Reversible fluorescence modulation through the photoisomerization of an azobenzene-bridged perylene bisimide cyclophane†
Guanghui
Ouyang
ab,
David
Bialas
a and
Frank
Würthner
 *a
*a
aInstitut für Organische Chemie and Center for Nanosystems Chemistry, Universität Würzburg, Am Hubland, 97074 Würzburg, Germany. E-mail: wuerthner@uni-wuerzburg.de
bCAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, ZhongGuanCun, North First Street 2, 100190, Beijing, China
First published on 2nd February 2021
Abstract
A new type of perylene bisimide (PBI) cyclophane in which two PBI chromophores were linked via an azobenzene (Azo) bridge was designed and synthesized. It was found that the integration of electron-donating azobenzene and electron-deficient PBI in a well-defined cyclophane enables the regulation of the fluorescence lifetime and quantum yield of the PBI units. With the aid of a high efficiency trans → cis transformation of the azobenzene moiety, the PBI–PBI chromophore distance can be effectively controlled as revealed by exciton coupling and density functional theory (DFT) computation, leading to additional non-radiative decay pathways and a pronounced fluorescence quenching due to cooperative adjustments of PBI–PBI and PBI–Azo interactions. Reversible fluorescence intensity switching for at least five cycles was successfully achieved with alternate UV and visible light irradiation. These well-controlled fluorescence modulation and photoswitching behaviours not only provide insights into interchromophoric interactions but also contribute to the development of stimuli-responsive luminescent macrocycles.
Introduction
Macrocycles and cyclophanes,1–3 developed by Cram, Lehn and Pederson for molecular recognition, constitute the first examples of synthetic supramolecular elements.4 More recently, to meet the developing requirements of smart supramolecular materials,5–9 dynamic macrocycles and cyclophanes responding to external stimuli have gained increasing attention due to their key roles in designing smart fluorescent materials,10–13 molecular machines,14 and controlled supramolecular transport systems.15,16 However, among the reported stimuli-responsive macrocycles, dynamic cyclophanes are less studied,17,18 partly due to their relatively rigid conformations and synthetic challenges from the build-up of strain.PBI-based cyclophanes are of particular interest due to the high photoluminescence quantum yield and robust photo and chemical stability of PBIs.19,20 However, because of the poor solubility and strong aggregation tendency of unsubstituted PBIs, the synthesis of PBI cyclophanes is quite challenging and only a few examples have been reported so far (Fig. 1a).21–25 Furthermore, due to the strong attraction of PBIs, there are no cavities provided by these cyclophanes for the complexation of guest molecules. Only in 2015, our group successfully reported a new type of PBI cyclophane bearing an open cavity by utilizing tetra-tert-butylphenoxy substituted perylene bisanhydride as an easily soluble precursor and toluene as the solvent and template for the cyclization reaction.26 Based on this high efficiency cyclization method, a PBI cyclophane library was established by our group using various CH2–arylene–CH2 linkers.27–31 The structure–property investigation of this PBI cyclophane library clearly clarified that the length of the linker could effectively control the interchromophoric distances and therefore significantly affect both PBI–PBI interactions and recognition properties for polycyclic aromatic hydrocarbon guest molecules.26,28,32 Inspired by these discoveries, we envisioned a dynamic PBI cyclophane by using a photoresponsive moiety as a linker, which is expected to reversibly regulate the PBI–PBI interchromophoric interactions and further modulate the fluorescence properties of the PBI units. Accordingly, the family of PBI cyclophanes could be enriched with a new supramolecular element that is the first one bearing a light-switchable architecture (Fig. 1b).
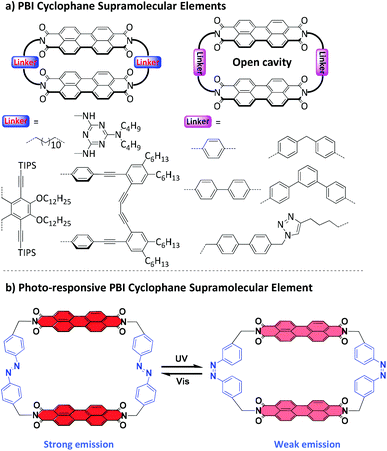 | ||
| Fig. 1 Schematic illustration of PBI cyclophane supramolecular elements. (a) Representative PBI cyclophane supramolecular elements with rigid linkers. (b) PBI cyclophane supramolecular element with photoresponsive linkers, which showed reversible structural and fluorescence switching under alternate UV and visible light irradiation. Notes: The bay-position substituents of the PBIs are omitted for clarity. For details of the PBI cyclophane structures illustrated in (a), the original literature reports21–25 should be consulted. | ||
Thus, we herein report a dynamic supramolecular macrocycle by the cyclization of two azobenzene moieties and two PBI luminophores into cyclophane APC (![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) zo-
zo-![[P with combining low line]](https://www.rsc.org/images/entities/char_0050_0332.gif) BI
BI ![[c with combining low line]](https://www.rsc.org/images/entities/char_0063_0332.gif) yclophane), whose fluorescence intensity can be reversibly regulated under UV and visible light irradiation. At the thermal equilibrium state, both azobenzene units are mainly in the trans-configuration. The relatively long molecular length of trans-Azo led to the formation of a large cavity for the cyclophane, resulting in weak exciton coupling between the PBI units.33,34 Under UV irradiation (λex = 350 nm) for several seconds, the azobenzene groups undergo a trans → cis transformation, resulting in the formation of a photostationary state of APC (PSS-APC). Such a light-initialized isomerization process effectively changed the molecular geometry and electronic properties of APC. As a result, the FLQY and fluorescence intensity of PSS-APC weakened compared with those of its trans–trans counterpart due to cooperative adjustments of PBI–PBI and PBI–Azo interactions. Back-switching from PSS-APC to the initial trans–transAPC (abbreviated as trans-APC) could be realized by visible light irradiation. Up to five cycles of fluorescence switching using alternate UV and visible light irradiation proved to be a macrocycle fluorescence switch with fatigue resistance, which might enable potential applications in smart photoresponsive fluorescent probes and controlled supramolecular transport systems.
yclophane), whose fluorescence intensity can be reversibly regulated under UV and visible light irradiation. At the thermal equilibrium state, both azobenzene units are mainly in the trans-configuration. The relatively long molecular length of trans-Azo led to the formation of a large cavity for the cyclophane, resulting in weak exciton coupling between the PBI units.33,34 Under UV irradiation (λex = 350 nm) for several seconds, the azobenzene groups undergo a trans → cis transformation, resulting in the formation of a photostationary state of APC (PSS-APC). Such a light-initialized isomerization process effectively changed the molecular geometry and electronic properties of APC. As a result, the FLQY and fluorescence intensity of PSS-APC weakened compared with those of its trans–trans counterpart due to cooperative adjustments of PBI–PBI and PBI–Azo interactions. Back-switching from PSS-APC to the initial trans–transAPC (abbreviated as trans-APC) could be realized by visible light irradiation. Up to five cycles of fluorescence switching using alternate UV and visible light irradiation proved to be a macrocycle fluorescence switch with fatigue resistance, which might enable potential applications in smart photoresponsive fluorescent probes and controlled supramolecular transport systems.
Results and discussion
APC was synthesized according to the route shown in Scheme 1 and S1 (ESI†). The two precursor components, azobenzene diamine 335 and perylene bisanhydride 5,36–38 were synthesized following literature-known procedures. Equal equivalents of 3 and 5 were then refluxed in anhydrous toluene under a nitrogen atmosphere for 16 h in the presence of an excess amount of imidazole. After purification by flash column chromatography and recycling gel permeation chromatography, APC was obtained as a red-brown solid in a yield of 25%. APC was characterized by its melting point, and 1H-NMR, 13C-NMR spectroscopy and high-resolution mass spectrometry (Fig. S1–4, ESI†).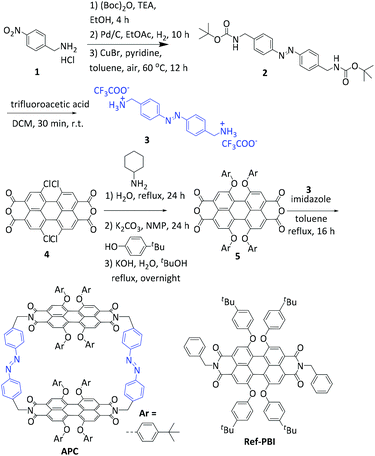 | ||
| Scheme 1 The synthetic route towards PBI cyclophane APC. In addition, the chemical structure of Ref-PBI27 is shown, which is used as a reference compound. | ||
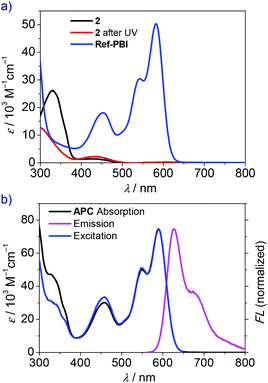
First, UV/Vis spectra of reference compounds 2 and Ref-PBI (for the chemical structure, see Scheme 1) were recorded to investigate the optical properties of the pristine linker and PBI moiety. The absorption maximum of Ref-PBI in dichloromethane is located at 588 nm with a vibronic progression at 547 nm (Fig. 2a, blue line, and Table 1). Furthermore, an additional absorption band is present at 454 nm, which can be assigned to the S0→ S2 transition.27 In contrast, reference azobenzene 2 shows significant absorption at shorter wavelengths with a maximum at 343 nm (Fig. 2a, black line), whereas Ref-PBI shows only weak absorption. Hence, a selective excitation of the azobenzene moiety can be realized in the presence of a PBI chromophore. The validity of this strategy is well proven by control experiments. As shown in Fig. S7 (ESI†), 350 nm UV irradiation of a mixture of 2/Ref-PBI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in molar ratio) led to a significant decrease of trans-azobenzene absorption at 343 nm, which is a signature for the trans→cis transformation of azobenzene.39 The photoisomerization efficiency of reference azobenzene 2 is calculated to be about 70% according to the evaluation of both NMR and UV-vis experiments (Fig. S5–7, ESI†).
1 in molar ratio) led to a significant decrease of trans-azobenzene absorption at 343 nm, which is a signature for the trans→cis transformation of azobenzene.39 The photoisomerization efficiency of reference azobenzene 2 is calculated to be about 70% according to the evaluation of both NMR and UV-vis experiments (Fig. S5–7, ESI†).
| Trans-APC | PSS-APCa | Ref-PBI 26 | |
|---|---|---|---|
| a Photostationary state APC after UV irradiation for 1 min. b Absolute FLQY method using an integrating sphere at an excitation wavelength of 510 nm. c The wavelength of the pulsed laser diode is 505.8 nm, and the emission intensity was monitored at 650 nm. d Determined according to kfl = Φfl/τfl and knr = 1/τfl − kfl. | |||
| λ abs(A0–0)/nm | 583 | 586 | 588 |
| λ abs(A0–1)/nm | 545 | 548 | 547 |
| A0–0/A0–1 | 1.44 | 1.41 | 1.66 |
| ε max(A0–0)/M−1 cm−1 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 670 |
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870 870 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| ε max(A0–1)/M−1 cm−1 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 510 |
52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
| λ em/nm | 626 | 626 | 620 |
Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) Stokes/cm−1 Stokes/cm−1 |
1196 | 1183 | 878 |
| FLQYb | 0.16 | 0.07 | 0.97 |
| Lifetimec | 2.6 ns | 2.6 ns | 6.5 ns |
| k fl [108 s−1]d | 0.62 | 0.27 | 1.49 |
| k nr [108 s−1]d | 3.23 | 3.58 | 0.05 |
The basic optical properties of PBI cyclophane APC were then examined by UV-vis absorption and fluorescence spectroscopy in CHCl3 due to its good solubility in this solvent. As shown in Fig. 2b, the characteristic absorption peaks of A0–0 and A0–1 vibronic transitions are located at 589 and 550 nm, respectively. The A0–0/A0–1 ratio, which can be used as an indicator for revealing H- or J-aggregates for PBI chromophores,34,40 amounts to 1.50. This value is smaller than that of Ref-PBI (1.66, Table 1) and indicates weak exciton–vibrational coupling between the PBI chromophores within the cyclophane. The fluorescence spectrum was obtained by excitation in the PBI S0→ S1 absorption region (λex = 550 nm), which showed an emission band in the wavelength region of 560–800 nm (Fig. 2b, magenta line).
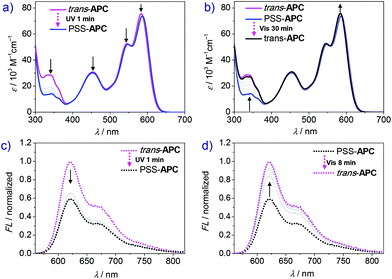
The photoisomerization experiments of APC were then studied by UV-vis absorption, fluorescence and NMR spectroscopy. As shown in Fig. S8a,† after 10 s UV irradiation (λex = 350 nm), the absorption peak at 328 nm ascribed to the trans-Azo moiety drops but remains almost unchanged in the following longer irradiation time (30 s and 60 s), which proves that a photostationary state is achieved under these irradiation conditions. The corresponding cis-Azo absorption peak, which should be observed at about 440 nm (Fig. 2a), is buried under the S0→ S2 absorption band of the PBI units (λmax = 455 nm). The PBI emission shows only a slight decrease after UV irradiation (Fig. S9, ESI†). The trans→cis transformation ratio of APC in chloroform was calculated to be about 22% based on NMR and UV-vis spectra (Fig. S10 and S11, ESI†). Although the photoisomerization shows good reversibility (Fig. S8c and d, ESI†), the relatively low conversion rate limits its potential application.41
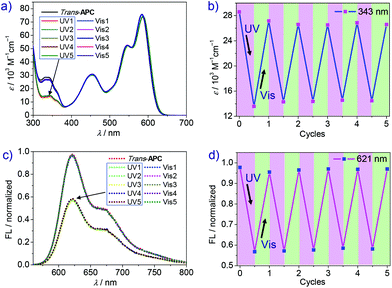
However, the trans → cis conversion ratio could be significantly enhanced by changing the solvent. Accordingly, time-dependent UV-vis spectra of cyclophane APC after variable UV irradiation time were recorded in dichloromethane. As shown in Fig. 3a, the absorption peak at 343 nm assigned to trans-Azo decreases significantly upon UV irradiation. After 70 s UV irradiation, the photostationary state is achieved, showing negligible absorption change compared with that after 60 s irradiation. However, due to the poor solubility of APC in dichloromethane at the NMR concentration scale (mM), the trans → cis transformation efficiency of APC could be only estimated by UV-vis spectroscopy (Fig. S12, ESI†), giving a value of 80%. Notably, the absorption maximum of PBI shows a small bathochromic shift from 583 to 586 nm, and the A0–0/A0–1 ratio is slightly reduced from 1.44 to 1.41 (Table 1) upon photoisomerization, which reveals an increase of the exciton coupling strength between the two PBI chromophores within the cyclophane due to the decrease of the PBI–PBI distance. In addition, the quantum yield of APC decreased from 16% to 7% after UV irradiation (Table 1) accompanied by a decrease of fluorescence emission intensity. As shown in Fig. 3c, fluorescence intensity decreases drastically in the first 10 s of irradiation and reaches the final photostationary state after 60 s, which shows a weakened fluorescence intensity compared with the initial state.
Back-switching from PSS-APC to trans-APC was studied by time-dependent absorption and emission spectroscopy to evaluate the reversible capability. After irradiating with visible light for 30 min (Fig. 3b), PSS-APC can be almost totally switched back to the initial thermodynamically stable trans-state. In our experiments, fluorescence intensity shows a faster recovery rate (only 8 minutes) than the recovery of the initial UV-vis absorption spectrum because of the lower concentration of cyclophane APC for fluorescence measurements, which is an order of magnitude smaller than the one applied in UV-vis spectroscopy. By alternate UV and visible light irradiation (Fig. 4), trans- and PSS-APC states were successfully switched for five cycles, revealing no indication of efficiency decrease or degradation as demonstrated by the absorption intensity at 343 nm ascribed to trans-azobenzene (Fig. 4b) as well as by the PBI emission band at 621 nm (Fig. 4d), which indicates a fatigue resistance photoswitch.
The optical properties of APC before and after UV irradiation are summarized in Table 1. The excitation spectrum (Fig. 2b, blue line) reveals that the azobenzene absorption also contributes to the PBI emission. In addition, the fluorescence quantum yield of APC by excitation of both the azobenzene part (λEx = 350 nm) and the PBI part (λEx = 510 nm) gave the same value of 16% (Table S1, ESI†), which reveals an energy transfer process from the excited trans-Azo* to PBI. This conclusion is in agreement with the work of Müllen and coworkers.42 They have proved an efficient energy transfer in an Azo–PBI dendrimer from the excited Azo* moiety to the PBI chromophore by fluorescence and ultrafast transient absorption spectroscopy. It was also shown that after energy transfer, the excited PBI undergoes a radiative relaxation to the ground state with the lifetime (several nanoseconds) as observed when only the PBI unit is excited at 570 nm. For our system, both the fluorescence quantum yield and lifetime of APC are obviously smaller than those of Ref-PBI (Table 1), which indicates an efficient nonradiative decay process.
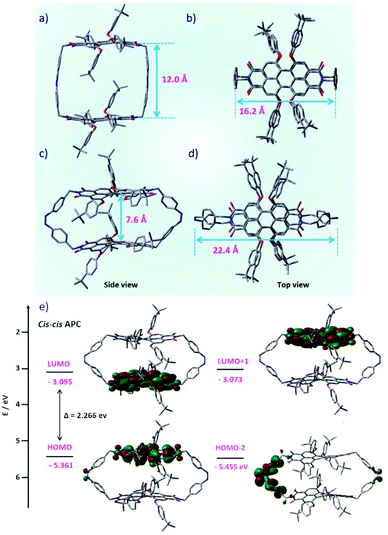
To gain insight into the molecular geometries and electronic structures of trans–transAPC and cis–cisAPC, theoretical calculations were carried out using density functional theory (DFT) at the B3LYP 6-31 G(d,p) level.43 The geometry-optimized structures are shown in Fig. 5. Accordingly, for the trans–trans isomer, the distance between the planes of the two PBI chromophores is about 12 Å (Fig. 5a), which explains the weak exciton coupling as is evident from the A0–0/A0–1 ratio in the absorption spectrum of trans-APC, which is slightly smaller than that of individual Ref-PBI (Table 1). For the cis–cis state, the distance between the two PBI planes is shortened to 7.6 Å (Fig. 5c), which is reflected in the further decrease of the A0–0/A0–1 ratio from 1.44 to 1.41 (Table 1). A comparison of the quantum yields of trans-APC and PSS-APC with the values obtained for PBI cyclophanes with a similar interchromophoric distance but without the presence of an azobenzene moiety28 reveals a strong decrease of the quantum yield for trans-APC and PSS-APC. Hence, the azobenzene moiety used by us leads to pronounced fluorescence quenching. We attribute this to an electron transfer from the azobenzene moiety to the photoexcited PBI moiety. An MO analysis (Fig. S14 and S15, ESI†) reveals pairwise orbitals due to slight differences in the respective PBI and Azo units. Both frontier orbitals, i.e., the HOMO (and HOMO−1) as well as LUMO (and LUMO+1) are located on the PBI subunits. However, the orbitals HOMO−2 and HOMO−3 of cis-APC, both of which have a large coefficient on the azobenzene moiety (Fig. 5e and Fig. S15, ESI†), are only ∼0.1 eV lower in energy than the HOMO (Fig. S13†), which is located at one of the PBI chromophores (Fig. 5e). Thus, considering conformational motions that are pronounced for both the cis-Azo and the tetraphenoxy-PBI44 subunits, an electron can be transferred from the azobenzene moiety to the PBI moiety after photoexcitation of PBI. However, the energy difference for trans-APC (∼0.5 eV, Fig. S13†) is larger than that for cis-APC. Therefore, while photo-induced electron transfer is the most likely origin of the strong fluorescence quenching of cis-APC, another quenching mechanism might be operative for trans-APC.
Because some PBI cyclophanes studied in our earlier work were excellent hosts for several polycyclic aromatic hydrocarbons26,45 and even for some alkaloids,29 we also performed UV-vis and fluorescence titration experiments with a variety of guests (Fig. S16 and S17†), e.g., naphthalene, 1,5-dimethoxynaphthalene, 1,1′-biphenyl, 1-phenylnaphthalene, anthracene, phenanthrene, and pyrene, for both trans-APC and PSS-APC states in dichloromethane. However, no evidence for binding was observed, which suggests that neither the cavities formed by trans- nor those by cis-azobenzene pillar units provide a suitable preorganization for the complexation of aromatic guest molecules.
Conclusions
In summary, an azobenzene-bridged perylene bisimide cyclophane was synthesized and characterized. Our spectroscopic studies in combination with theoretical calculations indicate a pronounced electronic communication between the azobenzene and PBI chromophores, as is evident from the significant fluorescence quenching of the PBI units. Notably, a photoisomerization of azobenzene is observed upon irradiation with UV light. The reversible photoswitching of the azobenzene chromophore under UV and visible light irradiation is accompanied by pronounced structural and electronic changes of APC, which significantly altered the cavity size of the macrocycle and the emission behaviour of the perylene bisimide chromophores. Accordingly, this work provides insights into the design of photoresponsive luminescent macrocycles and supramolecular elements based on PBI and azobenzene.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are thankful for the fund from the Chinese Academy of Sciences for supporting a visiting research of G. O. at Universität Würzburg.Notes and references
- J. M. Lehn, Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture), Angew. Chem., Int. Ed. Engl., 1988, 27, 89–112 CrossRef.
- Q. He, G. I. Vargas-Zúñiga, S. Kim, S. Kim and J. L. Sessler, Macrocycles as Ion Pair Receptors, Chem. Rev., 2019, 119, 9753–9835 CrossRef CAS.
- J. A. Faiz, V. Heitz and J. P. Sauvage, Design and Synthesis of Porphyrin-containing Catenanes and Rotaxanes, Chem. Soc. Rev., 2009, 38, 422–442 RSC.
- H. W. Schmidt and F. Würthner, A Periodic System of Supramolecular Elements, Angew. Chem., Int. Ed., 2020, 59, 8766–8775 CrossRef CAS.
- X. Yan, F. Wang, B. Zheng and F. Huang, Stimuli-responsive Supramolecular Polymeric Materials, Chem. Soc. Rev., 2012, 41, 6042–6065 RSC.
- C. D. Jones and J. W. Steed, Gels with Sense: Supramolecular Materials that Respond to Heat, Light and Sound, Chem. Soc. Rev., 2016, 45, 6546–6596 RSC.
- J. Boekhoven and S. I. Stupp, 25th Anniversary Article: Supramolecular Materials for Regenerative Medicine, Adv. Mater., 2014, 26, 1642–1659 CrossRef CAS.
- Y. Wang, H. Xu and X. Zhang, Tuning the Amphiphilicity of Building Blocks: Controlled Self–Assembly and Disassembly for Functional Supramolecular Materials, Adv. Mater., 2009, 21, 2849–2864 CrossRef CAS.
- D. B. Amabilino, D. K. Smith and J. W. Steed, Supramolecular Materials, Chem. Soc. Rev., 2017, 46, 2404–2420 RSC.
- B. Shirinfar, N. Ahmed, Y. S. Park, G. S. Cho, I. S. Youn, J. K. Han, H. G. Nam and K. S. Kim, Selective Fluorescent Detection of RNA in Living Cells by Using Imidazolium-Based Cyclophane, J. Am. Chem. Soc., 2013, 135, 90–93 CrossRef CAS.
- J. H. Tang, Y. Sun, Z. L. Gong, Z. Y. Li, Z. X. Zhou, H. Wang, X. P. Li, M. L. Saha, Y. W. Zhong and P. J. Stang, Temperature-Responsive Fluorescent Organoplatinum(II) Metallacycles, J. Am. Chem. Soc., 2018, 140, 7723–7729 CrossRef CAS.
- K. Mase, Y. Sasaki, Y. Sagara, N. Tamaoki, C. Weder, N. Yanai and N. Kimizuka, Stimuli–Responsive Dual–Color Photon Upconversion: A Singlet–to–Triplet Absorption Sensitizer in a Soft Luminescent Cyclophane, Angew. Chem., Int. Ed., 2018, 57, 2806–2810 CrossRef CAS.
- M. Dumartin, M. C. Lipke and J. F. Stoddart, A Redox-Switchable Molecular Zipper, J. Am. Chem. Soc., 2019, 141, 18308–18317 CrossRef CAS.
- S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Artificial Molecular Machines, Chem. Rev., 2015, 115, 10081–10206 CrossRef CAS.
- K. Kurihara, K. Yazaki, M. Akita and M. Yoshizawa, A Switchable Open/closed Polyaromatic Macrocycle that Shows Reversible Binding of Long Hydrophilic Molecules, Angew. Chem., Int. Ed., 2017, 56, 11360–11364 CrossRef CAS.
- H. Wu, Y. Chen, L. Zhang, O. Anamimoghadam, D. K. Shen, Z. C. Liu, K. Cai, C. Pezzato, C. L. Stern, Y. Liu and J. F. Stoddart, A Dynamic Tetracationic Macrocycle Exhibiting Photoswitchable Molecular Encapsulation, J. Am. Chem. Soc., 2019, 141, 1280–1289 CrossRef CAS.
- S. T. J. Ryan, J. del Barrio, R. Suardiaz, D. F. Ryan, E. Rosta and O. A. Scherman, A Dynamic and Responsive Host in Action: Light-Controlled Molecular Encapsulation, Angew. Chem., Int. Ed., 2016, 55, 16096–16100 CrossRef CAS.
- G. Szaloki, V. Croue, V. Carre, F. Aubriet, O. Aleveque, E. Levillain, M. Allain, J. Arago, E. Orti, S. Goeb and M. Salle, Controlling the Host-Guest Interaction Mode through a Redox Stimulus, Angew. Chem., Int. Ed., 2017, 56, 16272–16276 CrossRef CAS.
- F. Würthner, C. R. Saha-Möller, B. Fimmel, S. Ogi, P. Leowanawat and D. Schmidt, Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials, Chem. Rev., 2016, 116, 962–1052 CrossRef.
- C. Li and H. Wonneberger, Perylene Imides for Organic Photovoltaics: Yesterday, Today, and Tomorrow, Adv. Mater., 2012, 24, 613–636 CrossRef CAS.
- H. Langhals and R. Ismael, Cyclophanes as Model Compounds for Permanent, Dynamic Aggregates – Induced Chirality with Strong CD Effects, Eur. J. Org. Chem., 1998, 1915–1917 CrossRef CAS.
- J. Feng, Y. Zhang, C. Zhao, R. Li, W. Xu, X. Li and J. Jiang, Cyclophanes of Perylene Tetracarboxylic Diimide with Different Substituents at Bay Positions, Chem. – Eur. J., 2008, 14, 7000–7010 CrossRef CAS.
- K. E. Brown, W. A. Salamant, L. E. Shoer, R. M. Young and M. R. Wasielewski, Direct Observation of Ultrafast Excimer Formation in Covalent Perylenediimide Dimers Using Near-Infrared Transient Absorption Spectroscopy, J. Phys. Chem. Lett., 2014, 5, 2588–2593 CrossRef CAS.
- W. Kim, A. Nowak-Król, Y. Hong, F. Schlosser, F. Würthner and D. Kim, Solvent-Modulated Charge-Transfer Resonance Enhancement in the Excimer State of a Bay-Substituted Perylene Bisimide Cyclophane, J. Phys. Chem. Lett., 2019, 10, 1919–1927 CrossRef CAS.
- F. Schlosser, M. Moos, C. Lambert and F. Würthner, Redox–switchable Intramolecular π−π–Stacking of Perylene Bisimide Dyes in a Cyclophane, Adv. Mater., 2013, 25, 410–414 CrossRef CAS.
- P. Spenst and F. Würthner, A Perylene Bisimide Cyclophane as a “Turn–On” and “Turn–Off” Fluorescence Probe, Angew. Chem., Int. Ed., 2015, 54, 10165–10168 CrossRef CAS.
- D. Bialas, C. Brüning, F. Schlosser, B. Fimmel, J. Thein, V. Engel and F. Würthner, Exciton–Vibrational Couplings in Homo– and Heterodimer Stacks of Perylene Bisimide Dyes within Cyclophanes: Studies on Absorption Properties and Theoretical Analysis, Chem. – Eur. J., 2016, 22, 15011–15018 CrossRef CAS.
- J. Rühe, D. Bialas, P. Spenst, A.-M. Krause and F. Würthner, Perylene Bisimide Cyclophanes: Structure–Property Relationships upon Variation of the Cavity Size, Org. Mater., 2020, 02, 149–158 CrossRef.
- M. Sapotta, A. Hofmann, D. Bialas and F. Würthner, A Water-Soluble Perylene Bisimide Cyclophane as a Molecular Probe for the Recognition of Aromatic Alkaloids, Angew. Chem., Int. Ed., 2019, 58, 3516–3520 CrossRef CAS.
- M. Sapotta, P. Spenst, C. R. Saha-Möller and F. Würthner, Guest-mediated Chirality Transfer in the Host–guest Complexes of an Atropisomeric Perylene Bisimide Cyclophane Host, Org. Chem. Front., 2019, 6, 892–899 RSC.
- P. Spenst, R. M. Young, M. R. Wasielewski and F. Würthner, Guest and Solvent Modulated Photo-driven Charge Separation and Triplet Generation in A Perylene Bisimide Cyclophane, Chem. Sci., 2016, 7, 5428–5434 RSC.
- T. A. Barendt, W. K. Myers, S. P. Cornes, M. A. Lebedeva, K. Porfyrakis, I. Marques, V. Felix and P. D. Beer, The Green Box: An Electronically Versatile Perylene Diimide Macrocyclic Host for Fullerenes, J. Am. Chem. Soc., 2020, 142, 349–364 CrossRef CAS.
- J. Seibt, T. Winkler, K. Renziehausen, V. Dehm, F. Würthner, H. D. Meyer and V. Engel, Vibronic Transitions and Quantum Dynamics in Molecular Oligomers: A Theoretical Analysis with an Application to Aggregates of Perylene Bisimides, J. Phys. Chem. A, 2009, 113, 13475–13482 CrossRef CAS.
- F. C. Spano, The Spectral Signatures of Frenkel Polarons in H- and J-Aggregates, Acc. Chem. Res., 2010, 43, 429–439 CrossRef CAS.
- C. Zhang and N. Jiao, Copper-catalyzed Aerobic Oxidative Dehydrogenative Coupling of Anilines Leading to Aromatic Azo Compounds Using Dioxygen as An Oxidant, Angew. Chem., Int. Ed., 2010, 49, 6174–6177 CrossRef CAS.
- D. Dotcheva, M. Klapper and K. Müllen, Soluble Polyimides Containing Perylene Units, Macromol. Chem. Phys., 1994, 195, 1905–1911 CrossRef CAS.
- A. Nowak-Król and F. Würthner, Progress in the Synthesis of Perylene Bisimide Dyes, Org. Chem. Front., 2019, 6, 1272–1318 RSC.
- P. Osswald, M. Reichert, G. Bringmann and F. Würthner, Perylene Bisimide Atropisomers: Synthesis, Resolution, and Stereochemical Assignment, J. Org. Chem., 2007, 72, 3403–3411 CrossRef CAS.
- H. M. D. Bandara and S. C. Burdette, Photoisomerization in Different Classes of Azobenzene, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC.
- F. Würthner, T. E. Kaiser and C. R. Saha-Möller, J-aggregates: from Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials, Angew. Chem., Int. Ed., 2011, 50, 3376–3410 CrossRef.
- A. A. Beharry and G. A. Woolley, Azobenzene Photoswitches for Biomolecules, Chem. Soc. Rev., 2011, 40, 4422–4437 RSC.
- T. T. Nguyen, D. Turp, D. Wang, B. Nolscher, F. Laquai and K. Müllen, A Fluorescent, Shape-Persistent Dendritic Host with Photoswitchable Guest Encapsulation and Intramolecular Energy Transfer, J. Am. Chem. Soc., 2011, 133, 11194–11204 CrossRef CAS.
- M. J. Frisch, et al., Gaussian 16 Rev. C. 01 Search PubMed.
- E. Fron, G. Schweitzer, P. Osswald, F. Würthner, P. Marsal, D. Beljonne, K. Müllen, F. C. De Schryver and M. Van der Auweraer, Photophysical Study of Bay Substituted Perylenediimides, Photochem. Photobiol. Sci., 2008, 7, 1509–1521 RSC.
- P. Spenst, A. Sieblist and F. Würthner, Perylene Bisimide Cyclophanes with High Binding Affinity for Large Planar Polycyclic Aromatic Hydrocarbons: Host-Guest Complexation versus Self-Encapsulation of Side Arms, Chem. – Eur. J., 2017, 23, 1667–1675 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0qo01635g |
| This journal is © the Partner Organisations 2021 |