selectfluor, the reaction underwent a ring opening of 2H-indazoles to afford unsymmetrical ortho-alkoxycarbonylated azobenzenes in good yields.


 Please wait while we load your content...
Please wait while we load your content...