Recent progress in the synthesis of the furanosteroid family of natural products†
Abstract
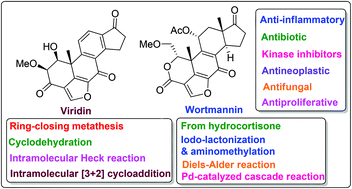
Furanosteroids are a class of novel pentacyclic fungal metabolites that share in common a furan ring, bridging at the 4 and 6 positions of the steroid skeleton. The strained furan ring is responsible for the rich biological activity attributed to these classes of compounds. Members of this class of natural products have attracted significant attention for many years due to their potent anti-inflammatory and antibiotic properties and more recently because of their potential antiproliferative activities. The unique steroidal scaffold of furanosteroids coupled with their exciting biological activities prompted tremendous efforts by chemists to develop efficient synthetic strategies for these targets. This review focuses on an overview of recent advances in the synthesis of furanosteroids and illustrates applications in medicinal chemistry over the period of 2005–present. In addition, a brief account of some significant furanosteroids and their structural congeners is presented with their sources and bioactivities in tabular format.
- This article is part of the themed collection: 2021 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...